INTRODUCTION
In the southeastern Baltic, only few radiocarbon (14C) ages related to the period of the Last Glacial-Interglacial Transition (LGIT) are available (Meadows et al. Reference Meadows, Eriksen, Zagorska, Dreves and Simpson2014; Girininkas et al. Reference Girininkas, Rimkus, Slah, Daugnora, Stančikaitė and Zabiela2016). This scarcity of 14C dates makes it challenging to construct a reliable chronological framework for the Final Palaeolithic/Early Mesolithic in the region. Obtaining new dates is thus an essential research priority. As stratified sites are rare in the region, accelerator mass spectrometry (AMS) dates obtained directly from osseous artifacts—even from stray finds—provide valuable data for the Final Palaeolithic/Early Mesolithic chronology and help to test existing artifact typologies and cultural histories. With this in mind, eight osseous artifacts housed at the Kaliningrad Regional Museum of History and Art were subjected to AMS dating.
The Kaliningrad Regional Museum of History and Art, the successor of Königsberg City Museum, houses a number of osseous artifacts that survived World War II. Prior to the 1940s, Königsberg City Museum owned a rich collection of organic archaeological artifacts, which had been collected as stray finds during the construction of drainage channels and the reclamation of swamps and marshes for land development in the second part of the 19th century and in the first part of the 20th century (e.g. Gaerte Reference Gaerte1929; Engel and La-Baume Reference Engel and La-Baume1937; Groß Reference Groß1937b, Reference Groß1937a, Reference Groß1938b, Reference Groß1938c, Reference Groß1938a, Reference Groß1939). Unfortunately, the museum burned down during World War II and was later completely demolished. The artifacts stored at the museum were damaged, destroyed, handled roughly or thrown away. But a few decades later, a number of artifacts were rediscovered in the ruins of the former museum (Königsberg Castle) and became museum objects once again. As a consequence, the condition of some of the finds is poor and detailed curatorial documentation is lacking: references to them can only be found in old publications.
Eight osseous artifacts from the collection that are believed to date to the Final Palaeolithic or Early Mesolithic based on raw material and/or typological assessment were chosen for AMS dating (Groß Reference Groß1939; Timofeev and Filippov Reference Timofeev and Filippov1981; Galiński Reference Galiński2013). These new AMS dates double the number of existing 14C dates; they provide improved fix-points for the chronology of the Final Palaeolithic/Early Mesolithic and shed new light on the earliest prehistory of the region.
MATERIALS AND METHODS
Osseous tools that had been collected at various sites in Russia (Kaliningrad district) and northeastern Poland (former Prussia) were sampled for AMS dating (Figures 1 and 2):

Figure 1 The sampled artifacts with the corresponding illustrations from previous publications: 1. AAR-26644, Antler shaft from Pluty, illustration after Okulicz Reference Okulicz1973 (17:d); 2. AAR-26645, Fragment of a lanceolate shaped bone point; corresponding illustration not found; 3. AAR-26646, Fragment of an antler axe found in the River Krutynia (former Cruttinna-Fluss), illustration after Šturms Reference Šturms1970 (4:4); 4. AAR-26647, Antler axe from Morąg (former Mitteldorf), illustration after Šturms Reference Šturms1970 (4:3); 5. AAR-26648, Fragment of an antler axe from Dobrowolsk (former Grenzfelde bei Pillkallen), illustration after Šturms Reference Šturms1970 (4:5); 6. AAR-26649, Fragment of a single-row slotted bone point; corresponding illustration not found; 7. AAR-26650, A uniserial harpoon from Orzysz (former Arys-See), illustration after Šturms Reference Šturms1970 (6:7); 8. AAR-26651, A worked reindeer antler found near Vysokoye (former Popelken), illustration after Šturms Reference Šturms1970 (2:2).
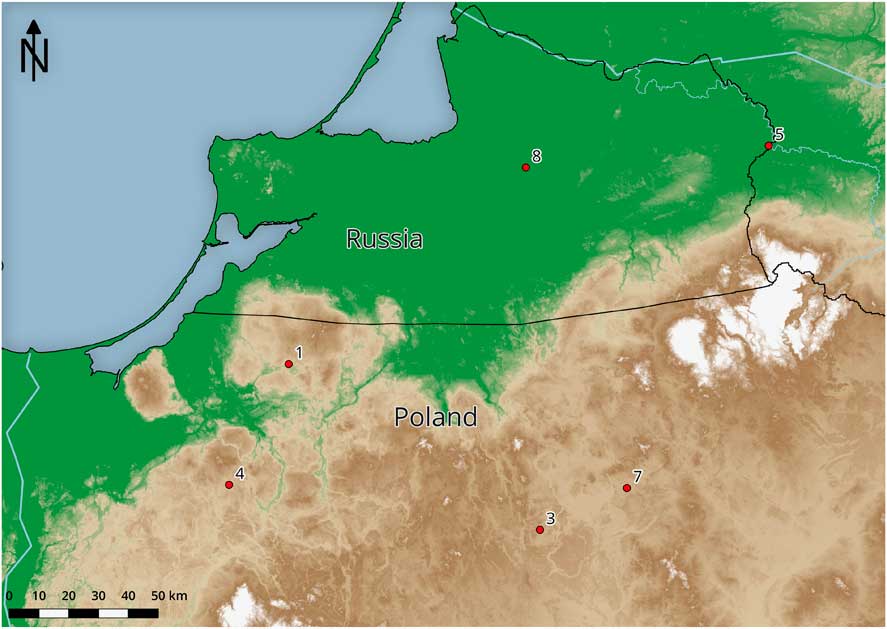
Figure 2 Map showing find locations of artifacts: 1. Pluty (AAR-26644); 2. Location for a lanceolate shape bone point is not identified (AAR-26645); 3. Krutynia (AAR-26646); 4. Morąg (AAR-26647); 5. Dobrowolsk (AAR-26648); 6. Location for a single-row slotted bone point is not known (AAR-26649); 7. Orzysz (AAR-26650); 8. Vysokoye (AAR-26651).
AAR-26644. Antler shaft (inv. no. 9951.2 1/15) or so-called bâton percé found close to Pluty in northeastern Poland in 1930 (Okulicz Reference Okulicz1973; Timofeev and Filippov Reference Timofeev and Filippov1981). Dimensions: L – 26.8 cm, W – 3.7 cm, T – 3.8 cm.
AAR-26645. Fragment of a lanceolate shaped bone point (inv. no. 16879.25). The tip of the point and the base of the tang are missing. It is not possible to identify the exact find location beyond the southeastern Baltic region (former Prussia). Close parallels are known from Kolosovka, Kaliningrad region (formerly Pentekinnen) and Kalniškiai, Lithuania (formerly Bachmann), as well as from Piecki (formerly Peitschendorf) and Koszajny (formerly Koschainen), Poland (Gaerte Reference Gaerte1929; Šturms Reference Šturms1970; Galiński Reference Galiński2013). Dimensions: L – 10.3 cm, W – 1.7 cm, T – 1 cm.
AAR-26646. Fragment of an antler axe (inv. no. 9951.3 1/15) of the problematically labeled “Lyngby type” (see Clausen Reference Clausen2004) found in the River Krutynia (formerly Cruttinna-Fluss) in 1933 (Groß Reference Groß1939; Timofeev and Filippov Reference Timofeev and Filippov1981). The larger part of the axe handle is missing. Dimensions: L – 21.5 cm, W – 16.2 cm, T – 4.5 cm.
AAR-26647. Antler axe (inv. no. 16879.1) from Morąg (formerly Mitteldorf), eastern Poland, found in a small bog at a depth of approximately 1.20 m while digging a drainage channel in 1934 (Groß Reference Groß1937b). Dimensions: L – 49.5 cm, W – 12 cm, T – 5.3 cm.
AAR-26648. Fragment of an antler axe (inv. no. 9951.4 1/15) from Dobrowolsk (former Pillkallen) found in an old riverbed at 2.5 m depth in 1933 (Groß Reference Groß1937b). Today, only the distal part of the handle remains. Dimensions: L – 34 cm, W – 5 cm, T – 3 cm.
AAR-26649. A fragment of a single-row slotted bone point (inv. no. 16879.17). The distal part with a tip and flint inserts are missing. The bone point is too fragmented to identify a secure match in the literature, but there are certain similarities to a specimen from Kornevo (formerly Zinten; see Šturms Reference Šturms1970). Dimensions: L – 12.1 cm, W – 1 cm, T – 0.5 cm.
AAR-26650. A uniserial harpoon (inv. no. 5007.16) found in Orzysz (former Arys-See) in Poland (Groß Reference Groß1939; Galiński Reference Galiński2013; Płonka Reference Płonka2017). When found, the harpoon was almost complete, 17 cm long with three well-pronounced barbs. Currently, the harpoon is broken into two parts, 10.5 and 4.5 cm long respectively, W – 2.1 cm, T – 0.8 cm.
AAR-26651. Worked reindeer antler (inv. no. 9951.1 1/15) found near Vysokoye (formerly Popelken). The antler has cut marks on the proximal end and a 14 cm long and 0.6 cm wide groove on one of the beams. Found on the bed of the River Melava in 1888 at ca. 2.35 m depth (Gaerte Reference Gaerte1929; Groß Reference Groß1939; Timofeev and Filippov Reference Timofeev and Filippov1981). Dimensions: L – 56 cm, W – 6 cm, T – 4 cm.
For sampling, pieces of bone or antler were sawn off with a jeweler’s saw or cut off with pliers. New saw blades were used for each sample. Pliers, tweezers and scalpels were cleaned with ethanol and precision wipes between samples. As some of the artifacts are exhibited in the museum, sampling was conducted as inconspicuously as possible. Furthermore, we avoided cut marks or diagnostic parts of the bone/antler to preserve as much information as possible for future studies. Consequently, some of the samples comprise broken edges, i.e. parts of the artifact where the collagen is expected to be less well-preserved than at the center of the artifact.
In the case of the slotted bone point, small pieces of tar were removed with a scalpel and tweezers for 14C dating. The sample amount needed for a 14C date is smaller for tar than for bone, and this sampling method does not affect the visual appearance of the tool. The antler/bone artifacts AAR-26646, 26647, 26648, 26650 and 26651 and the tar AAR-26649 had been treated with unknown preservative substances. They were therefore subjected to an extraction with organic solvents (hexane, acetone, ethanol) in an ultrasonic bath and rinsed thoroughly in MilliQ water before further pretreatment.
Bone and antler samples were demineralized over several days in 0.2M to 1M HCl at 4°C. The HCl was renewed at regular intervals (0.2M was used when the samples stayed in the acid over the weekend, 0.5M over a day and 1M for less than a day) until the dissolution was complete—i.e. CO2 effervescence had ceased and the solution did not show the density gradient caused by dissolved calcium phosphate. The demineralized bones were rinsed several times in MilliQ water before humic substances were removed with NaOH at 4°C until no more color change could be observed in the solution. After rinsing with MilliQ water to neutral, a weak HCl solution was applied to the samples and adjusted to pH 3. The samples were gelatinized (i.e., the collagen was dissolved) at 58°C overnight, and again over several days to extract as much collagen as possible. Samples were centrifuged and the supernatant (containing the dissolved collagen) was ultra-filtered in pre-cleaned Amicon Ultra Centrifugal Filters Ultracel -30K, made of regenerated cellulose (REF UFC803096, LOT R4CA36137). The<30kDa fraction is usually discarded but was kept in this study in addition to the>30kDa fraction for stable isotope measurements. In the case of AAR-26645, the sample was so small that it did not allow ultrafiltration and the entire collagen was dated. Finally, the extracted collagen was lyophilized.
The tar sample AAR-26649 from the slotted bone point 16879.17 was pretreated in 1M HCl for 1 hr to remove inorganic contamination such as carbonates, and in 1M NaOH for 3 hr to remove humic substances, both at 80°C. Lastly, the sample was subjected to 1M HCl at room temperature overnight to remove any CO2 absorbed during the NaOH treatment. Between and after reagents, the sample was rinsed in MilliQ water until neutral.
Samples were combusted in evacuated quartz tubes with 200 mg pre-cleaned CuO. They were graphitized using the H2 reduction method with an iron catalyst. AMS measurements were performed on the 1MV Tandetron AMS system from High Voltage engineering at the Aarhus AMS Centre (see Olsen et al. Reference Olsen, Tikhomirov, Grosen, Heinemeier and Klein2016 for details about graphitization and AMS measurements).
For stable isotope measurements, subsamples of bone collagen were weighed into tin capsules. They were combusted and analyzed with a Vario Cube elemental analyzer coupled to an IsoPrime stable isotope ratio mass spectrometer. δ13C and δ15N isotope ratios were determined relative to an internal standard (commercial gelatin) and reported as ‰VPDB and ‰AIR, respectively. Instrument precision was determined by the standard deviation of measurements of the internal standard to 0.10 ‰ in the case of δ13C and 0.10 to 0.14‰ in the case of δ15N. C:N atomic ratio, carbon and nitrogen fraction were measured with a precision of ±0.005 to±0.010.
RESULTS
The 14C and stable isotope results are given in Table 1. Sample AAR-26645 was so small that isotope measurements could not be performed. The tar sample AAR-26649 contained no measurable amounts of nitrogen, so δ15N, C:N ratio and nitrogen fraction are not available for this sample.
Table 1 14C dates and stable isotope values of the osseous artifacts. AMS dates were measured on the ultrafiltered (>30kDa) fractions, stable isotope data consists of averages of the>30kDa and<30kDa fractions. See text for details. 14C ages and uncertainties are rounded off to the nearest five 14C years. The last two columns indicate the expected dates based on artifact typology compared to the AMS dates.

All the samples show excellent quality of the bone and the extracted collagen. The Aarhus AMS Centre’s criteria for this are the following:
∙ Collagen yield (after ultrafiltration) at least 1–2%
∙ Carbon fraction around 35–45%
∙ Nitrogen fraction 13–16%
∙ C:N ratio close to 3.2
Samples AAR-26644, 26645, and 26649 had been expected to originate in the Early Holocene, and the other samples in the Late Pleistocene. Several samples are thus younger than expected.
Four of the five oldest samples were identified as reindeer. They have the highest δ13C and lowest δ15N values of the dataset. This agrees well with the expected environment and diet of Rangifer: as they feed on lichens, they tend to have higher δ13C and lower δ15N values than animals that feed on vascular plants (due to direct nitrogen fixation from the atmosphere). The 14C and stable isotope data also agree with a similar artifact from Lithuania, which was dated to 11,170±40 14C yr BP (BETA-403383, δ13C=–19.4‰, δ15N=1.0‰), and other δ13C values from the literature, e.g., –17.9 to –20.2‰ for Eastern Baltic reindeer (Ukkonen et al. Reference Ukkonen, Lõugas, Zagorska, Lukševica, Lukševics, Daugnora and Jungner2006; Girininkas et al. Reference Girininkas, Rimkus, Slah, Daugnora, Stančikaitė and Zabiela2016).
Curiously, two of the youngest artifacts are also described as reindeer in the literature: AAR-26644, an antler tool from Pluty, and AAR-26650, a uniserial harpoon from Orzysz (Timofeev and Filippov Reference Timofeev and Filippov1981; Galiński Reference Galiński2013). Both are substantially younger than the usual range of reindeer dates in the region (cf. Figures 4 and 6). The δ13C and δ15N values of these specimens indicate a diet based on vascular plants and a less cold climate and more developed soils. This is not impossible since, in the absence of lichens, reindeer will feed on vascular plants. Reindeer/caribou living in closed canopy environments tend to have lower δ13C values than those living in open environments (Drucker et al. Reference Drucker, Bridault, Hobson, Szuma and Bocherens2008). This is not only due to different diet. The δ13C values of lichen are also lower in forested than in open areas. For example, δ13C values measured on lichen from the Arctic tundra of Alaska were around –22.5‰, while from the boreal forest of Canada they were –25.0‰ (Drucker et al. Reference Drucker, Bridault, Hobson, Szuma and Bocherens2008). Furthermore, decreasing δ13C values during the Holocene, probably due to soil development or the canopy effect, have been observed for other species as well (Drucker et al. Reference Drucker, Bridault, Hobson, Szuma and Bocherens2008).
Figure 3 displays the 14C ages, δ13C and δ15N values of the six bone and antler samples for which stable isotope measurements were available. There are robust correlations between the different parameters. Decreasing δ13C values have been observed in previous studies of large herbivores, with developing forests causing the δ13C values of the vegetation to decrease due to soil development and the canopy effect (Drucker et al. Reference Drucker, Bridault, Hobson, Szuma and Bocherens2008 and references therein; Drucker et al. Reference Drucker, Bridault, Cupillard, Hujic and Bocherens2011). As the δ15N values of vegetation can be linked to soil conditions (Stevens et al. Reference Stevens, Jacobi, Street, Germonpré, Conard, Münzel and Hedges2008), the increasing δ15N values of the specimens in our study can be explained by the soils developing during the Holocene (Drucker et al. Reference Drucker, Bridault, Cupillard, Hujic and Bocherens2011). However, in our case, the trends are most probably due to the fact that the artifacts were made of different species: four were made of reindeer, and the other two of other species that lived on vascular plants.

Figure 3 Stable isotope values and 14C dates of the samples in this study, compared to the values from the literature (Ukkonen et al. Reference Ukkonen, Lõugas, Zagorska, Lukševica, Lukševics, Daugnora and Jungner2006; Girininkas et al. Reference Girininkas, Rimkus, Slah, Daugnora, Stančikaitė and Zabiela2016).
The calibrated 14C ages are shown in Figures 4 and 5. For comparison, Figure 4 also displays the age range of other artifacts from the southeastern Baltic region (Meadows et al. Reference Meadows, Eriksen, Zagorska, Dreves and Simpson2014; Girininkas et al. Reference Girininkas, Rimkus, Slah, Daugnora, Stančikaitė and Zabiela2016). In addition, the age ranges of reindeer remains from Estonia, Latvia and Lithuania (Ukkonen et al. Reference Ukkonen, Lõugas, Zagorska, Lukševica, Lukševics, Daugnora and Jungner2006) and from Europe (Sommer et al. Reference Sommer, Kalbe, Ekström, Benecke and Liljegren2014) are shown. The five oldest artifacts in our study span the entire range of reindeer remains in northeastern Europe (see also Figure 6), while the three youngest of our dates clearly fall outside the period of reindeer remains. This raises doubt with regard to the original osteological species determination of these artifacts. They will therefore be subjected to protein-based species identification.

Figure 4 Dating results from the study coupled with already existing dates from the eastern Baltic (Meadows et al. Reference Meadows, Eriksen, Zagorska, Dreves and Simpson2014; Girininkas et al. Reference Girininkas, Rimkus, Slah, Daugnora, Stančikaitė and Zabiela2016), and with the reindeer population distribution in the same area (Ukkonen et al. Reference Ukkonen, Lõugas, Zagorska, Lukševica, Lukševics, Daugnora and Jungner2006) and in Europe (Sommer et al. Reference Sommer, Kalbe, Ekström, Benecke and Liljegren2014). The uppermost panel shows the suggested Late Palaeolithic and Mesolithic culture-historical chronology for the region (Šatavičius Reference Šatavičius2001; Girininkas Reference Girininkas2009), while the lower panel shows Greenlandic ice core (NGRIP) δ18O temperature proxy values (Lowe et al. Reference Lowe, Rasmussen, Björck, Hoek, Steffensen, Walker and Yu2008; Rasmussen et al. Reference Rasmussen, Bigler, Blockley, Blunier, Buchardt, Clausen, Cvijanovic, Dahl-Jensen, Johnsen, Fischer, Gkinis, Guillevic, Hoek, Lowe, Pedro, Popp, Seierstad, Steffensen, Svensson, Vallelonga, Vinther, Walker, Wheatley and Winstrup2014).

Figure 5 Calibrated 14C ages of the five oldest artifacts from this study. The dates were calibrated in OxCal 4.3 (Bronk Ramsey Reference Bronk Ramsey2009) with the NHpine16 dataset (Hogg et al. Reference Hogg, Southon, Turney, Palmer, Ramsey, Fenwick, Boswijk, Büntgen, Friedrich, Helle, Hughen, Jones, Kromer, Noronha, Reinig, Reynard, Staff and Wacker2016a, Reference Hogg, Southon, Turney, Palmer, Bronk Ramsey, Fenwick, Boswijk, Friedrich, Helle, Hughen, Jones, Kromer, Noronha, Reynard, Staff and Wacker2016b), which is displayed in green. The IntCal13 calibration curve is shown in the background (in blue; please see electronic version for color figure.)

Figure 6 Distribution of 14C-dated reindeer remains from 25,000 to 10,700 cal BP (after Sommer et al.). Two circles at 8100 cal BP indicate the ages of a uniserial harpoon from Orzysz and an antler shaft from Pluty, showing that reindeer is unlikely to have been the raw material for these two tools. (Please see electronic version for color figure.)
When calibrating the older samples, we enter a part of the calibration curve IntCal13 that is not based on tree-ring measurements and therefore involves large uncertainties (Reimer et al. Reference Reimer, Bard, Bayliss, Beck, Blackwell, Bronk Ramsey, Buck, Cheng, Edwards, Friedrich, Grootes, Guilderson, Haflidason, Hajdas, Hatté, Heaton, Hoffmann, Hogg, Hughen, Kaiser, Kromer, Manning, Niu, Reimer, Richards, Scott, Southon, Staff, Turney and van der Plicht2013). The absolute tree ring chronology starts at 12,400 cal BP (10,450 cal BC), extended by a Swiss pine chronology to 12,594 cal BP (10,644 cal BC). The interval 14,000–12,594 cal BP of the calibration curve is only based on floating chronologies (Reimer et al. Reference Reimer, Bard, Bayliss, Beck, Blackwell, Bronk Ramsey, Buck, Cheng, Edwards, Friedrich, Grootes, Guilderson, Haflidason, Hajdas, Hatté, Heaton, Hoffmann, Hogg, Hughen, Kaiser, Kromer, Manning, Niu, Reimer, Richards, Scott, Southon, Staff, Turney and van der Plicht2013; Hogg et al. Reference Hogg, Southon, Turney, Palmer, Ramsey, Fenwick, Boswijk, Büntgen, Friedrich, Helle, Hughen, Jones, Kromer, Noronha, Reinig, Reynard, Staff and Wacker2016a). Older parts of the calibration curve are measured on macrofossils from lake sediments, marine foraminifera and corals, and speleothems (Reimer et al. Reference Reimer, Bard, Bayliss, Beck, Blackwell, Bronk Ramsey, Buck, Cheng, Edwards, Friedrich, Grootes, Guilderson, Haflidason, Hajdas, Hatté, Heaton, Hoffmann, Hogg, Hughen, Kaiser, Kromer, Manning, Niu, Reimer, Richards, Scott, Southon, Staff, Turney and van der Plicht2013). But even the parts of IntCal that are based on the absolute tree ring chronology are not unproblematic. For example, the 425-yr period between 12,325 and 11,900 cal BP (10,375–9,950 cal BC) only consists of 12 decadal 14C determinations (Reimer et al. Reference Reimer, Bard, Bayliss, Beck, Blackwell, Bronk Ramsey, Buck, Cheng, Edwards, Friedrich, Grootes, Guilderson, Haflidason, Hajdas, Hatté, Heaton, Hoffmann, Hogg, Hughen, Kaiser, Kromer, Manning, Niu, Reimer, Richards, Scott, Southon, Staff, Turney and van der Plicht2013; Hogg et al. Reference Hogg, Southon, Turney, Palmer, Ramsey, Fenwick, Boswijk, Büntgen, Friedrich, Helle, Hughen, Jones, Kromer, Noronha, Reinig, Reynard, Staff and Wacker2016a).
However, new 14C dates made on northern pine samples, covering the period 14,120–11,420 cal BP (12,170–9470 cal BC; Hogg et al. Reference Hogg, Southon, Turney, Palmer, Ramsey, Fenwick, Boswijk, Büntgen, Friedrich, Helle, Hughen, Jones, Kromer, Noronha, Reinig, Reynard, Staff and Wacker2016a; see also http://c14.arch.ox.ac.uk/NHPine16.14c), enable us to calibrate the dates with greater accuracy. Figure 5 shows the 14C dates of the five oldest artifacts calibrated with the NHpine16 dataset, on the background of the calibration curves IntCal13 (green) and NHpine16 (blue). However in our case, using NHpine16 does not change the calibrated dates themselves, only their precision, as our samples lie on parts of the curve where the deviation from IntCal13 is not substantial (Figure 5).
DISCUSSION
All dating attempts reported in this paper were successful and have provided five new Late Pleistocene and three new Early Holocene dates. Prior to this study, there was no secure evidence for hunter-gatherer presence in the region earlier than GI-1c-a (Siemaszko Reference Siemaszko1999; Ivanovaitė and Riede Reference Ivanovaitė and Riede2018). The worked antler from Vysokoye is now the earliest evidence of human presence in the Eastern Baltic after the Last Glacial Maximum (LGM). The obviously anthropogenically modified object suggests human presence in the southern part of the eastern Baltic at the end of GI-1e to GI-1d (Figure 4). Unfortunately, the worked antler is a stray find and cannot be related to any specific lithic assemblage, although there were attempts to connect the artifact to the Hamburgian culture (Rimantienė Reference Rimantienė1971; Okulicz Reference Okulicz1973; Szymczak Reference Szymczak1995). These attempts should be treated with caution, as modifications similar to the groove-and-split-technique found on the antler from Vysokoye are generally widespread antler-working techniques in the Final Palaeolithic and Mesolithic (Clark and Thompson Reference Clark and Thompson1954).
The antler axe from Dobrovolsk has provided the second-oldest date for the region within the period of GI-1c. It is the earliest dated implement of this type in the Eastern Baltic. It predates the Parupe antler axe from Lithuania by several hundred years (13,095–12,995 cal BP, Girininkas et al. Reference Girininkas, Rimkus, Slah, Daugnora, Stančikaitė and Zabiela2016) and is contemporaneous with the specimen from Klappholz LA 63 (Clausen Reference Clausen2004). The antler axes from Krutynia and Morąg are of younger age, i.e. the end of GS-1/beginning of the Holocene.
The presence of this tool form correlates well with the presence of a reindeer population in the area that ranges from 14,180–11,280 cal BP (Ukkonen et al. Reference Ukkonen, Lõugas, Zagorska, Lukševica, Lukševics, Daugnora and Jungner2006; see also Figure 4). A similar situation was observed in Northern Germany and Southern Scandinavia (Riede and Edinborough Reference Riede and Edinborough2012; Fischer et al. Reference Fischer, Clemmensen, Donahue, Heinemeier, Lykke-Andersen, Lysdahl, Fischer Mortensen, Olsen and Vang Petersen2013), highlighting that whenever Rangifer is present, tools made of their antlers can be expected to be found, by and large independently of which specific Final Palaeolithic technocomplex is present in the area. “Lyngby type” antler artifacts should thus be seen as a general and generic component of the Final Magdalenian (Federmessergruppen/arch-backed point groups) and its derivatives (Ahrensburgian/Swiderian) in Northern Europe.
The last of our Late Palaeolithic dates, obtained on the bone point AAR-26645, falls into GS-1. Until now, there have been no radiometric dates for this type of bone points from the region. Previous dating efforts relied on pollen analysis and suggested an age around the Late Pleistocene/Early Holocene boundary (Groß Reference Groß1939). Against this background, it has been suggested that this tool type could possibly be attributed to the Swiderian culture (Rimantienė Reference Rimantienė1996). The new and in this light surprisingly early AMS date has confirmed the object’s Late Pleistocene age, although it is again not possible to connect this tool type with any specific cultural taxonomical unit.
The antler shaft or so-called bâton percé from Pluty and the uniserial harpoon from Orzysz have provided younger dates than expected (Table 1). It has been suggested that the bâton percé from Pluty is made of reindeer antler and could date to the early Holocene (Timofeev and Filippov Reference Timofeev and Filippov1981); the newly obtained 14C date (8045–7870 cal BP) is much younger, however. This date corresponds to similar antler shafts from Poland and Southern Scandinavia, where these objects are dated to the Early Atlantic (Andersen Reference Andersen1981; Płonka Reference Płonka2003).
Surprisingly, the uniserial harpoon from Orzysz has also provided a young date of 8105–7935 cal BP. It has been suggested (by typological inference) that this harpoon with one row of well-pronounced barbs dates to the Late Palaeolithic-Early Mesolithic, and that reindeer bone was the raw material (Galiński Reference Galiński2013). The measured age, however, contradicts such an assessment. Uniserial harpoons with well-pronounced barbs are commonly thought to date to the Late Palaeolithic (Płonka Reference Płonka2017) and are often linked with the tanged point technocomplex (Kozłowski Reference Kozłowski2006), especially with the Ahrensburgian (Cziesla and Masojć Reference Cziesla and Masojć2007). Usually, harpoons with well-pronounced barbs are interpreted as being older than those with smaller barbs (Andersen and Petersen Reference Andersen and Petersen2009). However, previous dating efforts have already highlighted the wide temporal span of these barbed point and harpoon forms (Cziesla and Pettitt Reference Cziesla and Pettitt2003). While some of the directly dated objects of this artifact class are indeed of Late Pleistocene age (such as the harpoon from Węgliny, 14,145–13,780 cal BP; see Cziesla and Masojć Reference Cziesla and Masojć2007), the harpoon from Orzysz is much younger. The substantial temporal interval between the two harpoons of a rather similar design belies attempts at dating these by typological comparison (e.g. Galiński Reference Galiński2013). The dates obtained here raise the question of whether these two specimens could be made from reindeer bone and antler as previously suggested (Timofeev and Filippov Reference Timofeev and Filippov1981; Galiński Reference Galiński2013). It is thought that reindeer became extinct in the eastern Baltic in the transitional period from Late Pleistocene to Early Holocene (Ukkonen et al. Reference Ukkonen, Lõugas, Zagorska, Lukševica, Lukševics, Daugnora and Jungner2006; see above for a discussion of the information obtained by δ13C analyses, Figures 3, 4, and 6). In the future, protein-based species identification methods may be able to resolve such uncertainties.
The slotted bone point is dated to 7560–7430 cal BP and corresponds well with the wider chronology of the same object type in the region. By that time, slotted bone technology was already present in the Baltic region (Knutsson et al. Reference Knutsson, Knutsson, Molin and Zetterlund2016).
CONCLUSION
Our attempt at dating presumed Late Palaeolithic/Early Mesolithic osseous artifacts has been successful, providing eight new AMS dates—some surprising—for the southern part of the Eastern Baltic. These new dates not only significantly enlarge the existing 14C dataset for the region, but also include the hitherto earliest evidence for the presence of hunter-gatherers here. Attempts to explore the area after the last deglaciation can now be placed at the very end of GI-1e or the beginning of GI-1d. The dates of three antler axes and a bone point provide fix-points for hunter-gatherer presence in the region during the GI-1c-a and GS-1. The Late Pleistocene dates are concentrated at the end of the latter period, indicating a possible increase in the exploitation of the area by hunter-gatherers. The antler shaft, the uniserial harpoon and the slotted bone point date to the first half of the Holocene, indicating a Mesolithic affiliation. While the date for the slotted bone point fits well with the general chronology of this tool in the Baltic area, the two dates of the antler shaft from Pluty and the uniserial harpoon from Orzysz are much younger than expected on the basis of typological inference. These newly obtained dates also raise doubts as to whether reindeer bone/antler could have been used as the raw material for making these two specimens. The dating results of the antler shaft and the uniserial harpoon are not in agreement with previous attempts to date these items based on established typological assessment. This emphasizes the need to review existing classifications based on typology and type-based comparisons with other regions (see also Ivanovaitė and Riede Reference Ivanovaitė and Riede2018). As a result, the new dates reported here stimulate novel ways of viewing the Final Palaeolithic and Early Mesolithic chronology in the southeastern Baltic and shed a new light on the earliest prehistory of the region.
ACKNOWLEDGMENTS
BP is supported by the Danish National Research Foundation under the grant DNRF119 - Centre of Excellence for Urban Network Evolutions (UrbNet). FR and LI are supported by the Independent Research Fund Denmark grant # 6107-00059B.









