INTRODUCTION
Dinoflagellates are considered one of the main groups of marine phytoplankton (Matsuoka and Fukoyo Reference Matsuoka and Fukuyo2000; Dale Reference Dale2009; Naidu et al. Reference Naidu, Patil, Narale and Anil2012). They can form cysts and remain dormant in order to survive. Preserved cysts are great indicators of environmental variations (Fensome et al. Reference Fensome, Taylor, Norris, Sarjeant, Wharton and Williams1993). Dinocysts assemblages from coastal embayment and estuarine systems have been used as bioindicators of domestic and industrial pollution, and eutrophication events (Pospelova et al. Reference Pospelova, Chmura, Boothman and Latimer2002). They are also great indicators of paleoproductivity and paleoclimate during the Holocene (Mudie et al. Reference Mudie, Harland, Matthiessen and de Vernal2001; Naidu et al. Reference Naidu, Patil, Narale and Anil2012) and as bioindicators of sea level variations (van Soelen et al. Reference van Soelen, Lammerstma, Cremer, Donders, Sangiorgi, Brooks, Larson, Damsté, Wagner-Cremer and Reichart2010) in marine and estuarine sediments.
Since they can be preserved over long periods of time, dinocysts are used as material for palaeoenvironmental reconstructions in the Quaternary period (Matthiessen et al. Reference Matthiessen, Schreck, Schepper and Zorzi2018) and are important ecological indicators (Dale Reference Dale, Jansonious and McGregor1996). Bays and estuaries are geologically important areas in which sediments are deposited and preserved (Figueiredo et al. Reference Figueiredo, Toledo, Cordeiro, Godoy, Silva, Vasconcelos and Santos2014), providing a record and valuable data for micropaleontological studies focusing on the evolution and palaeoenvironmental reconstruction. There are many gaps in the fossil record of dinoflagellates in the sediments of bays and estuaries in Brazil (Oliveira et al. Reference Oliveira, Mendonça Filho, Oliveira and Iemini2007). However, they are widely studied on the continental shelf and slope regions (Santos et al. Reference Santos, Carvalho, Oliveira and Mendonça Filho2017). In the Brazilian State of Espírito Santo, dinocysts have been observed in pond and delta sediments (Ferrazo et al. Reference Ferrazo, Bauermann and Leipnitz2008; Lorente et al. Reference Lorente, Pessenda, Obooh-Ikuenobe, Buso Júnior, Cohen, Meyer, Giannini, Oliveira, Rosseti and Borotti Filho2014). Against this backdrop, the main objective of the present study was to reconstruct and interpret the hydrodynamics in the region surrounding the Vitória Bay during the Holocene.
STUDY AREA
Vitória Bay is located between 20º15'S–40º22'W and 20º20'S–40º16'W in the Southeast of Brazil (Figure 1). It is characterized as a semi-enclosed body of water bounded by an elevation of the Barreiras Formation to the north and by Pre-Cambrian elevations to the south. The bay is a well-sheltered estuarine system (Bastos et al. Reference Bastos, Vilela and Quaresma2010; Veronez et al. Reference Veronez, Bastos and Quaresma2009). Martin et al. (Reference Martin, Suguio, Flexor and Archanjo1996) point out that the municipality of Vitória is located in an area where crystalline outcrops reach the coastline, favoring the formation of a truncated coastline.

Figure 1 Localization map from the three sediment core (T_A, T_C and T_D) collection point located at Passagem channel, Espírito Santo State, Brazil (ArcGis 9.3; SIRGAS_2000_Zone_24S).
The total area of Vitoria Bay extends over the region from the Camburi beach in the Bay of Espírito Santo as far as the Santa Maria da Vitória (SMV) river, the largest tributary bringing continental water to the interior of the bay. The bay is connected directly to the ocean by two channels; the Porto channel and the Passagem channel (Lima et al. Reference Lima, Soares and Bonicenha1994). The Porto channel is the link between Vitória Bay and Espírito Santo Bay, with an average depth of 13 m and reaching 20 m in deeper areas (Corrêa et al. Reference Corrêa, Elias, Martins and Ketzer1993). The Passagem channel is a natural link between Espírito Santo Bay to the north and Victoria Bay to the south. It is approximately 10 km long and 80 m wide on average (Rigo and Chacaltana Reference Rigo and Chacaltana2006). The bathymetry of the area is variable, with depths during low tide ranging from 1 m to 9 m. The channel has average depth of 6 m over its entire length (Nascimento et al. Reference Nascimento, Chacaltana and Piccoli2013) and is an area of low hydrodynamics (Rigo and Chacaltana Reference Rigo and Chacaltana2006).
The vegetation around Vitória Bay is mainly mangrove, and the most conserved area is next to the Passagem channel. According to Rigo and Chacaltana (Reference Rigo and Chacaltana2006), this vegetation considerably affects water current speeds. Narrower areas inside Vitória Bay are conducive to more intense currents that control sedimentation along the estuary. In general, the distribution of the bottom sediment near Espírito Santo Bay is mainly sand of marine origin, and fluvial deposits are located in the areas near the mouth of the Santa Maria da Vitória river. Sandy sediment has built up in other areas and is associated with channel narrowing and the presence of carbonates (Veronez et al. Reference Veronez, Bastos and Quaresma2009).
MATERIAL AND METHODS
Sediment Core Collection
Three sediment cores (T_A, T_C and T_D) were collected with a vibracorer on the banks of the Passagem channel. The 203-cm T_A sediment core was collected at 20°15'13"S–40°17'38"W. This location is in the most sheltered part of the estuary. The 490-cm T_C sediment core was collected at 20°14'48"S–40°18'23"W, upstream of the estuary. The 430-cm T_D sediment core was collected at 20°27'65"S–40°31'06"W, downstream of the estuary (Figure 1). The cores were split into two halves and described in terms of thickness, grain size, structure, color, appearance, and the presence of shells. The cores were sliced into 10 cm pieces and stored at 4°C.
Particle Size Analysis
The analysis was based on the grain size scale proposed by Wentworth (Reference Wentworth1922). Sand fractions were analyzed by dry sieving and mud fractions by laser diffraction. The calculations for statistical grain size distribution parameters proposed by Folk and Ward (Reference Folk and Ward1957) were applied to the percentiles of each fraction.
Radiocarbon Dating
Preserved shells were selected from the three cores for 14C dating. In addition, organic sediment samples were selected from the T_A and T_C cores for radiocarbon dating. The shells were dated at the Center for Applied Isotope Studies, University of Georgia, USA, and organic sediment samples were dated at Beta Analytic Inc, Florida, USA. Calibrated ages (BP) were calculated by Calib 7.1 software, using the Marine13 curve at 2 sigma and ΔR = 67 ± 33 (Macario et al. Reference Macario, Alves, Chanca, Oliveira, Carvalho, Souza, Aguilera, Tenório, Rapagnã, Douka and Silva2016) (Table 1).
Table 1 Dated samples by the 14C method of the sediment cores (T_A, T_C and T_D) collected in the Passagem channel, Espírito Santo State, Brazil.

a Center for Applied Isotope Studies, University of Georgia, USA.
b Beta Analytic, Florida, USA.
Geochemical Analysis (TOC, δ15N, δ13C)
For geochemical analysis, approximately 1 g of dry sediment was taken at depth intervals of 30 cm from each sediment core (T_A, T_C, T_D) and weighed. The analysis was carried out at the Stable Isotope Facility, UC Davis, University of California, USA, in order to obtain isotopic values for δ15N, δ13C and TOC (total organic carbon).
Palynological Sample Preparation
To examine for dinocysts, 1 g of dry sediment for each 10 cm of depth was removed from the three sediment cores. The material was subjected to the standard chemical treatment proposed by Mertens et al. (Reference Mertens, Bradley, Takano, Mudie, Marret, Aksu, Hiscott, Verleye, Mousing and Smyrnova2012) for recent sediments. In order to determine the accumulation of cysts, one exotic spore of Lycopodium clavatum (Batchnr. 1031) with 20848 spores per tablet was added (Stockmarr Reference Stockmarr1971). Next, the samples were acidified with hydrochloric acid (10%) and then hydrofluoric acid (40%), and sieved through a 10-μm mesh. The cysts were counted and identified using standard reference catalogs (Lewis et al. Reference Lewis, Rochon and Harding1999; Rochon et al. Reference Rochon, de Vernal, Turon, Mathiessen and Head1999; Matsuoka and Fukuyo Reference Matsuoka and Fukuyo2000; Radi et al. Reference Radi, Pospelova, de Vernal and Barrie2007; Zonneveld and Pospelova Reference Zonneveld and Pospelova2015). Percentage and accumulation diagrams were produced using TILIA and CONISS software (Grimm Reference Grimm1987).
RESULTS
Particle Size Analysis
The T_A core consisted of a sediment of medium sand from the base up to a depth of 150 cm. This was followed by muddy sediment (silt) with plant fragments and preserved shells to the top of the core. From a depth of 50 cm to the top, the core consisted of sandy mud formed by fractions of medium sand to silt. The T_C core consisted of sandy mud from the base up to a depth of 480 cm. From this point onwards, it consisted of muddy sediment with preserved, fragmented shells up to a depth of 300 cm, followed by sandy mud with shell fragments, and from a depth of 290 cm to the top, a brown mud with fragments of plant material. The lithology of the T_D core was similar to that of the T_A core. From its base up to a depth of 30 cm, fine to medium sandy sediment was observed, with preserved, fragmented shells. From 30 cm to the top, the core consisted of sandy mud with fragments of plant material (Figures 2, 4 and 6).

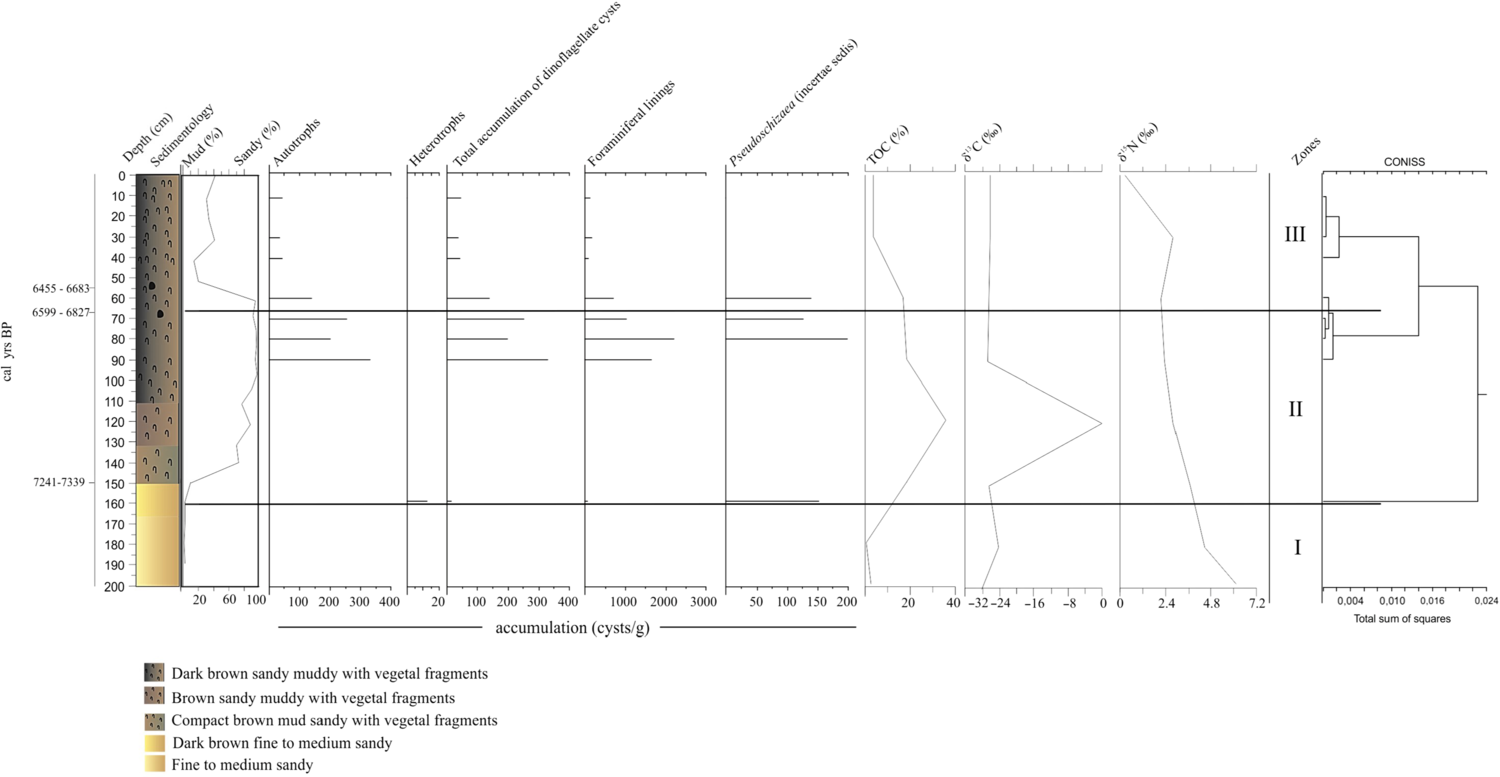
Figure 2 Accumulation diagram (cysts/g) from the identified dinoflagellate cysts; TOC (%) and isotopic data (C and N) in the analyzed sediment core (T_A).
Radiocarbon Dating
Eight 14C radiocarbon samples were obtained for the three sediment cores studied (Table 1). The oldest (7241–7339 cal yr BP) was for the T_A core at a depth of 150 cm. Similarly, the organic sediment in the T_C core was dated at 9396–9520 cal yr BP at a depth of 150 cm. The age of the T_D core was 6295–6505 cal yr BP at a depth of 370 cm, obtained by analyzing a preserved shell of Natica livida Pfeiffer.
Geochemical Analysis (δ15N, δ13C, TOC)
TOC in the T_A core ranged from 0.37 to 36%. For all cores analyzed, the highest percentage value was observed at a depth of 120 cm. δ13C results ranged from 28 to 0.0 ‰. High values were observed between depths of 200 and 150 cm (28.04 and 26.5 ‰). From 90 cm to the top, the most common value was 26.5‰. Values of δ15N were higher towards the base of the core, ranging from 6.39 to 4.46 ‰, subsequently dropping at locations closer to the top of the core (Figure 2).
TOC values in the T_C core ranged from 0.04 to 10%. The highest percentage value found in all cores analyzed was at a depth of 150 cm. δ13C values ranged from 14 to 27 ‰, with the highest value at the base of the sediment core. δ15N values ranged from 0.0 to 5.22 ‰, with the highest values at depths of 360 and 330 cm (Figure 4). TOC values from the base to the top of the T_D core ranged from 6.8 to 14.8%. The highest value (40.8%) was found at a depth of 30 cm. δ13C values were stable throughout the core (28.18 to 27.41 ‰) with only minor variations. The δ15N data indicated a variation of 5.8 to 4.8 ‰ from the base to the top of the core (Figure 6).
T_A SEDIMENT CORE
Based on the results obtained from the T_A core, three main zones were established (Figures 2, 3).

Figure 3 Frequency diagram (%) from the identified dinoflagellate cysts; TOC (%) and istopic data (C and N) in the analyzed sediment core (T_A).
Zone I: depth of 200–160 cm
No preserved dinocysts were observed in this zone (Figure 2).
Zone II: depth of 160–55 cm
The total accumulation of dinocysts was low at the base of this zone (depth of 160 cm, 12 cysts/g), and only heterotrophs with cysts of Brigantedinium spp. were preserved at the base of the zone. At a depth of 90 cm, there was an increase in the total accumulation of dinocysts but this tended to fall off towards the top of the zone (from 330 to 139 cysts/g) (Figure 2). Autotrophs were represented by Operculodinium centrocarpum (90–70 cm), decreasing by up to 50%, followed by Spiniferites ramosus at a depth of 70 cm (50%) (Figure 3).
Foraminiferal test linings were first detected at the base of this zone (63 palynomorphs/g), together with Pseudoschizaea (incertae sedis) (152 palynomorphs/g). At a depth of 90 cm, foraminiferal test linings reappeared and subsequently decreased in number (from 1650.3 to 696.8 palynomorphs/g). The highest accumulation was observed at a depth of 80 cm (2189 palynomorphs/g), along with the reappearance of Pseudoschizaea (incertae sedis), which showed the highest accumulation observed throughout the entire study (depth of 80 cm), falling off towards the top of the zone (from 199 to 139 palynomorphs/g) (Figure 2).
Zone III: depth of 55 cm to the top of the core
No preserved heterotrophic organisms were detected in this zone. Autotrophic taxa were observed at low accumulations, gradually decreasing, as in the previous zone. The number of cysts (41 to 44 cysts/g) remained consistent from a depth of 40 cm to the top of the zone (Figure 3). The predominant cycts were O. centrocarpum at a depth of 10 cm (Figure 3). Foraminiferal test linings followed the downward trend observed in the previous zone, with a slight increase towards the top of the core (83–132 palynomorphs/g) (Figure 3).
T_C SEDIMENT CORE
Based on the results obtained for the T_C core, three main zones were established (Figures 4, 5).

Figure 4 Accumulation diagram (cysts/g) from the identified dinoflagellate cysts; TOC (%) and istopic data (C and N) in the analyzed sediment core (T_C).

Figure 5 Frequency diagram (%) from the identified dinoflagellate cysts; TOC (%) and istopic data (C and N) in the analyzed sediment core (T_C).
Zone I: depth of 490–290 cm
The base of zone I (depth of 490 cm) showed low cyst accumulation with a tendency to increase towards the top of the zone (43 to 204 cysts/g). Throughout this zone, the highest occurrence of dinocysts recorded consisted of autotrophs (43–102 cysts/g), with the highest value found at a depth of 400 cm (480 cysts/g). Heterotroph taxa were not present at the base of this zone and began to appear at a depth of 470 cm (93 cysts/g), remaining consistent along zone I. The highest accumulation in this zone was found at a depth of 350 cm (284 cysts/g) (Figure 4). At the base of the zone there was a significant number of preserved Pseudoschizaea (incertae sedis). This taxon was the most abundant in the core at a depth of 480 cm (533.4 palynomorphs/g). No foraminiferal test linings were observed at the base of the zone. They began to appear from a depth of 480 cm, gradually increasing towards the top of the zone (11.9–3033.3 palynomorphs/g) (Figure 4).
O. centrocarpum was the predominant autotroph taxon, with variations from the base to the top of the zone (100 to 0%), followed by Lingulodinium machaerophorum that appeared between depths of 480 and 350 cm (33–8 cysts/g) (Figure 5). Other autotroph taxa were present in this zone at a lower frequency, and in particular Spiniferites spp. (33%), Spiniferites hyperacanthus (14.3–20%) and Tuberculodinium vancampoae (11–16%). The most frequent heterotroph taxon in this zone was Brigantedinium spp. (28–16%). Other cysts were also observed (Polykrikos kofoidii 16.7% and Protoperidinium sp. 14%). Incertae sedis (Pseudoschizaea) was well represented along this zone (2–25%), with the highest value at a depth of 480 cm. Foraminiferal test linings were consistently present (33–15%), with the highest value (96%) at a depth of 320 cm. (Figure 5).
Zone II: depth of 290–60 cm
In this zone there was a tendency for the total accumulation of dinocysts to increase compared to the top of the previous zone (300 cm depth) (204–716 cysts/g). However, the highest accumulation was found at a depth of 260 cm (2821 cysts/g) (Figure 4). This was attributed to the fact that autotroph taxa (2508 cysts/g) were highly preserved. Then there was a sudden drop and subsequent increase towards the top of the zone (193–657 cysts/g). In contrast, values for heterotroph taxa were lower than those of the autotrophs. In general, there was a gradual increase with small oscillations between depths of 290 and 90 cm (102–708 cysts/g), subsequently dropping towards the top of the zone (290–59 cysts/g). The highest accumulation was observed at a depth of 180 cm (1194 cysts/g) (Figure 4).
The highest values for foraminiferal test linings were found at the base of the zone, taking the core as a whole (3033 palynomorphs/g), with a subsequent drop (771cysts/g) and variations between depths of 270–60 cm (1229–179 cysts/g). The only occurrence of Botryococcus algae (266.9 palynomorphs/g) was observed at a depth of 170 cm. The highest value for Pseudoschizaea incertae sedis was found in this zone at a depth of 210 cm (209 palynomorphs/g) (Figure 4). The highest value for O. centrocarpum was found at a depth of 190 cm, with a tendency to drop between depths of 160 and 60 cm (15–91%); T. vancampoae was the next most frequent, with a high (50%) at a depth of 200 cm (Figure 5). Among the heterotroph taxa, Brigantedinium spp. was found throughout the entire zone. Cysts of Brigantedinium spp. showed a tendency to decrease between depths of 290 and 70 cm (16–50%), dropping further towards the top of the zone. Foraminiferal test linings were present throughout the zone, with higher values near the base at depths of 280–260 cm (80–77%) and at 190–170 cm (90–50%). Botryococcus algae were observed at a depth of 170 cm (50%) and the highest values for Pseudoschizaea (incertae sedis) were found at a depth of 210 cm (33%). (Figure 5).
Zone III: depth of 60 cm up to the top of the sediment core
The total accumulation of dinocysts decreased compared to the zone II, with a tendency to increase from a depth of 10 cm towards the top of the core (2366 to 4732 cysts/g) (Figure 4). Autotroph and heterotroph taxa showed the same pattern as that observed in the total accumulation. Fewer autotrophs were preserved at the base of this zone, compared with the zone II. An increase was observed from a depth of 10 cm towards the top of the core (887 to 1774 cysts/g). Higher values were also found for heterotrophs between a depth of 10 cm and the top of this zone (1124 to 2248 cysts/g). Foraminiferal lining values ranged from 179 to 0 palynomorphs/g along this zone. Pseudoschizaea (incertae sedis) was recorded at a depth of 40 cm (25 palynomorphs/g) (Figure 4).
In this zone, the most prominent autotroph dinocysts were O. centrocarpum and T. vancampoae. O. centrocarpum was found from the base to the top of the zone (25 to 0%) and the highest value was found at a depth of 20 cm (55%). Values for T. vancampoae ranged from 0 to 15% between a depth of 50 cm and the top of the core, with the highest value observed at a depth of 30 cm (62%). Among the heterotroph taxa, there was a tendency for Brigantedinium spp. to increase from 0 to 42% towards the top of this zone (Figure 5). Foraminiferal test linings were present, ranging from 47 to 0%, and Pseudoschizaea (incertae sedis) recorded (5%) at a depth of 40 cm (Figure 5).
4.5 T_D SEDIMENT CORE
Based on the results obtained for the T_D sediment core, three main zones were established (Figures 6, 7).

Figure 6 Accumulation diagram (cysts/g) from the identified dinoflagellate cysts; TOC (%) and istopic data (C and N) in the analyzed sediment core (T_D).

Figure 7 Frequency diagram (%) from the identified dinoflagellate cysts; TOC (%) and istopic data (C and N) in the analyzed sediment core (T_D).
Zone I: depth of 430–260 cm
A low accumulation of dinocysts (4 to 11 cysts/g) was observed throughout this zone. Both autotroph and heterotroph taxa were identified. Autotrophs ranged from 4 to 0 cysts/g, with the highest value at 300 cm (41 cysts/g). There were no preserved heterotrophs along this zone, except for one sample at the top of the zone (11 cysts/g). Values for foraminiferal test linings ranged from 47 to 303 palynomorphs/g. Pseudoschizaea (incertae sedis) was found in two samples and the highest value (6 palynomorphs/g) was found at 270 cm (Figure 6).
Among the autotroph taxa, O. centrocarpum was the most frequent, observed from the base to the top of this zone (depths of 430–260 cm). Operculodinium israelianum was observed in only one sample (320 cm), followed by Brigantedinium spp., the only representative of heterotroph taxa, observed at the top of the zone. Foraminiferal test linings were found from the base to the top of the zone, usually at a frequency of 100%. Values of 100% were found for Pseudoschizaea (incertae sedis) near the base and a lower percentage near the top of this zone (33%) (Figure 7).
Zone II: depth 260–130 cm
A low accumulation of dinocysts was observed in this zone. Values ranged from 11 to 23 cysts/g. However, the accumulation value peaked at a depth of 130 cm (121 cysts/g), compared to the rest of the zone (Figure 6). Cysts of autotroph taxa were recorded in this zone at depth of 190–130 cm, with low accumulation values (8 to 14 cysts/g). However, at a depth of 130 cm, a high accumulation value was identified within this group (121 cysts/g) (Figure 6). Heterotrophs were present in samples analyzed from the base to the top of the zone, but at lower accumulations (11 to 9 cysts/g) compared to autotroph taxa (Figure 6). The accumulation of foraminiferal test linings tended to gradually decrease (303–33 palynomorphs/g) throughout the entire zone (from 260 to 130 cm). Pseudoschizaea (incertae sedis) was also observed at a depth of 140 cm (3 palynomorphs/g) (Figure 6).
Spiniferites spp. was found at depths from 190 to 140 cm with values between 33 to 66%. Similarly, cysts of O. centrocarpum were present at depths of 170–130 cm, with values ranging from 100 to 33% (Figure 7). L. machaerophorum was observed only between depths of 150 and 130 cm, with values ranging from 33 to 20%. T. vancampoae was observed in two samples (depths of 190 and 160 cm) at frequencies of 33 and 100% respectively (Figure 7). Heterotroph taxa were represented in this zone by Brigantedinium spp., which was found in two samples (260 and 200 cm). Foraminiferal test linings were present throughout the zone, occurring in most analyzed samples at frequencies of 100 to 14% (from the base to the top of the zone). Only one sample contained Pseudoschizaea (incertae sedis) (140 cm, 33%) (Figure 7).
Zone III: depth 130 cm to the top of the sediment core
In this zone, total dinocyst abundance gradually increased towards the top of the core (23–65 cysts/g) (Figure 6). Autotrophs were found from the base of the zone up to a depth of 70 cm (14–11 cysts/g). A new occurrence of this group was observed at the top of the core (0 cm depth) (65 cysts/g) (Figure 6). Heterotrophs were found near the base of this zone (130 cm depth) but at low accumulation (9 cysts/g) and were subsequently observed near the top of the zone (60–20 cm; 32.4–76 cyst/g) (Figure 6). Foraminiferal test linings were recorded at low accumulation near the base of the zone (33 cysts/g), increasing slightly at a depth of 120 cm (200 cysts/g) and towards the top of the core (25–130 cysts/g) (Figure 6).
Autotroph taxa present in this zone included O. centrocarpum, found from the base of the zone up to 70 cm and ranging from 40 to 100% (Figure 7). Spiniferites spp. was observed in two samples (depth of 120 and 100 cm) with values of 50 and 33% respectively. S. hyperacanthus was also observed in this zone (33%), followed by T. vancampoae (100%) at the top of the sediment core. The heterotroph Brigantedinium spp. was found (Figure 7) in two samples (depth of 60 and 20 cm) with values of 100%. Low percentages (14%) of foraminiferal test linings were recorded near the base of the zone. These organisms increased in frequency at a depth of 120 cm (100%) and subsequently decreased towards the top of the core (40–20%) (Figure 7).
DISCUSSION
The oldest sedimentary record was observed in the T_C core (9396–9520 cal yr BP). In the stratum just below this date (490 cm depth), there was a low accumulation of dinocysts, associated with the non-preservation of foraminiferal test linings. A higher accumulation of Pseudoschizaea (incertae sedis) was found throughout the three analyzed sediment cores. This fact, together with the sandy mud lithology and low TOC value may indicate a fluvial influence (Freitas et al. Reference Freitas, Barreto, Bastos and Baptista-Neto2017). Scott (Reference Scott1992) points out that this incertae sedis (Pseudoschizaea) is characteristic of wetland environments. This interpretation is further corroborated by the less negative δ13C isotope values. The values recorded show that, based on the T_C sediment core, C4 (herbaceous) plants predominated, and the genus Cyperaceae was its predominant representative, characteristic of wetland areas (Sritrairat et al. Reference Sritrairat, Peteet, Kenna, Sambrotto, Kurdyla and Guilderson2012; Yang et al. Reference Yang, Siegwolf and Komer2015).
O. centrocarpum and L. machaerophorum (autotrophs) were well represented throughout zone I in the T_C core. O. centrocarpum is characterized as tolerant to different salinities, and is associated with transitional environments between the coastal and oceanic regions (Wall et al. Reference Wall, Dale, Lohmann and Smith1977; Dale Reference Dale2009; Marret and Zonneveld Reference Marret and Zonneveld2003; Zonneveld et al. Reference Zonneveld, Marret, Versteegh, Bogus, Bouimetarhana, Crouch, de Vernal, Elshanawany, Esper and Forke2013). This species is also observed in estuaries located in the North Atlantic (Price et al. Reference Price, Baustian, Turner, Rabalais and Chmura2017) and in areas influenced by warm currents in southern Brazil (Gu et al. Reference Gu, Zonneveld, Chiessi, Arz, Patzold and Behling2017).
On the other hand, L. machaerophorum is sensitive to nutrient increase. This increase was reported by Poliakova et al. (Reference Poliakova, Zonneveld, Herbeck, Jennerjahn, Permana, Kwiatkowski and Behling2017) as a result of continental nutrient inputs which are also observed in the warm water environments of temperate to tropical coastal regions (Zonneveld et al. Reference Zonneveld, Marret, Versteegh, Bogus, Bouimetarhana, Crouch, de Vernal, Elshanawany, Esper and Forke2013). In coastal areas, nutrient increases have been associated with environmental eutrophication (Saetre et al. Reference Saetre, Dale, Abdullahb and Saetre1997; Dale Reference Dale2009). Similarly, Brigantedinium spp. was found frequently in the T_C sediment core. Its occurrence could be associated with local anthropic activity (Zonneveld et al. Reference Zonneveld, Chen, Elshanawany, Fischer, Hoins, Ibrahim, Pittauerova and Versteegh2012) and increased food availability (Poliakova et al. Reference Poliakova, Zonneveld, Herbeck, Jennerjahn, Permana, Kwiatkowski and Behling2017). These data suggest a variation between a mesotrophic and eutrophic environment, in which sufficient nutrients are available for the establishment and proliferation of dinoflagellates.
Near the top of this zone (zone I - T_C core) there is a lithological change (sandy mud with shells fragments). The dating of a preserved shell of Caryocorbula cymella Dall revealed an age of 6928–7158 yr cal BP. This bivalve species is characteristic of euryhaline environments (Rios Reference Rios2009) and is also observed in estuarine-lagoon environments in Southeast Brazil (Martínez et al. Reference Martínez, Mahiques and Burone2013). The identification of malacological records in the sediment is very useful for correlating palaeoenvironmental and marine palaeontological interpretations (Murray-Wallace and Woodroffe Reference Murray-Wallace and Woodroffe2014). Core locations in which there were high accumulations of shells also showed the highest accumulations of foraminiferal test linings, which gradually increased. According to Stancliffe (Reference Stancliffe, Jansonius and Macgregor1996), these organisms are always related to conditions of higher salinity in the environment. However, Traverse (Reference Traverse2008) points out the lack of information regarding the taxonomy and ecology of these organisms. A high accumulation of these organisms was also observed by Pienkowski et al. (Reference Pienkowski, Mudie, England, Smith and Furze2011) in bottom sediments from an archipelago in Canada, and was associated with high local productivity.
The occurrence of shell fragments associated with higher deposition of foraminiferal test linings could be related to a rise in the sea level along the Brazilian coast when it crossed the present level approximately 7000 yr (Angulo et al. Reference Angulo, Lessa and Souza2006) (Figure 8). Lorente et al. (Reference Lorente, Pessenda, Obooh-Ikuenobe, Buso Júnior, Cohen, Meyer, Giannini, Oliveira, Rosseti and Borotti Filho2014) report a transgressive phase on the Espírito Santo State coast between 7521–4847 cal yr BP, deduced from the data on foraminiferal test linings and dinocysts found in a lake located some 23 km from the Atlantic Ocean. This same pattern of marine transgression from 7550 cal yr BP was also observed by França et al. (Reference França, Alves, Castro, Cohen, Rosseti, Pessenda, Lorente, Fontes, Buso, Giannini and Franciquini2015) from the analysis of δ13C and δ15N in sediments collected in Delta do Rio Doce plain. The authors point out that during this period there was a mixture of organic matter from marine and continental origin where the existing delta was originally an estuarine channel. The data obtained by these authors corroborate our findings, i.e. that around 6928–7158 cal yr BP the Espírito Santo coast was passing through a transgressive phase.

Figure 8 Summarized figure with the autotroph/heterotroph taxa percentage from T_A, T_C and T_D sediment cores; Brazillian sea level curve and the age model from Vitória Bay, Espírito Santo State, Brazil.
This transgressive phase evidenced by the data from the T_C sediment core was also observed in zone I of the T_A core (Figure 8). The core was taken from a sheltered area of the bay, further away from the final stretch of the Passagem Channel. It was possible to verify the absence of dinocysts along this zone, together with the occurrence of sandy sediment and low TOC values. Machado et al. (Reference Machado, Bastos, Freitas and Baptista Neto2018) point out that the grain size from medium to coarse sand in this sediment core may indicate a geological heritage. In general, continental palynomorphs (pollen and spores) and marine organisms (dinocysts) are highly preserved in reducing environments and at finer particle size (Traverse Reference Traverse2008).
The low TOC values observed may also indicate higher environmental energy, preserving less organic matter in the sediment (Tian et al. Reference Tian, Doblin, Johnston, Pei and Hu2018). Only at a depth of 160 cm were low accumulations of Brigantedinium spp. observed, and at similar levels to those of Pseudoschizaea (incertae sedis). At the beginning of zone II of the T_A core, there is also a significant increase in less negative values of δ13C. At a depth of 150 cm, a plant fragment was found and dated to 7241–7339 cal yr BP, corroborating the data obtained for the same timespan in the T_C core, when the Brazilian coast was passing through a transgressive period (Angulo et al. Reference Angulo, Lessa and Souza2006) (Figure 8).
The highest accumulation of dinocysts and foraminiferal test linings observed in the T_A core occurred near the top of zone II. At this stage, no preserved heterotrophs were found. O. centrocarpum and S. ramosus have been found within different temperature and salinity ranges (Zonneveld et al. Reference Zonneveld, Marret, Versteegh, Bogus, Bouimetarhana, Crouch, de Vernal, Elshanawany, Esper and Forke2013). Narale et al. (Reference Narale, Naidu, Anil and Godad2015) points out that Spiniferites species are important indicators of hypersaline conditions. The core samples containing the highest numbers of preserved cysts also contained the highest accumulation of foraminiferal test linings in this sediment core, indicating higher environmental salinity (Stancliffe Reference Stancliffe, Jansonius and Macgregor1996). This higher salinity, associated with the preservation of dinocysts and the dating of two shells of benthic organisms (Crassostrea sp. 6599–6827 cal yr BP and Neritina virginea Linnaeus 6455–6683 cal yr BP) indicate that the environment was undergoing a transgressive period that reached its peak around 5000 yr BP (Angulo et al. Reference Angulo, Lessa and Souza2006) (Figure 8).
Andrews (Reference Andrews1940) and Hendy et al. (Reference Hendy, Jones, Moreno, Zapata and Jaramillo2015) point out that the dated benthic organisms are characteristic of shallow estuarine environments. However, the occurrence of these species, observed in the present study, was also observed in the tidal region of Paranaguá Bay, located in southern Brazil (Boehs et al. Reference Boehs, Absher and Cruz-Kaled2004), suggesting that these organisms can occur in coastal areas and at shallow to medium depths (Ekdale Reference Ekdale1974; Gandara-Martins and Almeida Reference Gandara-Martins and Almeida2013). Similarly, the δ15N values could indicate the presence of mixed continental and oceanic waters, characteristic of estuarine environments (França et al. Reference França, Alves, Castro, Cohen, Rosseti, Pessenda, Lorente, Fontes, Buso, Giannini and Franciquini2015).
As in the T_A core, the frequent presence of dinocysts and foraminiferal test linings was also observed in the T_C core (zone II). The significant preservation of dinocysts associated with finer particle size could have favored the preservation of palynomorphs, even in a transgressive period (6928–7158 cal yr BP) (Angulo et al. Reference Angulo, Lessa and Souza2006) (Figure 8). Doblin and Dobbs (Reference Doblin and Dobbs2006) point out that dinocysts act as fine particles accumulated in low turbulence systems. Botryococcus algae may occur in environments ranging from freshwater to brackish (Traverse Reference Traverse2008). Guy-Ohlson (Reference Guy-Ohlson1992) points out that the significant preservation of this colonial algae could be associated with a shallow, undisturbed environment with stable climatic conditions prior to deposition. Cysts of autotrophs O. centrocarpum and T. vancampoae were the most frequent in this zone. Despite being considered cosmopolitan and tolerant of environmental variations (Wall et al. Reference Wall, Dale, Lohmann and Smith1977; Marret and Zonneveld Reference Marret and Zonneveld2003), O. centrocarpum is also reported as an important indicator of stronger marine influence (Hessler et al. Reference Hessler, Young, Holzwarth, Mohtadi, Luckge and Behling2013; Poliakova et al. Reference Poliakova, Zonneveld, Herbeck, Jennerjahn, Permana, Kwiatkowski and Behling2017).
However, T. vancampoae is characterized as a shallow water species (Poliakova et al. Reference Poliakova, Zonneveld, Herbeck, Jennerjahn, Permana, Kwiatkowski and Behling2017) occurring in subtropical to tropical coastal environments, in waters ranging from oligotrophic to eutrophic (Zonneveld et al. Reference Zonneveld, Marret, Versteegh, Bogus, Bouimetarhana, Crouch, de Vernal, Elshanawany, Esper and Forke2013), and is sensitive to nutrient increase which stimulates proliferation (Poliakova et al. Reference Poliakova, Zonneveld, Herbeck, Jennerjahn, Permana, Kwiatkowski and Behling2017). Brigantedinium spp. (heterotroph) was found from the base of zone II to a depth of 150 cm (T_C core). Species of this genus have been reported in different parts of the world as local anthropic activity indicators (Zonneveld et al. Reference Zonneveld, Chen, Elshanawany, Fischer, Hoins, Ibrahim, Pittauerova and Versteegh2012; Narale and Anil Reference Narale and Anil2016). On the other hand, its occurrence may be associated with higher nutrient availability originating from the continental water supply (Pospelova et al. Reference Pospelova, Esenkulova, Johannessen, O’Brien and Macdonald2010; Zonneveld et al. Reference Zonneveld, Susek and Fischer2010).
The association of O. centrocarpum with T. vancampoae and Brigantedinium spp. in the present study could be a further indication of the transgressive period during the Holocene on the coast of Brazil (Angulo et al. Reference Angulo, Lessa and Souza2006) (Figure 8). As from the dated period 6928–7158 cal yr BP (T_C core), higher salinity was observed in the environment, favoring the establishment of O. centrocarpum, and foraminiferal test linings. The expansion of Brigantedinium spp. cysts associated with the gradual increase of TOC could indicate greater availability of nutrients in the environment. Tian et al. (Reference Tian, Doblin, Johnston, Pei and Hu2018) point out that TOC is one of the most important parameters for the variation in dinocysts in the environment, due to the boosted preservation of organic matter, and availability of micro and macronutrients in the sediment.
In the T_A core, after 6599–6827 cal yr BP there is a gradual decrease in the occurrence of autotroph taxa. O. centrocarpum is recorded only near the top of the core. Radi et al. (Reference Radi, Pospelova, de Vernal and Barrie2007) emphasize that autotroph species are more common in coastal and shallow regions. The decrease in the frequency of O. centrocarpum together with the low preservation of foraminiferal test linings could indicate a decrease in local salinity, even in a period of marine transgression (Figure 8). The decrease in the accumulation of dinocysts in this core (T_A) could be associated with the sample collection point, inside the most sheltered part of the bay, under the strong influence of inflowing continental waters and in particular mangrove vegetation (Machado et al. Reference Machado, Bastos, Freitas and Baptista Neto2018). The reduction in the transparency of the water column due to the presence of suspended sediment was highlighted as one of the indicators for low primary production and subsequent deposition of dinocysts in an estuarine region of Bangladesh (Hoq et al. Reference Hoq, Abdul Wahab and Nazrul Islam2006).
The lithology of the T_D core was almost totally characterized by fine/medium sand along the three highlighted zones. Compared to the other cores (T_A and T_C) it was the youngest core according to the dating of the shell of Natica livida Pfeiffer (6295– 6505 cal yr BP) and the dating of a Bulla striata Bruguière shell (5459–5637 cal yr BP). The presence of these species in the T_D core suggests an environment more strongly influenced by the marine environment (Ekdale Reference Ekdale1974). In the coastal region of Espírito Santo State, these species of benthic organisms have been reported in sandy and sandy mud sediments (Castro and Santos Reference Castro and Santos1989), corroborating the data in our study. According to Le Roux and Rojas (Reference Le Roux and Rojas2007), both the degree of selection and the diameter of the sediment grains provide important indicators for palaeoenvironmental characterization of past environments, supplying information on the energy in the environment, and the distance and duration taken for the sediment to be transported (e.g. proximity to the source area).
Machado et al. (Reference Machado, Bastos, Freitas and Baptista Neto2018) point out that this sediment core is in the most dynamic part of the estuary, associated with high accumulation of bioclasts, the presence of shell fragments and almost total absence of organic matter, suggesting that deposition was strongly influenced by the marine environment. The preservation of dinocysts and foraminiferal test linings throughout the T_D core was the lowest compared to the other two cores (T_A and T_C). Matsuoka et al. (Reference Matsuoka, Yurimoto, Chong and Man2017) point out that accumulation values are low for dinocysts observed in tropical estuaries and shallow coastal regions. Among the many factors at play are the local sedimentation rate and chemical components in the sediment that inhibit the preservation of cysts in predominantly mangrove environments, as well as energy and local circulation (Furio et al. Reference Furio, Matsuoka, Mizushima, Baula, Chan, Puyong, Srivilai, Sidharta and Fukuyo2006; Baula et al. Reference Baula, Azanza, Fukuyo and Siringan2011; Hessler et al. Reference Hessler, Young, Holzwarth, Mohtadi, Luckge and Behling2013).
In general, the dinocysts species observed in the other cores (T_A and T_C) were also observed in the T_D core, especially O. centrocarpum, Spiniferites spp. and T. vancampoae (predominant in the sandy fraction) and Brigantedinium spp. Elshanawany and Zonneveld (Reference Elshanawany and Zonneveld2016) also found these species (with the exception of T. vancampoae) in oligotrophic environments, and reported that they could have achieved an advantage over the others by better using micro and macronutrients, resulting in a higher growth rate. In conjunction with these factors, there is a gradual rise in sea level from the dates obtained for this core (6295–6505 cal yr BP at a depth of 370 cm and 5459–5637 cal yr BP at 58 cm) (Figure 8), indicated by low TOC values. The presence of saline water in the estuarine system due to the marine transgression observed during the Holocene on the Espírito Santo shore could have favored the establishment of an oligotrophic environment, such as that observed on both the Gulf Coast of Aqaba and the Red Sea (Elshanawany and Zonneveld Reference Elshanawany and Zonneveld2016). Narale et al. (Reference Narale, Naidu, Anil and Godad2015) point out that Spiniferites species are good indicators of hypersaline conditions. Similarly, the low TOC values associated with coarser sediment could be directly correlated with the preservation and deposition of dinocysts (Tian et al. Reference Tian, Doblin, Johnston, Pei and Hu2018).
Contrary to what is observed in other tropical estuaries around the world, in our study autotrophs were better represented than heterotrophs. The occurrence of heterotrophs in tropical environments has been reported in different parts of the world from the analysis of surface sediments (Furio et al. Reference Furio, Matsuoka, Mizushima, Baula, Chan, Puyong, Srivilai, Sidharta and Fukuyo2006; Srivilai et al. Reference Srivilai, Lirdwitayaprasit and Fukuyo2012). On the other hand, it is worth noting that in estuarine environments and under mangrove vegetation, there is less of a tendency for dinocysts to occur and be preserved in sediments (Matsuoka et al. Reference Matsuoka, Yurimoto, Chong and Man2017). In conjunction with these factors, the influence of the marine transgression during the Holocene on the coast of Espírito Santo State could have initially favored the establishment of an oligotrophic, high-energy environment, in line with the data obtained from the T_D sediment core.
However, although the T_A core contains finer particles that the T_D core, cyst preservation was better from a depth of 90 cm, and this is associated with the low preservation of foraminiferal test linings and significant preservation of Pseudoschizaea (incertae sedis). These facts taken together could indicate that even in a transgressive period, this could characterize a tidal plain environment, since the sample location is in a more sheltered area of the bay (Machado et al. Reference Machado, Bastos, Freitas and Baptista Neto2018). The foraminiferal test linings as a salinity indicator in the environment (Stancliffe Reference Stancliffe, Jansonius and Macgregor1996) together with the presence of incertae sedis Pseudoschizaea (common in wetland or flooded environments) lends weight to the current interpretation. Furio et al. (Reference Furio, Matsuoka, Mizushima, Baula, Chan, Puyong, Srivilai, Sidharta and Fukuyo2006) point out that areas under mangrove influence and sulfate bioavailability in the environment can inhibit the deposition and germination of dinocysts.
The T_C core is also the oldest of the three cores, has finer particle size and is closest to the Santa Maria da Vitória (SMV) river. There is shell deposition and finer particle size at a depth of around 304 cm, dating from 6928–7158 cal yr BP, characterizing the period during which the sea level crossed the current level (Angulo et al. Reference Angulo, Lessa and Souza2006) (Figure 8). Despite the greater influence of the continental water inflow, there was ample evidence of the preservation of dinocysts and other palynomorphs (foraminiferal test linings and Botryococcus algae). The core also contained the highest total accumulation of dinocysts present in the three samples. Taylor et al. (Reference Taylor, Hoppenrath and Saldarriaga2008) point out that autotrophs can prey on other organisms at some stage in their life cycles, and are then characterized as mixotrophic. This could account for the greater preservation of autotrophic cysts in an area strongly influence by continental inflow, supplying significant quantities of organic matter to the interior of the estuarine system.
CONCLUSIONS
The combined study of stable isotopes and 14C dating associated with other palynomorphs (foraminiferal test linings, algae and the Pseudoschizaea) evidenced the changes in the Vitória Bay during the Holocene.
-
1. From about 9396 to 9520 cal yr BP, the palaeonvironmental evolution of the Vitória Bay was strongly influenced by the variation in sea level.
-
2. The influence of the marine transgression observed during the Holocene on the Espírito Santo State coast could have initially favored the establishment of an oligotrophic environment and a local high-energy environment, based on the sediment cores studied.
-
3. The oscillation in sea level significantly influenced the ingress and deposition of dinocysts into Vitória Bay.
ACKNOWLEDGMENTS
The authors would like to thank CAPES (Coordenação de Aperfeiçoamento Pessoal do Ensino Superior), FAPES (Fundação de Amparo à Pesquisa e Inovação do Espírito Santo), FAPERJ (Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro), CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) and Secretaria Nacional dos Portos for financial support.