INTRODUCTION
Lake sediments in volcanic regions record valuable information on past eruptions (Koshimizu et al. Reference Koshimizu, Uchiyama, Yamamoto, Aramaki, Fujii, Nakada and Miyaji2007; Van Daele et al. Reference Van Daele, Moernaut, Silversmit, Schmidt, Fontijn, Heirman, Vandoorne, De Clercq, Van Acker and Wolff2014; Obrochta et al. Reference Obrochta, Yokoyama, Yoshimoto, Yamamoto, Miyairi, Nagano, Nakamura, Tsunematsu, Lamair and Hubert-ferrari2018) and their environmental consequences (Yamamoto et al. Reference Yamamoto, Uchiyama, Miyairi and Yokoyama2018). Terrestrial plant remains are generally considered ideal 14C-dating materials in lake sediments because they contain carbon derived directly from atmospheric CO2 (Bertrand et al. Reference Bertrand, Araneda, Vargas, Jana, Fagel and Urrutia2012) and are rapidly delivered to sediment (Gierga et al. Reference Gierga, Hajdas, van Raden, Gilli, Wacker, Sturm, Bernasconi and Smittenberg2016); however, terrestrial plant remains are rarely found in volcanic region sediment as volcanisms can severely restrict vegetation cover. Total organic carbon (TOC) may be used as an alternative but it often contains uncertainties arising from the reservoir effect (Fontes et al. Reference Fontes, Gasse and Gibert1996), source organic matter heterogeneity (Uchida et al. Reference Uchida, Shibata, Ohkushi, Yoneda, Kawamura and Morita2005), and contribution of redeposited aged organic matter (Naeher et al. Reference Naeher, Suga, Ogawa, Schubert, Grice and Ohkouchi2016).
Mount Fuji is the largest active volcano in Japan and its activity began ca. 100,000 yr BP (Takada et al. Reference Takada, Yamamoto, Ishizuka and Nakano2016). To date, several studies have attempted to reconstruct its eruptive history using sediments from proximal volcanic-dammed lakes (Lakes Motosu, Shoji, Sai, Kawaguchi, and Yamanaka), namely the Fuji Five Lake (Taba et al. Reference Taba, Kosugi, Endo and Miyaji1990; Koshimizu et al. Reference Koshimizu, Uchiyama, Yamamoto, Aramaki, Fujii, Nakada and Miyaji2007). These lakes are distributed from the northeast to northwestern flank of the mountain and contain a large number of tephra layers within sediments that originated from Mount Fuji volcanism. However, most studies have been limited owing to the lack of fossil leaves, except for Lake Motosu where a high-fidelity age model was recently published based on high-density radiocarbon dating of terrestrial macrofossil and bulk organic matter (Obrochta et al. Reference Obrochta, Yokoyama, Yoshimoto, Yamamoto, Miyairi, Nagano, Nakamura, Tsunematsu, Lamair and Hubert-ferrari2018).
The development of compound-specific radiocarbon analysis (CSRA) (Eglinton et al. Reference Eglinton, Aluwihare, Bauer, Druffel and McNichol1996) has enabled 14C measurement of source-specific organic compounds (biomarkers) to become a promising tool for determining depositional ages in settings where traditional dating methods are impractical. For example, Uchikawa et al. (Reference Uchikawa, Popp, Schoonmaker and Xu2008) measured the radiocarbon in plant wax n-alkanes that were retrieved from lake sediments in Hawaii and found that they were in good agreement with the 14C ages of terrestrial macrofossils in nearby horizons. Furthermore, Hou et al. (Reference Hou, Huang, Brodsky, Alexandre, McNichol, King, Hu and Shen2010) also demonstrated that lignin phenols in late Quaternary lake sediments had 14C ages that are compatible with varve-counted and macrofossil-dated sediment horizons. In contrast, substantial contributions of pre-aged plant waxes in lake sediments from tropical lakes in Mexico and Lake Soppensee in Switzerland (Douglas et al. Reference Douglas, Pagani, Eglinton, Brenner, Hodell, Curtis, Ma and Breckenridge2014; Gierga et al. Reference Gierga, Hajdas, van Raden, Gilli, Wacker, Sturm, Bernasconi and Smittenberg2016; Douglas et al. Reference Douglas, Pagani, Eglinton, Brenner, Curtis, Breckenridge and Johnston2018) highlights the limitation when using terrestrial higher plant biomarkers as a dating tool in the environments, wherein significant amount of pre-aged materials have been transported from the catchment.
On the other hand, CSRA of short-chain (i.e., C14, C16, and C18) fatty acids derived from various eukaryotes and eubacteria and that of chlorophyll a degradation product (e.g., cyclopheophorbide-a-enol) has been applied to reconstruct chronologies in settings where significant contribution from relict organic carbon leads to anomalously old 14C ages (Ohkouchi et al. Reference Ohkouchi, Eglinton and Hayes2003; Yamane et al. Reference Yamane, Yokoyama, Miyairi, Suga, Matsuzaki, Dunbar and Ohkouchi2014). These compounds in surface sediments have Δ14C values that are consistent with those of dissolved inorganic carbon (DIC) in surface water (Uchida et al. Reference Uchida, Shibata, Kawamura, Kumamoto, Yoneda, Ohkushi, Harada, Hirota, Mukai and Tanaka2001; Ohkouchi and Eglinton Reference Ohkouchi and Eglinton2008; Kusch et al. Reference Kusch, Kashiyama, Ogawa, Altabet, Butzin, Friedrich, Ohkouchi and Mollenhauer2010a), suggesting that they likely represent a time when sediments were deposited without contribution from relict materials. To date, several studies have reported the 14C ages of the short-chain fatty acids in both marine and lacustrine environments (e.g., Uchida et al. Reference Uchida, Shibata, Ohkushi, Yoneda, Kawamura and Morita2005; Camuera et al. Reference Camuera, Jiménez-Moreno, Ramos-Román, García-Alix, Toney, Anderson, Jiménez-Espejo, Kaufman, Bright and Webster2018); however, their application may be limited, especially in lacustrine settings due to large discrepancies (>4000 yr BP) in ages with those from autochthonous emergent plants (C24 fatty acid; Camuera et al. Reference Camuera, Jiménez-Moreno, Ramos-Román, García-Alix, Toney, Anderson, Jiménez-Espejo, Kaufman, Bright and Webster2018).
In this study, we performed CSRA of organic compounds in surface sediments obtained from Lake Kawaguchi, central Japan. We compared radiocarbon contents (Δ14C) of short- (C16) and long-chain (C24, C26, and C28) fatty acids, chlorophyll a (Chl a) and its derivatives [132, 173-cyclopheophorbide-a-enol (CPhe a) and pheophytin a (Phe a)] with those of TOC, terrestrial plant remains (leaf and wood fragments), and DIC in surface water to reveal differential sources of organic compounds in the sediments, which could provide insight into their potential as a dating tool in volcanic lake environments.
MATERIALS AND METHODS
Study Site and Sampling
Lake Kawaguchi (35°31′N, 138°45′E; Figure 1) lies 830.5 m above sea level with a surface area of 5.7 km2 and a maximum depth of 14.6 m. The lake basin has a west-to-east orientation and is divided into three basins by the ridge of the Neogene basement rock. Lake Kawaguchi has no year-round, sustained river input except for the Terakawa and Okukawa rivers (Figure 1); instead, the lake is mainly fed by groundwater and seasonal surface runoff from its catchment, which is mainly caused by typhoons during the fall season, (area = 103.5 km2; Horiuchi et al. Reference Horiuchi, Lee, Watanabe and Fujita1992). The groundwater primarily enters the lake from the north and permeates into the underground valley approximately 5 km south of the lake (Kanno et al. Reference Kanno, Ishii and Kuroda1986). Contribution of volcanic CO2 to the lake water is unlikely because the volcanic activity of Mount Fuji has been dormant for more than 300 years without any marked CO2 outgassing around the mountain. There is no natural outlet; however, a small amount of water (0.485 m3/s: Jan.–Dec. 2016 average; unpublished data from Yamanashi Prefecture) continuously discharges through artificial drainage tunnels that were constructed in 1917 for flood control, irrigation, and power generation (Figure 1). The phytoplankton in Lake Kawaguchi is typically characterized by diatoms (Asterionella and Melosira) with an occasional dominance of cyanobacteria (Microcystis) during summer (Tanaka Reference Tanaka1992; Yoshizawa et al. Reference Yoshizawa, Yoshida and Hirabayashi2005).

Figure 1 Map showing the location of (a) the Fuji Five Lakes area, Yamanashi Prefecture, Japan, and (b) Lake Kawaguchi, and (c) a bathymetric map of Lake Kawaguchi with major inlet rivers and artificial drainage tunnels (solid arrows). Solid circles indicate sampling locations. These sites were selected because the sedimentation rate has been estimated based on the 210Pb and 137Cs dating in the adjacent cores.
Surface sediment (upper ca. 10 cm layer) was collected on August 1, 2016 (St 1; 35°30′49.30″N, 138°44′59.70″E, water depth = 8.5 m) and on March 19, 2017 (St 2; 35°30′23.91″N, 138°46′10.65″E, water depth = 10.4 m) using an Ekman–Birge grab sampler. Lake water was collected at St 2 on June 9, 2017 using a Van Dorn water sampler. Sampling locations are indicated by solid circles in Figure 1c. The water sample was obtained from the depth of 1.5–2 m below the surface to avoid precipitation influence and was immediately spiked with HgCl2 to inhibit microbial activity. Sediment and lake water samples were stored in a refrigerator (4°C) until they were analyzed.
Extraction and Separation of Organic Compounds
Fatty Acid Methyl Esters
Total lipids were ultrasonically extracted from the homogenized dried sediments (50–70 g) using dichloromethane (DCM)/methanol (MeOH) (95:5, v/v). Then, the extracts were combined and concentrated using a rotary evaporator under vacuum and blown with N2 gas to near dryness. Neutral fractions of the extracts were removed with n-hexane/DCM (10:1) after saponification with 1.0 M KOH in MeOH, and the remaining solution was then acidified with 6 M HCl and carboxylic acids were extracted using DCM. The acidic fraction was further treated with 5% HCl/MeOH to convert acids into their methyl esters. Fatty acid methyl esters (FAMEs) were then obtained using column chromatography with a silica gel (deactivated with 1% H2O) column and elution with n-hexane/DCM (1:2). Concentration of the methyl esters was determined using an Agilent 6890 gas chromatograph equipped with an on-column injector, DB-5 fused silica column (30 m × 0.32 mm i.d., 0.25-µm film thickness), and a flame ionization detector (GC-FID).
Isolation of the C16, C24, C26, and C28 FAMEs were conducted using the method described in Yamane et al. (Reference Yamane, Yokoyama, Miyairi, Suga, Matsuzaki, Dunbar and Ohkouchi2014) with some additional modifications. Briefly, FAMEs were introduced into a reversed-phase high performance liquid chromatography system (HPLC; Agilent 1100 series) equipped with two series-connected columns (Develosil C30-UG-5, 4.6 × 250 mm, 5 µm particle size, Nomura Chemical) with guard column (Develosil C30-UG, 4 × 10 mm, 5 µm particle size, Nomura Chemical), a Corona charged aerosol detector (CAD; Dionex) and each target compound is separately collected by a fraction collector. Two types of mobile phase [Acetonitrile/MeOH (1:2, v/v) with 0.5% pyridine (eluent A) and ethyl acetate with 0.5% pyridine (eluent B)] were used for isolation. The compounds were eluted isocratically with 100% eluent A for 25 min, followed by adding eluent B with a linear gradient up to 15% for 50 min, and then held isocratically until reaching 110 min. The remaining FAMEs (>C28) were flushed out with 100% eluent B until 126 min. Oven temperature was kept constant at 15°C from 0 to 25 min, increased at 2°C/min up to 50°C, and then held isothermally until reaching 126 min. The flow rate of the mobile phase was 1 mL/min. All the isolated fractions were subjected to silica gel column to remove any impurities derived from HPLC process. The purity of each compound was confirmed using GC-FID and GC-MS [Agilent 7890A/5975C GC-MSD with a VF-5ms fused silica column (30 m × 0.25 mm i.d., 0.1-µm film thickness)].
Chlorophyll a and Its Derivatives
Pigments were extracted and separated using the method described in Kusch et al. (Reference Kusch, Kashiyama, Ogawa, Altabet, Butzin, Friedrich, Ohkouchi and Mollenhauer2010a) with some additional modifications. Freeze-dried sediment was ultrasonicated in acetone at 0°C for 15 min followed by liquid–liquid separation (water:n-hexane = 3:1, v/v). The n-hexane layer was then dried under argon, and precipitated pigments were dissolved in dimethylformamide (DMF). The pigments were then isolated using an HPLC system (Agilent 1100 series) equipped with an Agilent Eclipse XDB-C18 column (250 × 4.6 mm; 5 µm) connected to an Agilent XDB-C18 guard column (12.5 × 4.6 mm; 5 µm), photodiode array detector (DAD), and fraction collector. Acetonitrile with 0.5% pyridine (eluent A) and ethyl acetate with 0.5% pyridine (eluent B) were used as mobile phases for isolation. The pigments were eluted isocratically with 75% eluent A for 5 min and then increased the ratio of eluent B with a linear gradient up to 50% for 50 min at a flow rate of 1.0 mL/min. The oven was kept constant at 30°C.
Purification of the isolated pigments was achieved using an Agilent Eclipse PAH column (250 × 4.6 mm; 5 µm) with a guard column (12.5 × 4.6 mm; 5 µm). The eluents used for this purification step is the same as for the first separation. Pigments were eluted isocratically using 20% eluent B for 5 min, followed by increasing of the ratio of eluent B with a linear gradient of up to 60% for 30 min and then up to 100% for 13 min at 1.0 mL/min. The oven temperature was kept at 15°C and 30°C for Phe a and CPhe a, respectively. The purified pigments were washed again with DCM/n-hexane and NaCl solution, and the DCM/ n-hexane layer was then dried under argon. The C/N ratios of the extracted pigments were measured using a FlashEA1112 automatic elemental analyzer connected to a Thermo Finnigan Delta plus XP (Ogawa et al. Reference Ogawa, Nagata, Kitazato, Ohkouchi, Ohkouchi, Tayasu and Koba2010) to confirm high purities of the isolated compounds.
Δ 14C Measurements
For the 14C measurements, purified compounds were transferred to quartz tubes and recovered as CO2 by combustion with CuO. Plant remains, i.e., a single piece of plant leaf debris and a wood fragment, were handpicked from sieved (>150 µm) sediment residues under microscope, which were then converted to CO2 in quartz tubes after acid–alkali–acid pretreatment (Miyairi et al. Reference Miyairi, Yoshida, Miyazaki, Matsuzaki and Kaneoka2004). DIC samples (250 mL) were acidified with 3 mL of 85% H3PO4 and purged with 0.8 atm of He gas to collect CO2 gas in glass tubes. Then, the evolved CO2 was purified cryogenically and reduced to graphite using the method described in Yokoyama et al. (Reference Yokoyama, Koizumi, Matsuzaki, Miyairi and Ohkouchi2010). The Δ14C values were measured using a single-stage accelerator mass spectrometer (AMS) at the Atmosphere and Ocean Research Institute, the University of Tokyo (Yokoyama et al. Reference Yokoyama, Miyairi, Aze, Yamane, Sawada, Ando, de Natris, Hirabayashi, Ishiwa and Sato2019). The Δ14C values are defined as follows (Stuiver and Polach Reference Stuiver and Polach1977):
The Δ14C values of FAMEs were corrected for the contribution of methyl carbon obtained from MeOH (Δ14C = −991‰; Yamane et al. Reference Yamane, Yokoyama, Miyairi, Suga, Matsuzaki, Dunbar and Ohkouchi2014) during the esterification using isotope mass balance. The HPLC procedural blank was measured for each compound using a sensitivity improved on-line system of FlashEA1112 automatic elemental analyzer connected to a Thermo Finnigan Delta plus XP (Ogawa et al. Reference Ogawa, Nagata, Kitazato, Ohkouchi, Ohkouchi, Tayasu and Koba2010), which was confirmed to be smaller than 0.10 µgC. The 14C dates were calibrated using Oxcal v4.2 calibration software (Bronk Ramsey Reference Bronk Ramsey2009) with the IntCal13 dataset (Reimer et al. Reference Reimer, Bard, Bayliss, Beck, Blackwell, Ramsey, Buck, Cheng, Edwards and Friedrich2013).
RESULTS AND DISCUSSION
We detected a homologous series of C12–C34 n-fatty acids in surface sediments from Lake Kawaguchi. The molecular distribution of n-fatty acids showed a strong even/odd carbon number predominance (CPI > 3.7; Figure 2), suggesting their biological origin. The n-fatty acids exhibited a bimodal distribution with maxima at C16 and C28 in St 1 and at C16 and C24 in St 2. Low molecular weight fatty acids (C12–C19) were generally attributed to various organisms including phytoplankton, bacteria and terrestrial higher plants, whereas high molecular weight fatty acids (> C24) originated from terrestrial higher plant waxes (Simoneit Reference Simoneit1977; Gagosian et al. Reference Gagosian, Peltzer and Zafiriou1981) as well as freshwater aquatic macrophytes (Ficken et al. Reference Ficken, Li, Swain and Eglinton2000).

Figure 2 Molecular compositions of n-fatty acids in surface sediments from Lake Kawaguchi. St 1 and St 2 indicate sample collection sites.
The Δ14C values of plant-derived long-chain (C24, C26, and C28) n-fatty acids ranged from −43‰ to −183‰, corresponding to 14C ages of 288–1554 yr BP, respectively (Table 1). In contrast, the Δ14C of a plant leaf fragment within St 2 sediments exhibited a significantly 14C-enriched value compared to these fatty acids (Δ14C = 0‰; Table 1). Vertical profiles of 210Pb and 137Cs in a core collected near St 2 have revealed a linear sedimentation rate for the upper 20 cm layer (51 mg/cm2/yr; Sakaguchi et al. Reference Sakaguchi, Yamamoto, Shimizu and Koshimizu2004), suggesting that our sediments were likely deposited after the late 1940s. Considering the 14C-depleted Δ14C prior to 1952 via the use of fossil fuel CO2 (~−23‰; Hua et al. Reference Hua, Barbetti and Rakowski2013) and a subsequent increases by above ground hydrogen bomb testing (Hua et al. Reference Hua, Barbetti and Rakowski2013; Hammer and Levin Reference Hammer and Levin2017), this plant leaf fragment was likely deposited just after 1955 when the atmospheric Δ14C began to rise to its maximum in the mid-1960s (Hua et al. Reference Hua, Barbetti and Rakowski2013). However, we cannot entirely rule out the possibility that plant leaf remains have some decadal-scale resident time due to a lack of its stratigraphic information.
Table 1 Summary of the Δ14C data for TOC, fatty acids, pigments and plant remains in surface sediment, and surface water DIC from Lake Kawaguchi.

a Determined by GC-FID for fatty acids and by HPCL-DAD for pigments.
b Estimated by CO2 gas pressure.
c Age range with 95.4% probability.
The Δ14C offset between wood fragment and plant leaf samples can be explained consistently by a longer residence time for wood fragments than plant leaves, as has suggested previously in the late Holocene lake sediment from western Nepal (Nakamura et al. Reference Nakamura, Yokoyama, Maemoku, Yagi, Okamura, Matsuoka, Miyake, Osada, Teramura and Adhikari2012). On the contrary, the 14C-depleted values for the long-chain acids apparently contradict the ages of the sediments. This suggests that the Δ14C of long-chain acids are likely affected by substantial contributions from pre-aged terrestrial materials in addition to those from contemporary plant leaf waxes with post-bomb Δ14C signals. Such input of pre-aged materials has been suggested in various marginal marine and lacustrine settings (Smittenberg et al. Reference Smittenberg, Hopmans, Schouten, Hayes, Eglinton and Sinninghe Damsté2004; Uchida et al. Reference Uchida, Shibata, Ohkushi, Yoneda, Kawamura and Morita2005; Kusch et al. Reference Kusch, Rethemeyer, Schefuß and Mollenhauer2010b; Feng et al. Reference Feng, Benitez-Nelson, Montluçon, Prahl, McNichol, Xu, Repeta and Eglinton2013; Douglas et al. Reference Douglas, Pagani, Eglinton, Brenner, Hodell, Curtis, Ma and Breckenridge2014). The depleted Δ14C for long-chain acids in St 1 can also be attributed to contribution of pre-aged terrestrial materials because the 210Pb and 137Cs profiles in an adjacent core has revealed linear sedimentation for the upper 10 cm since the late 1980s (123 mg/cm2/yr; Yamamoto et al. unpublished data).
Previous studies have reported that sedimentary n-fatty acids often exhibit a trend becoming older as their chain length increases (Uchida et al. Reference Uchida, Shibata, Kawamura, Kumamoto, Yoneda, Ohkushi, Harada, Hirota, Mukai and Tanaka2001; Ohkouchi et al. Reference Ohkouchi, Eglinton and Hayes2003; Kusch et al. Reference Kusch, Rethemeyer, Schefuß and Mollenhauer2010b; Feng et al. Reference Feng, Benitez-Nelson, Montluçon, Prahl, McNichol, Xu, Repeta and Eglinton2013), and such 14C depletion is typically explained by longer-chain homologues having higher resistance to degradation (e.g., Camacho-Ibar et al. Reference Camacho-Ibar, Aveytua-Alcázar and Carriquiry2003). The long-chain fatty acids in our samples had Δ14C values that showed a large scattering with depletion (44 to 108‰) in C26 fatty acid as compared to C24 and C28 homologues (Figure 3). Similar 14C-depletion for the C26 fatty acid has also been noted in surface sediment in the Santa Monica Basin (Pearson et al. Reference Pearson, McNichol, Benitez-Nelson, Hayes and Eglinton2001) and the shelf of the Washington margin (Feng et al. Reference Feng, Benitez-Nelson, Montluçon, Prahl, McNichol, Xu, Repeta and Eglinton2013); the former study suggested potential contamination from the column bleed originating from the thermal degradation of the GC column. Although we cannot completely rule out the possibility that the C26 at St 1 could have been affected by extraneous carbon, as suggested by the large difference between the CO2 pressure- and GC-FID-based estimates on the carbon amount, the high purity of the C26 acid is warranted at St 2 based on the good agreement between the amount of samples quantified as CO2 and via GC-FID (Table 1).
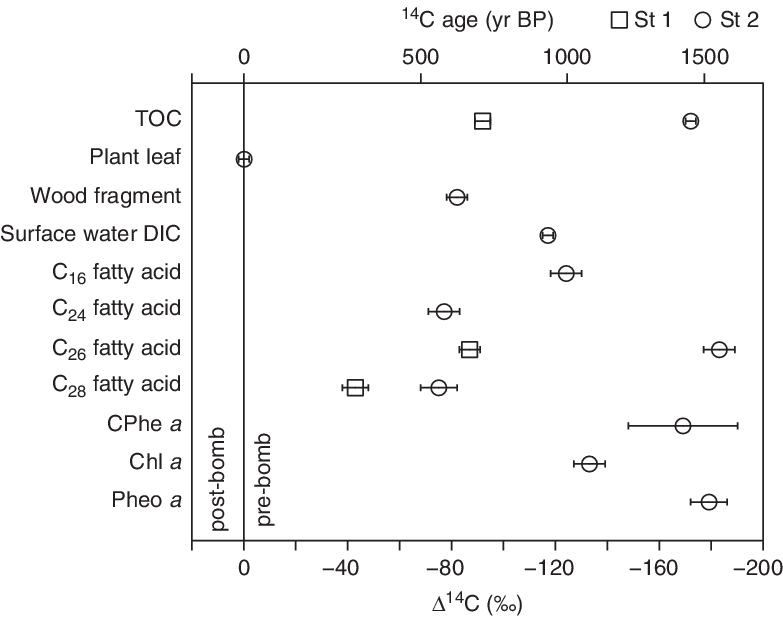
Figure 3 Δ14C values of individual fatty acids, pigments, terrestrial plant remains (leaf and wood fragments), total organic carbon in the surface sediments, and dissolved inorganic carbon in surface water from Lake Kawaguchi. Sediments from St 1 and St 2 were collected in August 2016 and March 2017, respectively. Lake water was collected in June 2017. CPhe a: 132, 173-cyclopheophorbide-a-enol, Chl a: chlorophyll a, Pheo a: pheophytin a.
One possible explanation for the 14C-depleted C26 fatty acid within the St 2 sediments could be carbon source heterogeneity among long-chain fatty acids. The C26 fatty acid has a Δ14C value that appears to mirror the TOC, whereas the Δ14C of the C24 and C28 fatty acids showed a close agreement with that of a wood fragment. This suggests that contribution from sources other than terrestrial plants is not negligibly small for the C26 fatty acid unlike the C24 and C28 acids. Generally, long-chain fatty acids are considered as terrestrial vascular plant biomarkers; however, these acids have been also found in bacteria (Schweizer Reference Schweizer, Ratledge and Wilkinson1988). Thus, a portion of the C26 fatty acid could have derived from heterotrophic microbes that intake TOC at the sediment-water interface. Alternatively, the Δ14C values of the C24 and C28 acids could be affected by additional aquatic sources, i.e., aquatic plants and microalgae (Ficken et al. Reference Ficken, Li, Swain and Eglinton2000; Feakins et al. Reference Feakins, Eglinton and deMenocal2007; van Bree et al. Reference van Bree, Peterse, van der Meer, Middelburg, Negash, De Crop, Cocquyt, Wieringa, Verschuren and Sinninghe Damsté2018) with younger age, whereas the C26 represents the age of the reworked organic materials within the lake or from the catchment. Moreover, deposition of aeolian dust particles to the sediment could potentially explain the above results because substantially 14C depleted long-chain fatty acids have been reported in aerosols from northern Japan during Asian dust events (~518‰; Matsumoto et al. Reference Matsumoto, Kawamura, Uchida, Shibata and Yoneda2001; Kawamura et al. Reference Kawamura, Matsumoto, Uchida and Shibata2010). Overall the Δ14C of long-chain fatty acids highlight the potential for large variability in the age and source of these compounds in lake sediments.
On the other hand, the C16 fatty acid in St 2 sediments displayed a similar Δ14C value (−124 ± 6‰) to surface water DIC (−117 ± 2‰), suggesting that this compound is likely derived from primary producers that utilize DIC as carbon sources in photosynthesis. The aquatic origin of the C16 acid is also supported by consistent Δ14C values with chlorophyll a within the sediments (−133 ± 6‰; Table 1). However, given that the lake DIC tracks the atmospheric Δ14C signals and the DIC Δ14C during the deposition of the St 2 sediments, we expect that the Δ14C of lake producers should be higher than the modern values reflecting post-bomb 14C signals (~935.5‰ at NH zone 2; Hua et al. Reference Hua, Barbetti and Rakowski2013). Considering the increasing trends in organic matter along with recent eutrophication in the lake (Hirabayashi et al. Reference Hirabayashi, Oga, Yoshizawa, Yoshida and Kazama2012) and low resistance of these compounds to microbial oxidation (Kawamura and Ishiwatari Reference Kawamura and Ishiwatari1984; Carpenter et al. Reference Carpenter, Elser and Elser1986), the Δ14C values for these compounds could be biased toward younger ages through post-depositional degradation and enhanced production of those with modern Δ14C values. Alternatively, the lake reservoir effect could be underestimated due to the paucity in the DIC Δ14C data, or atmospheric CO2 contributes minimally to the lake DIC due to the short residence time of the water in Lake Kawaguchi (~0.37 yr; Yamanashi Prefecture 1993). The weak influence of the atmospheric CO2 to lake DIC has been previously reported in Lake Haruna, Japan, where DIC originated entirely from the decomposition of organic materials (Yamanaka Reference Yamanaka2017). In general, the age of the lake DIC depends mainly on the input of exogenous old carbon (originated from carbonate rock, degradation of organic matter) and groundwater and on CO2 exchange between the lake water and atmosphere (Ishikawa et al. Reference Ishikawa, Tayasu, Yamane, Yokoyama, Sakai and Ohkouchi2015; Zhang et al. Reference Zhang, Ma, Qiang, Huang, Li, Guo, Henderson, Holmes and Chen2016). The input from carbonate rocks can be discarded in this lake due to their limited exposure in the catchment (Katada Reference Katada1954). However, the ultimate sources of DIC should be identified by measuring δ13C in future studies to better understand carbon cycling in Lake Kawaguchi.
Interestingly, we observed a large offset (~46‰) between the Δ14C values of Chl a and those of 132, 173-cyclopheophorbide-a-enol (CPhe a) and pheophytin a (Phe a), which are major degradation products of Chl a (Figures 3 and 4). The Δ14C value of Chl a is consistent with the Δ14C value of DIC in surface water, demonstrating that a significant proportion of Chl a is likely derived from contemporary phytoplankton communities. This suggests that 14C-depleted CPhe a and Phe a in the sediments are most likely explained by contributions from older pheopigments within the lake. In support of this, such contribution has been also suggested by a study on mesotrophic Lake Zurich, Switzerland, wherein degraded Chl a derivatives exhibit higher δ15N than Chl a (Naeher et al. Reference Naeher, Suga, Ogawa, Schubert, Grice and Ohkouchi2016). Alternatively, older pigments could be also supplied from soils around the lake as Phe a is one of the primary pigments in photosystem II (Kusch et al. Reference Kusch, Kashiyama, Ogawa, Altabet, Butzin, Friedrich, Ohkouchi and Mollenhauer2010a).

Figure 4 Structures of (a) chlorophyll a (Chl a) and (b, c) its derivatives [132, 173-cyclopheophorbide-a-enol (CPhe a) and pheophytin a (Phe a)].
It should be noted that large offsets in the TOC Δ14C values have been observed between the sites, i.e., the Δ14C of TOC in St 1 (−92 ± 3‰) exhibits more 14C-enriched values than the surface water DIC (−117 ± 2‰), whereas the Δ14C in St 2 (−172 ± 2‰) is significantly 14C-depleted compared to the DIC value (Figure 3). The averaged TOC concentrations for the upper 10 cm of sediment in the adjacent cores were quite similar between these sites (5.1% for St 1 and 4.8% for St 2; Yamamoto et al., unpublished data). However, St 1 was located at a shallower water depth on the slope of the large alluvial fan of the Okukawa river, and the higher sedimentation rate and more enriched Δ14C value at St 1 than that at St 2 suggests that the sediments in St 1 likely received more contribution from modern plant (terrestrial/aquatic) sources. On the other hand, the Δ14C of TOC in St 2 represents significant contribution from reworked/pre-aged organic matter. Anthropogenic input of fossil-fuel-based organic carbon and mobilization of aged carbon associated with anthropogenic land-use change could also affect the TOC Δ14C values (Griffith et al. Reference Griffith, Barnes and Raymond2009; Butman et al. Reference Butman, Wilson, Barnes, Xenopoulos and Raymond2015) because the catchment is quite densely populated with many fields and houses, especially in the eastern basin.
In lacustrine sediments, various methods have been proposed for improving chronologies based on TOC 14C dating (e.g., Bertrand et al. Reference Bertrand, Araneda, Vargas, Jana, Fagel and Urrutia2012; Hou et al. Reference Hou, D’Andrea and Liu2012; Obrochta et al. Reference Obrochta, Yokoyama, Yoshimoto, Yamamoto, Miyairi, Nagano, Nakamura, Tsunematsu, Lamair and Hubert-ferrari2018). Bowen et al. (Reference Bowen, Nielson and Eglinton2019) has recently reported a broad similarity in the 14C between simultaneously deposited TOC and algal organic matter in sediments of the Great Salt Lake, Utah, and suggested that TOC is little affected by allochthonous organic matter. However, the application of the TOC may be limited in the Lake Kawaguchi sediments owing to the variable contributions from the 14C-enriched and depleted organic materials between locations. The 14C ages of sedimentary organic compounds in Lake Kawaguchi were overall older than the plant leaf remain, i.e., the most precise age recorder in lacustrine sediments (Nakamura et al. Reference Nakamura, Yokoyama, Maemoku, Yagi, Okamura, Matsuoka, Miyake, Osada, Teramura and Adhikari2012). Nevertheless, the Δ14C of the C16 fatty acid showed similarity to that of DIC, which implies potential for applying CSRA as a dating tool in volcanic lake environments.
ACKNOWLEDGMENTS
We thank S. Nozawa for her help during sampling. We also thank two anonymous reviewers for constructive comments which improved the manuscript. This research was partly supported by Yamanashi Prefecture, the Cooperative Program (No. 146, 2016; No. 147, 2017; No. 151, 2018) of Atmosphere and Ocean Research Institute, The University of Tokyo, and JSPS KAKENHI Grant Numbers 15KK015, 17H01168 and 18K03769.







