INTRODUCTION
For forensic investigations, being able to estimate the year of birth and the year of death of a dead body is critical. Traditionally, amino acid racemization using the ratio of L-isomers to D-isomers of amino acid in the human body has been used in forensic investigation to estimate the age at death in adults (Holtkötter Reference Holtkötter2011). Recently, forensic studies that estimate the year of death and the year of birth by using radiocarbon (14C) dating techniques have been performed by many accelerator mass spectrometry (AMS) laboratories with various body tissues. For instance, Hodgins (Reference Hodgins2009), Taylor et al. (Reference Taylor, Suchey, Payen and Slota1989), Ubelaker (Reference Ubelaker2001), and Cardoso et al. (Reference Cardoso, Puentes, Soares, Santos and Magalhaes2012) conducted studies with human skeletons; Nakamura et al. (Reference Nakamura, Kojima, Ohta, Nishida, Rakowski, Ikeda, Oda and Niu2007) carried out research with human hair and molars (teeth); Speller et al. (Reference Speller, Spalding, Buchholz, Hildebrand, Moore, Mathewes, Skinner and Yang2012) conducted research with human skeletons and teeth; Santos et al. (Reference Santos, Torre, Boudin, Bonafini and Saverwyns2015) estimated the year of death with human hair and fingernails because fingernails are also a good source of keratin protein; and Garrido-Varas et al. (Reference Garrido-Varas, Ubelaker and Intriago-Leiva2013) examined it with soft tissue and bone.
14C dating has a notable advantage in forensic science for samples after 1955 AD because the rapid change of 14C concentration in the atmosphere during the bomb peak era provides a very small error range for the calibrated 14C age. The atmospheric 14C concentration increased rapidly between 1955 and 1964 AD due to atomic bomb tests, and then decreased after 1964 AD. In the Northern Hemisphere, Δ14C increased by 524‰ from 1963 to 1964 AD and decreased by 12‰ from 1980 to 1990 AD and by 6.5‰ in the following decade. The change rate of 0.65‰ per year is still a sufficiently large value compared to the typical precision of AMS measurements of 14C. However, the turnover time of cells in samples makes the determination of death and birth years difficult because this parameter varies substantially depending on the parts of the body where the cells come from. Thus, the sample selection is very important for obtaining accurate results.
The 14C ages of dental enamel and dentin from adult teeth indicate the years when the person was 1–7 yr old because enamel and dentin are maintained throughout the whole lifetime of an individual (Ubelaker and Parra Reference Ubelaker and Parra2011). The lens crystalline of the eye also persists through the whole lifespan (Lynnerup et al. Reference Lynnerup, Kjeldsen, Heegaard, Jacobsen and Heinemeier2008). Hence, the 14C dating of dental materials and lens crystalline provides information on the birth year. Meanwhile, the 14C ages of collagen extracted from the human femur present different ages because femur cells are consistently replaced throughout an individual’s lifespan. To estimate the year of death using these samples, the turnover time of the sample should be known.
The Korean Police Department estimates that more than 20,000 unidentified human remains are excavated in South Korea every year. The National Forensic Service (NFS) of Korea has studied the application of 14C dating techniques since 2014 to identify such bodies with the cooperation of the AMS laboratory of the Korea Institute of Geoscience and Mineral Resources (KIGAM). The Ministry of National Defense (MND) Agency for Killed In Action (KIA) Recovery & Identification, a project started immediately after the end of the war, continues to excavate the skeletal remains of victims of the Korean War (1950–1953 AD). Many human bodies were found at a site in Seoul several years ago, and estimating the time of their death is an important issue because there is a possibility that they may be victims of the Korean War. Femur shafts were the only remains for most of the bodies, though several bodies included teeth and hair.
In this preliminary work, the femur heads, femur shafts, teeth, hair, and spleen tissues obtained from unidentified human corpses were measured by AMS to estimate their year of death and year of birth. The bomb peak was very useful because separating the victims from those that died after the war was a major issue in this case.
The main difficulty in determining the ages of these samples was that many of the body samples were preserved in formaldehyde (CH2O) for decades, as is usual for preservation of biological samples. The effect of formaldehyde on various samples was evaluated and several pretreatment methods were used to remove the influence of formaldehyde on the 14C age.
SAMPLES
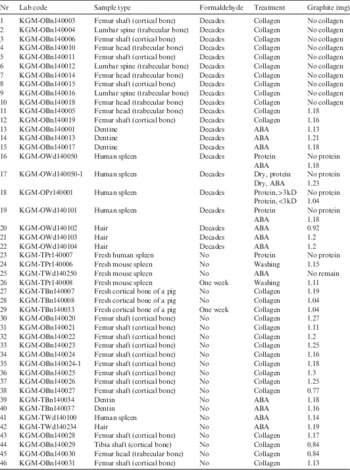
Twenty-two human samples preserved in formaldehyde were prepared to measure their 14C age. Seven of the samples were trabecular bones (femur heads and lumbar spines), and five were cortical bones (femur shafts). These bone samples give information on the year of death, and are the most frequently dated type of samples because they are preserved for a longer time and may contain sufficient amounts of collagen. There were three dentin samples of teeth, along with four spleen samples and three hair samples. All of these human remains were obtained from bodies of which the years of death were estimated to be in the 1960s except for one sample, which was obtained from 200-yr-old mummified remains.
Fresh human and mouse spleen samples and three fresh cortical bones of a pig preserved and not preserved in formaldehyde were prepared to evaluate the effect of formaldehyde.
An additional 17 human samples not preserved in formaldehyde were provided for age dating. Twelve of the samples were cortical bones (femur shafts and tibia shaft) and one was a femur head. The 14C ages of two fresh dentin samples, fresh human spleen tissue, and a hair sample were also measured. All the samples used in this work are listed in Table 1.
Table 1 Sample lists with chemical treatment methods and graphite sizes recovered after reduction. The graphite of each sample was made to a target without any remains. Reduction yields were over 95% for all samples, with the exception of samples 13 (94.1%) and 14 (91.5%).

CHEMICAL TREATMENTS
Bones (Collagen)
Bone samples were treated by a collagen-extraction method. To eliminate possible contamination, the bone surface was removed with 0.5 M HCl solution, rinsed by deionized water, and dried in an oven. The dried bone samples were powdered into 1–2 mm particles using a mortar agate, and 600 mg of bone powder was treated by a conventional ABA method using 0.5 M HCl solution and 0.1 M NaOH solution. Each step took 1 hr. The bone samples were then gelatinized by heating at 70°C for 12 hr in a pH 3 solution. The gelatin solutions were filtered using a 2.7-μm fiber filter to remove the remaining particles. The gelatin solutions were separated using a Centriprep (Merck Millipore Co.) filter into two parts, one with molecular weights below 30,000 dalton (30 kD) and the other with molecular weights above 30 kD. Those with molecular weights above 30 kD were dried by a freeze dryer.
Spleen
Two pretreatment methods, the protein extraction method and conventional ABA method, were applied to human spleen samples. A protein extraction method optimized for silk protein (Kim et al. Reference Kim, Southon, Imamura and Sparks2008) was tested for human spleen samples to remove the formaldehyde effect. The human spleen was dried and cut to a size of 1–2 mm. A 50-mL vial with 200 mg of spleen powder and 0.5 M HCl solution was shaken in a water bath at 60°C for 15 min. After three iterations of rinsing with deionized water, the solution was changed to 0.1 M NaOH, and the vial was shaken again at 60°C for 15 min. Finally, the spleen sample was shaken in 0.5 M HCl with the same conditions. After rinsing with deionized water, the spleen sample was moved to a 50-mL beaker, and 20 ml of 6 N HCl was added to the beaker. The beaker was then heated to 60°C for 20 hr. After heating, the solution was filtered using a 2.7-μm fiber filter to remove any remaining particles, and the filtered solution was diluted to a concentration of 1N with deionized water. The diluted solution was divided into three portions with molecular weights of below 3kD, 3–10 kD, and above 10 kD with a Centriprep filter. These three solutions were dried in an oven at 70°C. Because modern mouse spleens are so weak that nothing remains after the protein extraction and ABA method, these samples were simply washed with distilled water followed by drying.
Tooth (Dentin) and Hair
Because dental enamel is a very strong and stable material, enamel has a higher possibility of remaining in archaeological tooth samples than dentin. However, dentin contains a higher carbon content because 25% of its composition is collagen (Cook et al. Reference Cook, Dunbar, Black and Xu2006). Moreover, dentin is surrounded and protected by enamel and cementum so that the effects of environmental factors are minimized and normal hydration is maintained (Holtkötter Reference Holtkötter2011). Dentin is hence a better material for this work because most samples in forensic investigations are modern samples. The teeth were cut with a saw, and the dentin parts were collected from the cross section of the tooth samples using a scalpel. The dentin samples were ground into 1–2 mm powder and treated by the same ABA method as that applied to the bone samples. Soft tissues and hair samples were also treated using the same conventional ABA method as that for dentin samples.
Reduction
All the samples were combusted at 900°C in an elemental analyzer with an oxygen atmosphere to convert them to CO2 gas. The CO2 samples were collected in the cryogenic traps of a 24-fold automatic reduction system connected to the elemental analyzer. After several iterations of cryogenic purification, the CO2 samples were reduced to graphite with hydrogen and Fe catalyst at 600°C by using a KIGAM automatic reduction system (Hong et al. Reference Hong, Park, Kim, Woo, Kim, Choi and Kim2010). Graphite sizes were set to 1 mg, and the typical reduction time was 160 min. The typical reduction yield of all the samples was 95%. The graphite samples were measured for 14C with a 1 MV AMS of KIGAM.
RESULTS AND DISCUSSION
Formaldehyde Effect
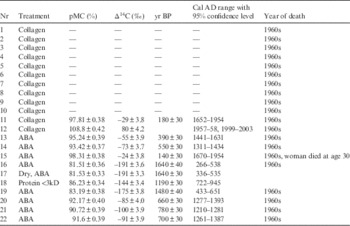
Results of AMS analyses performed on collagen extracted from 12 bone samples, which were obtained from humans who died in the 1960s, are presented in Table 2. Since the 1960s, all these samples were preserved in formaldehyde until recently. The Δ14C values in Table 2 were calculated by Equation (1), where fs is the fraction modern of the sample, and y is the year of the measurement, 2014 AD.
Table 2 Results of AMS measurements of human remains preserved in formaldehyde since the 1960s. IntCal 13 (Reimer et al. Reference Reimer, Bard, Bayliss, Beck, Blackwell, Bronk Ramsey, Buck, Cheng, Edwards, Friedrich, Grootes, Guilderson, Haflidason, Hajdas, Hatte, Heaton, Hoffmann, Hogg, Hughen, Kaiser, Kromer, Manning, Niu, Reimer, Richards, Scott, Southon, Staff, Turney and van der Plicht2013) and Bomb 13 NH2 (Hua et al. Reference Hua, Barbetti and Rakowski2013) data were used to calibrate the carbon ages.

Collagen with molecular weights above 30kD was not recovered from 10 of the 12 samples. The aldehyde group can combine with nitrogen and some other protein atoms, forming a cross-link CH2 –, called a methylene bridge (Kiernan Reference Kiernan2000). Cross-linked protein molecules were not dissolved by HCl during collagen extraction. The lack of collagen in the dissolved solution of bones is thought to be due to the collagen in the bone having been cross-linked by formaldehyde during the long preservation. Collagen was recovered from only two samples, samples 11 and 12. The 14C concentration of the trabecular bone, sample 11, indicates a pre-bomb era, which is prior to the year of death. However, the Δ14C value of the cortical bone, sample 12, indicates that the year of death is within the range of turnover time of cortical bone. The 14C ages of dentin samples 13 and 14 were much older than the collagen of bones, which means that teeth are more susceptible to attack by formaldehyde than bone. However, one dentin sample, sample 15, showed a Δ14C value of −24‰. This tooth was obtained from a woman who died in the 1960s, and her age at death is estimated to be 30 yrs old. Hence, her birth year should be in the 1930s, and the measured Δ14C value may agree with the year when her tooth was formed. Human hair samples 20–22 were more affected by dead carbon from formaldehyde than the tooth and bone.
The most severe effect is seen in soft tissues. All spleen samples (samples 16–19) treated by the protein extraction method failed to yield proteins with molecular weights of 3–10 kD as presented in Table 1. The 14C ages of the spleen tissues treated by the ABA method are much older than the estimated year of death. Samples 16 and 17 are the same samples, but sample 17 was dried in an oven at 40°C overnight before ABA treatment. The AMS measurement of these two samples presents identical results and it can be concluded that the formaldehyde effect is not removed by drying, even though formaldehyde is a volatile organic solution. This implies that formaldehyde forms chemical bonds with soft tissue. Hammer et al. (2012) and Fox et al. (Reference Fox, Johnson, Whiting and Roller1985) reported that the fixation with using formaldehyde causes an irreversible cross-linking process. Sample 18 obtained from the same sample treated by protein extraction had a molecular weight range below 3 kD. The influence of formaldehyde in this sample slightly decreased, but nonetheless remained. This is further evidence that formaldehyde bonds with the protein of the spleen.
To evaluate the effect of formaldehyde on various sample types, porcine bones and human and mouse spleen tissues were tested, and the results are summarized in Table 3. A fresh human spleen (sample 23) was treated by the protein extraction method, but protein in a molecular weight range of 3–10 kD could not be recovered from the tissue. The protein extraction method tested in this work was optimized to extract protein from silk, which mostly consists of sericin and fibroin, whereas spleen is derived from mesenchymal tissue, which contains reticular fiber (Vellguth et al. Reference Vellguth, Gaudecker and Muller-Hermelink1985). It appears that the dissolving conditions of this procedure are not suitable for the water-soluble protein of fresh spleen tissue. The conditions thus should be optimized for spleen protein. Fresh mouse spleen samples 24–26 were treated by the following three methods: (1) drying in an oven at 40°C overnight, (2) conventional ABA treatment, and (3) drying after preservation in formaldehyde for a week. The Δ14C of dried sample 24 was measured to be 13‰, which agrees well with the Δ14C value of 2014. Mouse spleen sample 25 was completely dissolved by the conventional ABA method. The tissue of the mouse spleen is likely weaker against alkali solution than that of the human spleen. Sample 26, which was preserved in formaldehyde for a week, presented a substantially different Δ14C of −93‰. The difference in the pMC values of samples 24 and 26 was 11%, which means that approximately 11% of carbon atoms contained in the spleen were exchanged with dead carbon atoms contained in formaldehyde within a week. Fox et al. (Reference Fox, Johnson, Whiting and Roller1985) reported that the binding process of formaldehyde with cells in rat kidneys is saturated within 18 hr at a room temperature of 37°C and less than 48 hr at 25°C.
Table 3 Results of formaldehyde tests with various biological samples. Intcal 13 and Bomb 13 NH2 data were used to calibrate the carbon age.

Collagen was successfully extracted from three cortical porcine bone samples: samples 27–29. A sufficient amount of collagen remained in sample 29, which was preserved in formaldehyde as shown in Table 1. It is thought that more than a week is needed to dissolve most of the collagen in cortical bone. However, the recovery of collagen decreased as a result of preservation in formaldehyde, as presented in Table 4. The recovery of the sample preserved in formaldehyde for a week was one-third that of the fresh samples. This is why collagen was not recovered from the bone samples preserved in formaldehyde for 50 yr. The Δ14C values of collagen samples (samples 27 and 28) extracted from two fresh porcine cortical bones were very close, but they were slightly different from the Δ14C value of sample 29, which was preserved in formaldehyde for a week. However, the difference was much smaller than that obtained for the spleen. The influence of preservation in formaldehyde for a week on soft tissue was 14 times larger than that for cortical bone. The large difference between the soft tissue and cortical bone can be explained by the difference in the permeability of formaldehyde into the biological tissue. The penetration of formalin into biological tissue is a physical process by which the solution diffuses into the specimen to reach the innermost layers of cells (Thavarajah et al. Reference Thavarajah, Mudimbaimannar, Elizabeth, Rao and Ranganthan2012). The physical density of bone is much higher than that of soft tissue, and the permeability of formaldehyde is also higher in soft tissue than bone samples.
Table 4 Comparison of collagen recovery of cortical bone preserved in formaldehyde for a week with the recoveries of fresh bones.

Estimation of the Year of Death and the Year of Birth
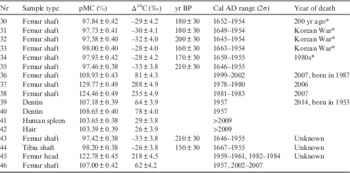
Because formaldehyde is chemically bonded with the organic materials of the samples and changes the molecular forms, only the ages of the samples not preserved in formaldehyde could be successfully measured. The results of the 14C measurements of human remains are presented in Table 5. All the samples listed in Table 5 were not preserved in formaldehyde. Sample 30 is a femur shaft obtained from mummified remains with an estimated age of 200 yr. The radiocarbon age of this sample agrees well with the estimation. Samples 31–33 are femur shafts from corpses estimated to be victims of the Korean War. Estimating the year of their death is difficult because the bomb peak cannot be applied to age calibration for these samples, as it is clear that they died before the bomb peak era. Their Δ14C values are similar to the Δ14C values for the 1930s–1940s. Ubelaker et al. (2011) reported that the turnover time of a femur is longer than 11 yr. On the other hand, Hedges et al. (Reference Hedges, Clement, Thomas and O’Connell2007) reported the turnover rate of femur collagen as 3–4%/yr for adult females and 1.5–3%/yr for adult males. The center value for females is equivalent to a turnover time of 28.6 yr. These ages precede the year of death of the bodies by 11–30 yr. The results appear to support the estimation that they are victims of the Korean War. The estimated year of death of the body from which sample 34 was obtained is around 1980 AD. The Δ14C of this collagen sample was very low compared with the estimate. The Δ14C of sample 35, which is collagen extracted again from the same bone as sample 34, was also measured to be similar. It is likely that the estimation for this sample is wrong considering that it is unlikely that collagen could be contaminated twice during the extraction procedure. The Δ14C values of samples 36–38 indicate age ranges of 1999–2002 AD, 1978–1980 AD, and 1981–1983 AD, respectively. The ages of samples 37 and 38 are within the turnover time from the year of death.
Table 5 Determining the results of years of death and birth of human remains. Intcal 13 and Bomb 13 NH2 data were used to calibrate the carbon ages.

* Estimated values.
Samples 39–42 are samples obtained from a male who was born in 1953 and died in 2014. The ages of the two dentin samples were measured to be 1957 AD. These results agree well with the year of birth of the male. Spleen and hair samples treated by the ABA method were measured to be originate from post 2009 AD, and these results also agree well with the year of death of the male because the turnover times of soft tissue and hair are very short. Keratin protein from hair is a good sample candidate for determining the year of death because of its short turnover time. However, the use of cosmetics and coloring dyes can affect the 14C age of hair (Santos et al. Reference Santos, Torre, Boudin, Bonafini and Saverwyns2015). The results obtained from this male corpse demonstrate the high confidence of the 14C age dating technique in forensic investigation. Samples 43 and 44 are femur shaft and tibia from bodies excavated in Seoul. Their Δ14C values imply that these corpses are victims of the Korean War. Samples 45 and 46 are the femur head and femur shaft from a corpse. Their Δ14C values were measured to be 218‰ and 62‰, respectively. The corresponding ages of the Δ14C of the femur head of sample 45 are 1959–1961 AD or 1982–1984 AD, while it is 1957 or 2002–2007 AD for the femur shaft of sample 46. The turnover rate of the femur head (trabecular bone) is slower than that of the femur shaft. Because the Δ14C of the femur head with a shorter turnover time is higher than that of the femur shaft with a longer turnover time, the bones formed before 1964 AD when the Δ14C of the bomb peak marked its maximum value. Hence, it is clear that 1959–1961 AD should be used for the femur head, and 1957 AD should be used for the femur shaft. The year of death of this person is estimated to be 1968–1987 AD with the turnover time of the femur shaft being 11–30 yr.
CONCLUSION
Victims of the Korean War were ascertained by year-of-death measurement using 14C dating. For samples preserved in formaldehyde, sample preparation was not easy because formaldehyde not only destroys organic components such as protein and collagen, but also chemically bonds to the components. Therefore, samples preserved in formaldehyde showed older ages due to chemical bonding with dead carbon in the formaldehyde made of fossil material. Chemical pretreatment methods to remove the influence of formaldehyde, such as collagen extraction and protein extraction, were tested in this work, but they failed to completely remove the formaldehyde influence, and the results were not satisfactory. The influence of formaldehyde was strongest in soft tissue, with decreasing effect on hair, dentin, trabecular bone, and cortical bone. Biological remains are frequently preserved in formaldehyde for long-term conservation, but in cases when 14C measurement is needed, preservation in formaldehyde should be avoided.
However, the 14C dating technique shows good performance for samples not affected by formaldehyde. In particular, two samples with different turnover times for a body can provide an easy way to reduce errors in measurement. To improve the accuracy in estimating the year of death, studies on the turnover times for various parts of the body should be performed. Research on the dependency of Δ14C on diet is also needed. KIGAM and NFS are planning a project to study these subjects together.
Acknowledgments
This work was supported by the Ministry of Science, ICT and Future Planning of Republic of Korea.