INTRODUCTION
Cyanobacteria are photosynthetic microorganisms, which were previously considered as a peculiar group of simple algae, hence their other name, blue-green algae. However, cyanobacteria lack a membrane-bound nucleus, chloroplasts, and other organelles found in eukaryotes, and are therefore classified with bacteria in the prokaryotic kingdom (Stanier and Bazine, Reference Stanier and Bazine1977; Baldauf and Palmer, Reference Baldauf and Palmer1990). Consequently, many bioconstructions previously considered as algal mats are nowadays interpreted as the products of cyanobacterial activity, and are called biomats (Carr and Whitton, Reference Carr and Whitton1982). Microbial mats are common in marine habitats, lakes, and coastal settings (Noffke et al., Reference Noffke, Christian and Wacey2013; Cristina et al., Reference Cristina, Barajas and Cantero2018). Their occurrences are documented in coastal areas of the USA (Cameron et al., Reference Cameron, Cameron and Jones1984), the North Sea (Gerdes and Krumbein, Reference Gerdes and Krumbein1987; Noffke et al., Reference Noffke, Gerdes, Klenke and Krumbein2001b), the Persian Gulf (Kendall and Skipwith, Reference Kendall and Skipwith1969; Kinsman and Park, Reference Kinsman and Park1976; Park, Reference Park1977), at Shark Bay in Western Australia (Logan et al., Reference Logan, Hoffman and Gebelein1974), and on the southern North Sea coast of Germany (Noffke et al., Reference Noffke, Gerdes, Klenke and Krumbein1996; Gerdes et al., Reference Gerdes, Klenke and Noffke2000). Along the Mediterranean coast, they have been observed in Aqaba Bay in Egypt (Aref, Reference Aref1998) and in the northwest of the Mediterranean Sea in the Ebro River Delta (Albert et al., Reference Albert, Cabeza, Masqué, Martínez-Alonso, Mir and Esteve1999).
In Holocene sediments at Sabkha Boujmel, southeastern Tunisia, these structures were first described as algal mats (Lakhdar, Reference Lakhdar1987), and then renamed as microbial mats by Noffke et al. (Reference Noffke, Gerdes, Klenke and Krumbein2001a). Living microbial mats have been studied at Bahar Alouane and Sabkha Boujmel (Gerdes et al., Reference Gerdes, Klenke and Noffke2000; Noffke et al., Reference Noffke, Gerdes, Klenke and Krumbein2001b). Gerdes et al. (Reference Gerdes, Klenke and Noffke2000) studied and compared temperate humid (Milleum, Germany) and subtropical arid (Bahar Alouane and Sabkha Boujmel, southeast Tunisia) peritidal environments. These authors reveal the basic types of microbial biostromes and biofilms, and demonstrate how biogeochemical microbial processes fundamentally control the development of sedimentary structures. Referring to their genesis, Noffke et al. (Reference Noffke, Gerdes, Klenke and Krumbein2001b) and Noffke (Reference Noffke2010) have introduced five categories of microbial activity, each of which forms a set of characteristic MISS.
This study aims to: (1) provide cartographic support by illustrating the spatial distribution of microbial mats on the southeastern Tunisian coast; (2) establish the relationship between the coastal morphology, tidal dynamics, and development of the mats; (3) describe and interpret the sedimentary structures resulting from the interplay of cyanobacterial activity, climate, and variability in physicochemical conditions. These features, which have been already presented by Porada et al., (Reference Porada, Bouougri, Ghergut, Schieber, Bose, Eriksson, Banerjee, Sarkar, Altermann and Catuneanu2007) and by Lakhdar and Soussi (Reference Lakhdar and Soussi2007), will be tentatively placed on a theoretical coastal profile, and the microbial mats compared with their fossil equivalents, which have been described in several regions of the world and through all geological ages, especially the Neoproterozoic (1000 Ma) (Porada and Löffler, Reference Porada and Löffler2000; Bouougri and Porada, Reference Bouougri and Porada2007) and in the Precambrian of central India (Singh and Wunderlich, Reference Singh and Wunderlich1978).
Geologic and oceanographic settings
Southern Tunisia has a vast coastal plain, which belongs to the Jeffara maritime lowland, lying between the Mesozoic Dahar reliefs to the west and the Mediterranean Sea to the east (Supplementary Figure 1). This plain comprises the el Jorf, Akara and Draa el Hnèche morphological units, which separate several depressions or bahiras (el Kantara, el Boughrara, and el Bibane) connected by tidal channels to the open sea (Fig. 1). These bahiras or lagoons are linked to several Sabkhas which correspond to Holocene-filled restricted lagoons (Perthuisot, Reference Perthuisot1975; Lakhdar, Reference Lakhdar1987; Marquer et al., Reference Marquer, Pomel, Abichou, Schulz, Kaniewski and Van Campo2008). The latter correspond to post-Villafranchian tectonic depressions submerged during the later Pleistocene and Holocene marine invasions.
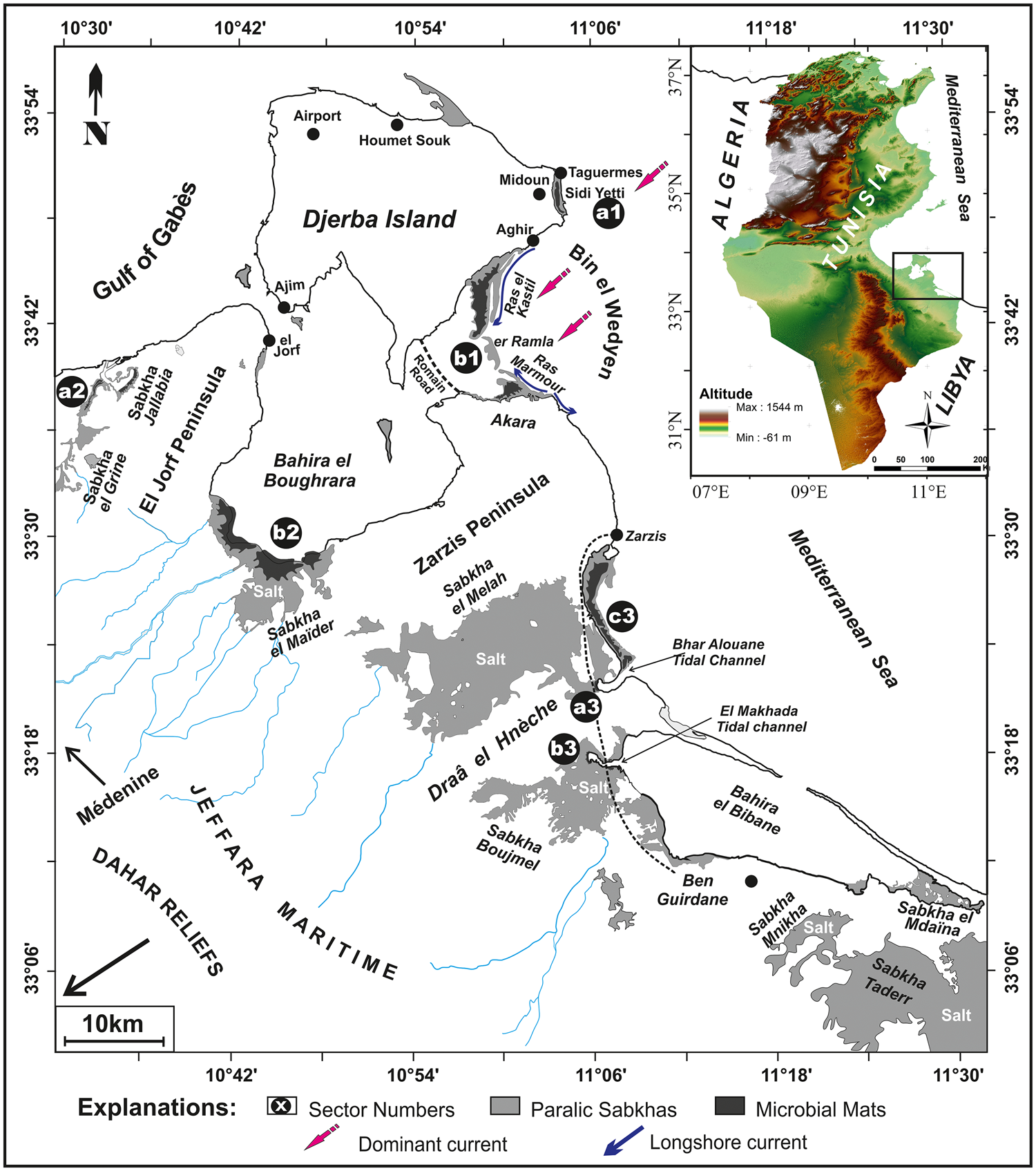
Figure 1. (color online) The study area and the distribution of the microbial mats along the southern Tunisian coast mapped areas.
The coastal dynamics of this western part of the Mediterranean Sea are dominated by waves reaching the shore, where they become steep and affect the seabed in the breaker zone (Masmoudi et al., Reference Masmoudi, Yaich and Yamoun2005; Sammari et al., Reference Sammari, Koutitonsky and Moussa2006). The most exposed coastlines are those on the west and north of Djerba Island and the northeast of Zarzis. Djerba Island is surrounded by a large shallow marine platform with a 10 m isobath located 5 km from the coastline (Supplementary Figure 1) (Paskof and Sanlaville, Reference Paskoff and Sanlaville1977; Ben Haj Ali, Reference Ben Haj Ali, Jedoui, Dali, Ben Salem and Memmi1985). The study area is part of a semi-diurnal tide system where Djerba Island acts as a shock absorber of wave action. The high tide amplitude varies between 0.40 and 2 m (Brahim et al., Reference Brahim, Atoui, Sammari and Aleya2014). During the equinoxes, the tidal amplitude can reach 1 m. The duration of each tide is 6 hours, and each tidal cycle includes approximately 10 to 11 days of spring tide and 4 to 5 days of neap tide. Longshore and rip currents develop under the action of the waves.
The study area is characterized by an arid climate, strongly influenced by the proximity of the sea, with annual rainfall ranging from 150 to 200 mm (Mekrazi, Reference Mékrazi1975; Ellouze et al., Reference Ellouze, Azri and Abida2009). The prevailing winter winds are from the western sector, while eastern sector winds predominate in the summer (Perthuisot, Reference Perthuisot1975).
The biomats, which are the subject of this study, are linked to the Sabkha as well as the spits and barrier island system of the Bin el Wedyen sector. During spring tides, the maximum flooding reaches the supratidal areas, keeping the cyanobacteria alive (Supplementary Figures 2a and 2b). It is important to note that the microbial mats situated within the moistened area are always more developed and denser, even during neap tides and low current energy.

Figure 2. (color online) Microbial mats distribution map of the Sidi Yetti coastline (a1) and Bin el Wedyen system (b1) showing organic matter analyzed sample positions (e1, e2, e3 and e4) and A–B; C–D profile cross sections.
METHODS
For the examination of the microbial mats' growth and death, several visits were made during spring and neap tides. They were mapped based on topographical maps and aerial photographs covering the study area. The Geographic Information System (GIS) software ArcView GIS 3.2 was used after scanning the cartographic documents to identify and map the microbial mats. A Global Positioning System (GPS) navigator (Garmin 12 with parallel channel) was used for the positioning (geographic coordinates of the MISS). These are corrected and then introduced under different themes.
Several excavations of 20 to 80 cm depth were dug using a shovel. When it was necessary, cores were taken from the surface sediments, by hammering in PVC tubes of 7.5 cm diameter. During the release of the core, the sediments were retained inside the tube by an “orange peel” bronze fixed at its base. We adopted the taxon determinations made by Gerdes et al. (Reference Gerdes, Klenke and Noffke2000), and the latest MISS classification presented by Noffke et al. (Reference Noffke, Gerdes, Klenke and Krumbein2001b).
Dissolved organic carbon (DOC) within the modern microbial mats was determined by wet chemical oxidation, according to standard NF EN 1484. The carbon dioxide (CO2) formed was measured using an infrared spectrophotometer. The phosphate content was determined by visible spectrophotometry at a wavelength of 720 nm, according to the method proposed by Michelsen (Reference Michelsen1957). Nitrogen (N) content was determined by the Kjeldahl (Reference Kjeldahl1883) method. The spectrophotometry and the structural analysis of organic complexes were determined by the absorption spectra (Quevauville et al., Reference Quevauviller, Thomas and Derbeken2006). The microbiological study was based on the seeding bacteria technique in a specific growing medium (Petri dish). Since bacterial proliferation is selective, after incubation counting was done by the most probable number method.
RESULTS
Spatial distribution of the microbial mats (base map)
Cyanobacterial mats are organized in continuous belts lying parallel to the shoreline. They can extend to the peritidal zone where they shrink in thickness, and extend to end in dendrites in the upper supratidal zone. From one area to another, important variations were observed in the extension of the microbial mat, its color and thickness, and the state of the associated MISS.
Area 1: Eastern Djerba Island / Open Marine Coastal System
Sidi Yetti protected lagoon setting (a1)
At Sidi Yetti, 5 km east of Djerba Midoun village, a tidal channel called es Saguia bypasses the bordering dunes of the back beach and then empties to the south in the lagoon-tidal flat area. The latter is partly occupied by microbial mats (Fig. 2a1) which lie on the eastern es Saguia tidal channel and extend behind the beach. The western tidal channel side, along the el Kantara-Aghir road, is bordered by a narrow supratidal zone about 15 m wide (Fig. 2a1). The east-west oriented AB simplified profile illustrates the area of development of the microbial mats with respect to the morphology of the system (Fig. 2). The first belt bordering the tidal channel is governed by a green biomat, while the second one, situated towards the north, is represented by pink species in the lower supratidal zone of the tidal plain.
Bin el Wedyen - barrier island and spits system (b1)
The Bin el Wedyen area is composed of the Ras el Kastil and Ras Marmour former littoral spits, separated by the er Ramla Island barrier which limits the exchange between the Bahira el Boughrara and the Mediterranean Sea (Fig. 2b1). The Ras el Kastil spit, which is 7 km long, corresponds to a sedimentary accumulation anchored to Djerba Island in the north but free towards the south (Oueslati, Reference Oueslati1995; Masmoudi et al., Reference Masmoudi, Yaich and Yamoun2005). The adjacent microbial mat is dominated by dark green species such as Microcoleus chthonoplastes, Aphanothece spp., and Lyngbya aestuarii (Gerdes et al., Reference Gerdes, Klenke and Noffke2000). It stretches from the subtidal–intertidal transition zone to the supratidal zone, where the microbial mats become a pink-orange color represented by Entophysalis granulose and Pleurocapsalean spp. (Gerdes et al., Reference Gerdes, Klenke and Noffke2000). South of the Ras Marmour spit, which is attached to the Zarzis Peninsula (Akkara) (Oueslati, Reference Oueslati1995; Masmoudi et al., Reference Masmoudi, Yaich and Yamoun2005), the microbial mat is dominated by green species consisting of Microcoleus chthonoplastes, Aphanothece spp., and Lyngbya aestuarii (Gerdes et al., Reference Gerdes, Klenke and Noffke2000) in the intertidal zone, and pink-orange species, represented by Entophysalis granulosa and Pleurocapsalean spp., in the lower supratidal zone.
Along these two spits, the microbial constructions seem to be less developed in the middle supratidal zone than in the lower supratidal and upper intertidal zones. The spaces left between halophilic vegetation along the shoreline are also occupied by these microbial mats. The NE-SW oriented CD profile illustrates the distribution of the microbial mats with respect to the morphology of the Bin el Wedyen system (Fig. 2).
Area 2: El Grine / Jallabia marginal restricted Sabkhas system
El Grine / Jallabia Sabkhas (a2)
The Sabkha el Grine is a paralic Sabkha connected to the adjacent el Kantara small lagoon. Here, the microbial mat substrate corresponds to a very fine sandy material accumulated on both sides of a north-south oriented el Grine tidal channel. The asymmetric occurrence of the microbial mat could be explained by the abundant terrigenous detrital material coming from the mainland. Secondary tidal channels, as well as areas left by tufts of samphire, are also covered by the microbial mats (Fig. 3a2). The mat itself is dominated at this site by species of pink-orange color (Entophysalis granulosa and Pleurocapsalim spp. ,Gerdes et al., Reference Gerdes, Klenke and Noffke2000), which generate an obviously cauliflower-like structure.

Figure 3. (color online) Microbial mats distribution map of el Grine-Jallabia Sabkhas (a2) and Bahira el Boughrara lagoon (b2).
At the Sabkha of Jallabia, the microbial mats are also dominated by a pink-orange color, and form a discontinuous band of very limited extension on the southern edge of the el Kantara lagoon. The latter communicates with the Mediterranean Sea through the el Grine tidal channel, which has two branches. The main one irrigates the lagoon, while the second pours into the Sabkha el Grine (Fig. 3a2). It is worth noting that a newly built road has obliterated this secondary channel and split the Sabkha el Grine into two parts. The microbial mats of the southern part of the Sabkha have almost disappeared and are only represented by a few relics.
Boughrara lagoon / Sabkha el Maider (b2)
El Boughrara lagoon (also named Bahira el Boughrara) communicates with the Mediterranean Sea through two inlets installed on both southern sides of Djerba Island (Fig. 1). The first one is situated between Aghir and Ajim, and the second extends from the Ras el Kastil to Ras Marmour spits. Recently, the latter inlet was also obliterated by the construction of the el Kantara Bridge (roman road: MC117) linking Zarzis to Djerba. Only a single 20 m wide pass has been laid out between the lagoon and the Mediterranean Sea (Fig. 2b1).
The tidal flat along the mainland side of the lagoon is colonized by a microbial mat extending parallel to the shore line from the subtidal to the supratidal zones. Here, the mat forms a discontinuous belt matching the shape of the lagoon's edge (Fig. 3b2). In this area, the microbial mat is characterized by a predominantly pink-orange coloration, and shows on its surface alveolar structures which may be due to gas exhaust. Once dried, it forms bulges which separate the mat from its underlying substrate. At this site, at several places in the upper intertidal zone, we observed accumulations of Posidonia leaves encrusted by cyanobacteria filaments. In front of the mouths of the distributaries reaching the Bahira el Boughrara, the microbial mats disappear because of the terrigenous detritus influx carried by the wadis.
Area 3: South of Zarzis / Ben Guirdane depression
Bahar Alouane tidal channel / Sabkha el Melah (a3)
At this site, the upper supratidal area is crossed by the Zarzis-Ben Guirdane road, and there remains only a narrow belt of the supratidal area and the intertidal zone (Fig. 4a3). The tidal creeks incising the latter are also lined by a microbial mat showing erosional structures caused by tidal currents. At Bahar Alouane, eastward of the Zarzis-Ben Guirdane road, along both sides of an artificial channel feeding the Sabkha el Melah with sea water, an important microbial mat can be observed. This is dominated by gray and locally greenish species, stretching along the intertidal zone. On the south shore of Bahar Alouane, isolated small ponds maintaining filamentous microbial communities are also observed.

Figure 4. (color online) Microbial mats distribution map of the Bahar Alouane (a3) and the Sabkha Boujmel (b3) areas.
El Makkada tidal channel / Sabkha Boujmel (b3)
The Sabkha Boujmel microbial mat was first described by Lakhdar (Reference Lakhdar1987). It was explored in detail by Gerdes et al. (Reference Gerdes, Klenke and Noffke2000), who described the microbiological signatures, and then by Noffke et al. (Reference Noffke, Gerdes, Klenke and Krumbein2001a), who studied the MISS and determined the major taxa and species of the biomats. Lakhdar et al. (Reference Lakhdar, Soussi, Ben Ismail and M'Rabet2006) demonstrated that the more recent microbial mats' eastward migration is an indicator of the progradation of the intertidal paleo-setting (Supplementary Figure 3). Here, the microbial mat bypasses the western edge of the el Makhada tidal channel, and extends into the upper subtidal zone (Fig. 4b3). The continuous seasonal examination of the microbial mat clearly indicates that it has been significantly reduced since 1987. This reduction could be attributed to the increase of the supratidal zone, which has replaced the intertidal zone on which the microbial mat preferentially proliferates. This is mainly caused by the spread of the detrital deposits carried by the adjacent wadis. However, the lower intertidal zone is characterized by a thick microbial mat, sometimes exceeding 4 cm in thickness.
South of Zarzis / Sabkha el Melah (c3)
This area shows the most important geographical extension of the microbial mat, which can reach about 6 km2 in surface area (Fig. 5c3). It covers an area extending from southern Zarzis town to the east, to the Bahar Alouane tidal channel to the west. Going from north to south, the topography of the coastline gently decreases landward and controls the flooding of the area by tidal currents becoming parallel to the mainland (Fig. 5). In the central part of this area, especially during spring time, a wide intertidal zone develops close to the mainland, where the microbial mat is dominated by filamentous greenish species, represented by Microcoleus chthonoplastes, Aphanothece sp., and Lyngbya aestuarii. Only small patches of microbial mats exist in the supratidal areas, where they are consistently of a pink-orange color composed of Entophysalis granulosa and Pleurocapsalean species (Gerdes et al., Reference Gerdes, Klenke and Noffke2000).

Figure 5. (color online) Microbial mats distribution map of South of Zarzis coastline (c3) and A–B profile cross sections.
Factors controlling the composition and distribution of microbial mats
The detailed mapping shows that from the continent towards the sea, the distribution and composition of the microbial mats are not random. Indeed, the dark blue-greenish color, characterizing the thick endobenthic microbial mats of the intertidal zone, is due to the abundance of highly mobile filamentous microbes represented by Microcoleus chthonoplastes, Lyngbya aestuarii and Phormidium fragilis (Noffke et al., Reference Noffke, Gerdes, Klenke and Krumbein2001a). These species are known to be able to drill up quickly through the sediments whenever this area is reworked by the tidal currents, as it constantly is (Decho, Reference Decho1990). However, the rose-to-pink color of the microbial mat in the supratidal zone is linked to the presence of a specific carotenoid pigment produced by coccoid cyanobacterial taxa, where Synechococcus sp. abounds (Gerdes et al., Reference Gerdes, Krumbein, Reineck, Krumbein, Paterson and Stal1994). It is thought that the abundance of secreted extracellular polymeric substance (EPS) protects these species from desiccation and osmotic pressure (Villbrandt, Reference Villbrandt1992).
The continuous seasonal examination of the microbial mats on the southeastern Tunisian coast demonstrates that their distribution is essentially controlled by the tidal dynamics, which ensure that peritidal areas are continuously supplied by marine waters rich in mineral salts and nutrients. In the different study areas, the microbial mats are organized in successive, usually parallel, belts of different colors. The first belt corresponds to the zone constantly flooded by the tide. It is colonized by thick mats of greenish, filamentous species. The second belt, which forms the lower supratidal and Sabkha zones, is flooded only during spring tides and storms, and is colonized by pink-orange coccoidal species. The bodies of water are routed through tidal channels both during the flood and the ebb. In some cases, the topography controls the dynamics of the water, resulting in a local zonation dependent on the position of the supratidal zone between the sea and the intertidal zone, as is the case at Sidi Yetti and south of Zarzis (Figs 2a1 and 5c3).
It is worth noting that the microbial mats studied by Gerdes et al. (Reference Gerdes, Klenke and Noffke2000) at Mellum Island in Germany, and belonging to the first belt, are dominated by Merismopedia punctata and Oscillatoria limosa, while Microcoleus chthonoplastes dominates the second belt.
Occurrences of MISS
The different MISS identified within the microbial mats are described and interpreted in the following sections, based on the classification by Noffke et al. (Reference Noffke, Gerdes, Klenke and Krumbein2001a). These structures arise from the interplay between growth, biostabilization, baffling and trapping, and binding processes, or from the effects of microbial activity (Noffke, Reference Noffke2010).
Structures resulting from microbial growth
Honeycomb structure
The honeycomb structure corresponds to a network of cells lining the surface mat (Fig. 6A), showing a striking similarity to the honeycombs described by Häntzschel and Reineck (Reference Häntzschel and Reineck1968). It has been observed at Bahira el Boughrara and Sabkha el Grine. The rectilinear boundaries of the cell structures are distinct from those resulting from gas leaks. A very intricate honeycombed ribbon-structure, related to a microbial fossil, was documented in the dry paleopools of Hidden Cave, NM, and exhibiting a typical hexagonal structure (Boston et al., Reference Boston, Spilde, Northup, Melim, Soroka, Kleina and Lavoie2001). Such a structure, induced by cell replication, is preserved by rapid in situ lithification of organic material.
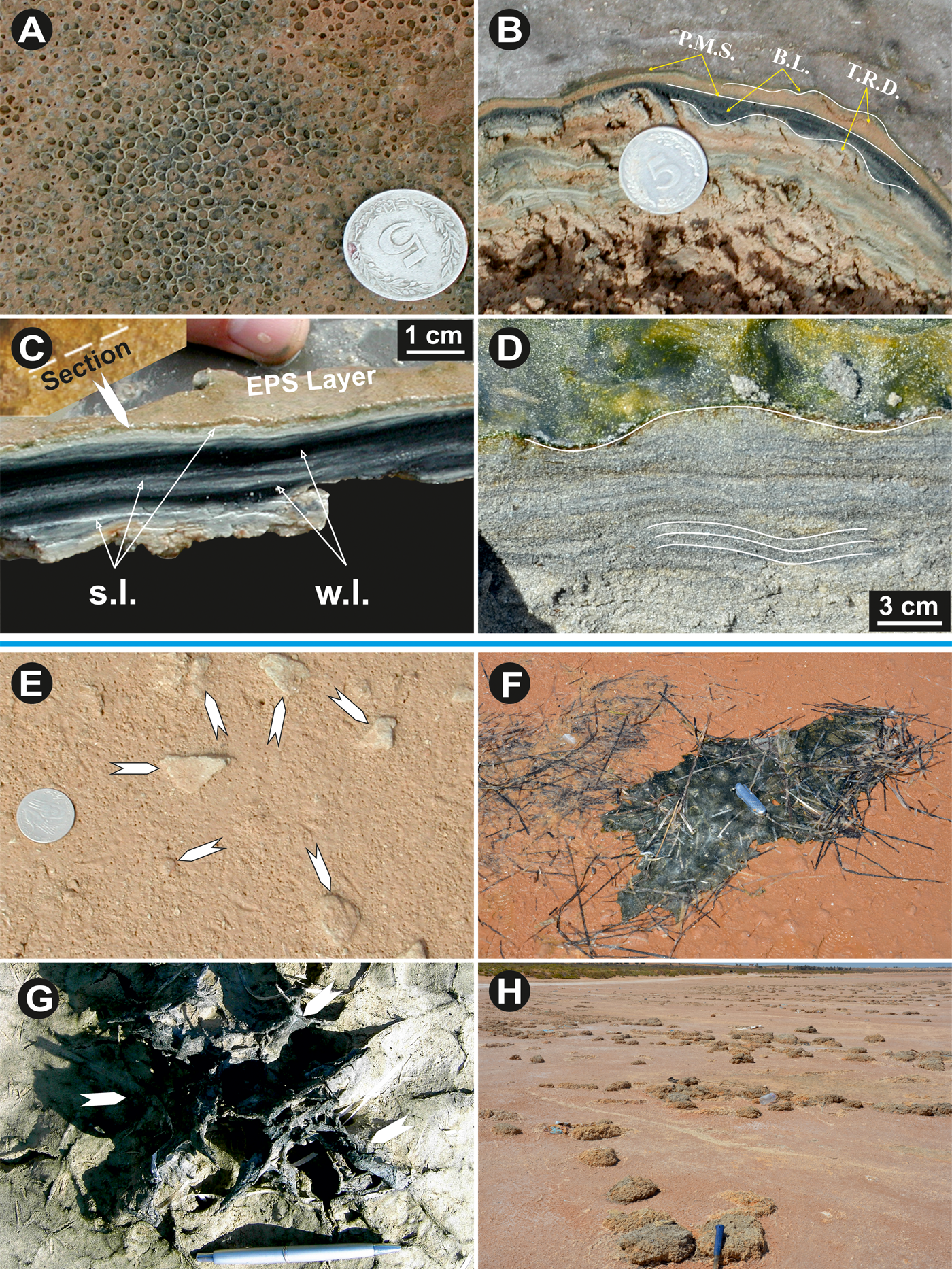
Figure 6. (color online) (A) Honeycomb structure (Site: Sabkha el Grine). (B) Vertical cross section showing alternation of microbial mats and sediment layers giving rise to a bedding growth (Bahira el Boughrara lagoon). (C) Living microbial mat vertical cross section on the el Makhada tidal channel edge (Sabkha Boujmel). EPS layers upon the mat surface and alternations of clear lamina (coccoid summer cyanobacteria) and dark lamina (filamentous winter cyanobacteria). (D) Intertidal ripple biostabilized by Oscilatoria. limosa making them visible because of the biofilm. (E) Mat fragments being buried (white arrows, upper intertidal Sabkha Boujmel area). (F) Mat chips amalgamated with high seas leashes and rejected backward (lower supratidal Boughrara). (G) Mats chips trapped by a halophile vegetation tuft (Ras el Kastil). (H) Meadow mat ships flap composed of amalgamated mat fragments with remains of high seas leashes (storm evidence), (upper supratidal Bahira el Boughrara). Coin diameter is 24 mm; P.M.S.: Planar Mat Surface; B.L.: Biolaminated Levelling; T.R.D.: Tidal Rippled Deposit; s.l.: summer laminae; w.l.: winter laminae.
Laminated leveling structure
The so-called laminated leveling structure is a continuous epibenthic mat lamina covering a prior rippled sedimentary surface (Fig. 6B) (Noffke, Reference Noffke2010; Noffke et al., Reference Noffke, Gerdes, Klenke and Krumbein2001a). A vertical cross-section through this structure shows that the microbial mat is composed of alternating dark, organic-rich, winter laminae, and white, organic-poor, summer laminae, built predominantly by the cyanobacterial species Synechnococcus sp. and Microcoleus chthonoplastes, respectively (Gerdes et al., Reference Gerdes, Klenke and Noffke2000) (Fig. 6C). Such stacks of organic-poor and organic-rich layers, where EPS amounts enhance carbonate mineral precipitation, are called biovarvites (Gerdes et al., Reference Gerdes, Krumbein, Reineck, G. Einsele, Ricken and Seilacher1991). The sand layers existing in between the laminae indicate burial phases of the previous mat biomass (Fig.s 6C and D).
Growth bedding forming biolaminites needs a sufficient time of about several weeks of non-deposition (Gerdes and Klenke, Reference Gerdes and Klenke2003). These structures have been documented at Gavish Sabkha, Gulf of Aqaba, Egypt (Gerdes, Reference Gerdes, Klenke and Noffke2000). Synechnococcus has UV-blocking pigments (carotenoids) which protect it in summer, while Microcoleus chthonoplastes is able to endure the poorer light conditions of the winter months (Noffke, Reference Noffke2010). They clearly indicate the alternation of a hot and arid seasonal climate.
Structure arising from microbial binding
Sinuous structures
This Sinuous structure corresponds to an organic, mesh-like network, controlled only by sedimentological processes where biomass production by cell replication does not play a great role, and the EPS may or may not be involved (Noffke, Reference Noffke2010). Trenches dug in tidal flats show sinuous-shaped laminae and very small ripple marks overgrown by Oscillatoria limosa cyanobacteria. Buried ripple marks, troughs, and lee faces are coated by a thin microbial mat. These ripple marks are thereafter protected from erosion and reworking by subsequent flooding, as they are relatively biostabilized. Sinuous structure is observed in trenches dug in the Sidi Yetti sector (Fig. 6D), showing an upper surface of linguoid ripple marks overgrown by a thin, dark blue-green microbial mat.
Structures arising from biostabilization
Biostabilization is the response of benthic cyanobacteria against hydrodynamic erosion. The epibenthic and endobenthic cyanobacteria and biofilms make soft sediments flexible and cause the sealing of the sedimentary surface. The simultaneous action of the EPS in reducing gas exchange between the deposits and the water or atmosphere above, and horizontally oriented cyanobacterial filaments, leads to a more erosion-resistant phase (Noffke, Reference Noffke2010).
Mat chips
Mat chips are cm-scale fragments of microbial mats. Noffke (Reference Noftke1997), studying mat chips on Mellum Island (southern North Sea), distinguished those formed by epibenthic microbial mats (type I) from those formed by endobenthic microbial mats (type II). Under the action of tidal currents, waves and storms, the margins of microbial mats can be detached from their siliciclastic bedrock (Fig. 8D), giving rise to mat chips. The fragments are blown randomly by the wind, and/or moved by floods and storms, to be trapped and re-sedimented in the intertidal (Fig. 6E) and/or supratidal areas (Fig. 6F). Chips can be trapped by bordering vegetation, as is the case at Ras el Kastil (Fig. 6G). Such structures have been described at Ras el Kastil, Sabkha Boujmel, Bahar Alouane and Bahira el Boughrara. These fragments can be mixed and amalgamated by high seas, and thrown towards the farthest supratidal zones during storms or spring tides, to form a “meadow” of chips initially encrusted by EPS and then cemented by evaporation of their saturated salt water content, as is the case at Bahira el Boughrara (Fig. 6H). Gerdes et al. (Reference Gerdes, Klenke and Noffke2000) found the same structure at Mellum Island. In the fossil record, mat chips constitute precious tools for recognizing storm activities and current directions (Noffke, Reference Noffke2010).
Mat curls (roll-up structures)
The term “mat curl”, designating bent or even rolled-up mat chips, was introduced by Noffke et al. (Reference Noffke, Gerdes, Klenke and Krumbein1996). Two types of mat curls are distinguished (Noffke, Reference Noffke2010). Since the mat and sand layers react differently to desiccation, the microbial mats shrink more than the drier, sandy layer underneath, giving rise to a mat curl or rolled-up structure (Noffke et al., Reference Noffke, Gerdes, Klenke and Krumbein1996; Noffke, Reference Noffke2010). These structures are the result of biostabilization, and their presence is good evidence of a strong storm or hurricane. The type I mat curls (Fig. 7A) indicate subaerial exposure of the tidal area, while the type II curls (Fig. 7B) indicate strong episodic storms that push seawater far into the supratidal zone (Noffke, Reference Noffke2010). The type II structures are widespread in all the studied zones, but have been identified especially in the Ras Kastil area.
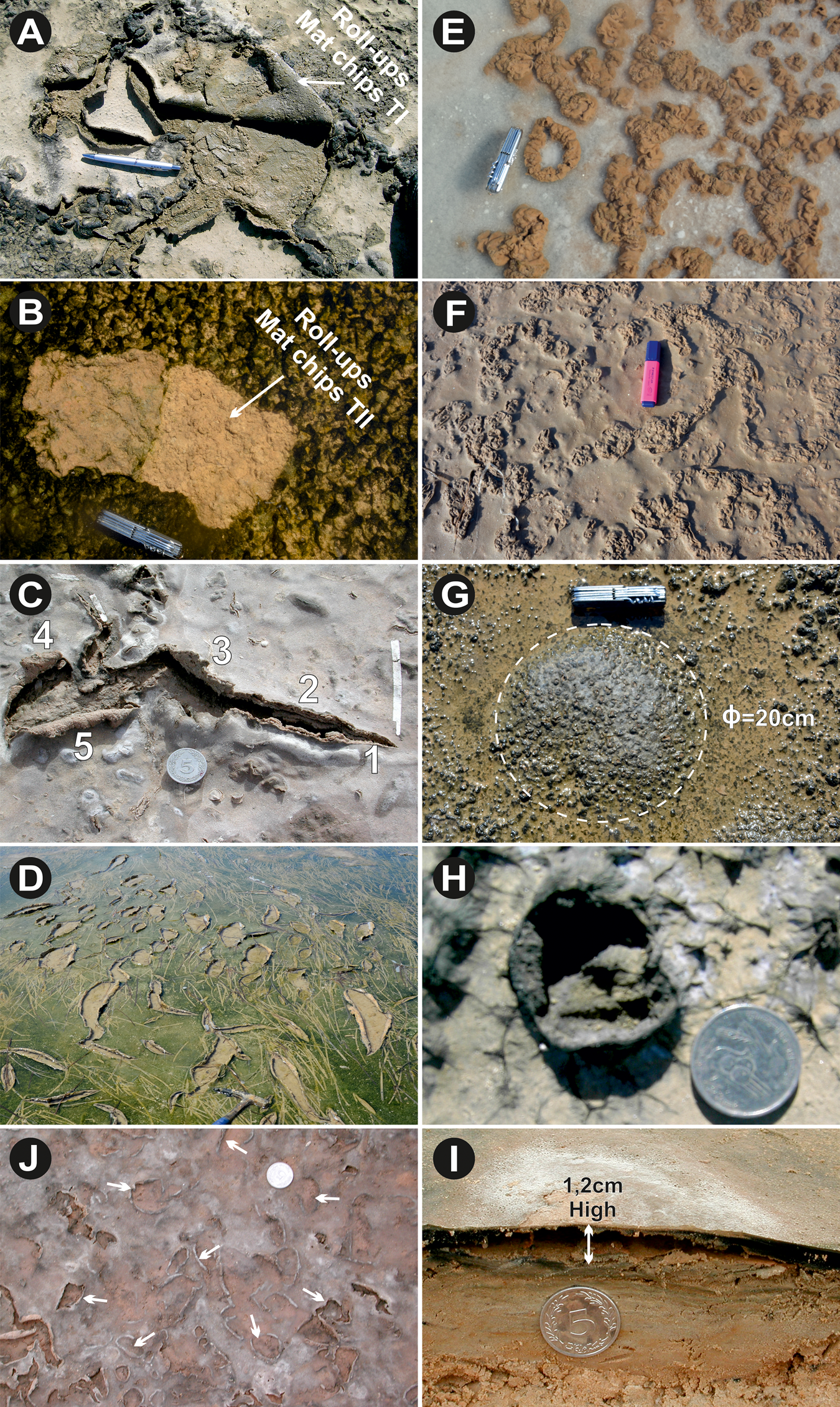
Figure 7. (color online) (A) Roll-ups mat chips type I: structure resulting from desiccation of the organic portion of the mat. (B) Roll-ups mat chips type II: structure caused during transport of the mat chips type II by bottom currents. (Both photos are from Ras el Kastil.) (C) Shrinkage developed in smooth epibenthic microbial mat showing different shrink stages (from 1 to 5; Bahar Alouane). (D) Thin microbial mat loosely attached to its substrate, shrink is probably initiated by bird's traces (Bahira el Boughrara). (E) and (F) Cauliflower structures consisting of protuberances in response to salt crystals developed by evaporation (respectively South of Zarzis and Bahar Alouane). (G) Soft epibenthic thick microbial mats forming gas dome (Ø=20 cm) (Ras Marmour). (H) Collapsed gas dome after drying (south of Zarzis). (I) Detached epibenthic microbial mats (about 1.2 cm high) from its base substrate showing an empty volume beneath (Bahira el Boughrara). (J) Up-curled biofilm margins taken off under the gas exhaust giving rise to jelley-rolls structure (white arrows). Coin diameter is 2.4 cm, knife length is 9 cm.
Shrinkage cracks
In southern Tunisia, the examination of the evolution of the microbial mats demonstrates that their shrinkage cracks appeared after two weeks of subaerial exposure and desiccation (Noffke et al., Reference Noffke, Gerdes, Klenke and Krumbein2001a). The elevated areas of the exposed microbial mats dry, and shrinkage begins in the hollow parts. During subsequent moistening, the microbial mats shrink more than the drier, sandy layers underneath, contributing to the formation of curls at their margins (Fig. 7C1,2,3). At the advanced phase, shrinkage fragments form overturned structures (mat curls, Fig. 7C4,5) (Gerdes et al., Reference Gerdes, Klenke and Noffke2000; Noffke et al., Reference Noffke, Gerdes, Klenke and Krumbein2001a). At the intertidal Boughrara area, a thin microbial mat, loosely attached to its substrate, shows a network of tears, probably initiated by birds' tracks (Fig. 7D).
Sediments stabilized by microbial mats produce desiccations that are different from those produced in clayey sediment. Indeed, based on a series of desiccation experiments, Kovalchuk et al. (Reference Kovalchuk, Owttrim, Konhauser and Gingras2017) have shown that abiotic, clay-rich sediments produce an orthogonal polygonal network of deep cracks, while in the biotic clay-rich sediments and microbial mats, crack propagation prevents the formation of a regular crack network. Sand layers in the fossil record may have shrunk during desiccation when they are associated with microbial mats, to leave partly incomplete polygons (Schieber, Reference Schieber, Eriksson, Altermann, Nelson, Mueller and Catuneau2004). Shrinkage cracks are strong indicators of endobenthic and epibenthic microbial mats of the upper intertidal to lower supratidal zones (Noffke, Reference Noffke2010).
Jelley-rolls structures
The jelley-rolls structures are formed where the microbial sedimentary support is slippery, thus preventing the biofilm from attaching to its substrate. Under the effect of hydrodynamic agents, it tears and detaches. The up-curled edges constitute what are called jelley-rolls forms (Noffke et al., Reference Noffke, Beukes and Hazen2006a). On siliciclastic substrates, the bursting of gas bubbles initiates tearing of the biomat (Fig. 7H).
Petees
The term “petees” was coined by Gavish et al. (Reference Gavish, Krumbein, Halevy, Friedman and Krumbein1985) to distinguish them from “tepees,” which are sharp-edged triangular forms of abiotic origin (Reineck et al., Reference Reineck, Gerdes, Claes, Dunaijtschik, Riege, Krumbein and Heling1990). Petees are cauliflower-like elevations of 0.5–2 cm height, occurring as individual upheavals on the surfaces of microbial mats, and are products of biostabilization (type II). Noffke (Reference Noffke2010) has illustrated that when seen in vertical section through a petee, the microbial mat forms a folded arch above the sediment, producing a hollow cavern. These petees characterize the supratidal zones, such as those situated south of Zarzis (Fig. 7E) and at Bahar Alouane (Fig. 7F).
Under semi-arid, hot climates, Sabkha-like tidal flats draw up ground water by evaporation, and dissolved salty minerals drawn up by capillary action reach the depositional surface upon the microbial mats, where they can precipitate. It is thought that the growing microbial mats and the precipitated salt crystals (gypsum, halite) cause the development of the petees (Noffke, Reference Noffke2010). Consequently, our observations, in addition to those made by Noffke et al. (Reference Noffke, Gerdes, Klenke and Krumbein2001a), indicate that the presence of petees might be interpreted as a record of both hydraulic and climatological conditions when they develop within ancient tidal flat systems.
Gas domes
As described by Noffke (Reference Noffke2010), gas domes are hemispherical or roundly conical elevations of 0.5 to 25 cm height and 2 to 30 cm diameter at their bases. They typically occur along the normal high water line (Logan et al., Reference Logan1970; Noffke, Reference Noffke2010).
At the interface of the sediment and the microbial mat, accumulating gas pressure can lift the epibenthic microbial mat from its substratum, forming millimeter-to-centimeter half-sphere or half-ellipsoid protuberances (Fig. 7G). These gas domes are constructed by Microcoleus chthonoplastes, whose mucilage prohibits the exchange of gases between tidal deposits and water or atmosphere (Logan et al., Reference Logan1970; Noffke et al., Reference Noffke, Gerdes, Klenke and Krumbein2001a). Analyses show that the hollow domes are filled with H2S, H2, CO, CO2, and CH4 gas, produced from the decay of the underlying organic matter (Noffke, Reference Noffke2010). When the domes explode and collapse, the gas escapes and only a kind of crater remains (Fig. 7H). Their encrustation by halite or gypsum makes their preservation possible. The remaining protruding lips form a network of craters which will be recolonized later by the next microbial mat generation. A vertical section through a gas dome shows an empty cavity (Fig. 7I). Gas domes are significant indicators for narrow facies zones in tidal settings. They indicate the normal high water line.
Sponge pore fabrics
Within the sandy layers underlying the microbial mat laminae, pores of 0.5–3 mm diameter (Figs. 8A and 8B), called sponge pore fabrics (Noffke et al., Reference Noffke, Gerdes, Klenke and Krumbein1996, Reference Noffke, Gerdes, Klenke and Krumbein2001b) can accumulate the gases produced from the decay of the above organic-rich plastic microbial mat, which acts as a seal prohibiting their upward migration and transfer to the water or atmosphere. The presence of sponge pore fabrics within white laminae alternating with black biomats in the ancient sedimentary record suggests the positions of the lower supratidal and high water lines.
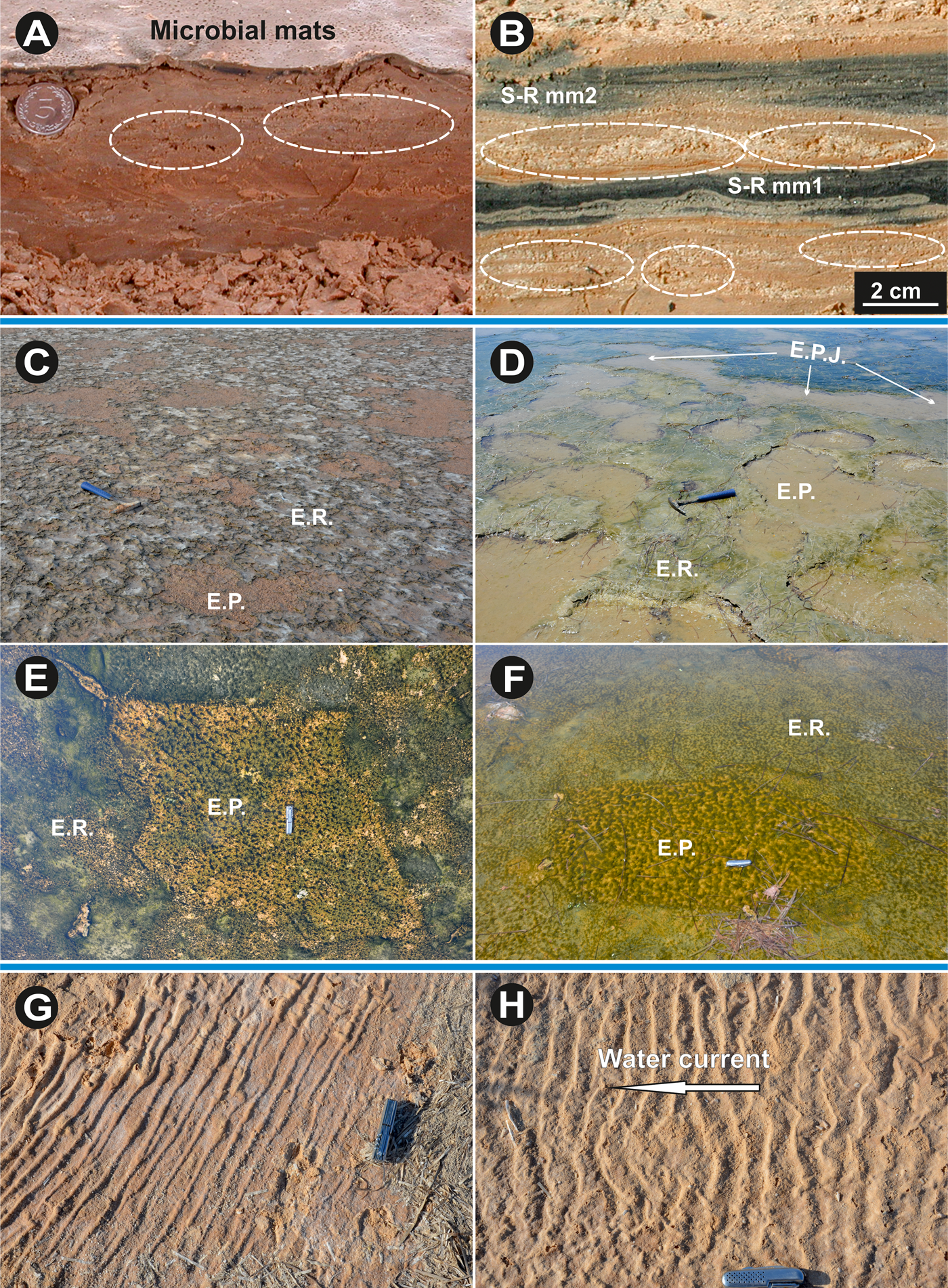
Figure 8. (color online) (A) Vertical cross-section through Bahira el Boughrara tidal flat shows microbial mat laminae alternating with sandy layers displaying sponge pore fabrics (pores). (B) Vertical cross-section through Sabkha Boujmel tidal flat shows two sub-recent microbial mat laminae alternating with sandy layers displaying sponge pore fabrics and salt crystals (pores). The pores form as the pressure of gases accumulating underneath the sediment-sealing microbial mat layer increases. (C) and (D) Erosional pockets and remnants patches of the el Grine lower supratidal and intertidal Bahira el Boughrara areas, respectively. (E) and (F) Photos show that pockets constitute favorable micro-environments for rapid recolonization by other cyanobacteria generations (south of Zarzis and Bahira el Boughrara, respectively). (G) Microbial slime agglutinate clay and gypsum causes elongated folds similar to table cloths (upper supratidal Bahar Alouane). (H) Asymmetrical sinuous current ripple marks stabilized by microbial mediated cohesive sand surface (Bahar Alouane). Coin diameter is 2.4 cm, knife length is 9 cm. S-R: Subrecent; E.P.: Erosional Pocket; E.R.: Erosional Remnant; E.P.J.: Erosional Pocket Join.
Structures controlled by microbial activity interacting with physical and sedimentary processes
Erosional remnants and pockets
The terms erosional remnants and erosional pockets were first introduced by Reineck (Reference Reineck1979), and then developed by Gerdes et al. (Reference Gerdes, Claes, Dunajtschik-Piewak, Riege, Krumbein and Reineck1993). The erosional pockets correspond to a more or less deeper-lying V-shaped, rippled depression of 8 to 80 cm in diameter, dug into the microbial mat by hydrodynamic agents such as strong tidal currents, waves, and storms. These structures, described by Noffke (Reference Noffke2010), are illustrated in Figures 8C and 8D. They are depressions resulting from tidal currents, with margins formed by the non-eroded endobenthic or epibenthic microbial mat (erosional remnants).
Erosional pockets constitute favorable micro-environments for rapid recolonization by later cyanobacteria generations, as shown in Figures 8E and 8F. These structures can occur either in isolation or in an assembly (Noffke, Reference Noffke1999; Gerdes et al., Reference Gerdes, Klenke and Noffke2000; Noffke et al., Reference Noffke, Gerdes, Klenke and Krumbein2001a). They develop particularly in the lower supratidal and upper intertidal zones, and allow the reconstruction of flood and ebb-currents from rocks formed in ancient tidal flats (Noffke, Reference Noffke2010).
Wrinkle structures
Hagadorn and Bottjer (Reference Hagadorn and Bottjer1997) described any crinkled sandy surface of biological or non-biological origin as a wrinkle structure. According to Noffke (Reference Noffke2000 and Reference Noffke2010), these structures occur in the fossil record only on the upper bedding planes, and they result from the lithification of endobenthic or epibenthic microbial mats. The crinkled surface of the living microbial mat, which resembles elephant skin (Fig. 9E), constitutes an exception to this (Gehling and Droser, Reference Gehling and Droser2009).
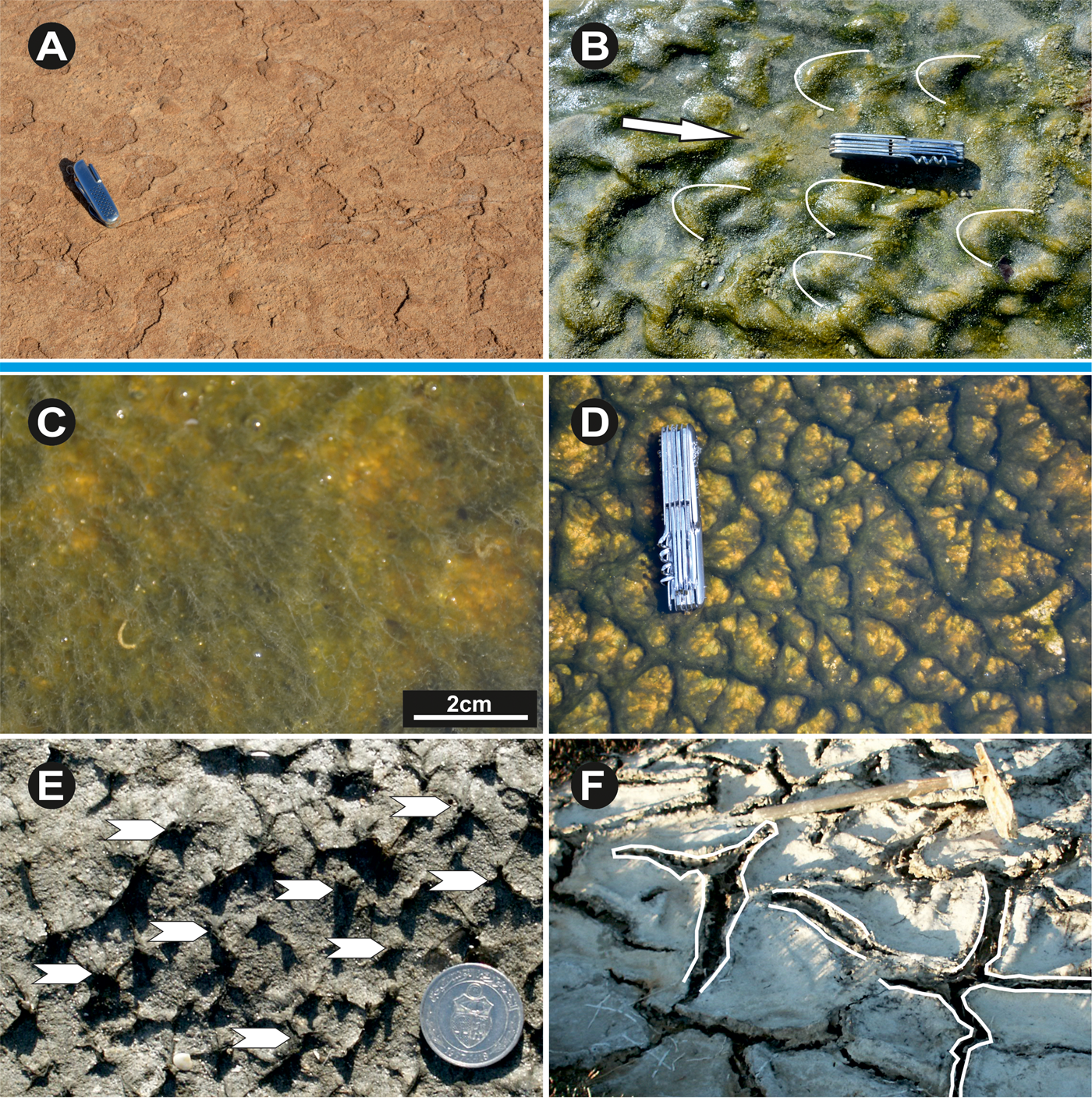
Figure 9. (color online) (A) Eroded microbial mats crests after cracking (Sabkha Jallabia). (B) Linguoid ripple marks coated by smooth filamentous microbial mats (Sidi Yetti area, white arrows indicate water current direction). (C) Floating filamentous cyanobacteria under the water surface and showing gas bubbles (Sidi Yetti). (D) Reticulated microbial mats or “elephant skin” (south of Zarzis and Bahira el Boughrara). (E) Pinnacles rooted in the basic microbial mats having 2–3 mm of high (white arrows). (F) Modern South of Zarzis tidal flat oscillation cracks. The mat surface displays a pattern of polygonal arranged cracks which are composed of two parallel running ridges constituting the margin of a mat polygon. The mat polygons expand laterally, when moist, and shrink when desiccating. Coin diameter is 2.4 cm, knife length is 9 cm., shovel length is 80 cm.
The development of wrinkle structures on sandstone bed surfaces where mud is absent requires the intervention of cyanobacteria, which form a hardened carapace by in situ salt precipitation, conserving all the protuberances generally formed by the agglutination of microbial slimes and evaporite minerals (Gerdes, Reference Gerdes, Schieber, Bose, Eriksson, Banerjee, Sarkar, Altermann and Catuneanu2007). Loading and dewatering processes during burial of microbial layers may be a likely explanation of this process (Noffke et al., Reference Noffke, Hazen and Nhleko2003). These structures occur in the higher-lying part of the Sabkhas, dominated by Aphanocapsa spp. and Gloeothece spp. coccoid cyanobacteria.
The upper bedding sediments crowned by these microbial mats can show undulating structures (Gavish et al., Reference Gavish, Krumbein, Halevy, Friedman and Krumbein1985) corresponding to microbial sludge (EPS) generating successive elongated folds similar to folded sheets (Fig. 8G). These structures can be sinuous or asymmetrical, caused by current ripple marks held in place by coccoid cyanobacteria and early cementation (Fig. 8H). In the Sidi Yetti area (Fig. 2), the lower supratidal zone shows linguoid ripple marks overgrown by a filamentous cyanobacteria species (Microcoleus chthonoplastes) (Fig. 9B). Under an arid to semi-arid climate, as is the case in southeastern Tunisia, the ripple marks may dry and crack (Fig. 9A).
Wrinkle structures are considered to be a useful tool in the detection of regression-transgression cycles in monotonic clastic series composed of sand, silt and mud (Noffke, Reference Noffke2010). It is thought that during sea level rises, when shallow marine environments and associated microbial mats develop, the presence of wrinkle structures indicates the turning points from regressions to transgressions (Noffke, Reference Noffke2010).
Tufts and pinnacles
Tufts are bundles of vertically oriented filamentous organisms that originally contribute to the basic microbial mat feature. It is frequently observed that when microbial growth shifts from a lateral to a vertical direction, in response to changes in physical and chemical environmental conditions, this can force the bundles of filaments to stand in tufts above the basal microbial mat (Gehling, Reference Gehling1999; Gerdes et al., Reference Gerdes, Klenke and Noffke2000) (Fig. 9C). According to Gerdes et al. (Reference Gerdes, Klenke and Noffke2000), such phenomena result from the filamentous bacteria (Microcoleus chthonoplaste, Lyngbya aestuarii) responding to changes in brightness and in the ionic composition of the water. As documented by Gerdes et al. (Reference Gerdes, Krumbein, Reineck, Krumbein, Paterson and Stal1994), these soft and flexible tufts sometimes act as hosts for coccoids and diatoms, which stabilize them in the form of stiffer pinnacles with millimeter to centimeter heights (Fig. 9D).
Elephant skin structure
During periods of flood or ebb tide, the water currents train the vertical cyanobacteria filaments horizontally, drawing a reticulate network that has a striking resemblance to the skin of an elephant (Fig. 9E). These structures were well documented in Shark Bay by Golubic (Reference Golubic and Walter1976).
Polygonal oscillation cracks
In southeastern Tunisia, some thick epibenthic microbial mats show locally a distinctive network made up of desiccation cracks and polygons. The polygons are 10 to 40 cm diameter, and each crack is between 3 and 8 cm wide. Each individual crack is composed of two parallel ridges marking the polygon edges, which are usually folded upward and composed of stacked sub-recent microbial mat laminae (Noffke et al., Reference Noffke, Gerdes, Klenke and Krumbein2001a). Because these structures arise from their upwardly folded margins, they were named polygonal oscillation cracks by Noffke et al. (Reference Noffke, Gerdes, Klenke and Krumbein2001a, and Reference Noffke, Beukes, Bower, Hazen and Swift2008). The margins of the U-shaped polygonal oscillation cracks form two parallel ridges arising from growth and biostabilization (Type II) (Fig. 9F). The presence of polygonal oscillation cracks is a useful indicator of the lower and upper supratidal settings, and of semi-arid to hot palaeoclimates.
Physicochemical characteristic of the microbial mats
In modern paralic environments, cyanobacteria develop at the water-sediment interface, and colonize surfaces in habitats poor in organic matter, such as siliciclastic sediments (Van Gemerden, Reference Van Gemerden1993). Microbial mats are therefore the result of bacterial activity creating a vertical physicochemical stratification, oxidizing at the surface and reducing at depth (Revsbech et al., Reference Revsbech, Jorgensen, Blackburn and Cohen1983). Thus, the cyanobacteria delimit within and beneath the mats an oxic zone and an anoxic zone, respectively (Donkor and Häder, Reference Donkor and Häder1991). The anoxic conditions are favorable to the preservation of the organic matter stock in the form of sequestered carbon. This is the most striking example of anoxia being developed close to the surface without a stratified water body.
The temporary or permanent extreme hyper-saline conditions result in the elimination of a large number of organisms that could play a role in the degradation of cyanobacterial communities, especially by grazing (Cornee, Reference Cornee1989). The production of cyanobacteria is limited by the phosphorus and nitrate contents, as well as by the light intensity and its depth of penetration. The inputs of these nutrients are linked to the available dissolved organic matter (DOM) as well as the water dynamics (Eyre and Ferguson, Reference Eyre and Ferguson2002).
At Ras el Kastil tidal flat (Bin el Wedyen sector), microbial mats host diverse bacterial colonies ranging from mesophiles to thermophiles and halophiles (Table 1). One of the characteristics of this hyper-saline environment is the very high production of organic matter. Indeed, the measurement of DOC, effectuated for the first time in the microbial mats of southeastern Tunisia, indicates a content ranging from 8.52 in the upper intertidal zone to 20.56% in the lower supratidal zone (Table 1). This lateral zoning of the dissolved organic compounds is in accordance with the nutrient contents (nitrogen and phosphates) of the water occupying these zones. However, the total nitrogen and phosphate contents range from 9.72% and 0.25 ppm in the upper intertidal zone, to 91.52% and 2.10 ppm in the lower supratidal zone, respectively. The high DOM is essentially of bacterial origin, as proven by the spectrophotometry absorptiometry technique (Table 1).
Table 1. Contents of dissolved organic carbon (DOC), total nitrogen (N), phosphates (P), and SUVA index in the Ras el Kastil upper intertidal-lower supratidal zones.

The specific UV absorbance (SUVA) index values calculated for the Ras el Kastil samples are all less than 1 (Table 1). This result corresponds to organic matter of a non-aromatic, hydrophilic nature, but it is composed mainly of non-humic substances due to high depolymerization and degradation of the dissolved organic compounds (Yakimenko et al., Reference Yakimenko, Khundzhua, Izosimov, Yuzhakov and Patsaeva2018). An important aspect to consider is the gas domes created by the H2S released under the epibenthic microbial mats, which is generated by anaerobic fermentation produced by sulfate-reducing bacteria (Jorgensen et al., Reference Jorgensen, Rothstein and Reznikoff1979).
INTERPRETATION AND DISCUSSION
MISS as reliable indicators for depositional environment interpretation
A theoretical profile, perpendicular to the shore line and crossing the entire tidal flat, shows the broad lateral distribution of MISS (Fig. 10). Three distinct zones are differentiated, based on the presence and type of microbial mats and associated MISS. From the open sea (east) to the mainland (west) they are: (1) the upper infratidal to lower intertidal zone, (2) the middle to upper intertidal zone, and (3) the lower to middle supratidal zone.
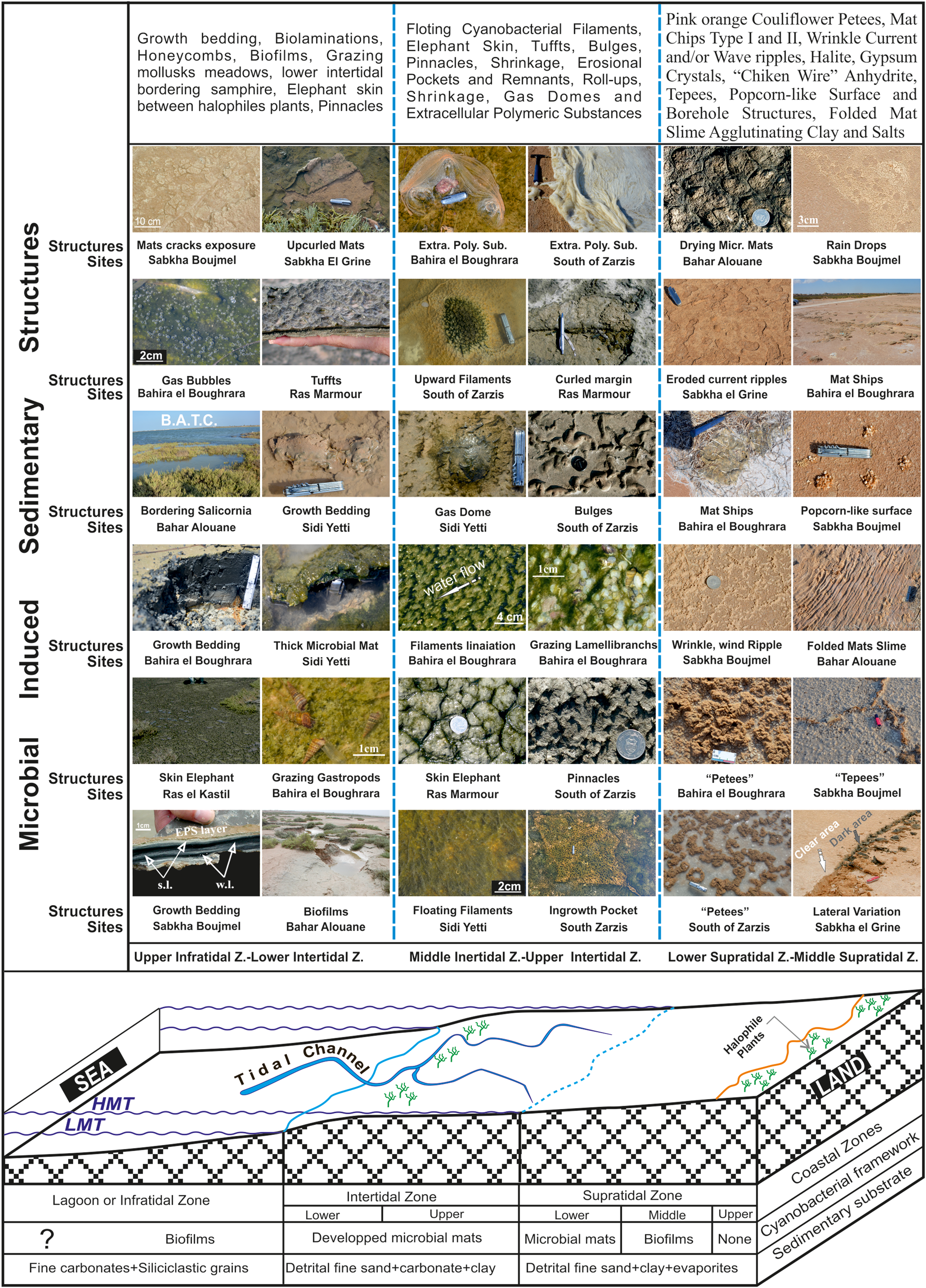
Figure 10. (color online) Hypothetic profile showing, from land to sea, littoral environment successions and mainly associated cyanobacterial structures. LMT: Low Mean Tide, HMT: High Mean Tide, s.l.; summer laminae, w.l.: winter laminae, B.A.T.C.: Bahar Alouane Tidal Channel. Coin diameter is 2,4 cm, knife length is 9 cm and Hammer length is 60 cm.
The upper infratidal to lower intertidal zone
In the upper infratidal zone, which is continuously invaded by sea water, there is preferential development of thick microbial mats of the biovarvite type. The best example is that at Sabkha Boujmel. However, in the lower intertidal zone, governed by strong flood and ebb tidal currents, and which is the first flooded and last exposed area during a tidal cycle, only biofilms are found. It is important to note that the lower intertidal zone, when colonized by a strip of halophilic vegetation, may comprises isolated ponds that are very suitable for the proliferation of thick epibenthic microbial mats, and which swarm with benthic organisms. The most frequent sedimentary structures encountered in the intertidal zone are growth bedding, biolaminations, honeycombs, biofilms, grazing mollusk meadows, elephant skin, and pinnacles.
The middle to upper intertidal zone
This zone is crossed by fairly strong tidal currents, which bring clay particles that can smother cyanobacteria, but do not detach the cyanobacteria from their substrate. This area is crowned by filamentous cyanobacteria with the ability to escape the high sedimentation rate by quickly digging upward through the sediments to settle on their surface. This repetitive competition between sedimentation and cyanobacterial growth generates endobenthic microbial mats. The main structures developed are floating cyanobacterial filaments, elephant skin, tufts, bulges, pinnacles, shrinkage, erosional pockets and remnants, roll-up structures (types I and II), gas domes, and extracellular polymeric substances.
The lower to middle supratidal zone
This area is characterized by sporadic submergence, a low sedimentation rate, and a large period of latency. Since this zone is the last to be flooded and the first to be exposed during a tidal cycle, it is reworked only for a short time. The sandy sediments of this area are crowned with thick epibenthic microbial mats. It is also the site of evaporitic sedimentation generated by evaporitic pumping phenomena. The different individualized structures are pink-orange cauliflower petees, mat chips (types I and II), wrinkle current and/or wave ripples, chicken wire anhydrite, tepees, popcorn-like surface and borehole structures, and folded mat slime agglutinating clay and gypsum.
As shown in the previous sections, and illustrated in Figure 10, the lateral distribution of the MISS is controlled by the interplay of hydrodynamic processes within the tidal flat and the effects of seasonal climatic variations, especially the dry and rainy periods. Sedimentological factors, such as the lithology of the substrate, the grain size, the sedimentation rate, and early diagenesis, strongly control the formation or inhibition of these MISS. Finally, physicochemical factors come into play through salinity, evaporation, pH, ionic charge, and DOC, as well as the availability of nitrogen and phosphate.
Modern and Holocene biomats in southeastern Tunisia
Trenches and cores were taken in tidal plains and Sabkha areas. These highlighted the presence of modern and Holocene degraded microbial mats (Lakhdar et al., Reference Lakhdar, Soussi, Ben Ismail and M'Rabet2006). These relatively old microbial mats are at varying depths, and may be continuous or discontinuous (Supplementary Figures 3 and Fig. 11). They may be single or multiple biomat layers alternating with silty-sand detrital deposits, including chicken wire anhydrite and gypsum crystals. A vertical section through a buried stack of horizontally oriented cyanobacteria laminae shows clear laminae formed by an extracellular polymeric substance-rich (EPS) clusters secreted by coccoidal species (Synechococcus sp., Aphanothece sp.), alternating with dark laminae formed by filamentous cyanobacteria species (Microcoleus chthonoplastes) (Gerdes and Krumbein, Reference Gerdes and Krumbein1987; Gerdes et al., Reference Gerdes, Krumbein, Reineck, G. Einsele, Ricken and Seilacher1991). This behavior seems to suggest a seasonal (summer-winter) climate variation (Fig. 11A).

Figure 11. (color online) (A) Cross section trough buried microbial mat showing alternations of clear layers (s.l.) formed by coccoidals cyanobacteria, and dark layers (w.l.) formed by filamentous cyanobacteria and gypsum /anhydrite pockets (white arrows) (supratidal Sabkha Boujmel). (B) Below dispatched dark areas at a few cm deep are discontinuous microbial mats (supratidal Sabkha el Grine). (C) Alternations of growing microbial mats (m-m) and probably flood periods draped sand layers (s-l) (tributary terrace at Sabkha el Grine). (D) The oldest microbial mat shows halophilic vegetation roots traces and burrows (white arrows). This mat is sealed by two more recent ones (m-m2 and m-m3) probably inserted by a flood layer (Sabkha el Grine). Coin diameter is 2.4 cm, knife length is 9 cm.
In the Boujmel and el Grine Sabkhas, the supratidal upper surface deposits locally display whitish and dark areas. Excavated trenches show that beneath the dark surfaces, there are biodegraded microbial mats enclosed within recent deposits (Fig. 11B). However, beneath the whitish areas, characterized by the presence of evaporite minerals such as gypsum and halite, microbial mats are absent. This variation in sediment surface color, over short distances, appears to be caused by the geochemistry of the water and the Sabkha sediments. The intense evaporation and high salinity stress seem to be determining factors in the growth of microbial mats.
Another phenomenon suggesting that climate can directly control the microbial mats' organization and development has been observed. During our examination of the biomats, we noted that a high sedimentation rate following a short flood phase can cause the rapid burial of the living microbial mat, which then dies due to the cyanobacteria being unable to escape towards the surface. Subsequent favorable conditions allow a second microbial mat generation (m-m2) to arise. This alternation of high sedimentation rates and microbial growth generates the organo-sedimentary structure illustrated in Figure 11C.
It is notable that a biodegraded microbial mat can undergo pedogenesis and/or intense bioturbation phenomena resulting from halophytes' root traces and burrows (Fig. 11D), as in the case of Sabkha el Grine. The oldest microbial mat (m-m1) is sealed by two more recent ones (m-m2 and m-m3), probably separated by flood deposits.
Ancient biolaminite sedimentary records
Microbial mat constructions are excellent paleoenvironmental and climatic indicators. Bouougri and Porada (Reference Bouougri and Porada2002) interpreted the Neoproterozoic West African Craton deposits (Anti-Atlas, Morocco) as having originated in an intertidal to supratidal setting that was subject to sporadic flooding by traction currents before their burial. The fossilized wrinkled structures that were found in the Early Archean (3.2 Ga) Moodies Group deposits in South Africa (Noffke et al., Reference Noffke, Beukes and Hazen2006a), and in the Meso-Archean (2.9 Ga) Former Pongola deposits and Witwatersr and Super groups, also in South Africa (Nofkke et al., Reference Noffke, Hazen and Nhleko2003; Nofkke et al., Reference Noffke, Hazen, Eriksson and Simpson2006b), are especially common in sediments separating the transgressive and regressive cycles.
Fossil microbial mats, also called biolaminites, have been described in several other regions of the world and from all geological ages. These include the Canadian Neoproterozoic reefs (Kelly et al., Reference Kelly, Narbonne and James2004), the marginal deposits of Neoproterozoic age of Morocco's Anti-Atlas (Bouougri and Porada, Reference Bouougri and Porada2002), the Neoproterozoic siliciclastic rocks of the center of Namibia (Porada and Löffler, Reference Porada and Löffler2000; Bouougri and Porada, Reference Bouougri and Porada2007), and Precambrian central India (Singh and Wunderlich, Reference Singh and Wunderlich1978).
The activity of microorganisms, identified in the Precambrian (from 650 to 4000 Ma), demonstrates their presence on earth since early in its history. They have been identified in sedimentary rocks of Early Archean age (ca. 3.48 Ga) in the Dresser Formation, Western Australia, where erosional pockets and remnants (Fig. 12Ao), mat chips (Fig. 12Bo), and pinnacles and reticulate ridges (Fig. 12Co) (Noffke et al., Reference Noffke, Christian and Wacey2013) are found. Also documented are palimpsest ripples (probably folded mat slime) from the 1.6 Ga Chorhat Sandstone, Vindhyan Supergroup, M.P., India, (Fig. 12Do) (Sarkar et al., Reference Sarkar, Banerjee, Samanta and Jeevankumar2006); elephant skin structure documented in the Upper Neoproterozoic Vingerbreek Member, Nudaus Formation, Nama Group, Farm Haruchas, Namibia (Fig. 12Eo) (Porada and Bouougri, Reference Porada and Bouougri2007); and gas domes from the Neoproterozoic Tizi n-Taghatine Group, Anti-Atlas belt, Morocco (Bouougri and Porada, Reference Bouougri and Porada2007) (Fig. 12Fo).
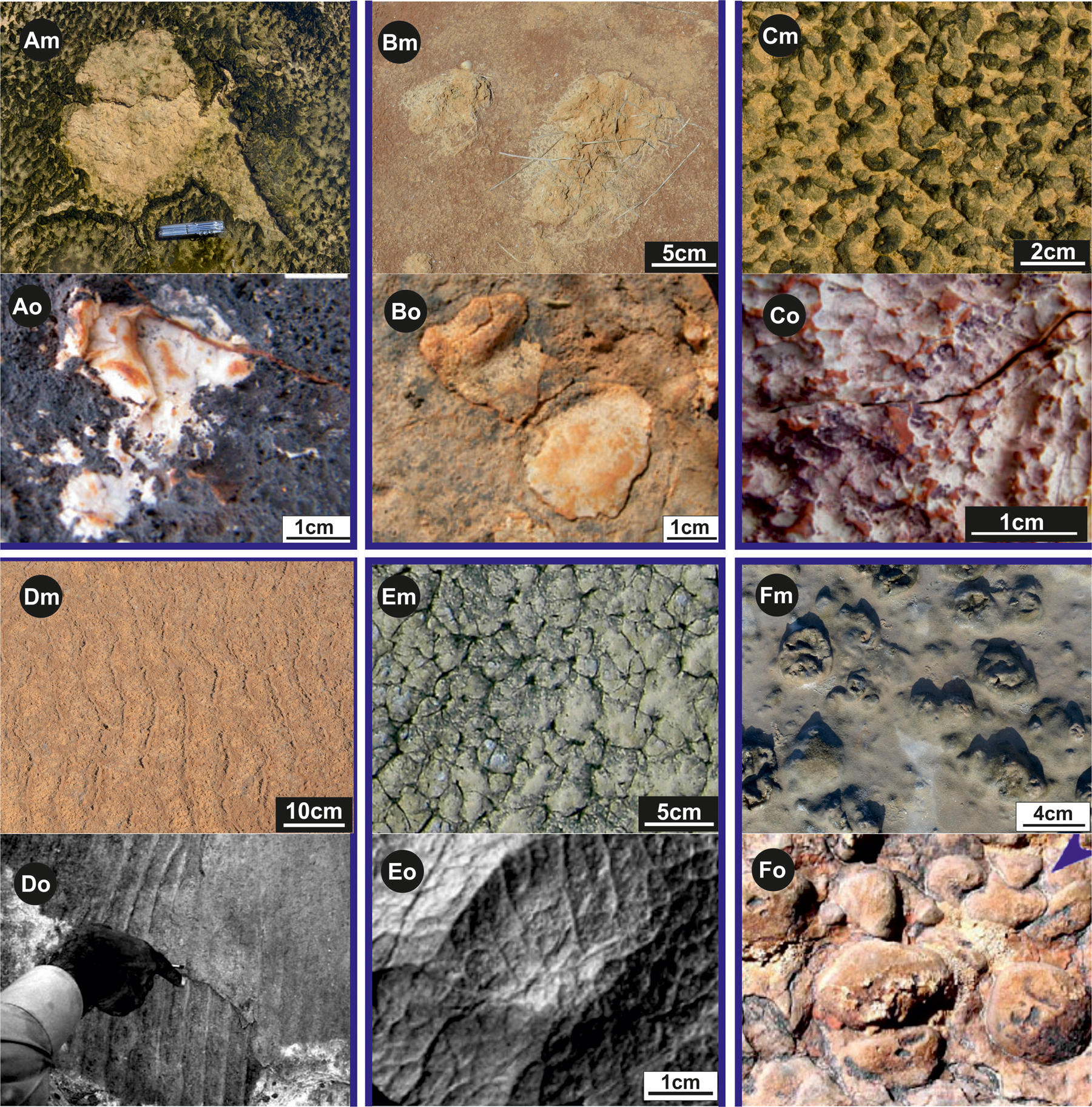
Figure 12. (color online) Macrostructures of modern microbial mat (Xm) with their possible old equivalents (Xo).
(Am) Modern erosional pocket and remnant (Ras el Kastil). (Bm) Two mat chips fragments (Bahira el Boughrara). (Cm) Pinnacles and reticulate ridges (Ras el Kastil). (Dm) Asymmetrical current ripple marks (Sabkha el Grine). (Em) Wrinkle structures (Ras el Kastil). (Fm) Gas domes (Bahar Alouane).
(Ao), (Bo) and (Co) are respectively erosional pocket and remnant, mat ships, pinnacles and reticulate ridge from Early Archean age (ca. 3.48 Ga), Dresser Formation, Western Australia (Noffke et al., Reference Noffke, Christian and Wacey2013). (Do) Palimpsest ripples probably folded mat slime in the 1.6 Ga Chorhat Sandstone, Vindhyan Supergroup, M.P., India (Sarkar et al., Reference Sarkar, Banerjee, Samanta and Jeevankumar2006). (Eo) and (Fo) are elephant skin structure (Upper Neoproterozoic Vingerbreek Member, Namibia) and gas domes (Neoproterozoic Tizi n-Taghatine Group, Anti-Atlas belt, Morocco) (Bouougri and Porada, Reference Bouougri and Porada2007), respectively.
The collected modern structures (Figs. 12Am, 12Bm, 12Cm, 12Dm, 12Em, and 12Fm) from southeastern Tunisia, when compared with their possible fossil equivalents, allow the interpretation, with little risk of error, the depositional environments.
CONCLUSIONS
The tidal flats of southeastern Tunisia, dominated by eolian siliciclastic sediments, are colonized over large areas by microbial constructions mainly formed by benthic cyanobacteria, which are dominated by species well adapted to the specific physicochemical and arid climatic conditions. The light permeates deeply enough, via silica grains and halite crystals, to allow the microbial mats to survive and flourish. Furthermore, the low sedimentation rate and wave/current action, and the surface hydrological cycles, generate optimal conditions for the development of endobenthic and epibenthic microbial mats.
Mapping of the microbial mats, and interpretation of the textural signatures identified within them, has revealed valuable information, and has allowed the creation of a catalog that can serve as a tool for the diagnosis of facies, and for the palaeo-environmental reconstruction of tidal flats and Sabkhas. MISS, by their great diversity and their spatiotemporal arrangement through the tidal plain, constitute high-resolution tools for characterizing the ecology of different segments of the coast.
Indicators of the middle to upper intertidal zone include endobenthic microbial mats (generated by filamentous cyanobacteria), elephant skin, tufts, bulges, pinnacles, shrinkage, erosional pockets and remnants, and type II roll-up structures. The lower supratidal zone is indicated by epibenthic microbial mats, cauliflower petees, type I mat chips, wrinkle current and/or wave ripples, folded mat slime agglutinating clay and gypsum, tepees, popcorn-like surface structures, and chicken wire anhydrite.
Fossil mat chips are valuable tools for reconstructing storm intensities and current directions. Type I mat curls indicate subaerial exposure of a tidal area, while type II curls indicate strong, episodic storms that push seawater far into the upper supratidal zone. When found at a site far from the coastline, mat chips clearly indicate strong storms or hurricanes. Alternating flood and drought periods are fossilized in the sediments that contain the Holocene microbial mats. Long periods of drought interspersed by flooding periods lead to the disappearance of the microbial mat.
Petees, chicken wire anhydrite structures, and halite and gypsum crusts record evaporitic pumping, and thus indicate the climatological conditions of ancient tidal flats. The laminated leveling structures and biovarvite structures indicate a hot and arid climate. Further detailed geochemical and molecular investigations on both the modern and fossilized biomats presented in this study will certainly help in deciphering the subtle variations in their composition, and therefore the climate variations that have affected this area through recent millennia. Organic geochemistry analyses demonstrate that DOC, nitrogen, and phosphate were present in greater quantities within the lower supratidal zone than in the upper intertidal zone.
ACKNOWLEDGEMENTS
This work was conducted with the support of the Ministry of Higher Education and Scientific Research of Tunisia, and was funded by the Research Laboratory: LR18 ES07 (Sedimentary Basins and Petroleum Geology). The authors thank Prof. Derek Booth and Dr. Louisa Bradtmiller, respectively the Senior Editor and Associate Editor, for their insightful comments and judicious recommendations. We are especially grateful to Prof. Nora Noffke, Fellow of the American Association for the Advancement of Science (AAAS), and an anonymous reviewer for their constructive remarks and comments, which helped to greatly improve the quality of the manuscript. Dr. Nabil Khelifi, Senior Editor, Springer, MENA, is greatly acknowledged for providing relevant references. The Elsevier Author Language Editing Service is also thanked for correcting and improving the English of the manuscript. Many thanks to the researchers of the LR18 ES 07 Laboratory for their continuous help and support. Thanks are extended to Sofièn Mekki for his precious financial help.
SUPPLEMENTARY MATERIAL
The supplementary material for this article can be found at https://doi.org/10.1017/qua.2020.91
















