INTRODUCTION
Located in southern continental Chile, close to the Pacific Ocean shore, the Mylodon Cave or Cueva del Milodón (CM, hereafter; also known as Caverna Eberhardt, Cave of Última Esperanza, or de Puerto Consuelo; Emperaire and Laming, Reference Emperaire and Laming1954, p. 175) is one of the most emblematic and studied paleontological sites in South America (Martinic, Reference Martinic1996). Numerous contributions, starting at the end of nineteenth century, were made on several issues, especially the local occurrence and potential coexistence of giant ground sloths (Mylodon) and humans (see Martin, Reference Martin2013, and references cited therein; Villavicencio et al., Reference Villavicencio, Lindsey, Martin, Borrero, Moreno, Marshall and Barnosky2016; Pérez et al., Reference Pérez, Toledo, Vizcaíno and Bargo2018).
Conversely, approaches to reconstruct Quaternary environmental evolution around CM based on biological proxy data are scarce. The first comprehensive study was made by Moore (Reference Moore1978) analyzing plant macrofossils recovered in the excavations made by Saxon (Reference Saxon1976, Reference Saxon1979). Pollen analyses were restricted to the Pleistocene “dung carpet” (Salmi, Reference Salmi1955; Markgraf, Reference Markgraf1985; Heusser et al., Reference Heusser, Borrero and Lanata1994). Finally, remarks about past environmental conditions were raised from the study of bird remains unearthed from the sequence of CM (Humphrey et al., Reference Humphrey, Péfaur and Rasmussen1993).
Overall, Holocene evidence from CM was eclipsed by the magnitude and preservation of Pleistocene remains. In fact, almost nothing is known about Holocene rodents from this cave (see Nordenskiöld, Reference Nordenskiöld1900 [1996]). A brief approach to CM micromammals and, subsidiarily, paleoenvironments was made by Simonetti and Rau (Reference Simonetti and Rau1989; see also Rau and Yáñez, Reference Rau and Yáñez1980). These authors studied a small sample putatively associated with Pleistocene levels, concluding it resembled the present-day micromammal community.
The goal of this paper is to study the entire small mammal sequence excavated by E. Saxon in 1976 from CM. In addition, we included samples in our analysis that were obtained more recently (Borrero excavations during 1989–2001), as well as the micromammal remains recovered in the neighboring rock shelter Dos Herraduras 1 (DH1, hereafter). After inferring the accumulating agents of the archaeological samples, the studied rodents were used to reconstruct the environmental conditions in southern Chile during the late Pleistocene–Holocene. In this context, we aimed to produce an integrative view about the paleoenvironmental value of several biological indicators retrieved from CM and associated sites in the Cerro Benítez sector.
METHODS
Studied localities
CM is a large cave (180 m long, 150 m wide, 30 m high; Favier Dubois and Borrero, Reference Favier Dubois and Borrero1997). DH archaeological locality is composed of three small rock shelters located ~700 m north MC (see Martin, Reference Martin2013, fig. 1.3). Both sites, as well as other well-known archaeological localities, such as Cueva Chica and Cueva del Medio, open on the southwestern slope of Cerro Benítez (51.56°S, 72.58°W; Stern et al., Reference Stern, Moreno, Villa-Martínez, Sagredo, Prieto and Labarca2011), south of the lacustrine and ridged mountain region that forms the Torres del Paine National Park (Región de Magallanes y la Antártica Chilena, Última Esperanza, Chile; Fig. 1; see Martin et al., Reference Martin, Todisco, Rodet, San Román, Morello, Prevosti, Stern and Borrero2015, and references therein; Todisco et al., Reference Todisco, Rodet, Nehme, Martin and Borrero2016).
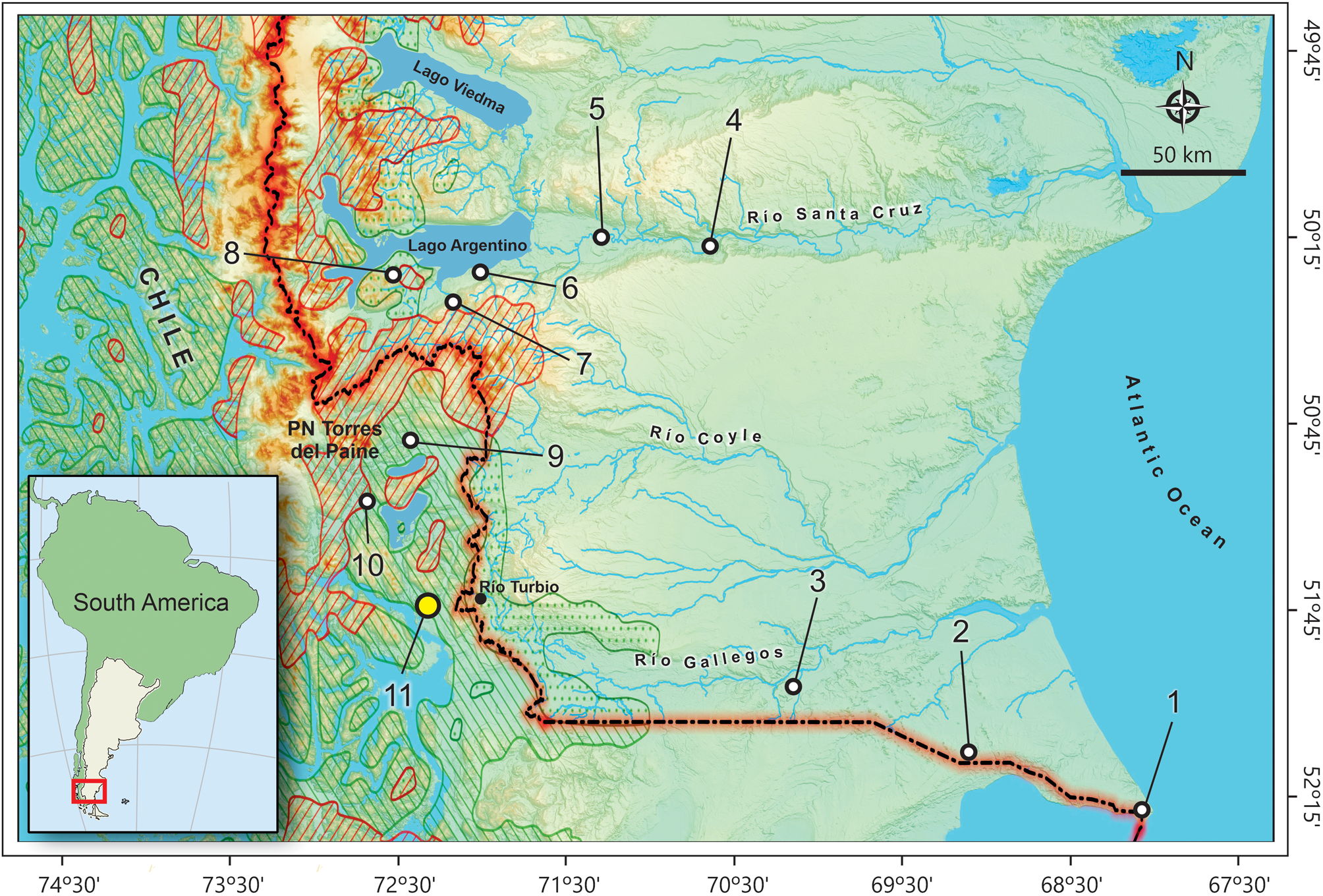
Figure 1. Map of the southernmost Argentina and Chile mainland depicting the geographic location of Cerro Benítez area (yellow circle) and recent owl pellet samples (white circles) included in the regional analysis (for the reference numbers, see Supplementary Table 2). Superimposed limits of the modern major vegetation zones (digitizing following Hasenack et al., Reference Hasenack, Souza da Silva, Weber and Hofmann2017, based on the vegetation map constructed by Kurt Hueck): Patagonian steppe (unmarked zone to the east); deciduous forest (stippled); evergreen forest (diagonally hatched in green); feldmark, ice, or Magellanic moorland (diagonally hatched in orange).
Fossil samples
Here, we studied rodent remains retrieved from both CM and DH1. The samples from Saxon excavations in CM, conducted during 1976, are housed at The Natural History Museum, Paleontological Collection (NHMUK, London, United Kingdom; Table 1). Additional samples from CM are from Borrero excavations (during 1989–2001), belonging to the collections of the Instituto de la Patagonia (Punta Arenas, Chile; Table 2). Each studied sample was manually sorted, and both isolated bones/teeth and complete/fragmented owl pellets separated. The latter were measured (when possible), photographed, and dissected following standard techniques (Marti, Reference Marti, Pendleton, Millsap, Kline and Bird1987). Taxonomic identifications were made on craniodental remains based on comparative recent material (Colección de Mamíferos del Centro Nacional Patagónico, Puerto Madryn, Chubut, Argentina) and literature (e.g., Osgood, Reference Osgood1943; Reise, Reference Reise1973). Rodent taxonomy used here follows Patton et al. (Reference Patton, Pardiñas and D'Elía2015). Recent revisions, such as the taxonomy advanced for the long-clawed mice Geoxus (see Teta and D'Elía, Reference Teta and D'Elía2016), are largely based on molecular data and provide insufficient detail to work with archaeological material; therefore, Geoxus is treated here at the generic level. Number of identified specimens (NISP), minimum number of elements (MNE), and minimum number of individuals (MNI) were calculated for each sample, following Grayson (Reference Grayson1984). In addition, the main attributes of each sample were recorded, including matrix overall features (e.g., owl pellet debris, plant material). We also studied all the rodent fossil remains from CM housed at NHMUK, which are few, isolated materials recovered by several travelers and scholars (e.g., J. Wolffsohn) and purchased or received by the NHMUK. As for most of the historical collections made in this site before 1950, these remains lack precise stratigraphical data (see Borrero and Martin, Reference Borrero and Martin2012; Pardiñas and Candela, Reference Pardiñas and Candela2017; Pérez et al., Reference Pérez, Toledo, Vizcaíno and Bargo2018).
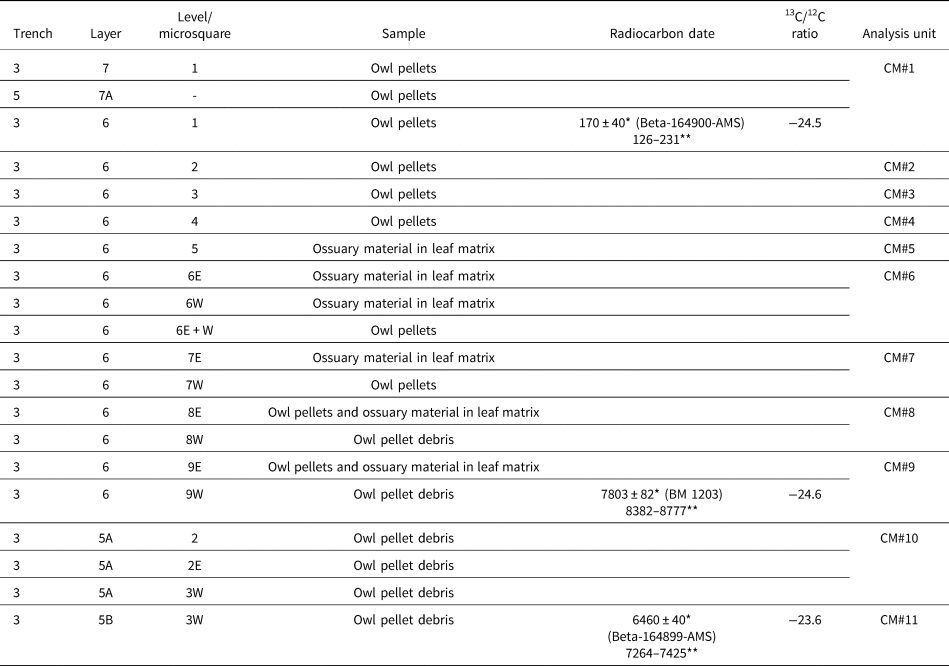
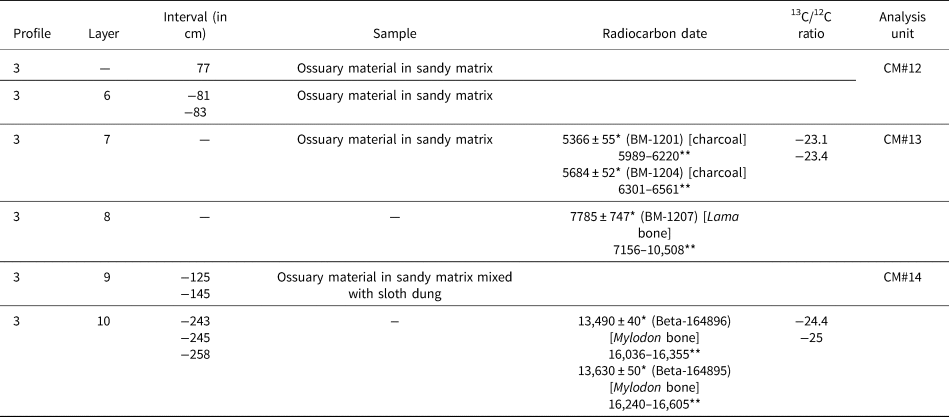
Table 1. Studied samples (arranged by layer from top to bottom of the archaeological sequence), chronology, and analysis units from Cueva del Milodón, Saxon's Reference Saxon1976 excavations. * = radiocarbon years before present (14C yr BP); ** = calibrated years, 2σ ranges (cal yr BP; using CALIB 8.2 program, in conjunction with Stuiver and Reimer, Reference Stuiver and Reimer1993).
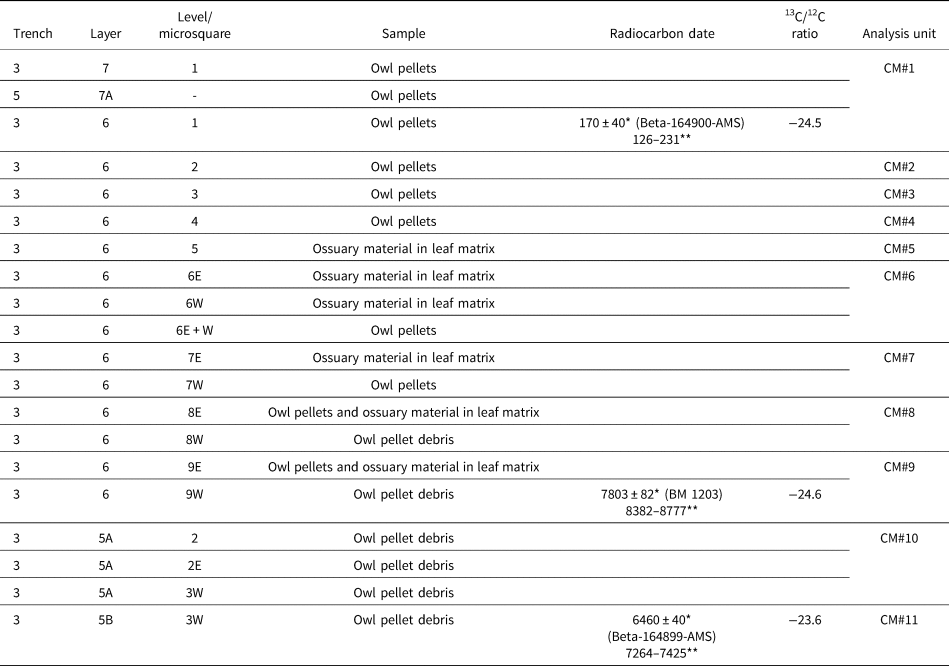
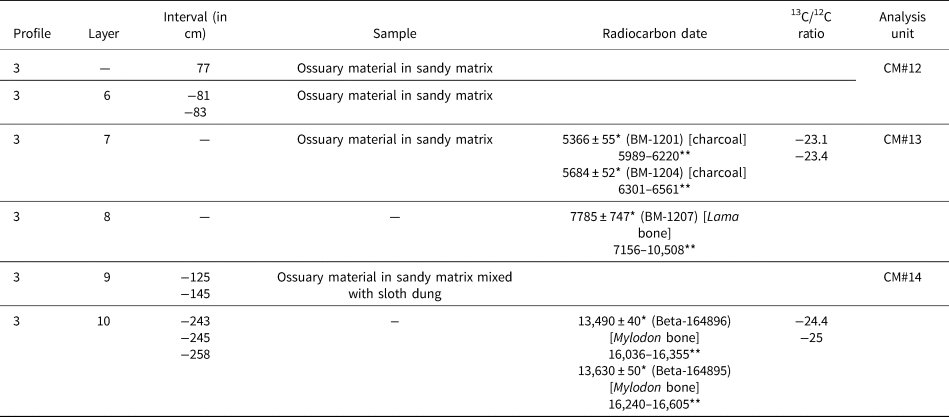
Table 2. Studied samples (arranged by layer from top to bottom of the archaeological sequence), chronology, and analysis units from Cueva del Milodón, Borrero's 2001 excavations. * = radiocarbon years before present (14C yr BP); ** = calibrated years, 2σ ranges (cal yr BP; using CALIB 8.2 program, in conjunction with Stuiver and Reimer, Reference Stuiver and Reimer1993).
Studied DH1 material was recovered by Massone et al. (Reference Massone, Borrero, Martin and Cárdenas1993; see also Borrero et al., Reference Borrero, Lanata and Cárdenas1991) and belongs to Instituto de la Patagonia collections. The excavations in DH1 were made in 10-cm-thick levels; we studied rodents retrieved from the first 3–13 levels of the rock shelter 1. Materials from two squares from DH rock shelter 3 were studied previously by Bárbara Saavedra in 1996, but the results remain unpublished.
Stratigraphy and chronology
Although stratigraphy and chronology of the lower levels of CM are firmly established (see compilations of radiocarbon dates in Martinic, Reference Martinic1996, Martin, Reference Martin2013, and Villavicencio et al., Reference Villavicencio, Lindsey, Martin, Borrero, Moreno, Marshall and Barnosky2016), Saxon samples of micromammal remains and owl pellets from Trench 3 require additional clarification. The sample code label refers to trench-layer-level (sometimes the latter associated with a letter in order to denote square microsector), according to Saxon field notes (L. Borrero, personal observation, 2019; see also Borrero et al., Reference Borrero, Lanata and Borella1988, fig. 1). Layers were labeled in increasing numbers, from bottom to top. Therefore, these samples can be arranged in an approximate stratigraphic order (Table 1; see also Moore, Reference Moore1978, table 1; Humphrey et al., Reference Humphrey, Péfaur and Rasmussen1993, table 3). The uppermost samples, belonging to layer 7 (but not layer 7 of Trench 2, according to Saxon, Reference Saxon1979), can be referred to historical times because they are constituted by owl pellets embedded in sediments removed by early excavations in CM during the end of nineteenth and beginning of twentieth centuries. In contrast, layers 6 and 5 conform to a succession of undisturbed sediments, mostly composed of leaves and other plant remains plus owl pellet debris and ossuary material (“windblown litter” according to Moore, Reference Moore1978).
Radiocarbon dates were performed on preserved owl pellets from two samples (Table 1), one belonging to the uppermost levels (layer 6, level 1) and the other to the lower part of the entire sequence (layer 5B, level 3W). In light of these radiocarbon results and the overall stratigraphic position, the studied samples from Saxon excavations probably represent latest early to middle Holocene and latest Holocene (Holocene limits, according to Walker et al., Reference Walker, Head, Berkelhammer, Björck, Cheng, Cwynar and Fisher2018). There are no rodents available for study from the Mylodon dung sequence excavated by Saxon (Reference Saxon1976, Reference Saxon1979) at his Trench 2. Samples from Borrero excavation, which is an extension of Saxon's Trench 2, are derived from mostly sandy layers labeled with increasing numbers from top to bottom. Radiocarbon dates indicate that layers 7 and 8 represent middle Holocene. Layer 9 lacks absolute ages; however, it can be confidently referred to late Pleistocene (Table 2).
DH1 stratigraphy and chronology were addressed by Massone et al. (Reference Massone, Borrero, Martin and Cárdenas1993; see also Martin, Reference Martin2013). The revealed sequence is composed of six stratigraphic units. The uppermost layer 1 is characterized by accumulations of introduced animal dung, scarce lithic artifacts, and faunal remains (levels 1 and 2). Layer 2 is composed of sandy sediment with presence of rodents, charcoal, and other faunal remains (level 3). Layer 3 is sandy brown sediment that contains the most important material proof of archaeological occupations in DH1, with abundant lithic artifacts, charcoal, and faunal remains (levels 4–14). Charcoal from level 9 was dated ca. 2769–3254 cal yr BP (A-7235). The deeper layers, 4–6, are sterile sediments. Layer 5 is a white volcanic ash (the R1 tephra dated ca. 12,685 ± 260 14C cal yr BP according to Stern, Reference Stern2008; see also Sagredo et al., Reference Sagredo, Moreno, Villa-Martínez and Kaplan2007). Therefore, rodents from DH1 studied here represent exclusively late Holocene.
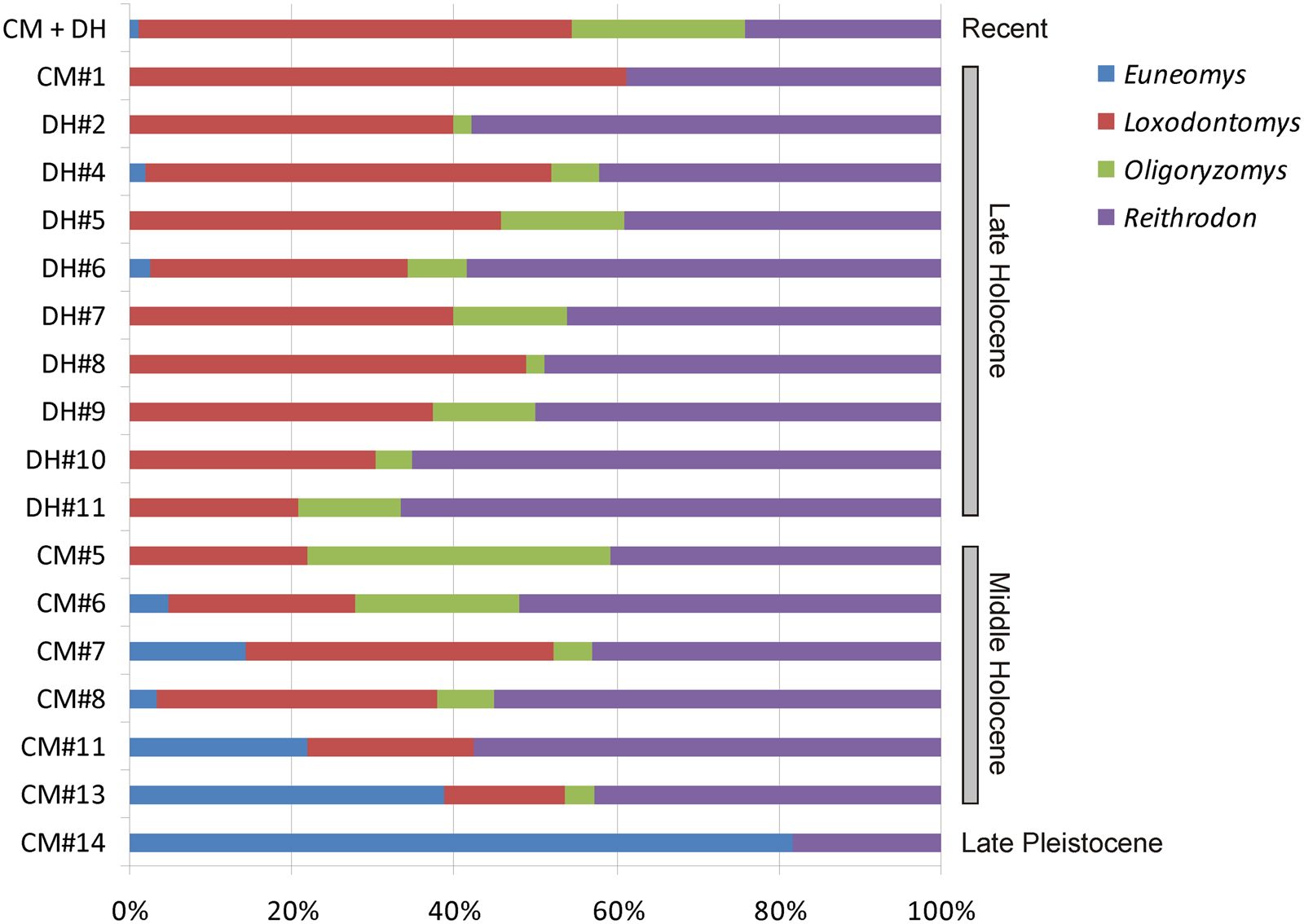
With all the available evidence at hand, we defined operational analysis units, in some instances pooling the NISP of original samples to increase sample size and to bring better support to paleoenvironmental reconstructions. For CM, these units also reflect sedimentary features (Tables 1, 2). For DH1, the correspondence between original samples and analysis units is almost direct, with the exception that uppermost samples 1(1) and 2(3) were pooled (DH#1); the remaining samples, from 3(4) to DH 3(13), correspond to analysis units DH#2 to DH#11, respectively.
The single sample here referred to late Pleistocene, CM#14, was extracted from a mixed sandy matrix where pulverized sloth dung was present, the latter from decomposition of the top of the underlying true “dung carpet” (Table 2). Regrettably, CM#14 has NISP < 30 and had to be excluded from statistical analyses. Saxon Trench 3 cut an ~2 m profile composed mostly of vegetational remains. Although chronological control of this organic sequence is poor (Table 1), we are confident that most of the studied samples (CM#5 to CM#11) represent middle Holocene, roughly bracketed between 7.8–5.6 ka (see also Moore, Reference Moore1978, table 1). In addition, the uppermost samples of the sequence (CM#1 to CM#4) are here referred to latest Holocene (Table 1). In this respect, our interpretation departs from Moore (Reference Moore1978, table 1), in which samples 3(6)1 to 3(6)3 are placed below a radiocarbon date of 2500 cal yr BP that was obtained at another locus of the cave, simply because we have a radiocarbon date (Table 1). Summarizing, to our best understanding, CM samples represent three differential and scattered “time windows”: a point in the late Pleistocene, a segment in the middle Holocene, and a short segment in the latest Holocene. Fortunately, the DH1 sequence added to the analysis a late Holocene segment coarsely covering the last 3 ka.
Recent samples
Two local samples of recent, fresh owl pellets were used to assess the local micromammal community in order to compare the studied fossil samples. The largest one was produced by the Great Magellan Owl, Bubo magellanicus, and collected inside DH1 by one of the authors (FMM) in January 1993. In addition, a smaller recent sample was taken from inside CM that was constituted by isolated fresh owl pellets collected beneath a roosting site from an indeterminate raptor bird (also by FMM, in 1993). The small-mammal assemblages derived from these two owl pellet samples are interpreted here as representing the present-day, small-mammal community that inhabits the area around the studied localities (i.e., the SSW slope of Cerro Benítez). A regional approach on small mammal living assemblages in southern Chile was made using several owl pellet analyses from localities in Torres del Paine National Park that was based on literature (Jaksic et al., Reference Jaksic, Rau and Yáñez1978; Yáñez et al., Reference Yáñez, Rau and Jaksic1978; Rau and Yáñez, Reference Rau and Yáñez1981; Iriarte et al., Reference Iriarte, Franklin and Johnson1990).
Taphonomy
Digestive corrosion (light, moderate, heavy, and extreme) on teeth, distal epiphysis of humeri, and proximal epiphysis of femora of rodents was evaluated considering four categories of predators based on the classification proposed by Andrews (Reference Andrews1990), Fernández et al. (Reference Fernández, Montalvo, Fernández-Jalvo, Andrews and López2017), and Montalvo and Fernández (Reference Montalvo and Fernández2019). The breakage of bones was assessed following Andrews (Reference Andrews1990) and Pardiñas (Reference Pardiñas2000). We also included an evaluation of the relative abundance of skeletal elements that considers the representation of each skeletal element in context of the MNI through MNEi/(Ei×MNI)×100 (Andrews, Reference Andrews1990). In addition, the potential effect of post-depositional processes (e.g., weathering, trampling, root action, manganese oxide, and wind dispersion) was considered (Korth, Reference Korth1979; Andrews, Reference Andrews1990; Pardiñas, Reference Pardiñas2000; Cheme Arriaga et al., Reference Cheme Arriaga, Montalvo and Sosa2012; Montalvo et al., Reference Montalvo, Cheme Arriaga, Tallade and Sosa2012). At the time when the samples excavated by Saxon were examined, neither digestive corrosion nor breakage degrees were registered; however, a complete quantification of skeletal remains was conducted, allowing us to calculate relative abundances.
Major vegetational units and rodent assemblages
As was described by Moore (Reference Moore1978; see also Markgraf, Reference Markgraf1985; Heusser, Reference Heusser2003), major vegetation types in the southern Patagonia mainland are distributed in approximate longitudinal belts, determined by topography and climate/rainfall gradient. From east to west, these vegetation types are the Patagonian steppe (grass and shrub steppes), the deciduous forest (mostly dominated by Nothofagus pumilio), the evergreen forest (where Nothofagus betuloides assumes dominance), and the Magellanic moorland; in addition, vegetation above the timberline has unique characteristics (feldmark).
Although rodent communities from the southern tip of continental Argentina and Chile were not addressed in detail, unpublished data plus the few available contributions (e.g., Allen, Reference Allen and Scott1905; Osgood, Reference Osgood1943; Pine et al., Reference Pine, Miller and Schamberger1979; Patterson et al., Reference Patterson, Gallardo and Freas1984; Reise and Venegas, Reference Reise and Venegas1987; Pardiñas et al., Reference Pardiñas, Udrizar Sauthier and Teta2009, Reference Pardiñas, Teta, Formoso, Barberena, Borrero and Borrazzo2011b) allow a superficial description of the current assemblages. The Patagonian steppe is characterized by the exclusive occurrence of Eligmodontia morgani, moderate proportions of Reithrodon auritus, and the absence of several cricetids, such as Abrothrix lanosa, Geoxus sp., and Paynomys macronyx. Deciduous forest rodent communities include a predominance of Loxodontomys micropus and Oligoryzomys longicaudatus and variable proportions of Abrothrix olivacea, A. hirta, and R. auritus (Reise and Venegas, Reference Reise and Venegas1987). Our knowledge about evergreen forest and Magellanic moorland rodent communities is very poor. The few collections made in these cold and many times inaccessible habitats suggest low specific richness with dominance of Oligoryzomys longicaudatus and Abrothrix olivacea in evergreen forest environments (Texera, Reference Texera1972, Reference Texera1973). The vegetational communities of prostrate dwarf shrubs, cushion plants, and bryophytes that characterize Magellanic moorland environments support a reduced set of rodents, mostly O. longicaudatus and A. lanosa (Guzmán Sandoval, Reference Guzmán Sandoval2010; Guzmán et al., Reference Guzmán, Ortiz and Cañón2015). Cerro Benítez is currently located in a deciduous forest environment. Local trappings around CM revealed A. hirta and A. olivacea (Reise and Venegas, Reference Reise and Venegas1987). Data from owl pellets, however, point to a rich assemblage composed of nine sigmodontines, but largely dominated by L. micropus (see below). However, 50 km N, where forest meets steppe, a mixture of taxa from closed and open environments typified the rodent community. This is the case for Torres del Paine. According to Reise and Venegas (Reference Reise and Venegas1987), trappings in ñire shrubs and pampa in Laguna Amarga retrieved a pool of five species (A. olivacea, A. hirta, L. micropus, O. longicaudatus, and R. auritus), while a swampy grass slope with bushes of N. pumilio plus dense N. antarctica produced abundant L. micropus and O. longicaudatus. The community revealed by owl pellets in Torres del Paine shows a codominance of A. olivacea, A. hirta, L. micropus, O. longicaudatus, and R. auritus, but also the occurrence of rocky specialists, such as Phyllotis, steppe elements, such as Eligmodontia (Jaksic et al., Reference Jaksic, Rau and Yáñez1978; Rau and Yáñez, Reference Rau and Yáñez1981; Iriarte et al., Reference Iriarte, Franklin and Johnson1990), and rare forms, such as A. lanosa and Paynomys macronyx (Rau et al., Reference Rau, Yáñez and Jaksic1978).
Paleoenvironmental reconstruction
Rarefaction curves were constructed with the aim to evaluate how species richness and diversity are potentially biased by differential sample sizes (Tipper, Reference Tipper1979). Selected archaeological samples, those with NISP > 30 (in order to avoid statistical biases) were used in further analyses (Formoso et al., Reference Formoso, Teta, Carbajo and Pardiñas2016). Paleoenvironmental significance of fossil samples was assessed using univariate and multivariate approaches. A first exploration was made inspecting fossil samples and recording species presence/absence and relative abundances (calculated as a percentage of the total NISP of all samples studied in order to avoid interdependence; Supplementary Table 1). Those species associated with contrasting habitats were highlighted and used to infer main vegetation units in the local area of Cerro Benítez. A taxonomical habitat index (THI) was calculated for each analysis unit, slightly modifying the THI proposed by Evans et al. (Reference Evans, Van Courvering and Andrews1981), as follows: THI = Σ ([pi x NISPi]/NISP)/S, where pi = proportion of the species i in a landscape unit; NISPi = number of identified specimens for the i species; NISP = NISP for the total sample; and S = total number of species in the sample (Pardiñas, Reference Pardiñas1999). This index was employed to indicate the overall representation of gross environmental/landscape units (i.e., grassland, shrubland, forest). The probability of occurrence of a species in each unit, expressed as a proportion, was derived from the trapping data provided by Iriarte et al. (Reference Iriarte, Franklin and Johnson1990, table 3; Supplementary Table 1) for Torres del Paine National Park (see also Johnson et al., Reference Johnson, Franklin and Iriarte1990). Abrothrix lanosa, Ctenomys, Geoxus, and Phyllotis were excluded from calculations because trapping data are unavailable. Although the informative value of the THI loses power when the pool of included species is small (Andrews, Reference Andrews1990; Pardiñas, Reference Pardiñas1999), this index constitutes a plausible tool to infer environmental evolution on a gross scale (Reed, Reference Reed2003). Finally, to make a regional environmental exploration, we constructed a data matrix of relative abundances (based on MNI), including available recent owl pellet samples from the southernmost South America mainland south of 50°S plus selected archaeological samples under study (Supplementary Table 2). Owl pellet data were largely derived from the database of the Colección de Material de Egagrópilas y Afines “Elio Massoia” (Centro Nacional Patagónico, Puerto Madryn, Chubut, Argentina) and complemented with literature. A correspondence analysis was conducted on this data matrix (MNI values ln transformed) using the software PAST (PAleontological STatistics), version 4.03 (Hammer et al., Reference Hammer, Harper and Ryan2001).
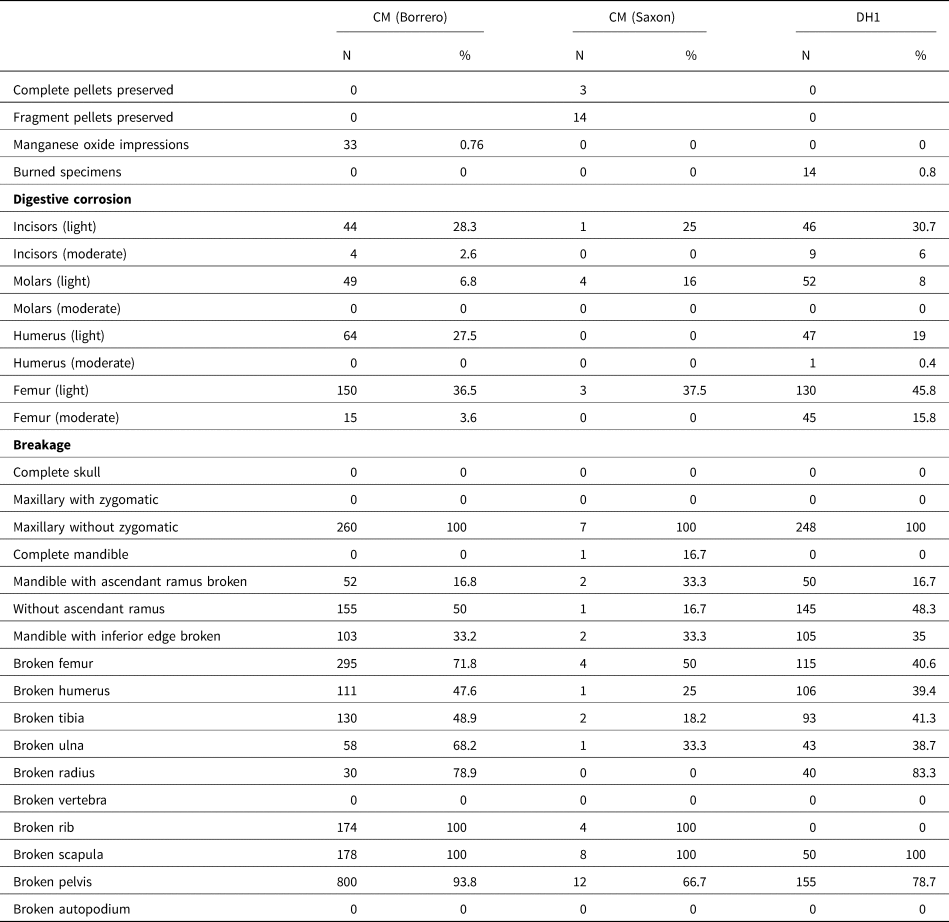
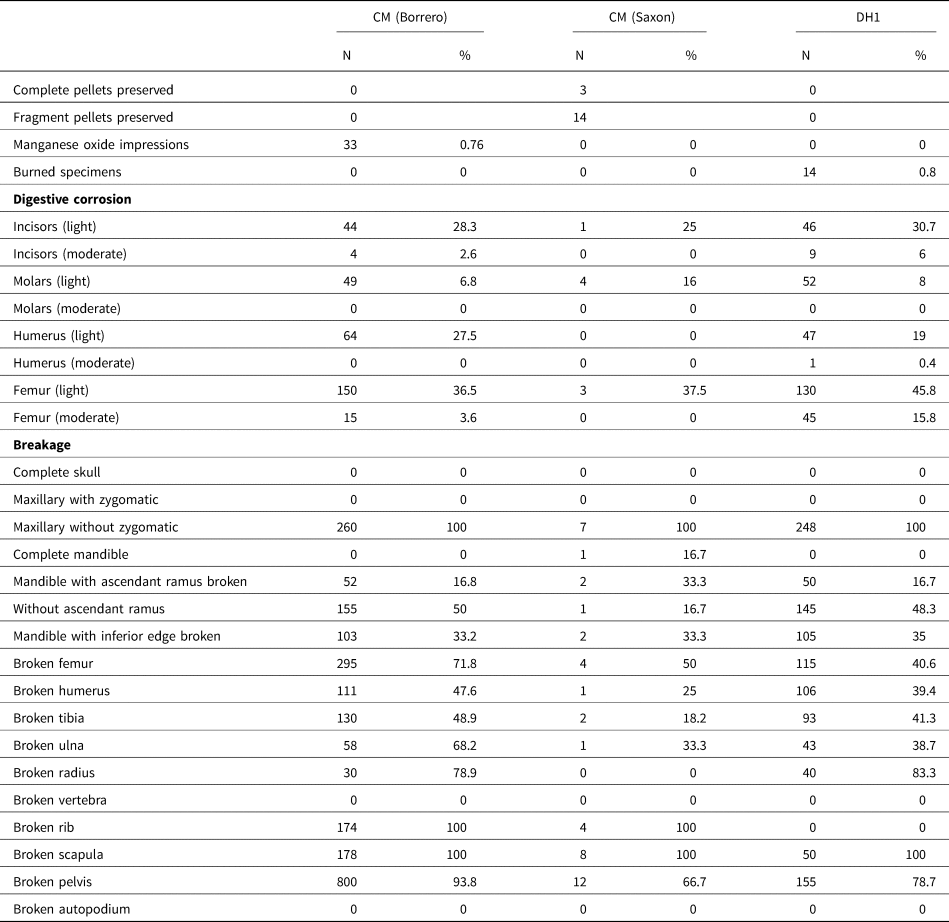
Table 3. Taphonomic attributes of pooled micromammal samples from Cueva del Milodón and Dos Herraduras 1 (Última Esperanza, Chile).
RESULTS
Taphonomy
Most of the studied samples from the Saxon excavation were complete or fragmented owl pellets. Owl pellets are distributed throughout the Holocene sequence of CM without perceptible variations in morphology or size (Fig. 2a). Regarding the latter, most of the entire pellets are crushed, so accurate measurements are impossible to make, but the pellets have lengths less than 50 mm (mean = 46 mm, n = 12).
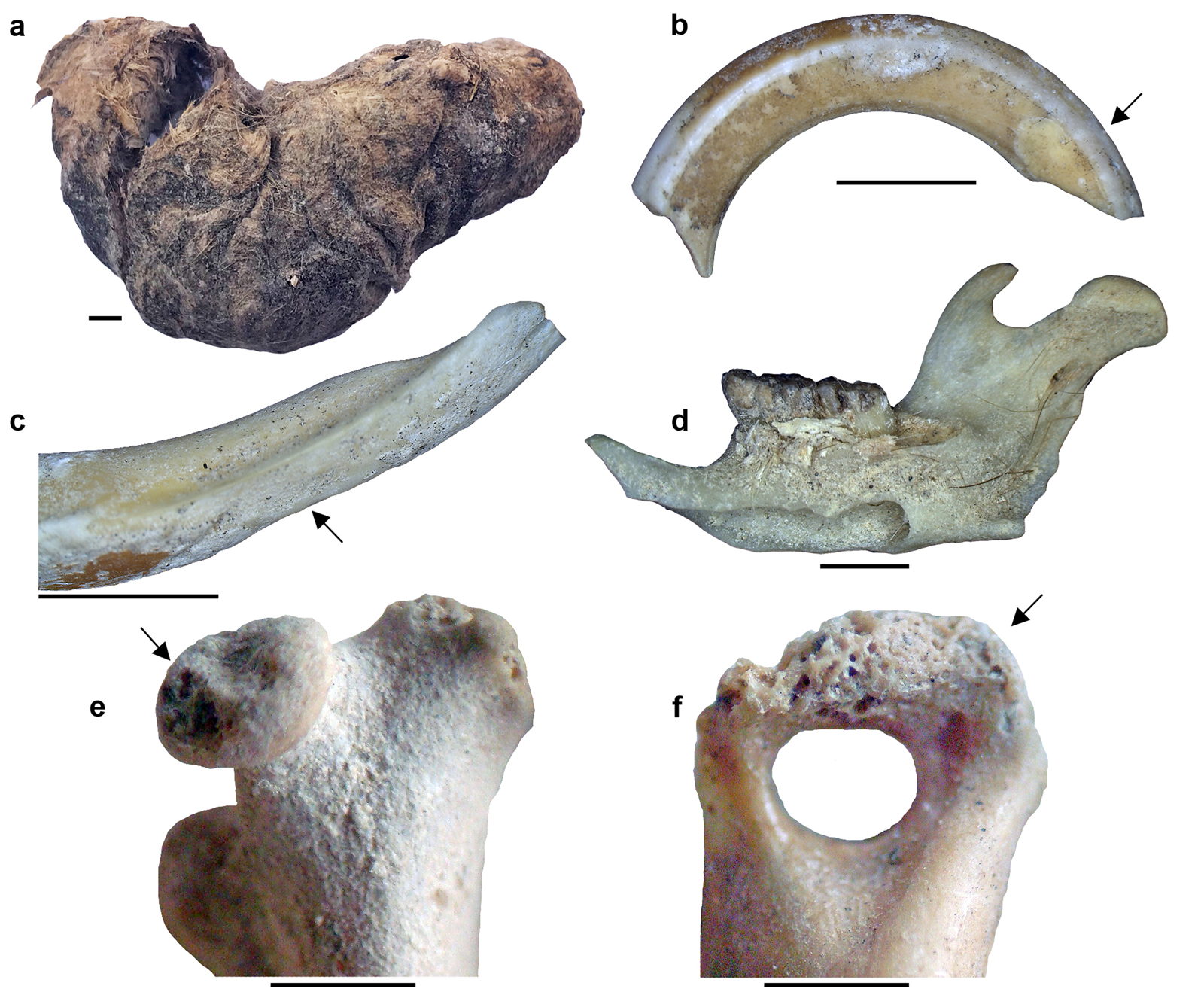
Figure 2. Evidence of owl trophic activity at Cueva del Milodón (CM) and Dos Herraduras 1 (DH1) archaeological sites, Cerro Benítez area, Chile. (a) Preserved owl pellet (sample 3[6]1, Saxon excavation); (b) upper incisor of sigmodontine rodent with light digestive corrosion (sample 3[7], Borrero excavation); (c) lower incisor of rodent with moderate digestive corrosion (sample 3[7], Borrero excavation); (d) right dentary of Loxodontomys micropus, derived from owl pellet, in lingual view showing attached hair remains (sample 3[6]4, Saxon excavation); (e) femur of rodent, proximal portion, with light digestive corrosion (DH1, artificial level 4); (f) humerus of rodent, distal portion, with moderate digestive corrosion (DH1, artificial level 9). Scales = 2.5 mm. Arrows indicate the sectors where the enamel (b and c), dentine (c) and bone tissue (e and f) were affected.
Table 3 shows the main taphonomic attributes recorded in selected studied samples from CM and DH1. Of the analyzed skeletal remains (i.e., femora, humeri, incisors, and molars), minor variations of marks produced by digestive corrosion per archaeological sample (CM Borrero samples: 21.5%; CM Saxon samples: 11.1%; DH samples: 24.4%) were found. In all samples, the category “light” was the most represented (Fig. 2b, e), followed by the category “moderate” (Fig. 2c, f), which reaches the highest values in DH1 samples (Table 3). In cases of digestive corrosion on teeth, low differences were observed per archaeological sample (CM Borrero samples: 11.1%; CM Saxon samples: 4.2%; DH samples: 13.4%). In addition, little variation was detected in the digestive corrosion of the proximal portions of the femora and distal portions of the humeri per archaeological sample (CM Borrero samples: 35.6%; CM Saxon samples: 25%; DH samples: 40.4%).
DH1 samples show a higher percentage of fracture (66.4%), followed by CM Borrero samples (54.2%) and CM Saxon samples (22.6%). The lowest values of breakage of the latter possibly occur because the bones were protected from post-depositional agents (see discussion) by the hair cover of the pellets. Complete crania were not recovered in any of the samples, and all of them corresponded to maxillae without the zygomatic arch. The rates of fractured mandibles were very high and exhibited similar representation among the different categories in CM and DH1 samples (Table 3). The fracture values of postcranial elements were higher in DH1 samples (50.9%) in comparison to the CM Borrero samples (47.2%) and CM Saxon samples (24.1%). Moreover, the vertebrae and autopodium elements were found complete in all samples. However, the most fragile elements (ribs, scapulae, and pelves) showed a high degree of breakage in all samples (Table 3).
The average relative abundance was higher in CM (Borrero samples: 46.66%; Saxon samples: 29.99% [Layers 5, 6: 26.00%; Layer 7: 34.00%]) compared to DH1 (29.80%). In these samples, a similar pattern of skeletal-part representation was observed, where the most abundant were the maxillae, mandibles, humeri, femora, and tibiae; the less abundant were the radii, vertebrae, ribs, and autopodial elements (Fig. 3). However, in CM, Saxon samples (Layers 5 and 6) and Borrero samples yielded greater proportions of pelves (Fig. 3).
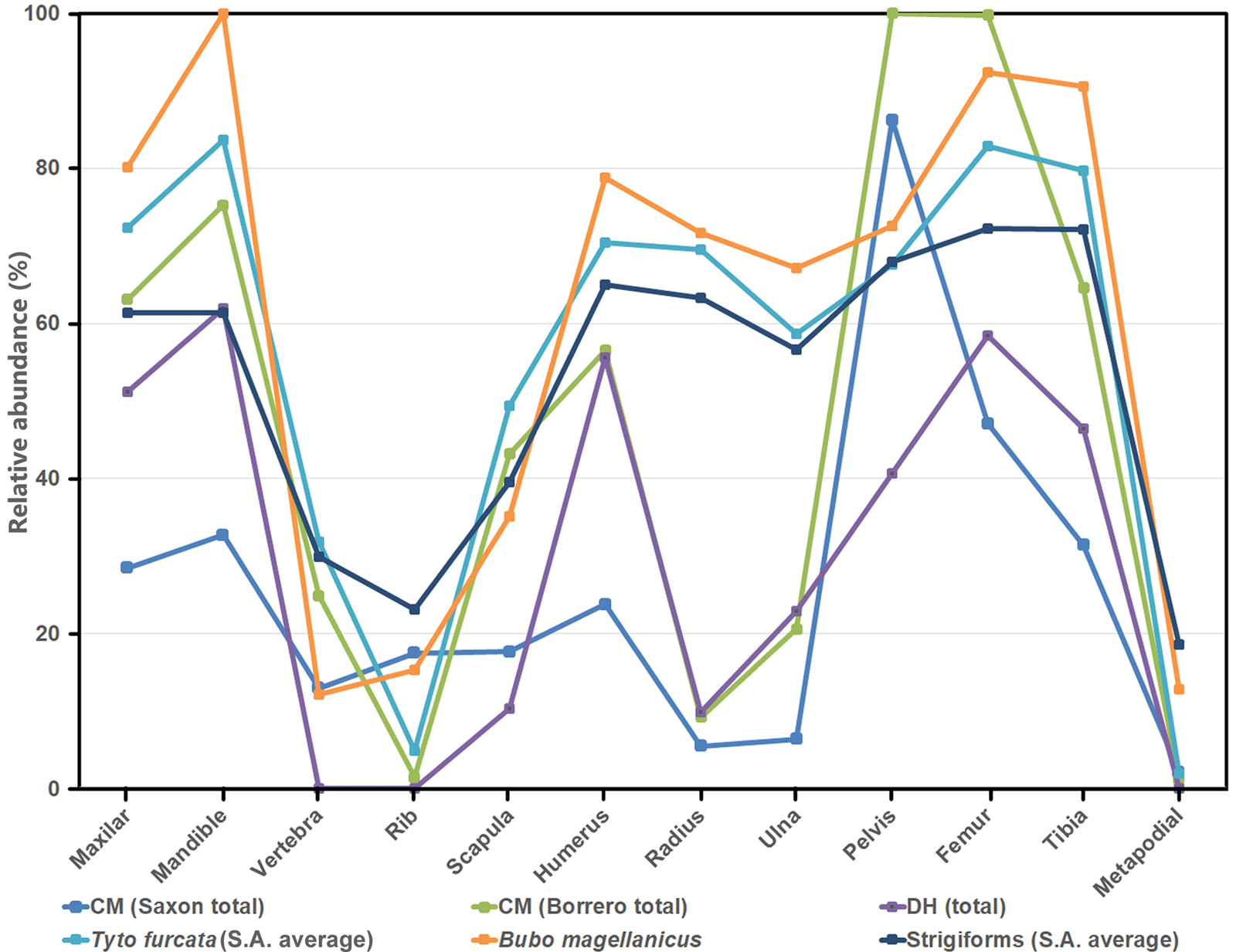
Figure 3. Relative abundance of skeletal elements for archaeological (Cueva del Milodón [CM] and Dos Herraduras 1 [DH1]) and modern samples (Tyto furcata, Quintana, Reference Quintana2015; Bubo magellanicus, Montalvo et al., Reference Montalvo, Fernández and Tallade2016, and average of Strigiformes samples of South America, Montalvo and Fernández, Reference Montalvo and Fernández2019).
Finally, none of the studied samples showed bones with signs of weathering, rodent marks, root traces, or polishing and rounding by the effects of hydraulic transport. Less than 1% of bones from CM Borrero and DH1 samples exhibited impregnation with manganese oxide and thermoalteration, respectively.
Taxonomy
The overall rodent assemblage recorded in the Cerro Benítez area, including both archaeological and recent samples, is composed of 10 species of cricetids and two caviomorph rodents (Table 4; Fig. 4). Cricetids are represented by small-sized abrotrichines, such as three species of the genus Abrothrix, two semifossorial forms (Geoxus and Paynomys), the widespread colilargo Oligoryzomys longicaudatus, and several medium-sized phyllotine and phyllotine-like species (the cony rat Reithrodon auritus, the southern pericote Loxodontomys micropus, the chinchilla rat Euneomys chinchilloides, and the leaf-eared mouse Phyllotis xanthopygus). The two caviomorph rodents detected are the subterranean Ctenomys magellanicus and the chinchillid Lagidium sp. The recorded micromammal assemblage conforms with previous data available from southern mainland Chile (e.g., Osgood, Reference Osgood1943; Reise and Venegas, Reference Reise and Venegas1987; Iriarte et al., Reference Iriarte, Franklin and Johnson1990; Johnson et al., Reference Johnson, Franklin and Iriarte1990).
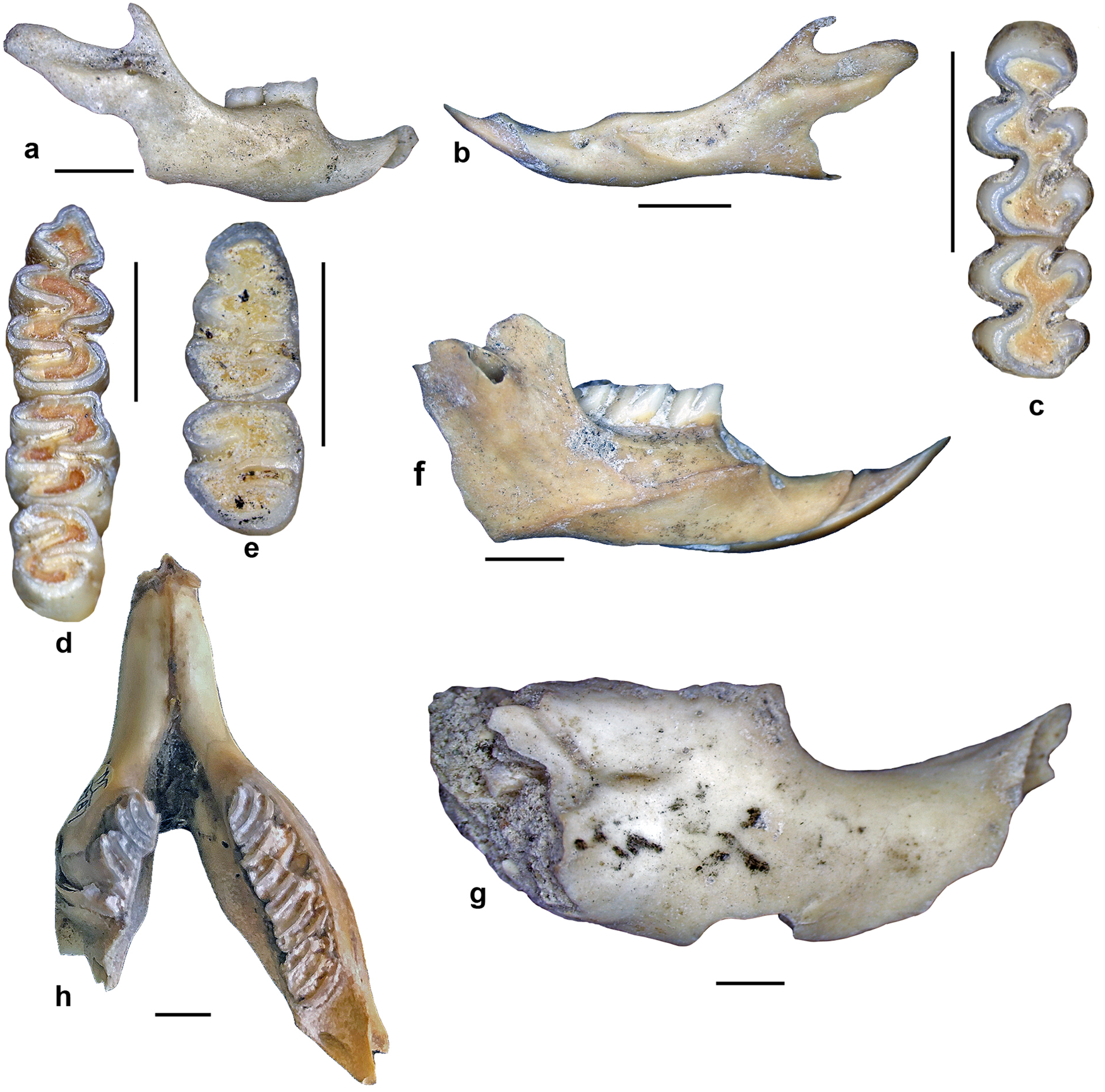
Figure 4. Selected craniodental remains of rodents excavated in Cueva del Milodón (CM, sample 3[7], Borrero excavation) or recorded in local recent owl pellets (Dos Herraduras 1, DH1), Cerro Benítez area, Chile. (a) Abrothrix olivacea, right dentary in labial view (CM); (b) Geoxus sp., left dentary in labial view (DH1); (c) Paynomys macronyx, left first and second upper molar in occlusal view (DH1); (d) Reithrodon auritus, right lower toothrow in occlusal view (CM); (e) Loxodontomys micropus, right first and second lower molar in oclussal view (CM); (f) Euneomys chinchilloides, right dentary in labial view (CM); (g) Ctenomys magellanicus, fragmentary right dentary in labial view (CM); (h) Lagidium sp., mandible in dorsal view (M 8787, historical collections NHMUK). Scales = 2.5 mm.
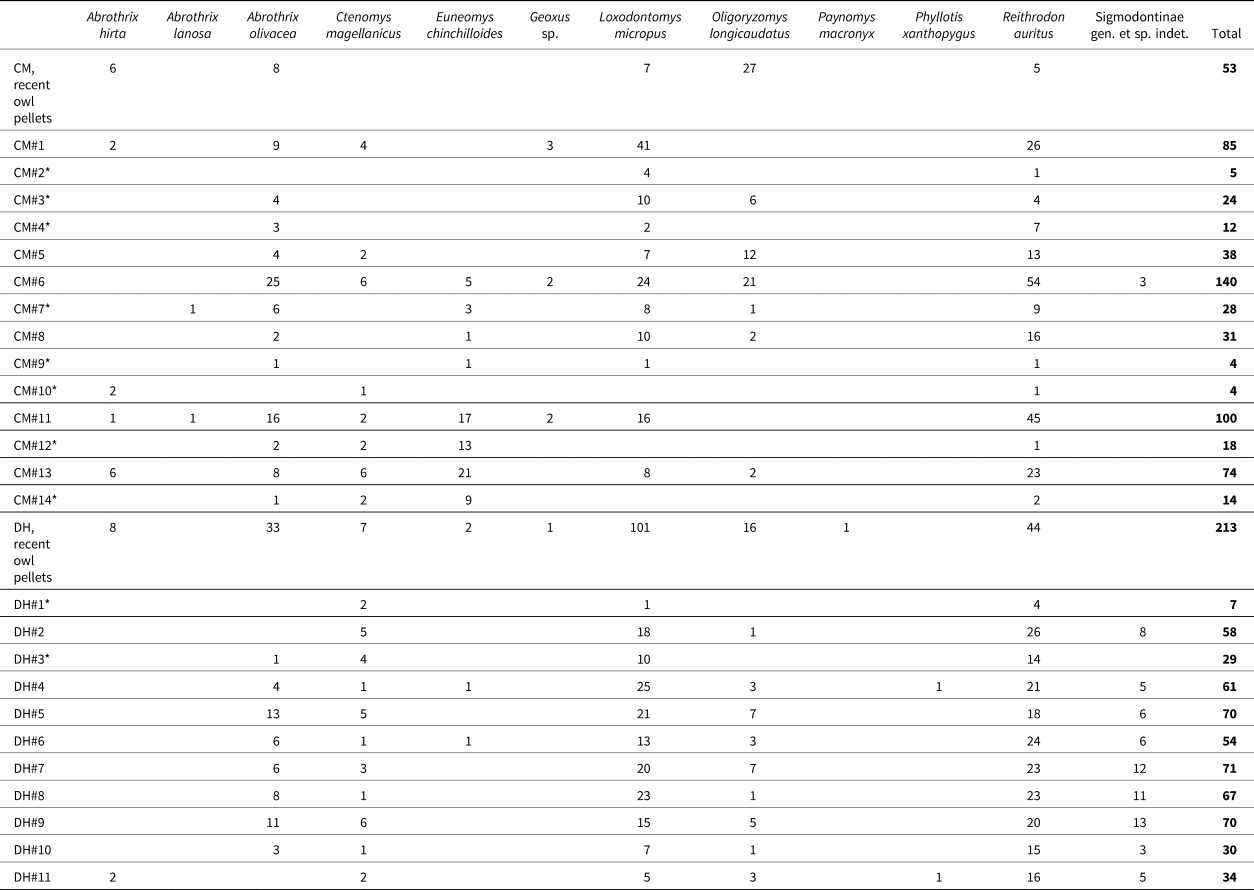
Table 4. Relative abundances (NISP) per taxa recorded in analyzed units from Cueva del Milodón (CM) and Dos Herraduras 1 (DH); those marked with an asterisk (*) were excluded from multivariate analyses.
We identified nine species of rodents in both the CM (based on a cranial NISP of 578 remains) and DH1 (NISP = 551) sequences (Table 4; Supplementary Tables 3, 4). The mole rat Paynomys macronyx was exclusively recorded in recent owl pellets; conversely, two cricetids, Abrothrix lanosa and Phyllotis xanthopygus, were limited to archaeological samples. In overall terms, past assemblages in both sites are characterized by almost continuous occurrences of A. olivacea, C. magellanicus, L. micropus, and R. auritus. Numerically, co-dominance between L. micropus and R. auritus is also evident (Table 4; Fig. 5).
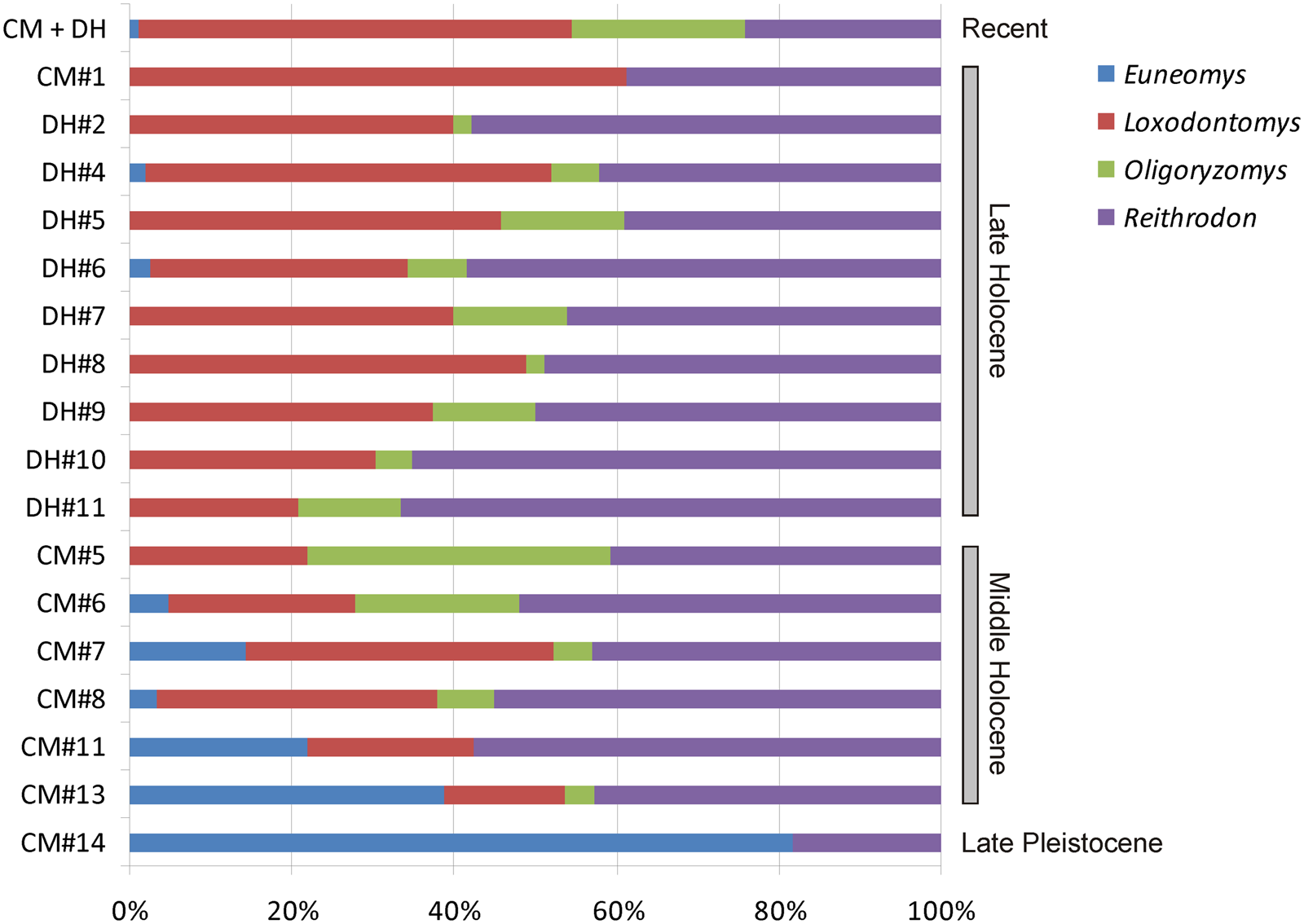
Figure 5. Temporal variation of the relative abundances (calculated as a percentage of the total NISP of all samples studied; see Supplementary Table 1) of selected rodent taxa from samples of Cueva del Milodón (CM) and Dos Herraduras 1 (DH1), Cerro Benítez area, Chile.
The record of Lagidium is based on isolated material collected in CM. A survey of the paleontological collections of the NHMUK revealed a fragmentary lower jaw (specimen number M8787, Fig. 4 h), a femur, and a tibia lacking the proximal portion but preserving the articulated part of the feet belonging to this chinchillid. These specimens are moderately well preserved and included attached soft tissues. These remains lack associated stratigraphical data but, judged by the degree of preservation of perishable tissues, probably were extracted from the Mylodon “dung carpet.”
Paleoenvironmental reconstruction: biases
Expectedly, correlation between sample sizes (measured by total NISP) and species richness (S) is high and significant (p = 0.95), whether calculated using the total of available samples (r2 = 0.69) or the total per each site (CM, r2 = 0.83; DH1, r2 = 0.64). However, when small samples (i.e., those of NISP < 30) are excluded from computations, these correlations drop (total samples, r2 = 0.54; CM = 0.78; DH1 = 0.57), and even disappear when the largest recent sample of DH1 (NISP = 213; S = 9) is discarded (total samples, r2 = 0.28; DH1 = 0.03). Rarefaction curves also reflect the interdependence between sample sizes and species richness (Supplementary Fig. 1), but a noteworthy point is that the observed differences in the value of S also could be linked with non-methodological biases. In fact, several of the unit analyses of CM (e.g., CM#6, CM#11, CM#13) show expected values of S > 6, suggesting higher species richness at some times in the past than in the present. In contrast, the rarefaction analysis for DH1 samples (Supplementary Fig. 1) tends to attenuate observed S differences. Summarizing, it is clear that small samples (NISP < 30) must be excluded in the paleoenvironmental reconstruction, especially in multivariate approaches, in order to avoid unrealistic results. However, even small in general terms, the studied samples can be used to infer past environments at a gross scale.
A second aspect that deserves closer scrutiny is the complex mosaic of data generated by different excavations, depositional environments (within the same cave and between the sites), and taphonomical histories. Environmentally significant differences between CM and DH1 can be ruled out, because both are very near each other (< 2 km), opened to the same general landscape, and approximately bracketed within the hunting territory of the same owl (see below). However, although the micromammal remains deposited at both sites were derived from bird raptor trophic activities (see above), depositional and preservational histories seem different among sectors excavated within CM and between CM and DH1. Saxon trench 3 was made mostly cutting dense layers of vegetation debris. These deposits have exceptional preservation of usually ephemeral biological material (such as hair, feathers, or leaves) mixed with bones and teeth. However, considering that wind retransportation and sorting was detected, the studied samples probably reflect selection biased to smaller taxa. We can surmise that most of the samples CM#7 to CM#9 are taphonomically impoverished of medium- and large-sized rodents (e.g., Ctenomys, Euneomys, Loxodontomys, Reithrodon). In sharp contrast, Borrero excavations were made mostly through sandy deposits with poor biological preservation. In this kind of sedimentary environment, micromammal assemblages are often biased to larger and heavier species. A single set of comparisons apparently favored these arguments. The ratio between medium- and large-sized (Ctenomys, Euneomys, Loxodontomys, Phyllotis, and Reithrodon) and small-sized (Abrothrix spp., Geoxus, and Oligoryzomys) is 3.0 for the total of CM samples, 2.7 for the samples from Saxon excavations, and 4.6 for the samples from Borrero excavations. Interestingly, this value for the DH1 samples is 4.7. The congruence of these two latter values suggests that the relationship 5:1 (medium-/large-sized versus small-sized rodents) shown by the samples of CM obtained by Borrero and those from DH1 are partially artefactual (i.e., these assemblages are taphonomically enriched with medium- and large-sized rodents).
Paleoenvironmental reconstruction: inferences
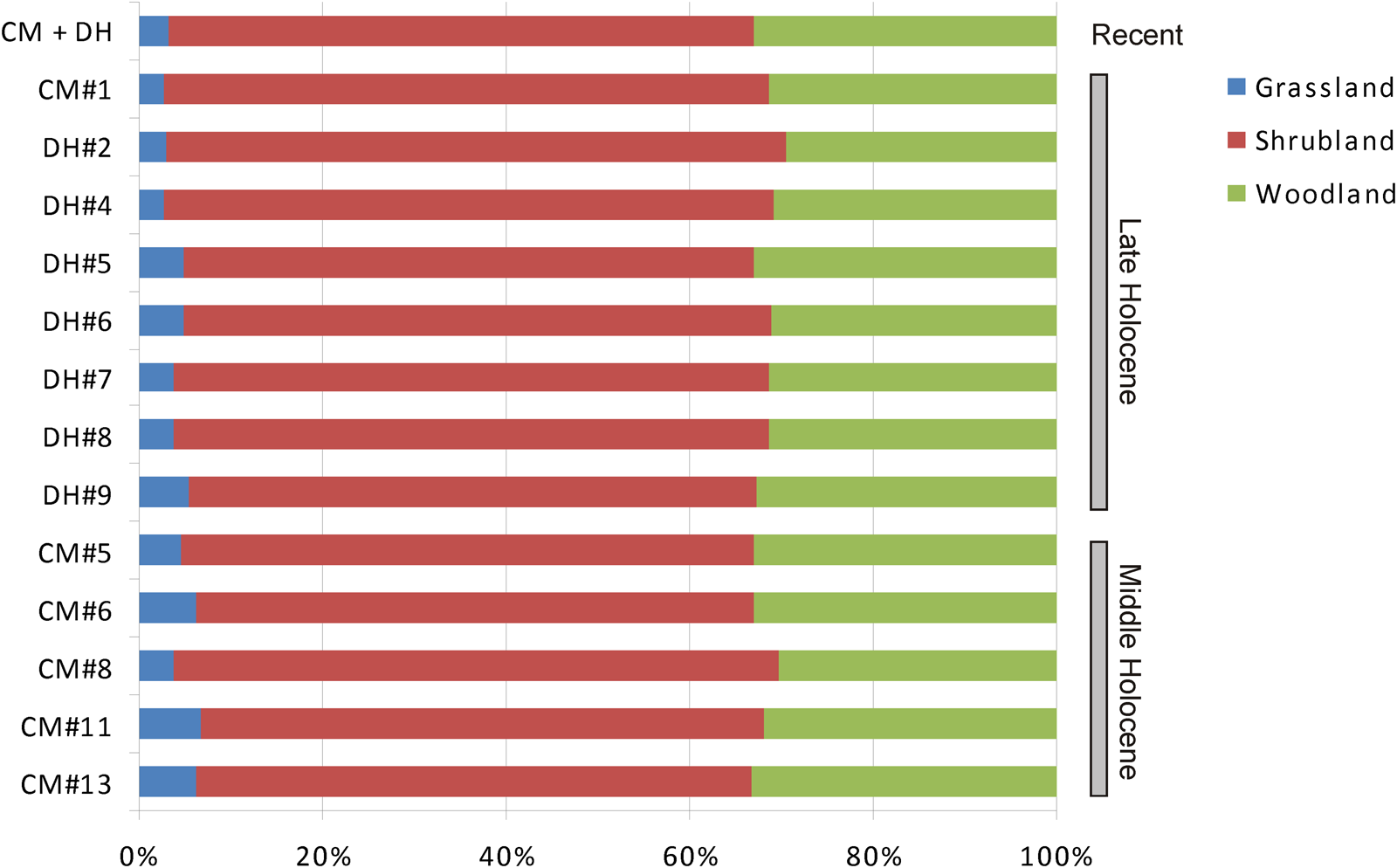
THI values from selected CM and DH1 units reveal a marked overall stability of gross environmental units across the middle and late Holocene. Minor changes that were detected (< 5%) imply a major proportion of open environments (grasslands) during the middle Holocene, and a perceptible increment of the shrubland during the late Holocene (Fig. 6). According to the THI values, most of the Holocene represented by CM and DH1 samples reflects a mosaic dominated by shrubland (ranging from 50–60%), followed by woodland (30–35%), and a scarce participation of grassland (< 10%). However, as was noted above, THI lost sensitivity when the pool of included species is small. This negative effect is magnified in the case of CM and DH1 because three significant species from an environmental point of view (A. lanosa, Geoxus sp., and P. xanthopygus) were not included.
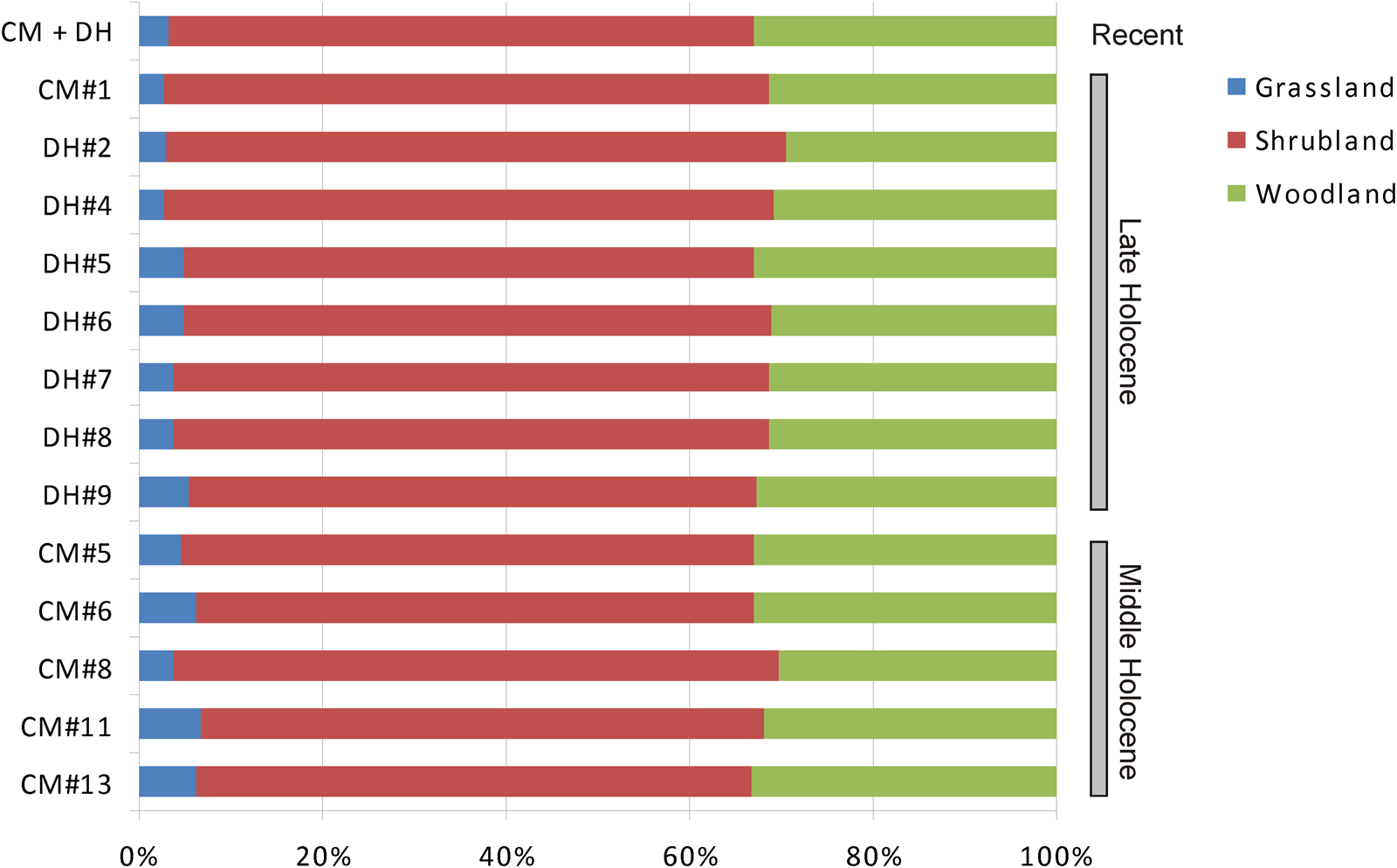
Figure 6. Holocene evolution of major habitat types according to values of the Taxonomical Habitat Index calculated from selected samples of Cueva del Milodón (CM) and Dos Herraduras 1 (DH1), Cerro Benítez area, Chile.
Direct evaluation of presence/absence and relative abundances of environmentally sensitive species reinforces the hypothesis that studied rodent samples are informative for reconstructing past conditions (Fig. 5). From CM#13 (middle Holocene), the abundance of L. micropus is increasing, reflecting a gradual but clear invasion of the shrubland associated with forest around the caves. This tendency continues throughout the late Holocene, with minor fluctuations. At the end of middle Holocene, O. longicaudatus peaks, surpassing L. micropus (in CM#5); this event can be interpreted as a moment of environmental disturbance favoring an opportunistic rodent such as O. longicaudatus. The same trend is reflected by the abundance of this rodent, coupled with that of A. olivacea, in the recent sample. Middle and late Holocene abundance of R. auritus, the best indicator of open environments, ranged from 40–60%, suggesting that grasslands near the caves experienced minor fluctuations during most of the Holocene. The presence of A. lanosa as an exclusive element during middle Holocene (in CM#11), even in a sample with low NISP (as CM#7), supports major habitat heterogeneity.
Correspondence analysis results reflect archaeological sample ordination mostly following axis 1 (Fig. 7). This indicates that the main structure of small mammal communities allied to the deciduous forest has remained moderately stable throughout the Holocene in the Cerro Benítez area. The main factor (Axis 1, 39.07% of the total variance) grouped some of the forest-related taxa (Geoxus sp., O. longicaudatus, L. micropus, P. macronyx, A. lanosa) with positive values, and grouped taxa typically allied to Patagonian steppe and rocky outcrops (Eligmodontia sp., E. chinchilloides, Microcavia australis, P. xanthopygus) with negative values. The analysis also supports the employed methodology because it reflects a low variability between modern owl samples taken from the same locality (Supplementary Table 2).
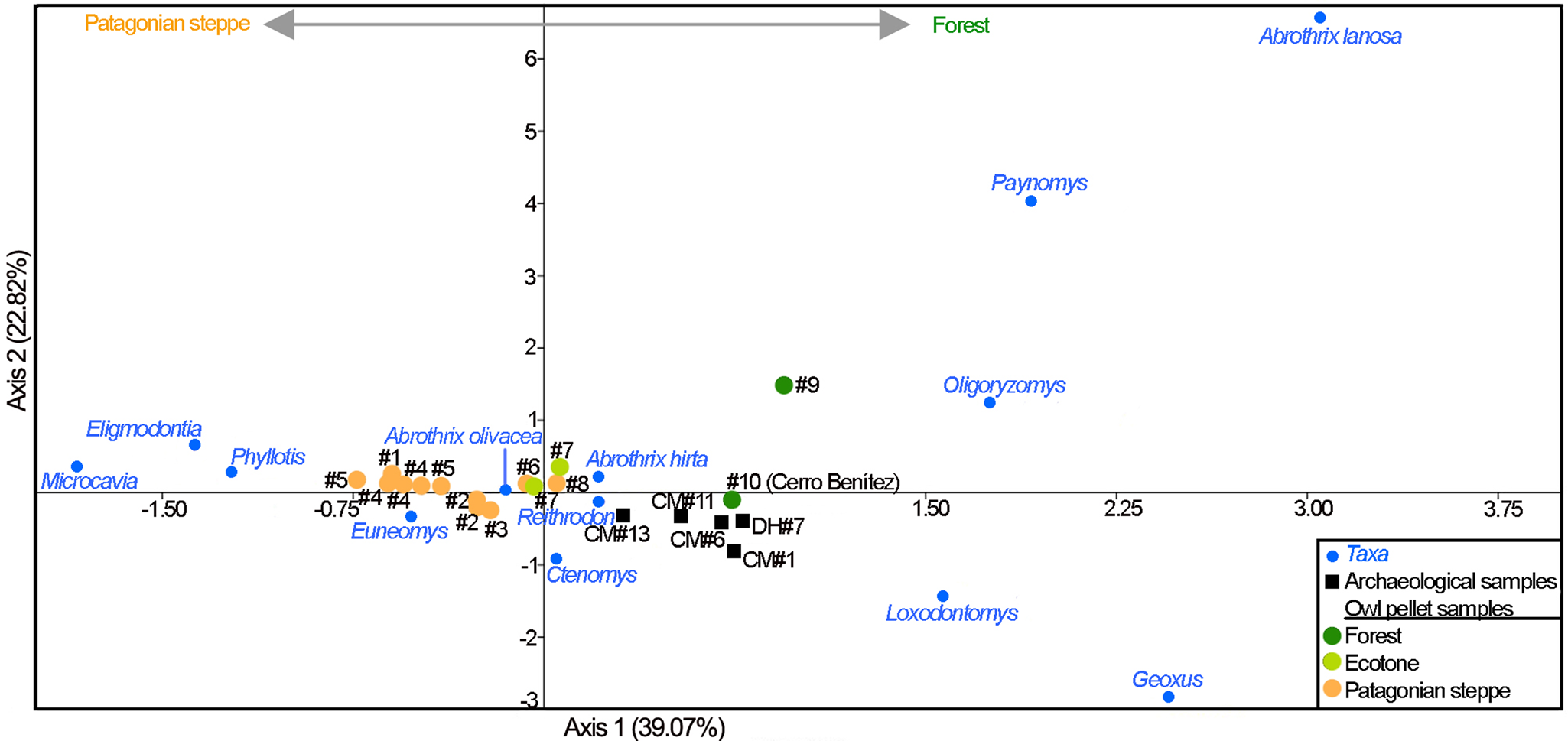
Figure 7. Results of correspondence analysis that examines relationships among species abundance between archaeological and modern samples at regional scale. Samples analyzed include modern and fossil assemblages from Cerro Benítez area (Cueva del Milodón [CM] and Dos Herraduras 1 [DH1]) as well as modern samples from an additional 10 localities in southernmost Argentina and Chile mainland in northern Patagonia (see Supplementary Table 2). Arrows at the upper center of the figure depict the direction of overall relationships among species abundance and the main habitats examined.
DISCUSSION
Taphonomy
The findings of Trejo and Ojeda (Reference Trejo and Ojeda2002) clearly indicate that there is a large overlap in owl pellet lengths among the four largest raptors recorded in southern Patagonia (Bubo magellanicus, Geranoaetus melanolecus, Strix rufipes, and Tyto furcata). Therefore, reliable inferences on the raptor species regarding CM owl pellet origin based on measurements, or even external features of the pellets, is unsupported. However, that the studied pellets belong to a nocturnal bird is indicated by scarce digestion of the bones and teeth, given that hawks and falcons produce high levels of corrosion and breakage (Errington, Reference Errington1930; Andrews, Reference Andrews1990; Montalvo and Fernández, Reference Montalvo and Fernández2019). In addition, S. rufipes probably can be mostly discarded because of the marked preference shown by this sylvan owl to nest in trees in the forest (del Hoyo et al., Reference del Hoyo, Elliott and Sargatal1999; Ippi and Rozzi, Reference Ippi and Rozzi2004). Because B. magellanicus was registered roosting and nesting inside CM during field work, it probably can be assumed to be one of the candidates for the pellets embedded in the “windblown litter” that was excavated by Saxon. However, the cave is large enough to support more than one species of raptor, and reutilization of roosting sites by several species has been recorded (e.g., del Hoyo et al., Reference del Hoyo, Elliott and Sargatal1999; Trejo et al., Reference Trejo, Figueroa and Alvarado2006). In addition, pellets produced outside the cave likely entered the cave owing to the strong winds and the surface plane being inclined to the interior, especially given that the external source of recorded plant remains is obvious (Moore, Reference Moore1978). In CM, the higher values of relative abundance of girdle elements (pelvis and scapula) with respect to the general models for strigiforms (Fig. 3) are consistent with the wind dispersion of bones proposed by Cheme Arriaga et al. (Reference Cheme Arriaga, Montalvo and Sosa2012) and Montalvo et al. (Reference Montalvo, Cheme Arriaga, Tallade and Sosa2012). CM opens to the west, which is the direction of the so-called westerlies, and dips toward the interior of the cave, promoting the selective dispersion of pellets and bones.
Interestingly, no Bubo remains are recorded among the moderate variety of birds registered in the CM sequence, including several diurnal and nocturnal raptors such as Buteo, Geranoaetus, Phalcoboenus, Milvago, Strix, and Tyto (Humphrey et al., Reference Humphrey, Péfaur and Rasmussen1993, table 4). Particularly abundant feathers and bones of Tyto furcata suggest the participation of this owl as one of the possible accumulating agents of small mammal assemblages detected in this cave. According to the taphonomical features of the osteological assemblages, the low values of digestive corrosion (light category) and the general pattern of relative abundance of elements showed by the studied samples of CM agree with assemblages generated by T. furcata (Andrews, Reference Andrews1990; Pardiñas, Reference Pardiñas2000; Quintana, Reference Quintana2015; Fernández et al., Reference Fernández, Montalvo, Fernández-Jalvo, Andrews and López2017; Montalvo and Fernández, Reference Montalvo and Fernández2019). In DH1, the higher levels of digestive corrosion (even of the moderate category) and the relative skeletal abundances are more in accordance with accumulations produced by Bubo magellanicus (see Montalvo et al., Reference Montalvo, Fernández and Tallade2016). Both raptor species are moderately frequent in southern Chile, where they usually nest in caves and rock shelters, have roughly similar home ranges, and exhibit an opportunistic trophic behavior, feeding mostly on small nocturnal mammals, such as those recorded at CM and DH1 (Jaksic et al., Reference Jaksic, Rau and Yáñez1978; Yáñez et al., Reference Yáñez, Rau and Jaksic1978; Rau and Yáñez, Reference Rau and Yáñez1981; Andrews, Reference Andrews1990; Iriarte et al., Reference Iriarte, Franklin and Johnson1990; Bellocq, Reference Bellocq2000). Dietary overlap between B. magellanicus and T. furcata is high, and significant differences in the taxonomic composition of the assemblages produced by these two owls can be discarded (Trejo et al., Reference Trejo, Kun, Sahores and Seijas2005). Differences in mean prey weight reported from samples of pellets of both raptors studied in Torres del Paine (Iriarte et al., Reference Iriarte, Franklin and Johnson1990) are due to the large effect of lagomorphs in the diet of Bubo. Because Lepus was introduced recently (last century), probably both owls preyed on the same basic pool of small mammal species during most of the Quaternary.
Paleoenvironmental reconstruction: late Pleistocene
Little can be said with confidence about late Pleistocene small mammal assemblages from the Cerro Benítez sector. Paradoxically, CM is a cave that preserved important evidence about large mammals and allowed numerous environmental regional inferences for the temporal segment from ca. 15–10 ky (e.g., Markgraf, Reference Markgraf1985, Reference Markgraf1993; Heusser et al., Reference Heusser, Borrero and Lanata1994; Borrero et al., Reference Borrero, Zárate, Miotti and Massone1998; Heusser, Reference Heusser2003; Sagredo et al., Reference Sagredo, Moreno, Villa-Martínez, Kaplan, Kubik and Stern2011; Stern et al., Reference Stern, Moreno, Villa-Martínez, Sagredo, Prieto and Labarca2011; Moreno et al., Reference Moreno, Villa-Martínez, Cárdenas and Sagredo2012; Martin et al., Reference Martin, San Román, Morello, Todisco, Prevosti and Borrero2013; Todisco et al., Reference Todisco, Rodet, Nehme, Martin and Borrero2016; Villavicencio et al., Reference Villavicencio, Lindsey, Martin, Borrero, Moreno, Marshall and Barnosky2016).
CM#14 is the oldest unit analyzed here and represents the late Pleistocene. Even though its craniodental preservation (NISP = 14) limits any substantive inference, the ratio of Euneomys/Reithrodon and the absence of Loxodontomys strongly reflects unforested environments around the cave. Euneomys is a rat associated with bare, open, and windy habitats in western Patagonia (Pearson, Reference Pearson1987; Pardiñas et al., Reference Pardiñas, Teta, D'Elía and Lessa2011a). Dominance of Euneomys is also detected in late Pleistocene rodent assemblages in northern Tierra del Fuego, based on faunal evidence from Tres Arroyos 1 (Latorre, Reference Latorre1998; Pardiñas et al., Reference Pardiñas, Martin, Borrero, Massone and Fernández2020). The same is true for definitively late Pleistocene rodent communities, near the Last Glacial Maximum, in northern Patagonia (Tammone et al., Reference Tammone, Hajduk, Arias, Teta, Lacey and Pardiñas2014). Through the entire sequence of CM, there is a perceptible decline of Euneomys from lower (e.g., CM#13, Euneomys NISP > 25% of total NISP) to upper levels, reaching relative abundances during the late Holocene that are similar to those shown by recent owl pellet samples (roughly < 3%; Fig. 5; Supplementary Table 1).
In this context, the CM sample studied by Simonetti and Rau (Reference Simonetti and Rau1989) deserves attention. According to these authors (Simonetti and Rau, Reference Simonetti and Rau1989, p. 3), the studied assemblage represents the late Pleistocene because “… fueron colectadas de los estratos A 1391 y NZ 1680, de acuerdo a la nomenclatura de Saxon (Reference Saxon1976)” (freely translated, “… they were collected from the strata A 1391 and NZ 1680, according to the nomenclature of Saxon [1976]”). We detect here confusion between laboratory codes for radiocarbon dates and putative strata (the same applies to the smaller sample from CM studied by Rau and Yáñez, Reference Rau and Yáñez1980). A-1391 is a radiocarbon date obtained on a Mylodon skin piece that was collected, dated, and reported by Long and Martin (Reference Long and Martin1974). NZ-1680 (sometimes cited as R-4299) is also a date from another Mylodon skin that was dated and reported by Long and Martin (Reference Long and Martin1974), but it probably was collected by R. Wellman. Both dated skin fragments lack stratigraphic provenance, which is a common condition of many remains extracted from CM (see Pérez et al., Reference Pérez, Toledo, Vizcaíno and Bargo2018). Nonetheless, the crucial point here is that the sample studied by Simonetti and Rau (Reference Simonetti and Rau1989) cannot be confidently associated with the stratigraphy advanced by Saxon (Reference Saxon1976, Reference Saxon1979). It is certainly a significant loss, because this sample has an MNI > 100 (including eight species of rodents) and shows a codominance between Reithrodon and Phyllotis, which is a configuration not recorded in any of our studied samples. In fact, Phyllotis xanthopygus, a rocky specialist that is widespread in Patagonia (Osgood, Reference Osgood1943; Kramer et al., Reference Kramer, Monjeau, Birney and Sikes1999), currently shows low abundances in the Última Esperanza region (typically < 5% of the mammals preyed upon by owls; Supplementary Table 2).
With the discarding of “Megamys” from CM (Pardiñas and Candela, Reference Pardiñas and Candela2017), only a single caviomorph rodent, Ctenomys magellanicus, confidently occurs in the late Pleistocene faunal assemblage (Hauthal, Reference Hauthal1899; Roth, Reference Roth1899; Emperaire and Laming, Reference Emperaire and Laming1954; this paper). This Ctenomys is widespread in southern Patagonia (Osgood, Reference Osgood1943) and probably has a long evolutionary history associated with the harsh environments of high south latitudes (Lessa et al., Reference Lessa, D'Elía, Pardiñas, Patterson and Costa2012). The species was recorded in a late Pleistocene assemblage from northern Tierra del Fuego, and occurs in several Holocene archaeological sequences on that island, as well as in southern Santa Cruz province (e.g., Borrero et al., Reference Borrero, Crivelli and Mengoni Goñalons1976; Pardiñas et al., Reference Pardiñas, Teta, Formoso, Barberena, Borrero and Borrazzo2011b, Reference Pardiñas, Martin, Borrero, Massone and Fernández2020). Although present across the entire sequence of CM and DH1, C. magellanicus was never abundant, probably because in both sites humans did not select this caviomorph as a dietary resource.
Summarizing, according to scarce rodent evidence, the late Pleistocene landscape surrounding CM and DH1 was mostly open and vegetation impoverished (dominance of Euneomys, absence of Loxodontomys), under cold and moist conditions (absence of Eligmodontia, a typical steppe element), probably similar to a feldmark. Moore (Reference Moore1978), after analyzing plant remains of the Mylodon “dung-carpet,” interpreted that sloths lived in a cold and humid (at least 800–1000 mm of annual precipitation) grassland. In contrast, Saxon (Reference Saxon1979, p. 335) hypothesized that “dry pampas prevailing at Mylodon cave 12,000 years ago can today be found less than 100 km to the east.” Rodents studied here support Moore's (Reference Moore1978) conjecture.
Holocene environmental evolution in Cerro Benítez: integrating biological evidence
The study of fossil rodents is largely unemployed as a proxy for reconstructing Quaternary environments (Rabassa, Reference Rabassa2008; Lowe and Walker, Reference Lowe and Walker2013; Gifford-Gonzalez, Reference Gifford-Gonzalez2018). Approaches to Pleistocene–Holocene landscape reconstructions in southernmost South America follow this general trend, being mostly dominated by palynological, glaciological, and, more recently, geochemical evidence (e.g., Heusser, Reference Heusser2003; Moreno et al., Reference Moreno, Villa-Martínez, Cárdenas and Sagredo2012; Recasens et al., Reference Recasens, Ariztegui, Gebhardt, Gogorza, Haberzettl, Hahn and Kliem2012; Borromei et al., Reference Borromei, Candel, Musotto, Cusminsky, Martínez, Coviaga, Ponce and Coronato2018; Zolitschka et al., Reference Zolitschka, Fey, Janssen, Maidana, Mayr, Wulf and Haberzettl2019). Certainly, a desirable goal is to increase the database of biological proxies, not only to gain a more accurate picture of environmental evolution, but also to understand how diverse biotic elements responded to past climatic changes (e.g., Labarca et al., Reference Labarca, González-Guarda, Lizama-Catalán, Villavicencio, Alarcón-Muñoz, Suazo-Lara and Oyanadel-Urbina2020).
The Holocene record of CM has a valuable and unusual element from plant remains because it contains Nothofagus leaves (Moore, Reference Moore1978). This fortunate situation, due to the exceptional preservation conditions in this cave (Favier Dubois and Borrero, Reference Favier Dubois and Borrero1997; Pérez et al., Reference Pérez, Toledo, Vizcaíno and Bargo2018), helps overcome the traditional impediment to reconstructing the connection between deciduous and evergreen forests that clouds Patagonian palynological approaches (e.g., Markgraf, Reference Markgraf1993; Heusser, Reference Heusser2003; Mancini et al., Reference Mancini, Bamonte, Marcos, Sottile, Echeverría and Prieto2018).
Valuable palynological evidence has been published over the last two decades in a growing process to construct a regional approach to late Pleistocene–Holocene evolution in southernmost Argentina and Chile (see Sottile et al., Reference Sottile, Bamonte, Mancini and Bianchi2012; Mancini et al., Reference Mancini, Bamonte, Marcos, Sottile, Echeverría and Prieto2018; and Prieto et al., Reference Prieto, Mancini, de Porras, Bamonte, Marcos and Prieto2018 for a synthesis). From the several available sequences studied, we selected those geographically and environmentally closer to Cerro Benítez for discussion in relation to the rodent assemblages. These are, roughly from north to south, Cerro Frías (Mancini, Reference Mancini2009; Echeverría, et al., Reference Echeverría, Sottile, Mancini and Fontana2014; Echeverría and Mancini, Reference Echeverría, Mancini and Prieto2018), Chorrillo Malo 2 (Mancini, Reference Mancini2002), Cerro Verlika 1 (Mancini, Reference Mancini2001, Reference Mancini2002), Vega Ñandú (Villa-Martínez and Moreno, Reference Villa-Martínez and Moreno2007), and Lago Guanaco (Moreno et al., Reference Moreno, Francois, Villa-Martínez and Moy2009). Previously known sequences from southern Chile, including Meseta Latorre 1 and 2 (Schäbitz, Reference Schäbitz1991) and Torres del Paine (Heusser, Reference Heusser1994a, Reference Heusserb, Reference Heusser2003), were also employed, plus the Holocene “pollen diagram” constructed by Markgraf (Reference Markgraf1993, fig. 6) for CM.
The macrobotanical remains approach developed by Moore (Reference Moore1978), based on the study and quantification of leaves, flowers, and seeds recovered in Trench 3 excavated by Saxon in CM, is the more appropriate proxy to examine. According to Moore's (Reference Moore1978) results, the increase of Nothofagus (mostly N. betuloides) was slow after the end of the “dung carpet” (roughly, late Pleistocene), and layers dated ca. 7.8 ka. This “soft transition” during the early Holocene is also marked in the pollen record of Meseta Latorre, located ~40 km northeast of CM (Schäbitz, Reference Schäbitz1991). The CM vegetational record showed a dramatic increase in N. betuloides abundance, which indicates establishment of an evergreen forest around the cave during the middle Holocene, with an acme in sample 3(6)5 (Moore, Reference Moore1978). A coeval and sudden increment of Nothofagus pollen is also verified in Meseta Latorre (Schäbitz, Reference Schäbitz1991). However, this abrupt forest colonization is varyingly recorded in the Torres del Paine area, highlighting the idiosyncratic effect of local vegetation communities on pollen records. According to Heusser (Reference Heusser1994b), pollen profiles studied near the north side of Lago Sarmiento contain no indication of a major shift in the forest/steppe during the Holocene (see also Heusser, Reference Heusser2003, p. 142–145). In contrast, a sudden appearance of Nothofagus forest is recorded in Vega Ñandú, suggesting a warming event at the beginning of the Holocene (Villa-Martínez and Moreno, Reference Villa-Martínez and Moreno2007). Also during the early Holocene, the spread of Nothofagus forest colonizing older late Pleistocene herbaceous steppes seems to be a common signature in the several profiles studied south of Lago Argentino (e.g., Cerro Frías, Chorrillo Malo 2, Península Avellaneda; see Mancini, Reference Mancini2002, Reference Mancini2009; Echeverría et al., Reference Echeverría, Bamonte, Marcos, Sottile and Mancini2017).
The forest landscape that developed during the middle Holocene in Cerro Benítez apparently did not constitute a dense N. betuloides forest since Nothofagus antarctica also integrated leaf-litter deposits, especially in the upper levels. Moore (Reference Moore1978) highlighted that the occurrence of the latter proves the presence of open, boggy areas around the cave. Adding these elements to the record of Maytenus in the same levels of CM, the macrobotanical evidence points to repeated transitions between parkland/woodland environments. In this context, the record of rodents, such as Abrothrix lanosa, supports a local patchy forest (Feijoo et al., Reference Feijoo, D'Elía, Pardiñas and Lessa2010). Along the same lines, the middle Holocene bird assemblage is mostly composed of forest and generalist species, lacking indicators of closed-canopy forest (Humphrey et al., Reference Humphrey, Péfaur and Rasmussen1993). A similar scenario can be surmised from the pollen record of Meseta Latorre, which, according to Schäbitz (Reference Schäbitz1991, p. 240) “in the following zone (ML3, 6900 to 5000 years B.P.), the curves of the three important taxa Nothofagus, Gramineae [sic] and Cyperaceae [sic] fluctuate with no clear trend.” North of CM, however, “Nothofagus started a multi-millennial expansion trend at 6800 cal yr BP, driven by the stabilization and prominent increase in westerly precipitation” (Villa-Martínez and Moreno, Reference Villa-Martínez and Moreno2007, p. 408).
Although Moore (Reference Moore1978) and Markgraf (Reference Markgraf1993) indicated the predominance of a deciduous Nothofagus pumilio forest characterizing CM vicinities during the late Holocene until the nineteenth century, this statement is undermined by the young chronology obtained here for sample 3(6)1. More probably, the colonization of N. pumilio was a very recent event, at least locally. In fact, the first properly taken pictures of CM show the presence of a dense deciduous forest fencing the mouth of the cave (Lehmann-Nitsche, Reference Lehmann-Nitsche1899, fig. 1). The macrobotanical record of CM is insufficient to describe late Holocene local vegetation, but most of the pollen records south of 50°S indicate the occurrence of Nothofagus forests with associated low shrub values, which indicate conditions closer to those of forests (e.g., Mancini, Reference Mancini2002, Reference Mancini2009; Villa-Martínez and Moreno, Reference Villa-Martínez and Moreno2007; Echeverría et al., Reference Echeverría, Bamonte, Marcos, Sottile and Mancini2017). A late Holocene moderate stability in Cerro Benítez is reflected by the rodents recorded in DH1, where Loxodontomys micropus and Reithrodon auritus were codominant during the last 3 ky. In addition, modern rodent assemblages also reflect widespread landscape degradation caused by human activities in historical times. Pollen signals for these latter effects are also recognized (but not in Meseta Latorre; Schäbitz, Reference Schäbitz1991), including an abrupt decline of Nothofagus forests and the expansion of Rumex acetosella in Torres del Paine (see Villa-Martínez and Moreno, Reference Villa-Martínez and Moreno2007). However, recent forest declination in south Lago Argentino mostly has been connected with the effects of global climatic events of short duration (e.g., Cerro Frías; Mancini, Reference Mancini2009). In any case, the marked current abundances of Oligoryzomys longicaudatus and L. micropus in Cerro Benítez can be connected with forest clearance and with concomitant shrubland expansion (see Tammone et al., Reference Tammone, Lacey and Pardiñas2020).
CONCLUSIONS
The rodent assemblages from CM and DH1 provide evidence for past changes in the vegetation and environment in Cerro Benítez area. Although this proxy has been rarely used to explore Quaternary environments in southern Argentina and Chile, it seems to be a valuable tool, especially when combined with other biological evidence, to reconstruct local conditions. Specifically for the studied area, the small mammal faunas lead to several conclusions. (1) The late Pleistocene rodent community appears to have been characterized by the dominance of E. chinchilloides and the virtual absence of L. micropus and O. longicaudatus, indicating mostly unforested habitats under cool and moist climatic conditions. The southernmost occurrence of the chinchillid Lagidium is probably associated with this kind of environment. The recorded rodent assemblage agrees with a local landscape that was probably similar to a feldmark. This condition is not repeated again across the studied sequence. (2) During latest early to middle Holocene, the occurrence of Nothofagus forest at the SSW slope of Cerro Benítez is firmly reflected by the record of several cricetids (e.g., Geoxus sp., Loxodontomys micropus, and Oligoryzomys longicaudatus). Decreasing numbers of E. chinchilloides from lower to upper levels suggest progressive reduction in open and bare environments. However, a parkland scenario is indicated by the occurrence of Abrothrix lanosa and variable abundances of Reithrodon auritus. (3) The late Holocene is characterized by a monotonous sequence that was codominated by L. micropus and R. auritus, suggesting more proximal Nothofagus forests. (4) During the last century, a marked increase of O. longicaudatus is verified, which indicates that the recent community was locally reshaped by human activities. (5) The sustained absence of Eligmodontia morgani, and the virtual absence of P. xanthopygus, is an eloquent indicator that the Patagonian steppe never reached the Cerro Benítez area and, therefore, that late Pleistocene–Holocene precipitation has always been >600 mm (annual mean).
Supplementary Material
The supplementary material for this article can be found at https://doi.org/10.1017/qua.2021.44
Acknowledgments
This contribution was developed over two decades and, as such, combines the field and laboratory efforts of many people. We want to express our gratitude to the colleagues that shared the excavations and findings in CM and DH1 (P. Cárdenas, J.L. Lanata, R. MacPhee, F. Morello, A. Prieto, M. San Román, S. Vizcaíno, and F. Prevosti), as well as those who collected and processed owl pellet samples from southern Argentina (A. Formoso, J. Sánchez, D. Podestá, D. Udrizar Sauthier, V. Roldán, and J. Pardiñas). The CONAF, the guards at Monumento Natural Cueva del Milodón, and the local owners M. Margoni and E. Helmer provided crucial logistic support. A. Currant granted the authors access to the NHMUK collections. J. Simonetti and J. Rau patiently handled consultations about the samples that they studied. Two anonymous reviewers and the editors, D. Booth and C. Marean, improved the manuscript.
Financial Support
Partial funding was derived from several grants, including British Council-Fundación Antorchas, FONDECYT 1180272, Agencia PICT 2008-547 and 2014-1039, and i-COOPB-20287 of Consejo Superior de Investigaciones Científicas de Cooperación Internacional. We are grateful to all of the aforementioned people and institutions.