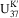INTRODUCTION
The Kuroshio Current, the largest warm current in the western Pacific, brings enormous amounts of heat and moisture from the tropical Pacific to the northwestern Pacific (Salisbury et al., Reference Salisbury, Shinohara and Richter2002). In the late Quaternary, the Kuroshio Current did not flow across the Okinawa Trough and instead extended into the far northwestern Pacific. The Kuroshio Current, along with the East Asian monsoon (EAM), profoundly influences the climate of the East Asia, a densely inhabited region with the total population of >2 billion. Due to large discharges of terrestrial materials from the Yangtze River, Yellow River, and other mountainous rivers, the Okinawa Trough has an exceptionally high sedimentation rate (up to 21 m per 1000 yr), making it an ideal locality for high resolution paleoenvironmental studies (Li et al., Reference Li, Jiang, Li, Li and Jiang2009; Ruan et al., Reference Ruan, Xu, Ding, Wang and Zhang2015). A number of studies (e.g., Jian et al., Reference Jian, Wang, Saito, Wang, Pflaumann, Oba and Cheng2000; Ujiie et al., Reference Ujiie, Hatakeyama, Gu, Yamamoto, Ishiwatari and Maeda2001; Lee et al., Reference Lee, Huh, Su and You2004; Sun et al., Reference Sun, Oppo, Xiang, Liu and Gao2005; Zhao et al., Reference Zhao, Huang and Wei2005; Kao et al., Reference Kao, Wu, Hsin and Dai2006; Xiang et al., Reference Xiang, Sun, Li, Oppo, Chen and Zheng2007; Diekmann et al., Reference Diekmann, Hofmann, Henrich, Futterer, Rohl and Wei2008; Zhao et al., Reference Zhao, Li, Cai, Wei, Hu, Dou, Wang, Xiang, Cheng, Dong and Zhang2015) have reported a series of abrupt changes in regional climate of the Okinawa Trough by examining marine sediment cores, most of which are consistent with well-defined late Quaternary climate events such as Heinrich events, Bølling-Allerød, Younger Dryas, 8.2 ka event, and the Little Ice Age. However, there are limited reports about the biological response to those climate changes in the Okinawa Trough (e.g., Xing et al., Reference Xing, Zhang, Liu, Zhao, Liu, Shi and Zhao2011; Zhao et al., Reference Zhao, Li, Li and Hu2012; He et al., Reference He, Zhao, Wang, Li and Li2013). This hinders our understanding of the biogeochemical cycle in the Okinawa Trough and its feedback on climate since phytoplankton, as primary producers, largely control the biological pump and, in turn, influence the atmospheric CO2 concentration (Harrison, Reference Harrison2000).
The Kuroshio Current is characterized by high temperature (28°C–29°C in the summer; 22°C–25°C in the winter off the eastern side of the island of Taiwan), high salinity (33.6‰–34.8‰), and low nutrients (<0.1 μmol/kg for NO3 −, <0.02 μmol/kg for PO4 3−, <1.0 μmol/kg for SiO2) (Chen, Reference Chen1996). In contrast, coastal currents such as the East China Sea current have a relatively lower temperature, lower salinity, and higher nutrients (Jian et al., Reference Jian, Wang, Saito, Wang, Pflaumann, Oba and Cheng2000). As a result, sea surface temperature (SST) in the Okinawa Trough is closely related to the intensity of the Kuroshio Current.
Presently, there are two commonly used organic geochemical paleothermometers, namely
![]() $${\rm U}_{{37}}^{{{\rm K}\prime}} $$
(a modified index for the unsaturated degree of alkenones with 37 carbon atoms) (Brassell et al., Reference Brassell, Eglinton, Marlowe, Pflaumann and Sarnthein1986; Prahl and Wakeham, Reference Prahl and Wakeham1987) and TEX86 (TetraEther indeX of tetraethers consisting of 86 carbon atoms) (Schouten et al., Reference Schouten, Hopmans, Schefuss and Sinninghe Damsté2002). The
$${\rm U}_{{37}}^{{{\rm K}\prime}} $$
(a modified index for the unsaturated degree of alkenones with 37 carbon atoms) (Brassell et al., Reference Brassell, Eglinton, Marlowe, Pflaumann and Sarnthein1986; Prahl and Wakeham, Reference Prahl and Wakeham1987) and TEX86 (TetraEther indeX of tetraethers consisting of 86 carbon atoms) (Schouten et al., Reference Schouten, Hopmans, Schefuss and Sinninghe Damsté2002). The
![]() $${\rm U}_{{37}}^{{{\rm K}\prime}} $$
index is defined as the relative abundance of long-chain unsaturated ketones (Brassell et al., Reference Brassell, Eglinton, Marlowe, Pflaumann and Sarnthein1986), biomarkers for certain Haptophyceae algae such as Emiliania huxleyi (Volkman et al., Reference Volkman, Eglinton, Corner and Forsberg1980) and Gephyrocapsa oceanica (Volkman et al., Reference Volkman, Barrerr, Blackburn and Sikes1995). These algae strictly reside in the euphotic zone, making
$${\rm U}_{{37}}^{{{\rm K}\prime}} $$
index is defined as the relative abundance of long-chain unsaturated ketones (Brassell et al., Reference Brassell, Eglinton, Marlowe, Pflaumann and Sarnthein1986), biomarkers for certain Haptophyceae algae such as Emiliania huxleyi (Volkman et al., Reference Volkman, Eglinton, Corner and Forsberg1980) and Gephyrocapsa oceanica (Volkman et al., Reference Volkman, Barrerr, Blackburn and Sikes1995). These algae strictly reside in the euphotic zone, making
![]() $${\rm U}_{{37}}^{{{\rm K}\prime}} $$
a reliable indicator for SST. TEX86 is based on the composition of isoprenoid glycerol dibiphytanyl glycerol tetraethers (GDGTs), membrane lipids of Thaumarchaeota, formerly assigned to Crenarchaeota (Schouten et al., Reference Schouten, Hopmans, Schefuss and Sinninghe Damsté2002; Sinninghe Damsté et al., Reference Sinninghe Damsté, Schouten, Hopmans, van Duin and Geenevasen2002) as well as other archaea (Schouten et al. Reference Schouten, Hopmans and Sinninghe Damsté2013 and references therein). The TEX86 in surface sediments shows a strong correlation with annual mean SST and therefore has been used as a proxy for reconstruction of paleo-SST ( Schouten et al., Reference Schouten, Hopmans, Schefuss and Sinninghe Damsté2002; Kim et al., Reference Kim, Schouten, Hopmans, Donner and Sinninghe Damsté2008; Kim et al., Reference Kim, Van der Meer, Schouten, Helmke, Willmott, Sangiorgi, Koç, Hopmans and Sinninghe Damsté2010; Tierney and Tingley, Reference Tierney and Tingley2015). However, studies of the Santa Barbara basin (Huguet et al., Reference Huguet, Schimmelmann, Thunell, Lourens, Sinninghe Damsté and Schouten2007), the gulf of California (McClymont et al., Reference McClymont, Ganeshram, Pichevin, Talbot, van Dongen, Thunell, Haywood, Singarayer and Valdes2012), the east equatorial Pacific (Seki et al., Reference Seki, Schmidt, Schouten, Hopmans, Sinninghe Damsté and Pancost2012), and the South China Sea (SCS) (Jia et al., Reference Jia, Zhang, Chen, Peng and Zhang2012) suggest that the TEX86 better correlates with subsurface temperature than surface temperature in these regions. This inconsistency is likely because Thaumarchaeota, the principal source for isoprenoid GDGTs, occur throughout the water column and vary spatially in the depth of their maximum productivity (Schouten et al., Reference Schouten, Hopmans and Sinninghe Damsté2013 and references therein).
$${\rm U}_{{37}}^{{{\rm K}\prime}} $$
a reliable indicator for SST. TEX86 is based on the composition of isoprenoid glycerol dibiphytanyl glycerol tetraethers (GDGTs), membrane lipids of Thaumarchaeota, formerly assigned to Crenarchaeota (Schouten et al., Reference Schouten, Hopmans, Schefuss and Sinninghe Damsté2002; Sinninghe Damsté et al., Reference Sinninghe Damsté, Schouten, Hopmans, van Duin and Geenevasen2002) as well as other archaea (Schouten et al. Reference Schouten, Hopmans and Sinninghe Damsté2013 and references therein). The TEX86 in surface sediments shows a strong correlation with annual mean SST and therefore has been used as a proxy for reconstruction of paleo-SST ( Schouten et al., Reference Schouten, Hopmans, Schefuss and Sinninghe Damsté2002; Kim et al., Reference Kim, Schouten, Hopmans, Donner and Sinninghe Damsté2008; Kim et al., Reference Kim, Van der Meer, Schouten, Helmke, Willmott, Sangiorgi, Koç, Hopmans and Sinninghe Damsté2010; Tierney and Tingley, Reference Tierney and Tingley2015). However, studies of the Santa Barbara basin (Huguet et al., Reference Huguet, Schimmelmann, Thunell, Lourens, Sinninghe Damsté and Schouten2007), the gulf of California (McClymont et al., Reference McClymont, Ganeshram, Pichevin, Talbot, van Dongen, Thunell, Haywood, Singarayer and Valdes2012), the east equatorial Pacific (Seki et al., Reference Seki, Schmidt, Schouten, Hopmans, Sinninghe Damsté and Pancost2012), and the South China Sea (SCS) (Jia et al., Reference Jia, Zhang, Chen, Peng and Zhang2012) suggest that the TEX86 better correlates with subsurface temperature than surface temperature in these regions. This inconsistency is likely because Thaumarchaeota, the principal source for isoprenoid GDGTs, occur throughout the water column and vary spatially in the depth of their maximum productivity (Schouten et al., Reference Schouten, Hopmans and Sinninghe Damsté2013 and references therein).
Biomarkers can also be used to reconstruct primary productivity and phytoplankton community structure (Meyers, Reference Meyers1997; Schubert et al., Reference Schubert, Villanueva, Calvert, Cowie, von Rad, Schulz, Berner and Erlenkeuser1998; Wakeham et al., Reference Wakeham, Peterson, Hedges and Lee2002). In marine environments, long-chain alkenones (e.g., heptatriaconta-15, 22-dien-2-one), brassicasterol (24-methylcholesta-5, 22-dien-3β-ol), long-chain diols (e.g., C30 1, 15-alkyl diol), and dinosterol (4α, 23, 24-trimethyl-5α-cholest-22-en-3β-ol) are mainly produced by haptophytes, diatoms, eustigmatophytes, and dinoflagellates, respectively (Boon et al., Reference Boon, Rijpstra, De Lange, De Leeuw, Yoshioka and Shimizu1979; Marlowe et al., Reference Marlowe, Green, Neal, Brassell, Eglinton and Course1984; Volkman, Reference Volkman1986; Volkman et al., Reference Volkman, Barrett and Blackburn1999). Such source specificity is useful for assessing the marine carbon cycle during the Quaternary glacial-interglacial cycles since different types of phytoplankton (e.g., silicic diatoms vs. calcitic coccolithophorids) have different efficiencies in the biological pump, (e.g., Harrison, Reference Harrison2000). However, it should be kept in mind that the abundance of biomarkers varies substantially among different organisms, and therefore, they are better used as qualitative, rather than quantitative, indicators. The biomarker-based studies for the Tasman Sea (Calvo et al., Reference Calvo, Pelejero, Logan and De Deckker2004), the Cariaco Basin (Werne et al., Reference Werne, Hollander, Lyons and Peterson2000), the Sea of Okhotsk (Seki et al., Reference Seki, Ikehara, Kawamura, Nakatsuka, Ohnishi, Wakatsuchi, Narita and Sakamoto2004), the middle Okinawa Trough (Xing et al., Reference Xing, Zhao, Zhang, Liu and Shi2008), and the sea off Cap Blanc (Zhao et al., Reference Zhao, Mercer, Eglinton, Higginson and Huang2006) suggest that phytoplankton community structures changed significantly during glacial-interglacial cycles. In contrast, studies for other regions such as the Arabian Sea (Schubert et al., Reference Schubert, Villanueva, Calvert, Cowie, von Rad, Schulz, Berner and Erlenkeuser1998) and the northern SCS (He et al., Reference He, Zhao, Wang, Li and Li2013) suggest relativity stable phytoplankton community structures. These results demonstrate that the glacial-interglacial pattern of phytoplankton community is site-specific.
Here, we conduct a comprehensive biomarker study for the Ocean Drilling Program (ODP) Core 1202B collected from the southern Okinawa Trough. Our objectives are threefold: 1) to reconstruct the variability of the Kuroshio Current since the last deglaciation using the gradient of temperature records from
![]() $${\rm U}_{{37}}^{{{\rm K}\prime}} $$
and TEX86; 2) to estimate phytoplankton community structures since the last glacial maximum (LGM); and, 3) to evaluate the relative importance of factors (e.g., Kuroshio Current, sea level, and nutrients) that may influence phytoplankton community structures.
$${\rm U}_{{37}}^{{{\rm K}\prime}} $$
and TEX86; 2) to estimate phytoplankton community structures since the last glacial maximum (LGM); and, 3) to evaluate the relative importance of factors (e.g., Kuroshio Current, sea level, and nutrients) that may influence phytoplankton community structures.
MATERIAL AND METHODS
Materials and age model
The ODP Site 1202B (24°48.24′N, 122°30′E; Fig. 1) is located in the southern Okinawa Trough approximately 80 km off northeast Taiwan at a water depth of 1274 m (Salisbury et al., Reference Salisbury, Shinohara and Richter2002). This region is under the strong influence of the EAM and the Kuroshio Current. The Core 1202B, with a total length of 140.5 m, was recovered during ODP Expedition 195 (Salisbury et al., Reference Salisbury, Shinohara and Richter2002). In this study, the top 85 m section was investigated, and samples (2 cm thick) were taken with plastic scoops at a 5 to ~20 cm resolution.

Figure 1 Map showing the location of Ocean Drilling Program (ODP) Site 1202B (24°48.24´N, 122°30´E). Gray lines represent the Kuroshio Current, fine gray lines represent branches of the Kuroshio Current, and blue lines represent the East China Sea coastal water (ECSCW). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
The age model was established based on 10 accelerator mass spectrometer (AMS) 14C dates on planktonic foraminifera (Wei et al., Reference Wei, Mii and Huang2005; Wu et al., Reference Wu, Tan, Zhou, Yang and Xu2012). The radiocarbon data are shown in Table 1. A constant 400 yr surface-ocean reservoir correction was used (Bard, Reference Bard1988; Hideshima et al., Reference Hideshima, Matsumoto, Abe and Kitagawa2001). A continuous timescale was achieved by linear interpolations between the age control points after converting the raw 14C dates into calendar age before present (BP=AD 1950) using the CALIB program (Stuiver et al., Reference Stuiver, Reimer, Bard, Beck, Burr, Hughen, Kromer, McCormac, Van der Plicht and Spurk1998). According to this age model, the mean resolution of our study period (20 ka to ~AD 1950) is ca. 50 yr.
Table 1 AMS radiocarbon ages of mixed planktonic foraminifera in sediments of ODP Core 1202B (southern Okinawa Trough).

a mbsf: meters below sea floor.
b 14C data for sediments 0–10 mbsf are from Wu et al. (Reference Wu, Tan, Zhou, Yang and Xu2012), whereas those for the sediments 10–80 mbsf are from Wei et al. (Reference Wei, Mii and Huang2005). The data are expressed as 14C age±1δ uncertainty.
Biomarker analysis
About 5 g of freeze-dried sediment sample was ultrasonically extracted in 20 mL dichloromethane:methanol (DCM:MeOH; 3:1 v/v) three times (15 min each). The combined extracts were centrifuged at 3500 rpm for 10 min and the supernatant was transferred into clean 250 mL pre-combusted glass flasks. The extracts were rotary evaporated until almost dry and transferred to 5 mL glass vials. After being completely dried under mild nitrogen streams, the extracts were re-dissolved in hexane (HEX) and separated into three fractions using silica gel columns, namely hydrocarbon (HEX:DCM; 9:1 v/v), alkenone (HEX:DCM; 1:1 v/v), and polar fractions (DCM:MeOH; 1:1 v/v). One aliquot of the polar fractions containing GDGTs and sterols was divided into two parts that were used for the analyses of sterols/diols and GDGTs, respectively.
TEX86 and branched and isoprenoid tetraether
The polar fractions eluted from silica gel columns were filtered through 0.45 μm PTFE filters, dried on a heating plate (40°C), and re-dissolved in 1 mL hexane/propanol (99:1 v/v). The GDGTs were analyzed on an Agilent 1200 liquid chromatography-atmospheric pressure chemical ionization-Agilent 6460 mass spectrometer (HPLC-APCI-MS) using the procedures described by Wu et al. (Reference Wu, Zhao, Pei, Ding, Yang and Xu2013). Separation was achieved on a Prevail Cyano column (150×2.1 mm, 3 μm). GDGTs were eluted with a mixture of hexane/propanol (99:1 v/v) for 5 min, and then a linear gradient to 1.8% isopropanol in 45 min, followed by a column wash with 1.8% isopropanol for 10 min. The APCI and MS conditions were: vaporizer pressure 4.2×105 Pa, vaporizer temperature of 400°C, drying gas flow 6 L/min, temperature 200°C, capillary voltage 3500 V, and corona current 5 μA (3.2 kV). GDGTs were quantified by comparison of the protonated-ion peak areas of GDGTs to an internal standard (IS; C46 GDGT) in selected ion monitoring mode. The structures of GDGTs are shown in Figure 2.

Figure 2 Structures of isoprenoid GDGTs (iGDGTs) and branched GDGTs (bGDGTs) mentioned in the study. Crenarchaeol’ (cren’) is a regioisomer of crenarchaeol (cren) with an anti-parallel configuration of the two glycerol moieties. IS, internal standard.
TEX86 is calculated based on the abundance of individual isoprenoid GDGTs (or iGDGTs) (Schouten et al., Reference Schouten, Hopmans, Schefuss and Sinninghe Damsté2002):
![]() ${\rm TEX}_{{86}} \, {\equals}\, {{2{\plus}3{\plus}{\rm cren}\prime} \over {1{\plus}2{\plus}3{\plus}{\rm cren}\prime}}$
which was converted into seawater temperature according to the global calibration of Kim et al. (Reference Kim, Schouten, Hopmans, Donner and Sinninghe Damsté2008): SST=56.2 × TEX86 - 10.8. Our previous study has demonstrated that the calibration of Kim et al. (Reference Kim, Schouten, Hopmans, Donner and Sinninghe Damsté2008) is suitable for the reconstruction of Holocene SST in the Okinawa Trough (Wu et al., Reference Wu, Tan, Zhou, Yang and Xu2012). Here, we extend the record to the last glacial maximum. Another GDGT indicator, the branched and isoprenoid tetraether (BIT) index, is based on the relative abundance of branched GDGTs (bGDGTs) over one iGDGT (crenarchaeol, Fig. 2) :
${\rm TEX}_{{86}} \, {\equals}\, {{2{\plus}3{\plus}{\rm cren}\prime} \over {1{\plus}2{\plus}3{\plus}{\rm cren}\prime}}$
which was converted into seawater temperature according to the global calibration of Kim et al. (Reference Kim, Schouten, Hopmans, Donner and Sinninghe Damsté2008): SST=56.2 × TEX86 - 10.8. Our previous study has demonstrated that the calibration of Kim et al. (Reference Kim, Schouten, Hopmans, Donner and Sinninghe Damsté2008) is suitable for the reconstruction of Holocene SST in the Okinawa Trough (Wu et al., Reference Wu, Tan, Zhou, Yang and Xu2012). Here, we extend the record to the last glacial maximum. Another GDGT indicator, the branched and isoprenoid tetraether (BIT) index, is based on the relative abundance of branched GDGTs (bGDGTs) over one iGDGT (crenarchaeol, Fig. 2) :
![]() $$ {\rm BIT}\, {\equals}\, {{{\rm I}{\plus}{\rm II}{\plus}{\rm III}} \over {{\rm I}{\plus}{\rm II}{\plus}{\rm III}{\plus}{\rm cren}}}$$
(Hopmans et al., Reference Hopmans, Weijers, Schefuss, Herfort, Sinninghe Damsté and Schouten2004). Unlike iGDGTs, bGDGTs have a 1, 2-di-O-alkyl-sn-glycerol configuration and are substantially more abundant in peat and soils than marine sediments, supporting the hypothesis that they are predominantly derived from soil bacteria (Sinninghe Damsté et al., 2000; Weijers et al., Reference Weijers, Schouten, Spaargaren and Sinninghe Damsté2006). Due to the source difference between bGDGTs and iGDGTs, BIT is a useful proxy for assessing the contribution of terrestrial (soil) organic carbon in aquatic environments. The value of the BIT index is close to 0 for marine sediments without terrestrial organic matter inputs, and higher than 0.9 for most soil/peat sediments (Hopmans et al., Reference Hopmans, Weijers, Schefuss, Herfort, Sinninghe Damsté and Schouten2004), although some alkaline soils have unusually low BIT values (Weijers et al., Reference Weijers, Schouten, Spaargaren and Sinninghe Damsté2006; Yang et al., Reference Yang, Ding, Wang, Jin, He, Qin and Xie2012). Duplicate analyses showed that instrumental errors were 0.005 for the TEX86 index, corresponding to a SST deviation of ±0.28°C, and 0.01 for the BIT index.
$$ {\rm BIT}\, {\equals}\, {{{\rm I}{\plus}{\rm II}{\plus}{\rm III}} \over {{\rm I}{\plus}{\rm II}{\plus}{\rm III}{\plus}{\rm cren}}}$$
(Hopmans et al., Reference Hopmans, Weijers, Schefuss, Herfort, Sinninghe Damsté and Schouten2004). Unlike iGDGTs, bGDGTs have a 1, 2-di-O-alkyl-sn-glycerol configuration and are substantially more abundant in peat and soils than marine sediments, supporting the hypothesis that they are predominantly derived from soil bacteria (Sinninghe Damsté et al., 2000; Weijers et al., Reference Weijers, Schouten, Spaargaren and Sinninghe Damsté2006). Due to the source difference between bGDGTs and iGDGTs, BIT is a useful proxy for assessing the contribution of terrestrial (soil) organic carbon in aquatic environments. The value of the BIT index is close to 0 for marine sediments without terrestrial organic matter inputs, and higher than 0.9 for most soil/peat sediments (Hopmans et al., Reference Hopmans, Weijers, Schefuss, Herfort, Sinninghe Damsté and Schouten2004), although some alkaline soils have unusually low BIT values (Weijers et al., Reference Weijers, Schouten, Spaargaren and Sinninghe Damsté2006; Yang et al., Reference Yang, Ding, Wang, Jin, He, Qin and Xie2012). Duplicate analyses showed that instrumental errors were 0.005 for the TEX86 index, corresponding to a SST deviation of ±0.28°C, and 0.01 for the BIT index.
Sterols and diols
Polar fractions containing sterols and diols were dried under mild N2 streams and derivatized with 50 μL N, O-bis(trimethyl-sily)-trifluroacetamide (BSTFA) and 50 μL pyridine (60°C, 1.5 h). After addition of 100 μL DCM, biomarkers were analyzed on an Agilent 7890A gas chromatograph (GC) coupled with a flame ionization detector (FID). A J&W HP-5ms column (30 m×0.32 mm×0.25 μm) was used to separate biomarkers. For some samples, an Agilent 7890A GC-5973C mass spectrometer (GC-MS) was used for biomarker identification. The detailed instrumental parameters have been described in Xu et al. (Reference Xu, Holmes and Jaffé2007).
Alkenones
Long-chain alkenones were analyzed on an Agilent 7890A GC-FID. An Agilent VF-200ms column (60 m × 250 μm × 0.25 μm) was used to separate alkenones according to the procedure of Longo et al. (Reference Longo, Dillon, Tarozo, Salacup and Huang2013). The GC oven was programed from 70°C (held 1 min) to 250°C at a rate of 20°C/min, further increased to 310°C at a rate of 2°C/min and was held at 310°C for 10 min. The
![]() $${\rm U}_{{37}}^{{{\rm K}\prime}} $$
index is calculated based on peak areas of alkenones:
$${\rm U}_{{37}}^{{{\rm K}\prime}} $$
index is calculated based on peak areas of alkenones:
![]() $${\rm U}_{{37}}^{{{\rm K}\prime}} \, {\equals}\, {{{\rm C}37 \colon 2} \over {{\rm C}37 \colon 2{\plus}{\rm C}37 \colon 3}}$$
where C37:2 and C37:3 are C37 alkenone with two and three double bonds, respectively. The SST was obtained according to the global calibration equation:
$${\rm U}_{{37}}^{{{\rm K}\prime}} \, {\equals}\, {{{\rm C}37 \colon 2} \over {{\rm C}37 \colon 2{\plus}{\rm C}37 \colon 3}}$$
where C37:2 and C37:3 are C37 alkenone with two and three double bonds, respectively. The SST was obtained according to the global calibration equation:
![]() $${\rm U}_{{37}}^{{{\rm K}\prime}} $$
=0.033 × SST + 0.044 (Muller et al., Reference Muller, Kirst, Ruhland, von Storch and Rosell-Mele1998). Duplicate analyses showed that the instrumental error was better than 0.01, corresponding to the SST deviation of ±0.3°C. Selected samples were analyzed on the Agilent GC-MS for compound identification. Due to a lack of authentic internal standards, we only report the relative abundance of sterols, diols, and alkenones.
$${\rm U}_{{37}}^{{{\rm K}\prime}} $$
=0.033 × SST + 0.044 (Muller et al., Reference Muller, Kirst, Ruhland, von Storch and Rosell-Mele1998). Duplicate analyses showed that the instrumental error was better than 0.01, corresponding to the SST deviation of ±0.3°C. Selected samples were analyzed on the Agilent GC-MS for compound identification. Due to a lack of authentic internal standards, we only report the relative abundance of sterols, diols, and alkenones.
RESULTS AND DISCUSSION
Temperature records of
 $${\bf U}_{{\bf 37}}^{{{\bf K}\bf \prime}} $$
and TEX86
$${\bf U}_{{\bf 37}}^{{{\bf K}\bf \prime}} $$
and TEX86
The raw data of
![]() $${\rm U}_{{37}}^{{{\rm K}\prime}} $$
and TEX86 are presented in supplementary tables (available online). The
$${\rm U}_{{37}}^{{{\rm K}\prime}} $$
and TEX86 are presented in supplementary tables (available online). The
![]() $${\rm U}_{{37}}^{{{\rm K}\prime}} $$
-derived temperature for the past 15 ka has been reported by Ruan et al. (Reference Ruan, Xu, Ding, Wang and Zhang2015). Here we extend the record back to 20 ka using the same analytical protocol. The SST based on the
$${\rm U}_{{37}}^{{{\rm K}\prime}} $$
-derived temperature for the past 15 ka has been reported by Ruan et al. (Reference Ruan, Xu, Ding, Wang and Zhang2015). Here we extend the record back to 20 ka using the same analytical protocol. The SST based on the
![]() $${\rm U}_{{37}}^{{{\rm K}\prime}} $$
(SSTUKʹ37) varied from 20.1°C to 27.3°C, and over 90% of samples fell in a range of 21.5°C to 26.5°C (Fig. 3A). The amplitude of this glacial-interglacial warming (ca. 5°C) is equal to the previous report of Zhao et al. (Reference Zhao, Huang and Wei2005) for the same core but at much lower resolution (ca. 200 to ~1500 yr). Meanwhile, the temperature based on TEX86 (TTEX86) oscillated from 17.6°C to 28.0°C, and fell in a range of 20.5°C to 26.5°C for 85% of samples (Fig. 3A). The amplitude of the glacial-interglacial warming in the TEX86 record (6.0°C) is slightly larger than that in the
$${\rm U}_{{37}}^{{{\rm K}\prime}} $$
(SSTUKʹ37) varied from 20.1°C to 27.3°C, and over 90% of samples fell in a range of 21.5°C to 26.5°C (Fig. 3A). The amplitude of this glacial-interglacial warming (ca. 5°C) is equal to the previous report of Zhao et al. (Reference Zhao, Huang and Wei2005) for the same core but at much lower resolution (ca. 200 to ~1500 yr). Meanwhile, the temperature based on TEX86 (TTEX86) oscillated from 17.6°C to 28.0°C, and fell in a range of 20.5°C to 26.5°C for 85% of samples (Fig. 3A). The amplitude of the glacial-interglacial warming in the TEX86 record (6.0°C) is slightly larger than that in the
![]() $${\rm U}_{{37}}^{{{\rm K}\prime}} $$
record, which was attributed to lower TTEX86 values during the period of 14.0 ka to 11.4 ka (Fig. 3A).
$${\rm U}_{{37}}^{{{\rm K}\prime}} $$
record, which was attributed to lower TTEX86 values during the period of 14.0 ka to 11.4 ka (Fig. 3A).

Figure 3 (A) Variability of
![]() $${\rm U}_{{37}}^{{{\rm K}\prime}} $$
-temperature (blue) and TEX86-temperature (orange) in sediments from Ocean Drilling Program (ODP) Core 1202B for the past 20 ka. Both lines are for 3-point smoothing. (B) Temperature gradients between
$${\rm U}_{{37}}^{{{\rm K}\prime}} $$
-temperature (blue) and TEX86-temperature (orange) in sediments from Ocean Drilling Program (ODP) Core 1202B for the past 20 ka. Both lines are for 3-point smoothing. (B) Temperature gradients between
![]() $${\rm U}_{{37}}^{{{\rm K}\prime}} $$
and TEX86 records, expressed as ΔTalkenone-GDGT. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
$${\rm U}_{{37}}^{{{\rm K}\prime}} $$
and TEX86 records, expressed as ΔTalkenone-GDGT. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Overall, SSTUKʹ37 and TTEX86 display similar temporal patterns for the past 20 ka, which stayed at low levels prior to 15 ka, further decreased and reached the lowest level during the period from 14.5 ka to 12 ka. Afterward, the temperature increased and remained at high levels until the present. Besides these similarities, apparent discrepancies were observed between these two organic geochemical proxies. In the deglaciation, SSTUKʹ37 was consistently higher than TTEX86. In order to express this gradient, we define a parameter as: ΔT =SSTUKʹ37–TTEX86. Prior to the Holocene, ΔT varied from 0°C to 3.5°C with an average of 1.5°C (Fig. 3B). The deviations between temperature derived from
![]() $${\rm U}_{{37}}^{{{\rm K}\prime}} $$
and TEX86 are usually attributed to a potential difference in seasonality (e.g., Castañeda et al., Reference Castañeda, Schefuß, Pätzold, Sinninghe Damsté, Weldeab and Schouten2010; Lopes dos Santos et al., Reference Lopes dos Santos, Spooner, Barrows, De Deckker, Sinninghe Damsté and Schouten2013) or in the depth habit of the source organisms (e.g., Kim et al., Reference Kim, Romero, Lohmann, Donner, Laepple, Haam and Sinninghe Damsté2012). As source organisms for long-chain alkenones, Haptophyceae such as E. huxleyi and G. oceanica strictly live in the euphotic zone, making
$${\rm U}_{{37}}^{{{\rm K}\prime}} $$
and TEX86 are usually attributed to a potential difference in seasonality (e.g., Castañeda et al., Reference Castañeda, Schefuß, Pätzold, Sinninghe Damsté, Weldeab and Schouten2010; Lopes dos Santos et al., Reference Lopes dos Santos, Spooner, Barrows, De Deckker, Sinninghe Damsté and Schouten2013) or in the depth habit of the source organisms (e.g., Kim et al., Reference Kim, Romero, Lohmann, Donner, Laepple, Haam and Sinninghe Damsté2012). As source organisms for long-chain alkenones, Haptophyceae such as E. huxleyi and G. oceanica strictly live in the euphotic zone, making
![]() $${\rm U}_{{37}}^{{{\rm K}\prime}} $$
a robust indicator for SST (Muller et al., Reference Muller, Kirst, Ruhland, von Storch and Rosell-Mele1998). A number of studies for the SCS, the East China Sea, and the Okinawa Trough (e.g., Pelejero et al., Reference Pelejero, Grimalt, Heilig, Kienast and Wang1999; Ijiri et al., Reference Ijiri, Wang, Oba, Kawahata, Huang and Huang2005; Zhou et al., Reference Zhou, Li, Jia, Zhu, Chi, Cao, Sun and Peng2007; Blumenberg et al., Reference Blumenberg, Seifert, Kasten, Bahlmann and Michaelis2009; Jia et al., Reference Jia, Zhang, Chen, Peng and Zhang2012; Ruan et al., Reference Ruan, Xu, Ding, Wang and Zhang2015) support that
$${\rm U}_{{37}}^{{{\rm K}\prime}} $$
a robust indicator for SST (Muller et al., Reference Muller, Kirst, Ruhland, von Storch and Rosell-Mele1998). A number of studies for the SCS, the East China Sea, and the Okinawa Trough (e.g., Pelejero et al., Reference Pelejero, Grimalt, Heilig, Kienast and Wang1999; Ijiri et al., Reference Ijiri, Wang, Oba, Kawahata, Huang and Huang2005; Zhou et al., Reference Zhou, Li, Jia, Zhu, Chi, Cao, Sun and Peng2007; Blumenberg et al., Reference Blumenberg, Seifert, Kasten, Bahlmann and Michaelis2009; Jia et al., Reference Jia, Zhang, Chen, Peng and Zhang2012; Ruan et al., Reference Ruan, Xu, Ding, Wang and Zhang2015) support that
![]() $${\rm U}_{{37}}^{{{\rm K}\prime}} $$
represents SST. Particularly, the results of Ruan et al. (Reference Ruan, Xu, Ding, Wang and Zhang2015) and Zhao et al. (Reference Zhao, Huang and Wei2005) support the hypothesis that
$${\rm U}_{{37}}^{{{\rm K}\prime}} $$
represents SST. Particularly, the results of Ruan et al. (Reference Ruan, Xu, Ding, Wang and Zhang2015) and Zhao et al. (Reference Zhao, Huang and Wei2005) support the hypothesis that
![]() $${\rm U}_{{37}}^{{{\rm K}\prime}} $$
in sediments records mean annual SST in the Okinawa Trough. Yamamoto et al. (Reference Yamamoto, Shimamoto, Fukuhara, Tanaka and Ishizaka2012) examined seasonal and depth variation in flux of GDGTs and TEX86 values in sinking particles in the western North Pacific, and their result shows that the flux-weighted average TEX86-based temperature corresponds roughly to mean annual SST. However, the TEX86 may also reflect subsurface temperature since archaea can live throughout the water column and may maximize their production in subsurface water (Karner et al., Reference Karner, DeLong and Karl2001; Tierney and Tingley, Reference Tierney and Tingley2015). By analyzing GDGTs in surface sediments from the SCS, Jia et al. (Reference Jia, Zhang, Chen, Peng and Zhang2012) found that TEX86 correlated better with annual mean sea temperature at 30–125 m (r=0.89) than with temperature in the 0–30 m mixed layer (r=0.69). Li et al. (Reference Li, Zhao, Tian and Li2013) also found a difference between SSTUKʹ37 and TTEX86 in the northern SCS for the past 35.6 ka and attributed it to integrated subsurface temperature recorded in TEX86. Given these observations, we propose that the TEX86 is an indicator for subsurface temperature in the southern Okinawa Trough, and the variation of ΔT is related to different depth temperature registered in the proxies of
$${\rm U}_{{37}}^{{{\rm K}\prime}} $$
in sediments records mean annual SST in the Okinawa Trough. Yamamoto et al. (Reference Yamamoto, Shimamoto, Fukuhara, Tanaka and Ishizaka2012) examined seasonal and depth variation in flux of GDGTs and TEX86 values in sinking particles in the western North Pacific, and their result shows that the flux-weighted average TEX86-based temperature corresponds roughly to mean annual SST. However, the TEX86 may also reflect subsurface temperature since archaea can live throughout the water column and may maximize their production in subsurface water (Karner et al., Reference Karner, DeLong and Karl2001; Tierney and Tingley, Reference Tierney and Tingley2015). By analyzing GDGTs in surface sediments from the SCS, Jia et al. (Reference Jia, Zhang, Chen, Peng and Zhang2012) found that TEX86 correlated better with annual mean sea temperature at 30–125 m (r=0.89) than with temperature in the 0–30 m mixed layer (r=0.69). Li et al. (Reference Li, Zhao, Tian and Li2013) also found a difference between SSTUKʹ37 and TTEX86 in the northern SCS for the past 35.6 ka and attributed it to integrated subsurface temperature recorded in TEX86. Given these observations, we propose that the TEX86 is an indicator for subsurface temperature in the southern Okinawa Trough, and the variation of ΔT is related to different depth temperature registered in the proxies of
![]() $${\rm U}_{{37}}^{{{\rm K}\prime}} $$
and TEX86.
$${\rm U}_{{37}}^{{{\rm K}\prime}} $$
and TEX86.
Compared to the deglaciation, the ΔT value in the Holocene is substantially smaller (average 0.15 °C), suggesting similar reconstructed temperatures from
![]() $${\rm U}_{{37}}^{{{\rm K}\prime}} $$
and TEX86 (Fig. 3B). Our result is different from that of Shintani et al. (Reference Shintani, Yamamoto and Chen2011), who found that TTEX86 was higher than SSTUKʹ37 in the Holocene for a core from the SCS. The change of ΔT between the deglaciation and the Holocene is unlikely to be caused by proxy calibration error since the same calibration equation was applied throughout the whole timescale in our study. The terrestrial input may lead to ΔT change because soil-associated iGDGTs, despite minor amounts in comparison to those in marine sediments, can be transported into seas and thereby alter TEX86 (Weijers et al., Reference Weijers, Schouten, Spaargaren and Sinninghe Damsté2006). In order to evaluate this effect, Hopmans et al. (Reference Hopmans, Weijers, Schefuss, Herfort, Sinninghe Damsté and Schouten2004) proposed a branched versus isoprenoid tetraethers (BIT) index because branched and isoprenoid GDGTs are mainly produced in terrestrial and marine realms, respectively. In ODP Core 1202B, the BIT values varied from 0.04 to 0.39 and were significantly higher in the deglaciation (mean±std; 0.22±0.07) than in the Holocene (0.07±0.02; P<0.01). This pattern is to be expected, since lower sea level during the last glaciation (up to –120 m) caused river mouths (e.g., Yangtze River) to be much closer to ODP Site 1202B (Fig. 1) and therefore more soil-associated bGDGTs were discharged into the Okinawa Trough, resulting in higher BIT values. Despite that, the BIT value was still lower than 0.3 in 90% of the samples (Fig. 4B). Because the concentration of iGDGTs is typically an order of magnitude lower than that of bGDGTs in soils, Weijers et al. (Reference Weijers, Schouten, Spaargaren and Sinninghe Damsté2006) concluded that the influence of terrestrial inputs on TEX86 is negligible when the BIT value is lower than 0.3. Therefore, terrestrial inputs, despite apparent fluctuations, cannot explain such a large ∆T change between the deglacial and the Holocene.
$${\rm U}_{{37}}^{{{\rm K}\prime}} $$
and TEX86 (Fig. 3B). Our result is different from that of Shintani et al. (Reference Shintani, Yamamoto and Chen2011), who found that TTEX86 was higher than SSTUKʹ37 in the Holocene for a core from the SCS. The change of ΔT between the deglaciation and the Holocene is unlikely to be caused by proxy calibration error since the same calibration equation was applied throughout the whole timescale in our study. The terrestrial input may lead to ΔT change because soil-associated iGDGTs, despite minor amounts in comparison to those in marine sediments, can be transported into seas and thereby alter TEX86 (Weijers et al., Reference Weijers, Schouten, Spaargaren and Sinninghe Damsté2006). In order to evaluate this effect, Hopmans et al. (Reference Hopmans, Weijers, Schefuss, Herfort, Sinninghe Damsté and Schouten2004) proposed a branched versus isoprenoid tetraethers (BIT) index because branched and isoprenoid GDGTs are mainly produced in terrestrial and marine realms, respectively. In ODP Core 1202B, the BIT values varied from 0.04 to 0.39 and were significantly higher in the deglaciation (mean±std; 0.22±0.07) than in the Holocene (0.07±0.02; P<0.01). This pattern is to be expected, since lower sea level during the last glaciation (up to –120 m) caused river mouths (e.g., Yangtze River) to be much closer to ODP Site 1202B (Fig. 1) and therefore more soil-associated bGDGTs were discharged into the Okinawa Trough, resulting in higher BIT values. Despite that, the BIT value was still lower than 0.3 in 90% of the samples (Fig. 4B). Because the concentration of iGDGTs is typically an order of magnitude lower than that of bGDGTs in soils, Weijers et al. (Reference Weijers, Schouten, Spaargaren and Sinninghe Damsté2006) concluded that the influence of terrestrial inputs on TEX86 is negligible when the BIT value is lower than 0.3. Therefore, terrestrial inputs, despite apparent fluctuations, cannot explain such a large ∆T change between the deglacial and the Holocene.

Figure 4 (color online) Relative abundance of biomarkers and other proxies for Ocean Drilling Program (ODP) Core 1202B. The biomarkers are normalized as the percentages of each proxy over the total peak areas of brassicasterol, dinosterol, C30 1,15-diol, and alkenones. (A) Global sea level curve from U/Th dated coral-based record (Peltier and Fairbanks, Reference Peltier and Fairbanks2006). (B) Branched and isoprenoid tetraether (BIT) values. (C) Brassicasterol (%). (D) Dinosterol (%). (E) C30 1, 15-diol (%). (F) Alkenones (%). The dashed line separates the deglaciation and the Holocene.
Li et al. (Reference Li, Zhao, Tian and Li2013) also reported glacial-interglacial ΔT fluctuations from 0.3 to 4.0°C in the northern SCS. However, their data showed lower ΔT values during glacial periods than interglacial periods. This pattern is attributed to the variation of Asian monsoon from the last glacial to the Holocene. During interglacial periods, when the East Asian summer monsoon (EASM) was strong and the East Asian winter monsoon (EAWM) was weak, stronger stratification of upper water and shoaling of the mixed layer depth (MLD) resulted in larger ΔT values. In contrast, during glacial periods EAWM became stronger and EASM was weaker, resulting in stronger mixing of upper water, deepening of MLD, and smaller ΔT values. Unlike the SCS, the Okinawa Trough was subjected to a stronger influence of the Kuroshio Current in the Holocene, leading to a deeper MLD (Wang et al., Reference Wang, Saito, Oba, Jian and Wang2001). Previous studies of foraminifera, carbon and oxygen isotopes, mineral composition, and digital models all suggest that the Kuroshio Current was much weaker in the last glaciation compared to the Holocene and its main axis was even displaced out of the Okinawa Trough (e.g., Ujiie and Ujiie, Reference Ujiie and Ujiie1999; Kawahata and Ohshima, Reference Kawahata and Ohshima2002; Ujiie et al., Reference Ujiie, Ujiie, Taira, Nakamura and Oguri2003; Kao et al., Reference Kao, Wu, Hsin and Dai2006). Because the Kuroshio Current has a high seawater temperature and a deep thermocline (up to 250 m; Wang et al., Reference Wang, Saito, Oba, Jian and Wang2001; Salisbury et al., Reference Salisbury, Shinohara and Richter2002), an intensified Kuroshio Current in the Holocene would give rise to a deepening of the mixed layer and a reduced temperature gradient (ΔT) between surface water (
![]() $${\rm U}_{{37}}^{{{\rm K}\prime}} $$
for 0–30 m) and subsurface water (TEX86 for 30–80 m), whereas, during the glacial period, a weaker Kuroshio Current led to a shoaling of the mixed layer and higher ΔT values. Thus, our results suggest that ΔT is controlled by different mechanisms in the Okinawa Trough compared to the SCS. In the Okinawa Trough, ΔT is a useful indicator for the intensity of the Kuroshio Current.
$${\rm U}_{{37}}^{{{\rm K}\prime}} $$
for 0–30 m) and subsurface water (TEX86 for 30–80 m), whereas, during the glacial period, a weaker Kuroshio Current led to a shoaling of the mixed layer and higher ΔT values. Thus, our results suggest that ΔT is controlled by different mechanisms in the Okinawa Trough compared to the SCS. In the Okinawa Trough, ΔT is a useful indicator for the intensity of the Kuroshio Current.
The largest ΔT (4.6°C) occurred in the boundary of the last deglaciation/Holocene (~11.5 ka; Fig. 3B). The detailed examination showed that SSTUKʹ37 started to increase at ~12ka, whereas TTEX86 remained at the low level until ~11ka. The delay of warming in the TEX86 record supports our assumption that the TEX86 and
![]() $${\rm U}_{{37}}^{{{\rm K}\prime}} $$
represent subsurface temperature and surface temperature, respectively, in the Okinawa Trough.
$${\rm U}_{{37}}^{{{\rm K}\prime}} $$
represent subsurface temperature and surface temperature, respectively, in the Okinawa Trough.
Phytoplankton community structures since 20 ka
Brassicasterol, dinosterol, C30 1,15-diol, and C37:2 alkenone are biomarkers for diatoms, dinoflagellates, eustigmatophytes, and haptophytes, respectively (Meyers, Reference Meyers1997; Volkman et al., Reference Volkman, Barrett, Blackburn, Mansour, Sikes and Gelin1998). Figure 4 shows the relative abundance of biomarkers in sediments from the ODP Core 1202B, which varied from 3.2% to 20% for brassicasterol, 3.6% to 53% for dinosterol, 13% to 53% for C30 1, 15-diol, and 6.4% to 75% for C37:2 alkenone during the past 20 ka. From the last deglaciation to the Holocene, the relative abundance of haptophytes, reflected by long-chain alkenones, significantly increased from 28.6±12.1% to 45.2±15.8% (P<0.01). In contrast, the relative abundance of diatoms (reflected by brassicasterol) and dinoflagellates (reflected by dinosterol) substantially decreased from 13.2±2.3% to 9.2±3.1% (P<0.01) and from 27.8±9.8% to 16.1±7.2% (P<0.01), respectively. Unlike these algae, the contribution of eustigmatophytes, indicated by long-chain diols, did not present a significant change from the glaciation (30.4±5.2%) to the Holocene (29.5±8.7%; P=0.22). Therefore, our biomarker record reveals an apparent change of phytoplankton community structures during the deglacial/Holocene transition, in which coccolithophorids became more abundant at the expense of diatoms and dinoflagellates.
The principal factors that influence phytoplankton community structures are nutrients, salinity, and temperature in the euphotic zone. In marine environments, coccolithophorids usually thrive in low-nutrient, warm environments, whereas diatoms thrive in high-nutrient, cool environments (Chen et al., Reference Chen, Chen and Chung2007; Falkowski and Oliver, Reference Falkowski and Oliver2007) and tropical upwelling zones (Werne et al., Reference Werne, Hollander, Lyons and Peterson2000). In our study, higher BIT values in the deglaciation (0.14 to 0.39) suggest larger amounts of fluvial terrestrial inputs (Hopmans et al., Reference Hopmans, Weijers, Schefuss, Herfort, Sinninghe Damsté and Schouten2004), which brought more nutrients (e.g., silica and iron) into the Okinawa Trough. Meanwhile, stronger winter monsoons during the dry and cold glacial stages carried more dust and associated nutrients from the interior Asian continent to sea (Maher et al., Reference Maher, Prospero, Mackie, Gaiero, Hesse and Balkanski2010). Both fluvial and dust transport result in higher nutrient delivery into the Okinawa Trough during the deglacial periods than in the Holocene, stimulating phytoplankton growth (particularly diatoms and dinoflagellates).
Besides nutrients, salinity also plays a role in phytoplankton growth. Coccolithophorids are more abundant in deep seas than in brackish coastal seas (Giraudeau, Reference Giraudeau1992). For example, E. huxleyi, a major source organism of long-chain alkenones, has never been found in seawater with the salinity below 11 psu (Bukry et al., Reference Bukry, King, Horn and Manheim1970; van der Meer et al., Reference van der Meer, Sangiorgi, Baas, Brinkhuis, Sinninghe Damsté and Schouten2008). In the East China Sea and Yellow Sea, coccolithophorid fossils are rarely found in sediments due to the strong influence of the Yangtze River and Yellow River (Zhao et al., Reference Zhao, Li and Xing2009). Thus, during the last deglaciation when the highly saline Kuroshio Current was weak and the briny coastal current intensified, lower salinity in the Okinawa Trough might have limited the production of coccolithophorids (Tada et al., Reference Tada, Irino and Koizumi1999; Ujiie and Ujiie, Reference Ujiie and Ujiie1999), which explains the decline in abundance of long-chain alkenones (Fig. 4). However, the continuous presence of long chain alkenones in the Core 1202B suggests a minor change in salinity in the south Okinawa Trough since the last glacial maximum.
Overall, diatoms and dinoflagellates were more abundant during the cold, nutrient-rich, lower saline deglacial stage, whereas coccolithophorids took advantage of warmer temperature, lower nutrients, and higher salinity in the Holocene. This kind of phytoplankton community shift during the glacial-interglacial transition may have an important feedback on climate via the regulation of atmospheric CO2 since silica diatoms have a higher efficiency in the biological pump than calcitic coccolithophorids. Diatoms, with a large cell size and being capable of voluminous production of the transparent exopolymer particles, could settle to the sediments via rapidly sinking aggregates (Smetacek, Reference Smetacek1985; De La Rocha and Passow, Reference De La Rocha and Passow2007). Coccolithophorids, with their relatively small cell size and high surface area/volume ratio, appear to most commonly sink within fecal pellets of coccoliths or whole coccolithophorids (Honjo, Reference Honjo1976, Reference Honjo1982; De La Rocha and Passow, Reference De La Rocha and Passow2007). From the euphotic zone to the deep sea, compared to coccolithophorids, more particulate organic carbon from diatoms may be exported to the sediments under the same primary production and degradation conditions.
These observations suggest that, in order to better understand the interactions amongst glacial-interglacial climate cycles, atmospheric CO2, and marine productivity and phytoplankton community structures, more biomarker-based studies for different marine environments are needed.
CONCLUSIONS
We have presented a comprehensive, high resolution biomarker study for ODP Core 1202B in order to reconstruct the paleoenvironment and biological responses to the variability of the Kuroshio Current in the Okinawa Trough since the last glacial maximum, upon which three conclusions have been reached. First, the temperature records of
![]() $${\rm U}_{{37}}^{{{\rm K}\prime}} $$
and TEX86 present similar trends and amplitudes for the past 20,000 yr. However, consistently higher SSTUKʹ37 than TTEX86 in the deglaciation suggests that different depth temperatures are recorded by TEX86 (subsurface temperature) and
$${\rm U}_{{37}}^{{{\rm K}\prime}} $$
and TEX86 present similar trends and amplitudes for the past 20,000 yr. However, consistently higher SSTUKʹ37 than TTEX86 in the deglaciation suggests that different depth temperatures are recorded by TEX86 (subsurface temperature) and
![]() $${\rm U}_{{37}}^{{{\rm K}\prime}} $$
(surface temperature). Second, the relative abundance of biomarkers reveals a change of phytoplankton community structures from a diatom/dinoflagellate-dominated community in the last deglaciation to a coccolithophorid-dominant community in the Holocene. This shift is controlled by the combined effect of nutrients, salinity, and temperature in the Okinawa Trough. Third, the Kuroshio Current changed significantly since the LGM. It was much weaker in the last deglaciation, leading to relatively large temperature differences between
$${\rm U}_{{37}}^{{{\rm K}\prime}} $$
(surface temperature). Second, the relative abundance of biomarkers reveals a change of phytoplankton community structures from a diatom/dinoflagellate-dominated community in the last deglaciation to a coccolithophorid-dominant community in the Holocene. This shift is controlled by the combined effect of nutrients, salinity, and temperature in the Okinawa Trough. Third, the Kuroshio Current changed significantly since the LGM. It was much weaker in the last deglaciation, leading to relatively large temperature differences between
![]() $${\rm U}_{{37}}^{{{\rm K}\prime}} $$
and TEX86 records. However, when the Kuroshio Current intensified in the Holocene, similar temperature records were observed for
$${\rm U}_{{37}}^{{{\rm K}\prime}} $$
and TEX86 records. However, when the Kuroshio Current intensified in the Holocene, similar temperature records were observed for
![]() $${\rm U}_{{37}}^{{{\rm K}\prime}} $$
and TEX86. Our result demonstrates the gradient between TUKʹ37 and TTEX86 is a useful indicator for reconstruction of the Kuroshio Current in the Okinawa Trough.
$${\rm U}_{{37}}^{{{\rm K}\prime}} $$
and TEX86. Our result demonstrates the gradient between TUKʹ37 and TTEX86 is a useful indicator for reconstruction of the Kuroshio Current in the Okinawa Trough.
ACKNOWLEDGMENTS
This project was financially supported by NSFC (41476062; 41676058). We are grateful to Huan Yang for GDGT analyses. Two anonymous reviewers are thanked for valuable comments. This research used samples provided by the Ocean Drilling Program (ODP) and its precusors. ODP is sponsored by the U.S. National Science Foundation (NSF) and participating countries under management of Joint Oceanographic Institutions (JOI), Inc.