INTRODUCTION
The late Cenozoic marine successions of western Emilia (northern Italy) represent an unparalleled sedimentary archive for defining the role of climate and tectonic dynamics in shaping sediment flux, landscape, and biotic interactions (e.g., Dominici, Reference Dominici2001, Reference Dominici2004; Gunderson et al., Reference Gunderson, Kodama, Anastasio and Pazzaglia2012; Crippa and Raineri, Reference Crippa and Raineri2015; Crippa et al., Reference Crippa, Angiolini, Bottini, Erba, Felletti, Frigerio and Hennissen2016, Reference Crippa, Baucon, Felletti, Raineri and Scarponi2018; Russo et al., Reference Russo, Artoni, Scarponi and Serventi2017; Cau et al., Reference Cau, Laini, Monegatti, Roveri, Scarponi and Taviani2019). In this respect, the successions cropping out along the banks of the Arda and Stirone Rivers represent a historical landmark for these kinds of studies. Indeed, studying the fossils from these successions, Lyell (Reference Lyell1833) confirmed his concept of the Pliocene, and Mayer-Eymar (Reference Mayer-Eymar1858) coined the term “Piacenzische Stufe” (“Piacenzian Stage”) to indicate the “blue shales” (“Argille azzurre”) so widespread in this area. Since then, the rich and well-preserved invertebrate fossil fauna from both the Arda and Stirone River beds has been the focus of numerous studies that promoted the development of the late Cenozoic geologic timescale, especially during the last century (e.g., Barbieri, Reference Barbieri1958; Papani and Pelosio, Reference Papani and Pelosio1962; Raffi, Reference Raffi1970; Caprotti, Reference Caprotti1972, Reference Caprotti1973, Reference Caprotti1974, Reference Caprotti1976; Pelosio and Raffi, Reference Pelosio and Raffi1974, Reference Pelosio and Raffi1977). Although several of these studies were performed on the Pliocene part of these successions, only a few were recently undertaken on the Pleistocene deposits (e.g., Dominici, Reference Dominici2001, Reference Dominici2004; Monegatti et al., Reference Monegatti, Raffi, Roveri and Taviani2001; Pervesler et al., Reference Pervesler, Uchman, Hohenegger and Dominici2011; Crippa, Reference Crippa2013; Crippa and Raineri, Reference Crippa and Raineri2015; Crippa et al., Reference Crippa, Angiolini, Bottini, Erba, Felletti, Frigerio and Hennissen2016, Reference Crippa, Baucon, Felletti, Raineri and Scarponi2018). Bio- and magnetostratigraphic analyses to constrain the age of the Arda and Stirone River sections were carried out nearly exclusively on Pliocene sediments (e.g., Barbieri, Reference Barbieri1958, Reference Barbieri1967; Mary et al.,Reference Mary, Iaccarino, Courtillot, Besse and Aissaoui1993; Channell et al., Reference Channell, Poli, Rio, Sprovieri and Villa1994; Gunderson et al., Reference Gunderson, Kodama, Anastasio and Pazzaglia2012; Cau et al., Reference Cau, Franchi, Roveri and Taviani2015). Recently, Crippa et al. (Reference Crippa, Angiolini, Bottini, Erba, Felletti, Frigerio and Hennissen2016) provided an integrated biostratigraphy (calcareous nannofossils and foraminifera) of the Pleistocene part of the Arda River section that led to the identification of three nannofossil (CNPL7, CNPL8, and CNPL9) and one foraminiferal (Globigerina cariacoensis) biozones, all of Calabrian age. Monesi et al. (Reference Monesi, Muttoni, Scardia, Felletti, Bona, Sala, Tremolada, Francou and Raineri2016) confirmed the Calabrian age of the Arda section based on magnetostratigraphy. Conversely, the Pleistocene succession cropping out along the Stirone River lacks a robust biostratigraphic framework.
Marine successions of western Emilia are also important because of the occurrence of the “northern guest” bivalve Arctica islandica (Fig. 1). The term “northern guest” was first used by Suess (Reference Suess1883–1888) to define organisms, such as A. islandica, which are thriving nowadays at higher latitudes in the Northern Hemisphere; they migrated into the Mediterranean Sea through the Strait of Gibraltar because of the onset of the cooling in the Calabrian (Malatesta and Zarlenga, Reference Malatesta and Zarlenga1986; Raffi, Reference Raffi1986; Martínez-García et al., Reference Martínez-García, Rodríguez-Lázaro, Pascual and Mendicoa2015). The first occurrence of the “northern guests” in the Mediterranean Sea has a historical importance; this biotic event was one of the main criteria used to mark the Pliocene-Pleistocene boundary (Pelosio and Raffi, Reference Pelosio and Raffi1974; Aguirre and Pasini, Reference Aguirre and Pasini1985; Raffi, Reference Raffi1986), when placed at the former Global Stratotype Section and Point (GSSP) at Vrica (Calabria, Italy). The Pliocene-Pleistocene boundary was then revised and lowered to 2.58 Ma at the base of the Gelasian Stage (e.g., Gibbard et al., Reference Gibbard, Head and Walker2010; Cohen et al., Reference Cohen, Harper and Gibbard2018). According to Raffi (Reference Raffi1986) and Rio et al. (Reference Rio, Raffi, Backman and Van Couvering1997), the lowest stratigraphic level where A. islandica occurred is reportedly in the Arda and Stirone River sections (see Table 1 reporting Pleistocene Mediterranean distribution of A. islandica); however, these authors did not report a detailed biostratigraphic analysis of these successions. Raffi (Reference Raffi1986) dated the first occurrence of A. islandica into the Mediterranean Sea at the top of the Olduvai magnetic subchron at 1.67 Ma. This age determination changed after 1995, when a new astronomically tuned timescale was proposed to define the magnetostratigraphic boundaries (Van Couvering, Reference Van Couvering and Van Couvering1997). The new age for the top of the Olduvai subchron and the corresponding first occurrence of A. islandica in the Mediterranean Sea is 1.77 Ma (Van Couvering, Reference Van Couvering and Van Couvering1997). In order to thoroughly understand the timing and the processes of the climatic changes (i.e., cooling) occurring during the early Pleistocene, and in particular from the Calabrian onward, it is important to accurately constrain the timing of the first occurrence of A. islandica in the Mediterranean Sea. Up to now, a detailed study on this topic has been lacking.
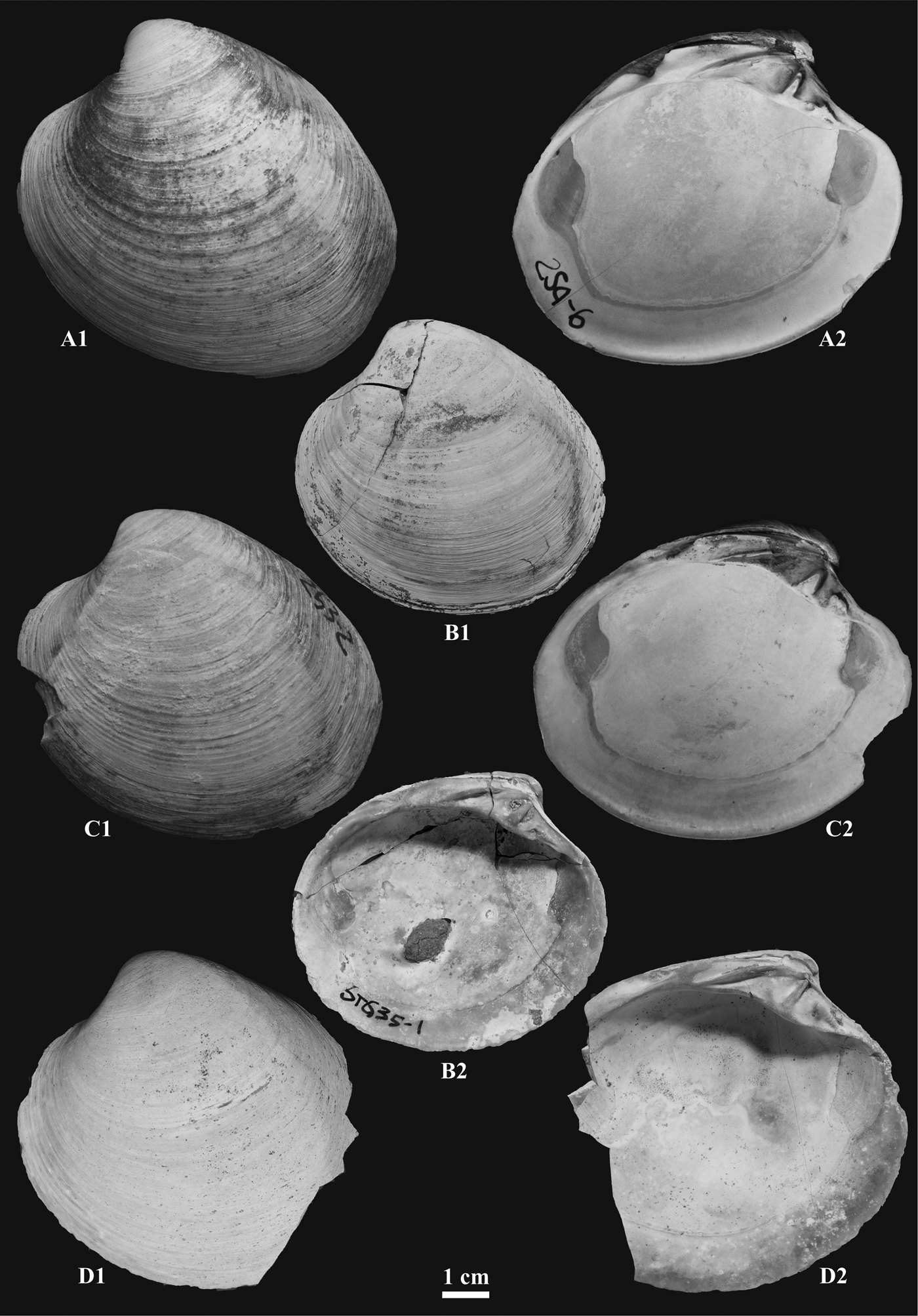
Figure 1. External (A1, B1, C1, D1) and internal (A2, B2, C2, D2) parts of left valves of specimens of Arctica islandica collected from the Arda River (A1, A2; C1, C2) and Stirone River (B1, B2; D1, D2) marine successions.
Table 1. Occurrence of Arctica islandica in the lower Pleistocene of the Mediterranean Sea. Subdivision of the Calabrian (early Pleistocene) into three substages (Santernian [lower Calabrian], Emilian [middle Calabrian], and Sicilian [upper Calabrian]) according to Ruggieri and Sprovieri (Reference Ruggieri and Sprovieri1975, Reference Ruggieri and Sprovieri1977). This subdivision, which refers to Italian marine stages, is not formally used yet, but several old and even recently published papers adopt it; here, it is used together with the formally accepted one (Cohen et al., Reference Cohen, Harper and Gibbard2018). Age in Ma reported only when explicitly cited in the literature.
The goals of this article are threefold: (1) to better describe the stratigraphic setting, particularly for the Stirone section, in order to correlate the marine successions of western Emilia by means of an integrated approach relying on facies analysis, calcareous nannofossils, and foraminifera biostratigraphy fitted into the magnetostratigraphic framework available from the literature; (2) to calibrate the first occurrence of A. islandica in the Mediterranean Sea; and (3) to map the occurrence of the species in the area during the early Pleistocene.
GEOLOGIC SETTING
The investigated successions crop out in northern Italy near the town of Castell'Arquato (Arda River section) and 3 km northwest of Salsomaggiore Terme (Stirone River section). These deposits belong to the Castell'Arquato wedge-top basin (CAB), which developed from the late Messinian (~6 Ma; upper Miocene) to the Pleistocene after the northeastward migration and fragmentation of the Po Plain–Adriatic foredeep (Roveri and Taviani, Reference Roveri and Taviani2003; Ghielmi et al., Reference Ghielmi, Minervini, Nini, Rogledi and Rossi2013; Fig. 2A). The CAB is located at the foothills of the northwestern sector of the Apennines; it is bounded to the south by the emerged front of Ligurian units (Monegatti et al., Reference Monegatti, Raffi, Roveri and Taviani2001) and to the north by the Salsomaggiore structure, one of the outermost thrust fronts of the Apennine orogenic wedge (Artoni et al., Reference Artoni, Papani, Rizzini, Calderoni, Bernini, Argnani, Roveri, Rossi, Rogledi and Gennari2004, Reference Artoni, Bernini, Papani, Rizzini, Barbacini, Rossi, Rogledi and Ghielmi2010).

Figure 2. (color online) (A) Geologic sketch map of northern Italy showing the study area (modified after Crippa et al., Reference Crippa, Angiolini, Bottini, Erba, Felletti, Frigerio and Hennissen2016, Reference Crippa, Baucon, Felletti, Raineri and Scarponi2018). (B) Enlarged geologic map showing the position of the Stirone River section (A-A’).
The basal part of the CAB succession comprises deep-sea sediments deposited when marine conditions were restored in the Mediterranean Sea after the end of the Messinian salinity crisis (Ceregato et al., Reference Ceregato, Raffi and Scarponi2007; Calabrese and Di Dio, Reference Calabrese and Di Dio2009); upward they pass to epibathyal to shelf-related facies (Pliocene to early Pleistocene in age). Then, through a strong regressive upward trend, alluvial deposits of the middle Pleistocene record the establishment of a continental environment with vertebrate faunas and freshwater mollusks (Cigala Fulgosi, Reference Cigala Fulgosi1976; Pelosio and Raffi, Reference Pelosio and Raffi1977; Ciangherotti et al., Reference Ciangherotti, Crispino and Esu1997; Esu, Reference Esu2008; Esu and Girotti, Reference Esu and Girotti2015). The late Messinian to Pleistocene sedimentary infill of the CAB is represented by a depositional sequence controlled by tectonics; beds form a regular monocline dipping toward the north/northeast, and no major faults are present in the area, allowing a good correlation of the cropping out stratigraphic units (Monegatti et al., Reference Monegatti, Raffi, Roveri and Taviani2001; Cau et al., Reference Cau, Laini, Monegatti, Roveri, Scarponi and Taviani2019). This sequence can be further subdivided into four lower rank unconformity bounded units, which are related to well-known Pliocene-Pleistocene tectonic phases of compressional deformation and depocenter migration toward the foreland of the Apennine thrust-fold belt (Roveri and Taviani, Reference Roveri and Taviani2003; Artoni et al., Reference Artoni, Bernini, Papani, Rizzini, Barbacini, Rossi, Rogledi and Ghielmi2010). In turn, these tectono-bounded stratigraphic units are subdivided into smaller-scale (higher-frequency) sequences.
The well-exposed Stirone succession crops out along the incised banks of the Stirone River (Fig. 2B), and it extends downstream the Pieve di San Nicomede locality (base at 44°50′38.87″N, 9°58′38.37″E; top at 44°50′46.96″N, 9°59′28.00″E). A detailed sedimentologic and biostratigraphic analysis of this section is presented in this article. For the sedimentologic, paleontological, and biostratigraphic reconstruction of the Arda River section, we refer to Crippa et al. (Reference Crippa, Angiolini, Bottini, Erba, Felletti, Frigerio and Hennissen2016, Reference Crippa, Baucon, Felletti, Raineri and Scarponi2018). For taphonomy and paleoecology of both sections, see Dominici (Reference Dominici2001, Reference Dominici2004).
MATERIALS AND METHODS
Facies analysis
The Stirone succession (Figs. 2 and 3) is 138 m thick; a detailed stratigraphic section has been measured with a resolution of 1 cm. Thickness of beds and depositional intervals, geometry of bed-bounding surfaces, presence of mud clasts, sedimentary structures, and paleocurrent indicators were all recorded. Bed amalgamation is frequent in the studied succession, and thus individual layers were measured through detecting the subtle grain-size breaks associated with amalgamation surfaces. Grain-size measurements were made using a hand lens and grain-size comparator. Bed thickness measurement was performed mainly with a Jacob's staff (e.g., a 1.5-m-high rod provided with a clinometer and a flat disc on top).
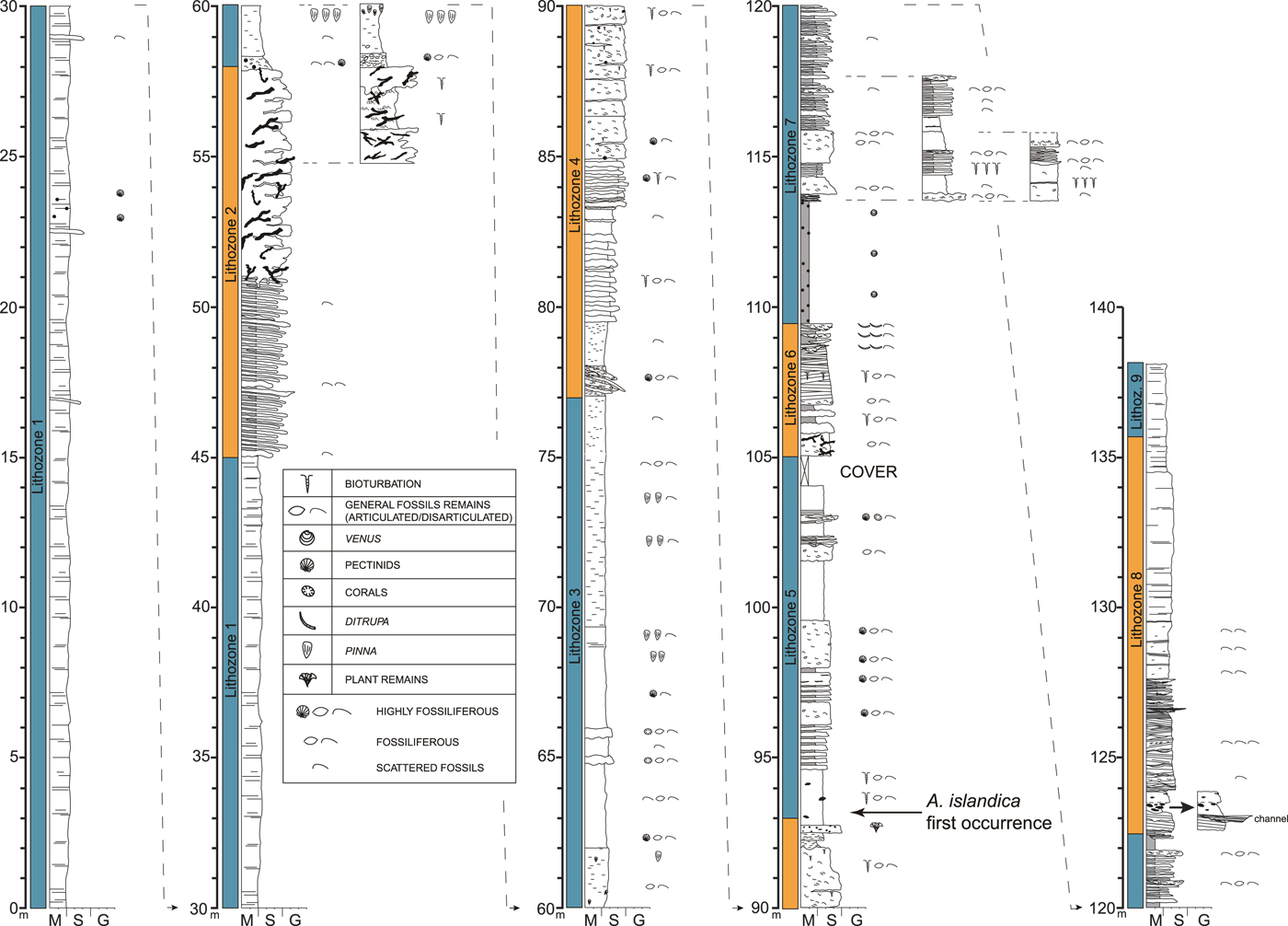
Figure 3. (color online) Detailed stratigraphic log of the Stirone River section. The nine lithozones identified in the succession are shown. M, mud; S, sand; G, gravel.
Calcareous nannofossils
A total of 30 samples from the Stirone section were analyzed for calcareous nannofossils (Supplementary Table 1). The study was performed on smear slides under polarizing light, at 1250× magnification. Smear slides were prepared following the methodology described by Monechi and Thierstein (Reference Monechi and Thierstein1985). For each sample, nannofossils were quantitatively characterized by counting at least 300 specimens. In addition, two random traverses were studied to detect rare and/or marker species. Specimen preservation is moderate throughout the entire studied interval (Supplementary Table 1). We applied the biozonation scheme by Backman et al. (Reference Backman, Raffi, Rio, Fornaciari and Pälike2012) and the identification of Gephyrocapsa specimens based on the informal taxonomic concepts established by Raffi et al. (Reference Raffi, Backman, Rio and Shackleton1993): “small” Gephyrocapsa refers to specimens <4.0 µm in size, “medium” Gephyrocapsa refers to specimens ≥4.0 µm, and “large” Gephyrocapsa refers to specimens >5.5 µm.
Foraminifera
A total of 21 samples were analyzed for planktonic and benthic foraminifera. Samples were processed using standard laboratory procedures for soft sediments and washed under water through >63 µm, >125 µm, and >250 µm sieves and then dried. Residues contain a variable amount of quartz, mica, ostracods, mollusk and echinoid fragments, vegetable debris, iron oxides, and aggregated grains. Foraminifera are generally common in all samples, with benthic taxa occurring in greater specimen abundance than planktonic ones; both groups decrease in number in the upper part of the stratigraphic section. Taxonomic concepts for foraminifera identification applied in this study follow Colalongo and Sartoni (Reference Colalongo and Sartoni1977), Agip (Reference Agip1982), Kennett and Srinivasan (Reference Kennett and Srinivasan1983), Iaccarino (Reference Iaccarino1985), Iaccarino et al. (Reference Iaccarino, Premoli Silva, Biolzi, Foresi, Lirer, Turco and Petrizzo2007), and Holbourn et al. (Reference Holbourn, Henderson and MacLeod2013). Foraminiferal biozonation follows Iaccarino et al. (Reference Iaccarino, Premoli Silva, Biolzi, Foresi, Lirer, Turco and Petrizzo2007) and Cita et al. (Reference Cita, Gibbard and Head2012). The most significant taxa for biostratigraphy were photographed using a Cambridge S-360 Scanning Electron Microscope (SEM) with lanthanum hexaboride (LaB6) cathodes at the University of Milan (Milan, Italy).
Calabrian Stage subdivision in the Mediterranean
The subdivision of the Calabrian Stage (early Pleistocene) into three Italian marine substages—Santernian (lower Calabrian), Emilian (middle Calabrian), and Sicilian (upper Calabrian)—was first proposed by Ruggieri and Sprovieri (Reference Ruggieri and Sprovieri1975, Reference Ruggieri and Sprovieri1977) and then modified by Cita et al. (Reference Cita, Capraro, Ciaranfi, Di Stefano, Marino, Rio, Sprovieri and Vai2006). Ruggieri and Sprovieri (Reference Ruggieri and Sprovieri1979) and Ruggieri et al. (Reference Ruggieri, Rio and Sprovieri1984) subsequently introduced the stage/age Selinuntian as a replacement for the Calabrian, which included the three abovementioned substages (Van Couvering, Reference Van Couvering and Van Couvering1997; Cita, Reference Cita2008). However, the Selinuntian was not defined following the procedures prescribed by the International Commission on Stratigraphy (ICS), so it was considered a nomen nudum (Vai, Reference Vai1996; Cita, Reference Cita2008; Cita et al., Reference Cita, Capraro, Ciaranfi, Di Stefano, Lirer, Maiorano, Marino, Raffi, Rio, Sprovieri, Stefanelli and Vai2008). At the end of 2007, the Calabrian Stage was redefined with the Vrica section as type-section (Cita, Reference Cita2008); for historical background and a deep review on Italian marine stages, see Cita (Reference Cita2008) and Cita et al. (Reference Cita, Capraro, Ciaranfi, Di Stefano, Marino, Rio, Sprovieri and Vai2006, Reference Cita2008, Reference Cita, Gibbard and Head2012).
The definition of the Santernian, Emilian, and Sicilian was essentially based on invertebrate paleontology, initially from the study of mollusks, but also of corals, ostracods, foraminifera, and calcareous nannofossils (Cita, Reference Cita2008). This subdivision of the Calabrian corresponds in part to the three successive migration floods of “northern guests” and to the gradual extinction of taxa of tropical affinity in the Mediterranean Sea (Ruggieri et al., Reference Ruggieri, Rio and Sprovieri1984; Raffi, Reference Raffi1986). According to Ruggieri and Sprovieri (Reference Ruggieri and Sprovieri1975, Reference Ruggieri and Sprovieri1977), the lower boundary of the Santernian, Emilian, and Sicilian corresponds respectively to the first occurrence of Arctica islandica, Hyalinea balthica, and Truncorotalia truncatulinoides excelsa. This subdivision is not formally used yet (Cohen and Gibbard, Reference Cohen and Gibbard2016), but it is still widely adopted in the Italian literature on the marine Quaternary (see references in Table 1); here, in order to give clear and precise indications of the age of the sections described, we use it together with the formally accepted one (Cohen et al., Reference Cohen, Harper and Gibbard2018).
RESULTS
Facies analysis and interpretation of the Stirone section
The study of facies associations of the Stirone section allowed us to define nine lithozones (Fig. 3), each representing a different depositional environment. The vertical and lateral arrangement of different lithofacies reflects cyclic depositional changes between suspended-load- and bed-load-dominated facies associated with different velocity and fallout rates. Sedimentary characteristics and process interpretation, complemented with macroinvertebrate assemblages, are provided subsequently.
Lithozone 1 (0–45 m) is composed of massive to laminated mudstones forming ungraded to normally graded beds of a few millimeters up to 100 cm in thickness and containing marine shelf-related bivalves (e.g., Amusium cristatum, Aequipecten opercularis, Corbula gibba, Glossus humanus, Pelecyora brocchi, and Venus nux), commonly in life position. The biosedimentary features observed suggest sedimentation under low hydrodynamic energy and low accumulation rates, such those typical of an offshore transition environment, dominated by suspension settling. In turn, graded beds suggest deposition by traction plus fallout by waning-waxing hyperpycnal flows generated in nearby flood-dominated fan-delta systems.
Lithozone 2 (45–58 m) is initiated by 6 m of alternating massive to laminated very fine-grained bioclastic limestones and mudstones. Typically, limestone beds are up to 20 cm thick and show either lenticular or pinch-and-swell geometry with a sharp base and top. Above follows 7 m of intensely burrowed (Thalassinoides ichnofabric) silty sandstones organized in meter-thick beds (Fig. 4A and B). Macrobenthic remains are sparse and mostly represented by fragments of pectinids (e.g., A. opercularis). The lower part of lithozone 2 is interpreted as the product of deposition in an offshore transition environment cyclically receiving fine-grained bioclastic materials. Although bioturbation has obliterated any sedimentary structure, the dominant grain size of the upper half of lithozone 2 suggests deposition in a shoreface environment.
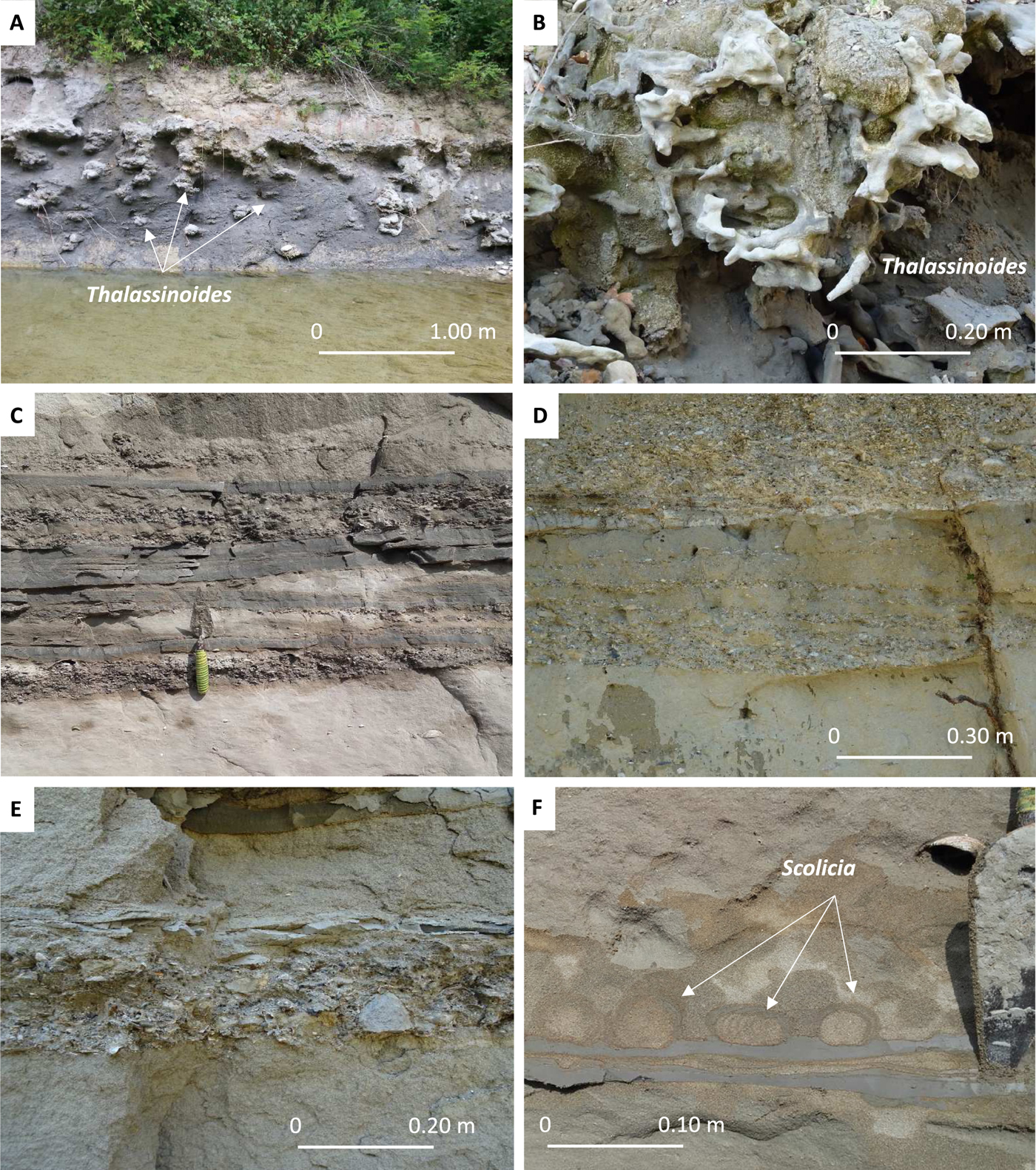
Figure 4. (color online) (A, B) Intensely burrowed silty sandstones: Thalassinoides ichnofabric dominated by vertical burrows filled by bioclastic sands. (C) Normally graded beds of fine sandstone and mudstone intercalated with accumulations of shells. (D, E) Normally graded beds of medium and coarse sandstone with large-scale lamination to small-scale cross lamination. Accumulations of shells and small floating clay chips are commonly found dispersed within the sandstone. (F) Scolicia ichnofabric overlying sand/mud couplets and reworking a mud layer.
Lithozone 3 (58–77 m) is similar to lithozone 1 apart from the lowermost 0.4 m, which consists of a closely packed unit of macrobenthic invertebrate shells, and from the macroinvertebrate content, which is more abundant and diversified with respect to lithozone 1. Within this lithozone, faunal assemblages are categorized as neighborhood and life assemblages (sensu Brenchley and Harper, Reference Brenchley and Harper1998), and the most common mollusks are Pinna sp., Nucula placentina, Glycymeris inflata, Anomia ephippium, Lembulus pella, Acanthocardia echinata, Loripinus fragilis, Azorinus chamasolen, and Serratina serrata. Less abundant brachiopods (e.g., Terebratula terebratula and Aphelesia bipartita) and corals (e.g., Flabellum sp.) are also present.
The sedimentary features of this lithozone suggest an offshore transition environment characterized by low hydrodynamic energy and low accumulation rates, dominated by suspension settling; this is also confirmed by the fossil assemblages represented by taxa commonly retrieved in offshore transition to inner shelf settings.
Lithozone 4 (77–93 m) mostly consists of variously cemented biocalcarenitic beds organized into a tripartite facies association. The lower unit is composed of horizontal strata (i.e., bottomsets) containing locally abundant articulated bivalves (such as G. inflata, Glycymeris glycymeris, Pecten jacobaeus, A. opercularis). The middle unit shows clinostratified geometries (i.e., foresets) with mainly reworked shells. The upper unit has flat-laying bedding (i.e., bedsets), showing shell pavements mainly represented by concave-down pectinids (e.g., P. jacobaeus and A. opercularis). In some places, planar lamination and thin horizons made of densely packed anomiid valves are also encountered.
The internal bedding geometry of this unit (i.e., superimposition of bottomset, foreset, and topset) suggests a basinward-prograding body developed in a relatively high-energy shelf environment. The reduced amount of siliciclastic component may reflect either low clastic input or winnowing by bottom currents. Similar Pliocene-Quaternary deposits from the CAB (Cau et al., Reference Cau, Taviani, Manzi and Roveri2013, Reference Cau, Roveri and Taviani2017, Reference Cau, Laini, Monegatti, Roveri, Scarponi and Taviani2019) and other Mediterranean sites (Massari and Chiocci, Reference Massari and Chiocci2006) have been interpreted likewise.
Lithozone 5 (93–105 m) is mainly composed of normally graded beds of fine sandstone (Fig. 4C–E), with structures ranging from horizontal or large-scale wavy lamination to small-scale cross lamination (wavy, sigmoidal, and/or climbing ripple structures). Bioturbations are frequent (Fig. 4F) and can obliterate any primary sedimentary structure. Locally, flaser, wavy, and lenticular beds with thickness in the range of a few millimeters to several centimeters are intercalated in the sandstones. Small floating clay chips are commonly found homogenously dispersed within the sandstone or clustered in the topmost part of beds. Accumulations of closely packed and mostly concave-down shells are also common and may form thick, sharp-based, and normally graded lags highlighting the base of sandstone beds and showing tabular to lenticular bed geometry. At the base of lithozone 5 (93.15 m), Arctica islandica first occurs in the section.
This lithozone is mostly composed of fine-grained sediments transported as suspended load, which form thick and complex intervals that can be massive or displaying traction plus fallout sedimentary structures. The lithofacies’ vertical arrangement reflects cyclical and gradual changes between suspended-load- and bed-load-dominated facies, which are the result of near-continuous deposition from a quasi-steady hyperpycnal flow (i.e., long-lived turbulent flow; Zavala et al., Reference Zavala, Arcuri, Di Meglio, Gamero Diaz, Contreras, Slatt and Zavala2011; Marini et al., Reference Marini, Felletti, Milli and Patacci2016) that tends to dominate the medial parts of a river-delta system. Closely packed and mostly concave-down shells suggest that these lags most likely represent transported assemblages (sensu Brenchley and Harper, Reference Brenchley and Harper1998).
Lithozone 6 (105–109.5 m) is represented by very thin to thick beds (0.3–1 m) of coarse sandstones internally displaying low-angle (less than 20°) tabular cross stratification with asymptotic relationships with bed top and base. A shallow-water macroinvertebrate fauna of neighborhood assemblage (sensu Brenchley and Harper, Reference Brenchley and Harper1998) is present here (e.g., Glycymeris insubrica, Dosinia lupinus, Chamelea gallina, and Ensis ensis). The diagnostic sedimentary structures along with a stock of nearshore bivalves point toward a high-energy littoral environment (shoreface settings).
Lithozone 7 (109.5–122.5 m) is initiated by 4.5 m of mudstones with abundant marine bivalves in life assemblages (sensu Brenchley and Harper, Reference Brenchley and Harper1998) in which Venus nux, Acanthocardia paucicostata, and Ostrea edulis are the most abundant species, followed upward by 8.5 m of structureless to laminated sandstones (horizontal, hummocky, tabular, or oblique cross laminations) locally interbedded with flaser, wavy, and lenticular beds (millimeters to centimeters thick). Floating clay chips and accumulations of shells are common features in the sandstone intervals, where they form thick lags at the base of massive or laminated strata. Internal erosional surfaces are frequent; carbonaceous remains and wood fragments are also common within sandstones. Soft-sediment deformation diapir-like structures (Fig. 5A and B) made of fine material (argillaceous fine sands and silts), which intrudes and deforms the overlying beds, have been observed at 116 m. In a few cases, plastic intrusions reach the surface and the sweeping of mud and sand produces the “sand volcanoes” (centimeter scale) set out as flattened cones along fissures. At 117 m, a small block of chemoherms is found containing the typical lucinid fauna. At the top of the interval, A. islandica is abundant with articulated valves in life position (Fig. 6).

Figure 5. (color online) (A, B) Soft-sediment deformation structures. These ductile deformation structures can be related to seismic shocks on water-saturated sediments. (C, D) Medium-grained sands with low-angle cross stratification, documenting a foreshore to upper-shoreface environment. (E, F) Flasers, wavy and lenticular bedding, from a few millimeters to several centimeters thick, intercalated with sandstones.

Figure 6. (color online) Specimens of Arctica islandica occurring in lithozone 7. (A, C, D) Articulated specimens in life position and not filled inside by the sediment; note the well-preserved organic ligament in panels C and D. (B) Accumulation bed with abundant disarticulated concave-down valves.
Lithozone 7 is mostly composed of fine-grained sediments transported as suspended load deposited in the medial parts of a river-delta system. The abundance of Venus nux at the base of the lithozone suggests a low-energy offshore transition setting; according to Taviani et al. (Reference Taviani, Roveri, Impiccini and Vigliotti1997) and Dominici (Reference Dominici2001), the Venus nux assemblage is indicative of water depths in the range of 20–40 m. Today, this species is commonly reported from deeper-water settings in the Mediterranean Sea (70–80 m; Raffi and Serpagli, Reference Raffi and Serpagli1993). The observed soft-sediment deformation structures formed in loose, unconsolidated to semiconsolidated fine-grained, water-saturated muddy and sandy deposits and were induced chiefly by liquefaction and/or fluidization processes. These ductile deformation structures can be related to seismic shocks on water-saturated sediments (Berra and Felletti, Reference Berra and Felletti2011). The presence of chemoherms is here recorded for the first time in this part of the section, whereas they are well documented in Pliocene sediments of western Emilia (Monegatti et al., Reference Monegatti, Raffi, Roveri and Taviani2001; Cau et al., Reference Cau, Franchi, Roveri and Taviani2015). These chemoherms were interpreted, by Monegatti et al. (Reference Monegatti, Raffi, Roveri and Taviani2001) and Cau et al. (Reference Cau, Franchi, Roveri and Taviani2015), to have formed in a deep-water environment affected by expulsion of hydrocarbon-rich fluids generated by the tectonic pulses of the Salsomaggiore structure.
Lithozone 8 (122.5–134.5 m) is represented by coarse-grained sands with intercalated massive and cross-stratified conglomerates with abundant coarse- to fine-grained sandstone matrix, especially in the lower part of the lithozone (Fig. 5C and D). Individual sets of cross beds commonly show thicknesses between 0.5 and 1.5 m and asymptotic relationships with top and base. Bounding surfaces between bedsets are erosional, and the foreset inclinations do not usually exceed 15°. Flaser and massive mudstone beds up to several centimeters thick are intercalated within these sandstones (Fig. 5E and F), as well as large mud clasts floating in a medium- to coarse-grained sandstone matrix.
This lithozone documents a foreshore to upper shoreface environment characterized by high hydrodynamic energy and high sedimentation rate. Simultaneously, the presence of massive and cross-stratified conglomerates suggests the existence of high-density flows that occur mainly in a flood-generated delta-front sandstone lobe (mouth bar).
Lithozone 9 (134.50–138 m) consists of laminated silty mudstone with diagnostic freshwater/ipohaline mollusks, such as Dreissena polymorpha, Theodoxus groyanus, Lithoglyphus sp., Melanopsis wilhelmi, and Tanousia stironensis (see also Esu, Reference Esu2008; Esu and Girotti, Reference Esu and Girotti2015).
The presence of such a large stock of freshwater mollusks points toward a coastal plain setting, characterized by a standing body of fresh to low-salinity water.
Biostratigraphy
Calcareous nannofossils
The nannofossil assemblages are composed of in situ specimens and reworked Cretaceous and Cenozoic species. The total nannofossil abundance (average number of specimens in one field of view, which is equal to 0.02 mm2) is 5.7. Only one sample (86.25 m) was found to be barren of nannofossils. The interval between 75.70 m and 87.35 m is characterized by the lowest total abundance (lower than 3.5 specimens/field of view). The taxa considered to be in situ constitute between 20% and 45% of the assemblages and include relatively small placoliths (e.g., reticulofenestrids and “small” Gephyrocapsa <4.0 µm in size), Pseudoemiliania lacunosa, Calcidiscus macintyrei, Helicosphaera sellii, and, in some intervals, “medium” Gephyrocapsa ≥4.0 µm and/or “large” Gephyrocapsa >5.5 µm. Reworked Cretaceous species make up, on average, 30% of the assemblages (Supplementary Fig. 1). Reworked Cenozoic species are present throughout the section and constitute, on average, 35% of the total nannofossil assemblage. Some of the most representative and/or biostratigraphically important nannofossil taxa found in the studied samples are shown in Figure 7.

Figure 7. (color online) Calcareous nannofossils identified in the Stirone section under light microscope in smear slides. Scale bar is 5 µm. PL, plain light; XPL, cross-polarized light. (A) Gephyrocapsa “small,” XPL (95.25 m). (B) Gephyrocapsa “medium,” XPL (112.8 m). (C) Gephyrocapsa “large,” XPL (113.5 m). (D) Pontosphaera discopora, XPL (64.75 m). (E) Helicosphaera sellii, XPL (57.65 m). (F) Syracosphaera pulchra, XPL (57.65 m). (G) Discoaster sp., PL (91.85 m). (H) Discoaster sp., PL (58.1 m). (I) Prediscosphaera cretacea, XPL (64.75 m). (J) Nannoconus sp., XPL (64.75 m).
The nannofossil analysis allowed the identification of the CNPL7 to CNPL9 zones described as follows (Fig. 8):
Zone CNPL7 (from 0 to 98.25 m) was determined by the presence of “small” Gephyrocapsa <4.0 µm and the absence of Discoaster brouweri. In this interval, the assemblage is characterized by specimens of H. sellii, C. macintyrei, and P. lacunosa.
Zone CNPL8 (from 98.25 to 121.50 m) was defined by the First Occurrence (FO) of Gephyrocapsa ≥4.0 µm at the base. “Large” Gephyrocapsa >5.5 µm (dated 1.59 Ma) was found from 112.80 m up to 121.50 m where its last occurrence (LO) marks the top of zone CNPL8.
Zone CNPL9 (from 121.50 to 138 m) was characterized by specimens of Gephyrocapsa (<4.0 µm) occurring concomitantly with specimens of P. lacunosa and H. sellii. The presence of P. lacunosa and H. sellii and the absence of Reticulofenestra asanoi suggests that the top of the Stirone section extends into the lowermost part of zone CNPL9 (below the LO of H. sellii and the FO of R. asanoi, dated at 1.14 Ma).
In this work, we used the biozonation scheme by Backman et al. (Reference Backman, Raffi, Rio, Fornaciari and Pälike2012), which is a development of previous calcareous nannofossil biozonations of the Cenozoic (e.g., Martini, Reference Martini1971; Bukry, Reference Bukry, Edgar, Saunders, Bolli, Boyce, Broecker, Donnelly and Gieskes1973, Reference Bukry, Larson, Moberly, Bukry, Foreman, Gardner, Keene, Lancelot, Luterbacher, Marshall and Matter1975, Reference Bukry1978; Okada and Bukry, Reference Okada and Bukry1980). Based on the identified nannofossil zones, the age of the Stirone section is early Pleistocene (Gelasian-Calabrian).

Figure 8. (color online) Biostratigraphic correlation between the Arda and Stirone River sections and main biovents. For both sections, the foraminiferal and calcareous nannofossil biozones, the magnetostratigraphic data available from the literature (Gunderson et al., Reference Gunderson, Kodama, Anastasio and Pazzaglia2012; Monesi et al., Reference Monesi, Muttoni, Scardia, Felletti, Bona, Sala, Tremolada, Francou and Raineri2016), and the stratigraphic logs are shown. The boundaries between calcareous nannofossil biozones are taken as tie points between the two sections. Main bioevents are the first occurrences of Arctica islandica, Globigerina cariacoensis, Uvigerina mediterranea, Uvigerina bradyana, Neogloboquadrina pachyderma, Hyalinea balthica, Bulimina elegans marginata, Bulimina etnea, and “small”, “medium” and “large” Gephyrocapsa.
Foraminifera
Benthic foraminifera are abundant, and preservation is moderate to good. However, assemblages show severe resedimentation being composed of mixed populations containing shoreface–upper offshore transition (i.e., infralittoral; Rotalidae, Elphididae, Astigerinidae, Nonionidae, and Miliolidae) and lower offshore transition-offshore taxa (i.e., circalittoral; Buliminidae, Bolivinidae, and Cassidulinidae) and more lagoon-brackish to nearshore species (i.e., Ammonia beccarii, Elphidium crispum, and Elphidium aculeatum). Planktonic foraminifera show their maximum abundance (35% of the total foraminifera) in the middle part of the section (from STF47, 39 m, to STF13, 75.75 m) with intercalated shoreface–upper offshore transition benthic taxa, whereas the freshwater influence increases upward as testified by the dominance of Ammonia spp. and Elphidium spp. (from STF33, 92.40 m, to STF54, 130.50 m). Selected species are illustrated in Figure 9.
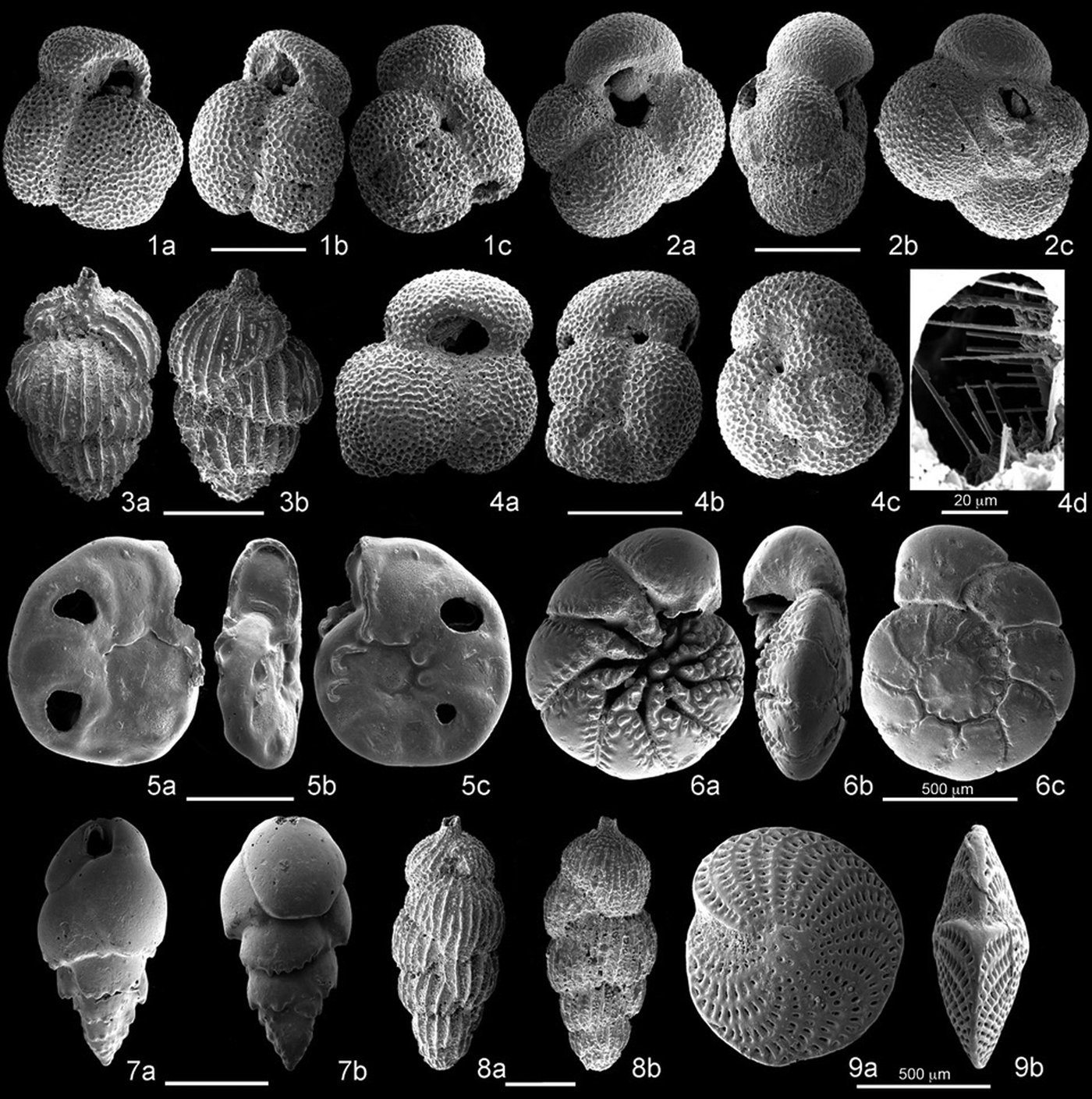
Figure 9. (1a–c) Globigerinoides obliquus extremus, sample STF9. (2a–c) Globigerina cariacoensis, sample STF9. (3a–b) Uvigerina mediterranea, sample STF13. (4a–d) Globigerinoides elongatus, sample STF13. (5a–c) Hyalinea cf. H. balthica, sample STF52. (6a–c) Ammonia beccarii, sample STF7. (7a–b) Bulimina elegans marginata, sample STF9. (8a–b) Uvigerina bradyana, sample STF9. (9a–b) Elphidium crispum, sample STF7. Scale bars 200 µm except where otherwise stated.
In general, planktonic foraminifera are rare to frequent, small in size, and moderate to poorly preserved. The assemblages are composed of the common Pleistocene taxa Globigerina bulloides, Globigerinoides elongatus, Globigerinoides obliquus extremus, Globigerinoides ruber, Globigerina falconensis, and Orbulina universa. The presence of Miocene and Pliocene marker species such as Globigerinoides parawoodi, Globigerina venezuelana, Globorotalia sphericomiozea, Globorotalia puncticulata, and Neogloboquadrina atlantica indicates reworking from older stratigraphic levels.
However, the presence of rare specimens of Globigerina cariacoensis and Neogloboquadrina pachyderma left-coiled, and the benthic foraminifera Uvigerina mediterranea, Uvigerina bradyana, and Bulimina elegans marginata from the base of the section (STF43, 18 m), allow assignment of the Stirone section to the early Pleistocene (Calabrian Stage) Globigerina cariacoensis zone (Cita et al., Reference Cita, Gibbard and Head2012 and references therein) (Fig. 8). The highest occurrence of Globigerinoides obliquus extremus is placed at 113.50 m (STF38), whereas only one specimen resembling the benthic foraminifera Hyalinea balthica is recorded at 121.50 m (STF52). The top of the section cannot be constrained using foraminifera because of the rarity of both benthic and planktonic foraminifera and the absence of marker bioevents (e.g., temporary disappearance of N. pachyderma left-coiled and lowest occurrence of Truncorotalia truncatulinoides excelsa). Nevertheless, our findings are in agreement with previous studies from the Arda section (Crippa et al., Reference Crippa, Angiolini, Bottini, Erba, Felletti, Frigerio and Hennissen2016) and other stratigraphic sections in the Mediterranean area (e.g., Colalongo and Sartoni Reference Colalongo and Sartoni1979; Ragaini et al., Reference Ragaini, Cantalamessa, Di Celma, Didaskalou, Impiccini, Lori, Marino, Potetti and Ragazzini2006; Cosentino et al., Reference Cosentino, Cipollari, Di Bella, Esposito, Faranda, Giordano and Gliozzi2009; Di Bella, Reference Di Bella2010, Maiorano et al., Reference Maiorano, Capotondi, Ciaranfi, Girone, Lirer, Marino, Pelosi, Petrosino and Piscitelli2010; Baldanza et al., Reference Baldanza, Bizzarri and Hepach2011, among many others). Therefore, based on foraminiferal biostratigraphy and geochronology data from the Vrica section (Calabrian GSSP; Cita et al., Reference Cita, Gibbard and Head2012 and references therein), the age of the studied stratigraphic interval is likely between 1.78 Ma and about 1.1 Ma (see Cita et al., Reference Cita, Gibbard and Head2012; Gradstein et al., Reference Gradstein, Ogg, Schmitz and Ogg2012).
DISCUSSION
Correlation of the Arda and Stirone River sections
Lithostratigraphic correlation
A large-scale lithostratigraphic correlation between the Arda and Stirone River sections has been previously attempted by Channell et al. (Reference Channell, Poli, Rio, Sprovieri and Villa1994) and Monegatti et al. (Reference Monegatti, Raffi, Roveri and Taviani2001), with a specific focus on the Pliocene part of the successions. In this regard, Channell et al. (Reference Channell, Poli, Rio, Sprovieri and Villa1994) observed that during the lower Zanclean, both sections were prone to mud deposition in a slope setting (i.e., “Argille azzurre”), whereas a change in the sedimentary regime is recorded from the upper Zanclean through the Gelasian with the establishment of an outer shelf environment in the Castell'Arquato area. Such differentiation in the study area into a slope environment (Stirone) and an adjacent shelf (Arda) during the late Pliocene is further substantiated by Monegatti et al. (Reference Monegatti, Raffi, Roveri and Taviani2001).
The large-scale lithostratigraphic correlation framework resulting from the present study suggests this configuration lasted through the Calabrian (1.78–1.14 Ma) even if less pronounced. Indeed, the Arda River section records biosedimentary dynamics developed closer to the basin margin with respect to the Stirone River section and to previous time intervals. However, both sections developed within a strongly fluvio-influenced marginal marine setting, recording improvised and strong changes in the depositional style.
All lithofacies/lithozones identified along the Arda (see Crippa et al., Reference Crippa, Baucon, Felletti, Raineri and Scarponi2018) and Stirone Rivers (except for lithozone 4) represent the sedimentary product of—and are strongly influenced by—a high-gradient fluvial network, originated when the rivers discharged a sustained and relatively dense turbulent mixture of freshwater and sediments (i.e., hyperpycnal flows; Bates, Reference Bates1953) into the receiving standing body of seawater; this is in agreement with the findings of Dominici (Reference Dominici2001, Reference Dominici2004). The influence of the fluvial systems extended for kilometers away from the rivers’ mouths. The fossil assemblages are mainly represented by sets of oligotypic to low-diversity macrobenthic remains, commonly represented by life assemblages (e.g., lithozone 1, lithozone 7), or lenticular-shaped, hydraulically sorted, or chaotically mixed fossils (e.g., lower most part of lithozone 3, lithozone 5).
The difference in sediment thickness observed between the two sections (CNPL8 biozone: 120 m in the Arda section, 23 m in the Stirone section) may be explained considering a lateral position, and for its lower part, a slightly deeper-water setting of the Stirone section with respect to the river mouth, with a consequent lower sedimentation rate. Indeed, the Arda River succession is characterized by an overall aggradational (cycles 0 to 2) to progradational stacking pattern (cycles 3 to top section), developed within offshore transition to alluvial depositional settings (Crippa et al., Reference Crippa, Baucon, Felletti, Raineri and Scarponi2018). Along the Stirone River section, lithozone 4, with its pectinid rich, bioclastic deposits (i.e., biocalcarenites), represents a prolonged phase of low clastic input or winnowing by bottom currents indicative of offshore transition settings; it constitutes the turning point from a slightly deepening upward (lithozones 1 to 4) to an overall progradational stacking pattern that led to emplacement of a thick marginal marine to coastal plain succession along the Stirone River section (lithozones 5 to 9). The onset of the progradational stacking pattern (i.e., Stirone lithozone 5 and Arda unit 2 in Crippa et al. [Reference Crippa, Baucon, Felletti, Raineri and Scarponi2018]) in both sections is highlighted by the first occurrence of Arctica islandica, an indicator of climate deterioration in the Mediterranean region during the Calabrian (Raffi, Reference Raffi1986).
The overall regressive trend of the Arda and Stirone River sections can be directly related to deformation phases of the local fronts of the Apennines. As underlined by Dominici (Reference Dominici2001, Reference Dominici2004), the sedimentary dynamics along both sections are controlled by recurring tectonic pulses on which, at smaller scale, climatic fluctuations are superimposed. Comparable sedimentary dynamics were already documented for a few other sedimentary successions outcropping along the northeastern margin of the Apennines, such as the Enza River section (e.g., Gunderson et al., Reference Gunderson, Pazzaglia, Picotti, Anastasio, Kodama, Rittenour and Frankel2014). However, correlation at the scale of genetically related packages of strata (high-frequency cycles) between the two sections is very complex within such highly dynamic depositional settings represented by the sedimentary staking of meter-thick layers of hyperpycnal flows associated with widespread erosional features.
Biostratigraphic correlation
The integrated biostratigraphic analysis of calcareous nannofossils and foraminifera in the Arda (Crippa et al., Reference Crippa, Angiolini, Bottini, Erba, Felletti, Frigerio and Hennissen2016) and Stirone sections allows us to identify three nannofossil zones (CNPL7, CNPL8, and CNPL9) and one foraminiferal zone (Globigerina cariacoensis), suggesting that the age of the studied stratigraphic intervals is Calabrian (early Pleistocene) (Fig. 8). Calcareous nannofossils suggest that the base of these sections is between 1.93 Ma (LO of D. brouweri) and 1.71 Ma (first common occurrence of “small” Gephyrocapsa). Foraminifera analysis further constrains the age of the base of the sections; the presence of rare specimens of Globigerina cariacoensis and Neogloboquadrina pachyderma left-coiled and of the benthic foraminifera Uvigerina mediterranea and Uvigerina bradyana indicates that both the Arda and Stirone River successions deposited during the early Pleistocene (Calabrian Stage) Globigerina cariacoensis zone (base at 1.78 Ma). The overlap of these biozones suggests that the base of the sections should be dated between 1.78 and 1.71 Ma. The top of the sections is here interpreted to be limited to the lowermost part of the nannofossil zone CNPL9; thus it is not younger than 1.14 Ma (FO of R. asanoi).
Other important bioevents are recorded in the two sections. A. islandica first occurs in both sections in the upper part of the CNPL7 biozone (a few meters below the top of this biozone), after the first occurrence of N. pachyderma left-coiled. H. balthica occurs before the first occurrence of A. islandica in the Arda section, but after in the Stirone one, where only one specimen resembling this benthic foraminifer has been found. Generally, in lower Pleistocene successions, the first occurrence of H. balthica is recorded after that of A. islandica (e.g., Rio et al., Reference Rio, Raffi, Backman and Van Couvering1997), but in the Arda section, we observe the opposite. To explain this, we need to consider the discontinuity of the benthic fossil record through the section, which is not complete and may be controlled by the Signor-Lipps and Jaanusson effects (Jaanusson, Reference Jaanusson and Bassett1976; Signor and Lipps, Reference Signor and Lipps1982). Indeed, here we refer to the first occurrence of both species and not to their first appearance. The occurrence of H. balthica before A. islandica in the CNPL7 biozone in the Arda section—and not in the upper part of the CNPL8 biozone (~Emilian substage) as generally accepted—suggests that the Calabrian substages cannot be used in their original significance based on the successive occurrence of A. islandica, H. balthica, and T. truncatulinoides excelsa.
The calcareous nannofossil and foraminiferal biostratigraphic data are not perfectly tuned with magnetostratigraphic data available from the literature (Gunderson et al., Reference Gunderson, Kodama, Anastasio and Pazzaglia2012; Monesi et al., Reference Monesi, Muttoni, Scardia, Felletti, Bona, Sala, Tremolada, Francou and Raineri2016). Both Gunderson et al. (Reference Gunderson, Kodama, Anastasio and Pazzaglia2012) and Monesi et al. (Reference Monesi, Muttoni, Scardia, Felletti, Bona, Sala, Tremolada, Francou and Raineri2016) indicated a Calabrian age for the Arda and Stirone sections, and this is in general agreement with our biostratigraphic data. However, looking at this in more detail, we observe that in both sections the lower boundary of the Olduvai subchron (1.94–1.77 Ma) does not fit with biostratigraphic data (Fig. 8). In fact, the lower boundary of this magnetic subchron (1.94 Ma) is recorded several meters stratigraphically above the bases of both sections, which, based on calcareous nannofossil and foraminiferal biostratigraphy, should be younger than 1.78 Ma. The top of the Olduvai (1.77 Ma) occurs stratigraphically below the top of the CNPL7 (1.71 Ma) in the Stirone section and thus fits quite well with both the age of the top of the CNPL7 biozone and the other bioevents (e.g., Arctica islandica first occurrence). However, this is not the case for the Arda section, where the top of the Olduvai subchron coincides with the top of the CNPL7 biozone. Magnetostratigraphic analysis thus assigned an older age to the base of the sections compared with biostratigraphy (i.e., 1.94 vs. 1.78 Ma). At the top of the Stirone succession, the position of the Jaramillo subchron (base at 1.07 Ma, top at 0.99 Ma) is in agreement with our biostratigraphic data, as it occurs stratigraphically above the base of the CNPL9 biozone (dated at 1.25 Ma); on the other hand, in the Arda section this magnetostratigraphic interval corresponds to the boundary between CNPL8 and CNPL9 nannofossil biozones (1.25 Ma; Fig. 8), thus suggesting a younger age for the upper part of the section.
One possible explanation of these discrepancies can be found in the different measurement of the thickness of the sections reported by different authors; this could be the case for the Stirone River succession (Gunderson et al., Reference Gunderson, Kodama, Anastasio and Pazzaglia2012 and this study), where different authors produced different stratigraphic logs, but not for the Arda River section, where the log used by Crippa et al. (Reference Crippa, Angiolini, Bottini, Erba, Felletti, Frigerio and Hennissen2016) and Monesi et al. (Reference Monesi, Muttoni, Scardia, Felletti, Bona, Sala, Tremolada, Francou and Raineri2016) was the same. In the first case, it is difficult to precisely locate the base of the Olduvai of Gunderson et al. (Reference Gunderson, Kodama, Anastasio and Pazzaglia2012) in the Stirone section, mainly because of the different recorded thicknesses and the lack of a detailed figured stratigraphic log in Gunderson et al. (Reference Gunderson, Kodama, Anastasio and Pazzaglia2012, their fig. 2b), which may have been helpful to correlate the two logs using lithologic markers. In the case of the Arda section, this discrepancy may be explained by making reference to the short reversed polarity interval found in the Vrica section at the top of the Olduvai subchron (the ß interval in Zijderveld et al., Reference Zijderveld, Hilgen, Langereis, Verhallen and Zachariasse1991; Roberts et al., Reference Roberts, Florindo, Larrasoaña, O'Regan and Zhao2010; Cita et al., Reference Cita, Gibbard and Head2012), which may be tentatively correlated with the reversed interval occurring between 50 and 90 m in the Arda section (Monesi et al., Reference Monesi, Muttoni, Scardia, Felletti, Bona, Sala, Tremolada, Francou and Raineri2016). Another possibility to consider would be microfossil reworking; this can be taken into account to explain the discrepancies at the top of the Arda section, but not the ones concerning the base of the Olduvai subchron. Reworked samples would result in a younger age for the base of the section, enhancing even more the lack of correspondence between magneto- and biostratigraphic data; however, reworking as the only cause should be excluded, as otherwise we would not have recorded such a complete sequence of bioevents and biozones, which strengthens our biostratigraphic data.
Although some of the discrepancies between bio- and magnetostratigraphic data can be considered irrelevant for the age determination of these sections (i.e., those regarding the part of the sections containing the Jaramillo subchron and the top of the Olduvai subchron), the ones concerning the base of the sections are difficult to reconcile. They suggest an age for the base of the sections that differs by about 200 ka (>1.94 Ma according to magnetostratigraphy vs. ≤1.78 Ma according to biostratigraphy). Unless considering the possibility of correlating the base of the sections with the abovementioned short reversed polarity interval (ß interval) at the top of the Olduvai subchron at Vrica, this issue remains unsolved.
Arctica islandica in the early Pleistocene of the Mediterranean Sea
The ocean quahog Arctica islandica is a shallow infaunal bivalve that today is found on both sides of the North Atlantic in temperate and boreal waters (e.g., Witbaard, Reference Witbaard1997; Dahlgren et al., Reference Dahlgren, Weinberg and Halanych2000). However, fossil evidence indicates that its paleobiogeographic range extended southward during phases of climate deterioration—that is, cooling starting in the Calabrian (ca. 1.8 Ma; Raffi, Reference Raffi1986; Table 1)—colonizing the Bay of Biscay and the Mediterranean Sea. In a revision of the available information, complemented with data from this study, on the first occurrence of A. islandica in the early Pleistocene of the Mediterranean Sea (Table 1, Fig. 10), two important outcomes are immediately observable and worthy of note: (1) nearly all lower Pleistocene marine on-land successions recording the widespread occurrence of A. islandica are located in Italy, with the exception of Rhodes, Greece (Zaccaria, Reference Zaccaria1968), and Mallorca, Spain (Pomar Gomà and Cuerda Barceló, Reference Pomar Gomá and Cuerda Barceló1979); and (2) the oldest outcrops are located in northern Italy.
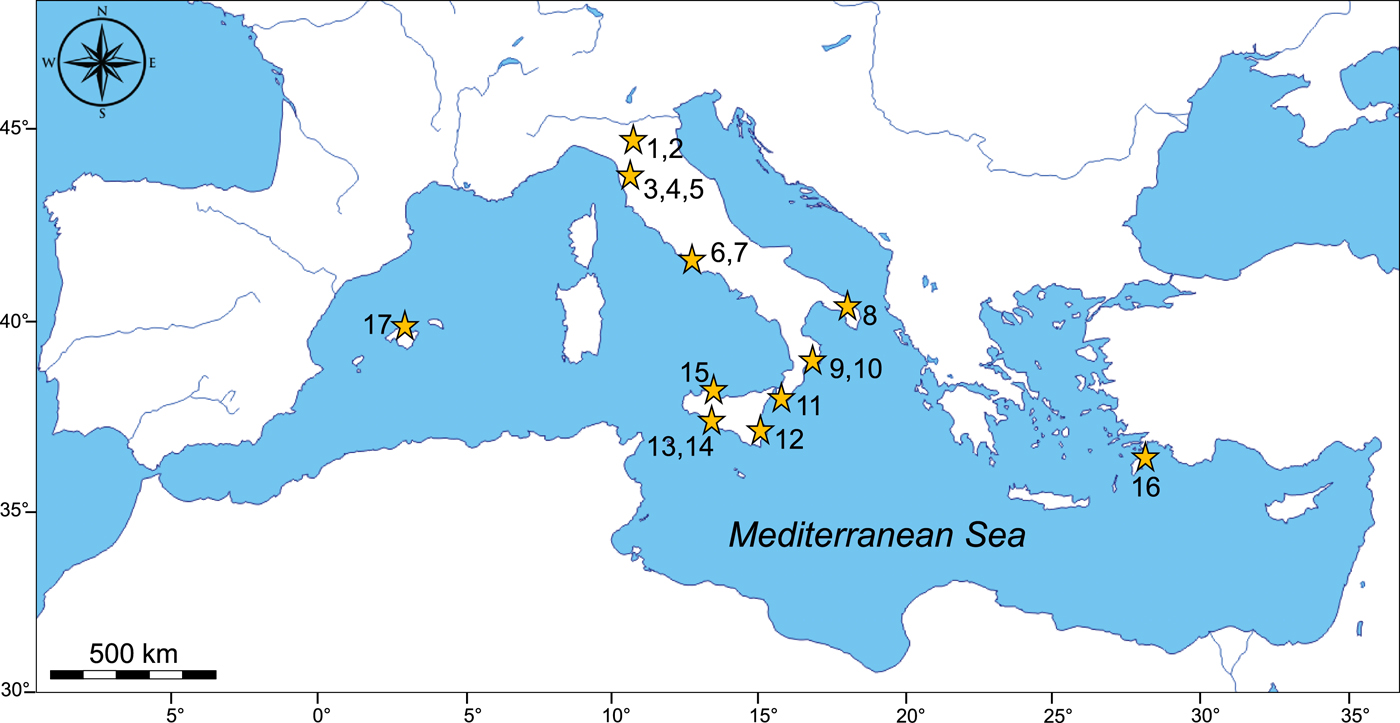
Figure 10. (color online) Map showing the lower Pleistocene outcrops with Arctica islandica in the Mediterranean Sea: 1, Santerno Valley, Bologna; 2, Arda and Stirone Rivers, Piacenza and Parma; 3, Collesalvetti, Pisa; 4, Val Cecina, Livorno; 5, Pisa hills, Valdarno, Pisa; 6, Monte Mario, Rome; 7, Tacconi quarry, Rome; 8, Cutrofiano quarry, Lecce; 9, Santa Maria di Catanzaro, Catanzaro; 10, Valle di Manche, Crotone; 11, Monasterace, Reggio Calabria; 12, Ogliastro quarry, Siracusa; 13, Capo Rossello, Agrigento; 14, Belice Valley, Agrigento; 15, Puleo Quarry, Palermo; 16, Rhodes, Greece; 17, Mallorca, Spain. See Table 1 for corresponding references for each locality.
These two considerations can be explained taking into account the present ecological range and the biology of the species and assuming that it has not changed since the Pleistocene (see Table 2). Unlike other areas in the Mediterranean Sea, at the beginning of the Calabrian the shallow marine settings of the northern paleo-Adriatic (northern Italy) satisfied the ecological requirements (type of substratum, salinity and oxygenation levels, temperature and water depth; Table 2) for the establishment of widespread populations of A. islandica. During the Santernian (lower Calabrian), northern Italy was a wide gulf wedging out westward, mainly characterized by terrigenous shallow-water settings, with fully marine and well-oxygenated conditions, apart from occasional events of salinity dilution (Kukla et al., Reference Kukla, Collins and Bender1979; Bedini et al., Reference Bedini, Bertolini, Braschi, Cotrozzi, Gani and Niccoli1981; Sarti et al., Reference Sarti, Ciampalini, Consoloni and Cerrina Feroni2007, Reference Sarti, Testa and Zanchetta2008; Crippa et al., Reference Crippa, Angiolini, Bottini, Erba, Felletti, Frigerio and Hennissen2016, Reference Crippa, Baucon, Felletti, Raineri and Scarponi2018; this study). These settings were characterized by strong seawater seasonality and low winter paleotemperatures conditions (Crippa et al., Reference Crippa, Angiolini, Bottini, Erba, Felletti, Frigerio and Hennissen2016), satisfying those required for the successful establishment and settlement of the first populations of A. islandica (Raffi, Reference Raffi1986).
Table 2. Summary of the main ecological and biological parameters for the ocean quahog Arctica islandica. Asterisk (*) indicates rare specimens have been reported up to 256 m, but the common occurrence is in less than 100 m. Nicol (Reference Nicol1951) reported a water depth up to 500 m from commercial catches; however, according to Merrill and Ropes (Reference Merrill and Ropes1969), the author did not state whether the catch contained live organisms or only shells.

According to Kukla et al. (Reference Kukla, Collins and Bender1979), A. islandica first occurred about 2.00 Ma ago in the Santerno Valley (northern Italy), based on magnetostratigraphic evidence and radiometric age (helium/uranium method) obtained on corals. The same or a slightly older age (≥2 Ma, based on magnetostratigraphy; Table 1) was suggested also by other authors (Arias et al., Reference Arias, Azzaroli, Bigazzi and Bonadonna1980; Bedini et al., Reference Bedini, Bertolini, Braschi, Cotrozzi, Gani and Niccoli1981). However, Azzaroli et al. (Reference Azzaroli, Colalongo, Nakagawa, Pasini, Rio, Ruggieri, Sartoni, Sprovieri and Van Couvering1997) observed that the magnetostratigraphic interpretation of Kukla et al. (Reference Kukla, Collins and Bender1979) was not in agreement with biostratigraphic data (see Azzaroli et al., Reference Azzaroli, Colalongo, Nakagawa, Pasini, Rio, Ruggieri, Sartoni, Sprovieri and Van Couvering1997 for a detailed discussion). Also, Arias et al. (Reference Arias, Azzaroli, Bigazzi and Bonadonna1980) did not record the occurrence of shells of A. islandica in their sections, but rather they estimated the age of its first occurrence through a comparison with other Italian successions, mainly based on magnetostratigraphic correlations without biostratigraphic data to support it. Crippa and Raineri (Reference Crippa and Raineri2015), Crippa et al. (Reference Crippa, Angiolini, Bottini, Erba, Felletti, Frigerio and Hennissen2016, Reference Crippa, Baucon, Felletti, Raineri and Scarponi2018), and this study recorded the first occurrence of rare specimens of A. islandica in the Arda and Stirone sections a few meters below the top of the CNPL7 biozone (dated at 1.71 Ma), but the species becomes more abundant in the CNPL8 calcareous nannofossil biozone (1.71–1.25 Ma; Backman et al., Reference Backman, Raffi, Rio, Fornaciari and Pälike2012). The same distribution pattern was also noted by other authors (e.g., Pelosio and Raffi, Reference Pelosio and Raffi1974; Kukla et al., Reference Kukla, Collins and Bender1979).
A. islandica is not present in outcrops of Santernian age of southern Italy, where this time interval is mainly represented by deep-water (bathyal) muddy/marly successions (e.g., Ruggieri and Sprovieri, Reference Ruggieri and Sprovieri1975; Di Geronimo and La Perna, Reference Di Geronimo and La Perna1997 and references therein); as an example, the Vrica section, GSSP for the Calabrian Stage, recorded during the Santernian the deposition of epibathyal, silty marl claystone (Cita et al., Reference Cita, Capraro, Ciaranfi, Di Stefano, Lirer, Maiorano, Marino, Raffi, Rio, Sprovieri, Stefanelli and Vai2008). In these areas, A. islandica populations are found only in the Emilian-Sicilian (middle–upper Calabrian); from this time on, the widely preserved on-land marine successions of southern Italy deposited in shallow-water settings and showed the optimal conditions for the proliferation of the species (Tables 1 and 2). Indeed, Arctica islandica in nearly all southern Italy successions (Emilian or Sicilian in age) occurs in coarse to fine sands deposited at water depth ranging from 20 to 80 m (Table 1 and references therein); this is in agreement with the present day highest densities and common occurrence of the species (see Table 2).
Outside the Italian peninsula, there may be other areas satisfying the ecological requirements for the establishment of A. islandica, but, to our knowledge, lower Pleistocene outcrops recording the occurrence of this species are rarely cited (outcrops not studied/found yet or inaccessible). Pomar Gomá and Cuerda Barceló (Reference Pomar Gomá and Cuerda Barceló1979) observed the presence of this species in the Sicilian (upper Calabrian) of Mallorca (Spain), whereas Zaccaria (Reference Zaccaria1968) documented the occurrence of rare specimens in the Calabrian of Rhodes (Greece), but no information is given about which of the three Calabrian substages (sensu Ruggieri and Sprovieri, Reference Ruggieri and Sprovieri1975, Reference Ruggieri and Sprovieri1977) is represented. Also, submarine successions with Arctica islandica from France (Lyon Gulf; Gadel and Mongin, Reference Gadel and Mongin1973) and Spain (Cap Creus; Mars, Reference Mars1958, Reference Mars1963) are all referred to the Würmian (upper Pleistocene).
Thus, our findings support the hypothesis that the lowest recorded occurrence of A. islandica in the Mediterranean Basin is in the Arda and the Stirone River sections (i.e., a few meters below the top of the CNPL7 biozone); here, this species successfully settled with widespread populations, subsequently establishing in southern Italy and in other Mediterranean sites, then becoming extinct in the Mediterranean Sea during the early Holocene (ca. 9.8 ka; Froget et al., Reference Froget, Thommeret and Thommeret1972).
CONCLUSIONS
This study allows us to date and correlate the Arda and Stirone Rivers, two lower Pleistocene key sections in northern Italy, and to calibrate and map the first occurrence of the bivalve Arctica islandica in the Mediterranean Sea during the early Pleistocene. We conclude the following:
The Stirone River section is Calabrian in age (1.78–1.14 Ma) based on the identification of three nannofossil (CNPL7, CNPL8, and CNPL9) and one foraminiferal (Globigerina cariacoensis) biozones; the same age was recorded in the nearby Arda River section (Crippa et al., Reference Crippa, Angiolini, Bottini, Erba, Felletti, Frigerio and Hennissen2016). The biostratigraphic data do not fit perfectly with magnetostratigraphic data available in the literature.
The Stirone River succession corresponds to the subaqueous extension of a fluvial system affected by hyperpycnal flows; a lateral position with respect to the river mouth is suggested. A general regressive trend is observed through the section, which can be directly related to the tectonic activity and to early Pleistocene climatic change.
The occurrence in both the Arda and Stirone marine successions of the “northern guest” A. islandica indicates a climatic deterioration (i.e., cooling) from the Calabrian, which is an expression of the climatic change occurring during the early Pleistocene, leading to the onset and establishment of middle and upper Pleistocene continental glaciations.
The original data here presented complemented with previous findings seem to support that the stratigraphically lowest level where A. islandica first occurred in the Mediterranean was in the Arda and Stirone River successions (in both sections a few meters below the top of the CNPL7 calcareous nannofossil biozone). The paleoenvironmental conditions present in this region satisfy the ecological requirements for the establishment and the proliferation of the species (i.e., water depth up to 100 m, sandy substratum, normal water salinity, and high seawater seasonality); only subsequently did the species successfully become established also in southern Italy and in other areas of the Mediterranean Sea.
This and previous studies (e.g., Crippa et al., Reference Crippa, Angiolini, Bottini, Erba, Felletti, Frigerio and Hennissen2016) strengthen the importance of applying integrated biostratigraphic analyses to obtain robust and reliable data concerning the age of a section, especially in shallow-water settings, avoiding or compensating for biases specific to the use of only one tool. Also, it highlights the importance of using multidisciplinary approaches in past environmental and climatic reconstructions in different geologic contexts.
SUPPLEMENTARY MATERIAL
The supplementary material for this article can be found at https://doi.org/10.1017/qua.2019.20.
ACKNOWLEDGMENTS
We would like to thank A. Rizzi (University of Milan) for SEM analyses. “Parco dello Stirone e del Piacenziano” and “Museo Geologico G. Cortesi” (Castell'Arquato) are acknowledged for the permissions to enter the conservation areas. L. Angiolini is warmly thanked for constructive discussions and suggestions. Two anonymous reviewers are also thanked for their useful comments and suggestions, which improved the quality of this manuscript.














