INTRODUCTION
Time averaging, the age mixing of noncontemporaneous fossils, is an inherent quality of most geologic strata and important for understanding sedimentary and taphonomic processes (Kowalewski, Reference Kowalewski1996). When unknown, time averaging may complicate the interpretation of geologic horizons, because accumulations of fossils from different time intervals can be mixed in a single stratum, giving the erroneous appearance of contemporaneity (Kidwell and Bosence, Reference Kidwell, Bosence, Allison and Briggs1991). Age mixing can also generate false patterns of fossil distribution through time. For example, an assemblage that spans a broad period of time can give the mistaken appearance of comparative contemporaneity between taxa. Quantifying time averaging is, therefore, an essential step before interpreting sedimentological, paleoecological, and/or paleoclimatic patterns from geologic deposits (Kowalewski, Reference Kowalewski1996; Kowalewski et al., Reference Kowalewski, Goodfriend and Flessa1998; Bush et al., Reference Bush, Powell, Arnold, Bert and Daley2002).
The degree of age mixing is primarily dependent on factors like fossil production (genesis) rates, destructive (decay) rates, and sedimentation (burial) rates (e.g., Tomašových et al., Reference Tomašových, Kidwell, Barber and Kaufman2014). As a result, the time resolution across depositional settings and fossil assemblages can be substantially variable, ranging from short (years to decades) to long (millennial to multimillenial) time scales (e.g., Tomašových et al., Reference Tomašových, Kidwell, Barber and Kaufman2014; Albano et al., Reference Albano, Filippova, Steger, Kaufman, Tomašových, Stachowitsch and Zuschin2016, Reference Albano, Gallmetzer, Haselmair, Tomašových, Stachowitsch and Zuschin2018). More importantly, not all taxonomic groups preserved jointly within a deposit will necessarily exhibit equivalent degrees of age mixing. For instance, specimens with durable hard parts (e.g., mollusks) may exhibit significant (multimillennial) time averaging, while less durable skeletons (e.g., echinoderms) are usually accumulated across more limited time spans (years to decades) due to higher taphonomic decay rates (Kowalewski et al., Reference Kowalewski, Casebolt, Hua, Whitacre, Kaufman and Kosnik2018). Similarly, the remains of short-lived organisms (such as those with a subdecadal life span) are more likely to be significantly time averaged than long-lived organisms due to the shorter life span (higher genesis rates). When fossils of short-lived organisms are examined, the likelihood of significant age mixing within a deposit increases (Kidwell and Bosence, Reference Kidwell, Bosence, Allison and Briggs1991; Goodwin et al., Reference Goodwin, Flessa, Téllez-Duarte, Dettman, Schöne and Avila-Serrano2004). Consequently, understanding the extent of time averaging can notably improve paleoecological and paleoenvironmental reconstructions from fossil assemblages.
Time averaging can be defined by scale, that is, the range of years across which age mixing has occurred; and by structure, that is, the distribution of specimen frequency through the observed age range of a fossil assemblage (Meldahl et al., Reference Meldahl, Flessa and Cutler1997; Kowalewski et al., Reference Kowalewski, Goodfriend and Flessa1998). The standard method to evaluate the scale and structure of time averaging is by directly dating individual fossils within a geologic stratum and then using the ages of fossils to infer the extent and shape of age mixing (e.g., Flessa et al., Reference Flessa, Cutler and Meldahl1993; Kowalewski and Bambach, Reference Kowalewski, Bambach and Harries2008). Much of our knowledge of scale and structure of time averaging of fossils is based on Holocene (last 11.7 ka) remains of marine bivalves (e.g., Kidwell, Reference Kidwell1998; Kidwell et al., Reference Kidwell, Best and Kaufman2005; Dominguez et al., Reference Dominguez, Kosnik, Allen, Hua, Jacob, Kaufman and Whitacre2016; Vidović et al., Reference Vidović, Nawrot, Gallmetzer, Haselmair, Tomašových, Stachowitsch, Ćosović and Zuschin2016; Schnedl et al., Reference Schnedl, Haselmair, Gallmetzer, Mautner, Tomašových and Zuschin2018), brachiopods (Carroll et al., Reference Carroll, Kowalewski, Simoes and Goodfriend2003; Krause et al., Reference Krause, Barbour, Kowalewski, Kaufman, Romanek, Simoes and Wehmiller2010), and more recently echinoderms (Kosnik et al., Reference Kosnik, Hua, Kaufman, Kowalewski and Whitacre2017; Kowalewski et al., Reference Kowalewski, Casebolt, Hua, Whitacre, Kaufman and Kosnik2018). Estimates of time averaging in continental fossil assemblages have received less focus. Some published studies of time averaging in terrestrial fossils from both the Holocene and the mid–late Pleistocene include small mammal assemblages (Terry and Novak, Reference Terry and Novak2015) and land snails (Yanes et al., Reference Yanes, Kowalewski, Ortiz, Castillo, de Torres and de la Nuez2007).
Land snail shells are abundant, easily identifiable, sensitive to climate change, and well preserved in many Quaternary continental settings. Accordingly, fossil shells have been increasingly used as paleoecological and paleoclimatic proxies (e.g., Goodfriend et al., Reference Goodfriend, Cameron, Cook, Courty, Fedoroff, Livett and Tallis1996; Yanes et al., Reference Yanes, Al-Qattan, Rech, Pigati, Dodd and Nekola2019). However, to prevent false or biased biological and paleoclimatic inferences from land snail assemblages, it is important to establish a robust chronological context and assess the degree of age mixing (Yanes et al., Reference Yanes, Kowalewski, Ortiz, Castillo, de Torres and de la Nuez2007).
A well-established and widely used method for dating Quaternary shells is amino acid racemization (AAR) calibrated with radiocarbon dating (e.g., Miller and Brigham-Grette, Reference Miller and Brigham-Grette1989; Goodfriend, Reference Goodfriend1992; Kowalewski et al., Reference Kowalewski, Goodfriend and Flessa1998; Kidwell et al., Reference Kidwell, Best and Kaufman2005; Yanes et al., Reference Yanes, Kowalewski, Ortiz, Castillo, de Torres and de la Nuez2007; Kosnik et al., Reference Kosnik, Kaufman and Hua2008). Amino acid dating is based on the principle that all amino acids (except glycine) have two mirrored orientations of chirality (d and l). While alive, virtually all living organisms, including snails, produce amino acids in the l orientation. Upon death, the d/l ratio increases from 0 toward equilibrium at 1.0 during the process of racemization, or epimerization for isoleucine/allo-isoleucine (e.g., Hare et al., Reference Hare, Hoering and King1980; Wehmiller and Miller, Reference Wehmiller and Miller2000). Different amino acids racemize/epimerize at different rates (e.g., Goodfriend, Reference Goodfriend1991; Goodfriend and Meyer, Reference Goodfriend and Meyer1991). Because the rate of racemization/epimerization is dependent on both taxon and temperature, it is necessary to calibrate the method for the locality and taxon to be investigated by using an independent dating method, such as radiocarbon dating, which uses radiocarbon present in samples to determine a numerical age that can be then calibrated to calendar years. The relatively new carbonate-target accelerator mass spectrometry (AMS) radiocarbon dating method described by Bush et al. (Reference Bush, Santos, Xu, Southon, Thiagarajan, Hines and Adkins2013) is rapid and cost-effective and has been increasingly used for dating Holocene marine carbonate-bearing fossils and calibration of AAR ages (e.g., Dominguez et al., Reference Dominguez, Kosnik, Allen, Hua, Jacob, Kaufman and Whitacre2016; Kosnik et al., Reference Kosnik, Hua, Kaufman, Kowalewski and Whitacre2017; Kowalewski et al., Reference Kowalewski, Casebolt, Hua, Whitacre, Kaufman and Kosnik2018).
For this study, we investigated previously undescribed snail-rich colluvial deposits from the Desertas Islands (Madeira, Portugal). This locality offers a wealth of paleobiological and paleoenvironmental information to assess regional climate and biotic evolution throughout its well-documented geological history (e.g., Cook et al., Reference Cook, Cameron and Lace1990) and diverse land snails throughout the fossil record spanning the past 150 ka (e.g., Cameron and Cook, Reference Cameron and Cook1992; Cook, Reference Cook2008). The native land snail fauna of the archipelago, studied intensively since the early nineteenth century, is species rich (more than 250 species described) and mainly endemic (Waldén, Reference Waldén1983; Cook et al., Reference Cook, Cameron and Lace1990; Cameron and Cook, Reference Cameron and Cook1999; Abreu and Teixeira, Reference Abreu, Teixeira, Borges, Abreu, Aguiar, Carvalho, Jardim, Melo, Oliveira, Sérgio, Serrano and Vieira2008). As a result, these land snails have been the subject of multiple taxonomic, ecological, and morphological studies. However, the majority of land snail studies examine the Madeira Archipelago as a whole (e.g., Waldén, Reference Waldén1983; Cameron and Cook, Reference Cameron and Cook1989; Cook, Reference Cook1996, Reference Cook2008) or the human-populated islands of Madeira (Cook et al., Reference Cook, Cameron and Lace1990; Prada and Serralheiro, Reference Prada and Serralheiro2000) and Porto Santo (Cameron et al., Reference Cameron, Cook, Goodfriend and Seddon2006). Land snail studies focusing on the Desertas Islands are rare, and the records of fossil land snail shells have been largely overlooked (e.g., Wollaston, Reference Wollaston1878; Cameron and Cook, Reference Cameron and Cook1999).
This study focuses on determining the age of fossil shells and quantifying the scale and structure of age mixing of snail assemblages using AAR calibrated with both graphite-target and carbonate-target AMS radiocarbon methods. We test the hypothesis that snail assemblages preserved in Quaternary colluvial deposits exhibit multimillennial-scale age mixing (beyond the radiocarbon dating and AAR calibration errors) and that the structure will be variable (e.g., right-skewed, left-skewed), as observed in late Pleistocene paleosols and dunes rich in land snails from the Canary Islands (Yanes et al., Reference Yanes, Kowalewski, Ortiz, Castillo, de Torres and de la Nuez2007). This research also tests the viability of the carbonate-target AMS radiocarbon dating for land snail shells and establishes the first chronology for these assemblages using multiple geochronological methods (i.e., AAR, graphite-target and carbonate-target AMS radiocarbon). Our results provide new age data from Quaternary terrestrial invertebrate assemblages that will complement and expand upon the intensive work conducted in Holocene marine assemblages over the last few decades.
STUDY AREA, MATERIALS, AND METHODS
Geographical and environmental setting
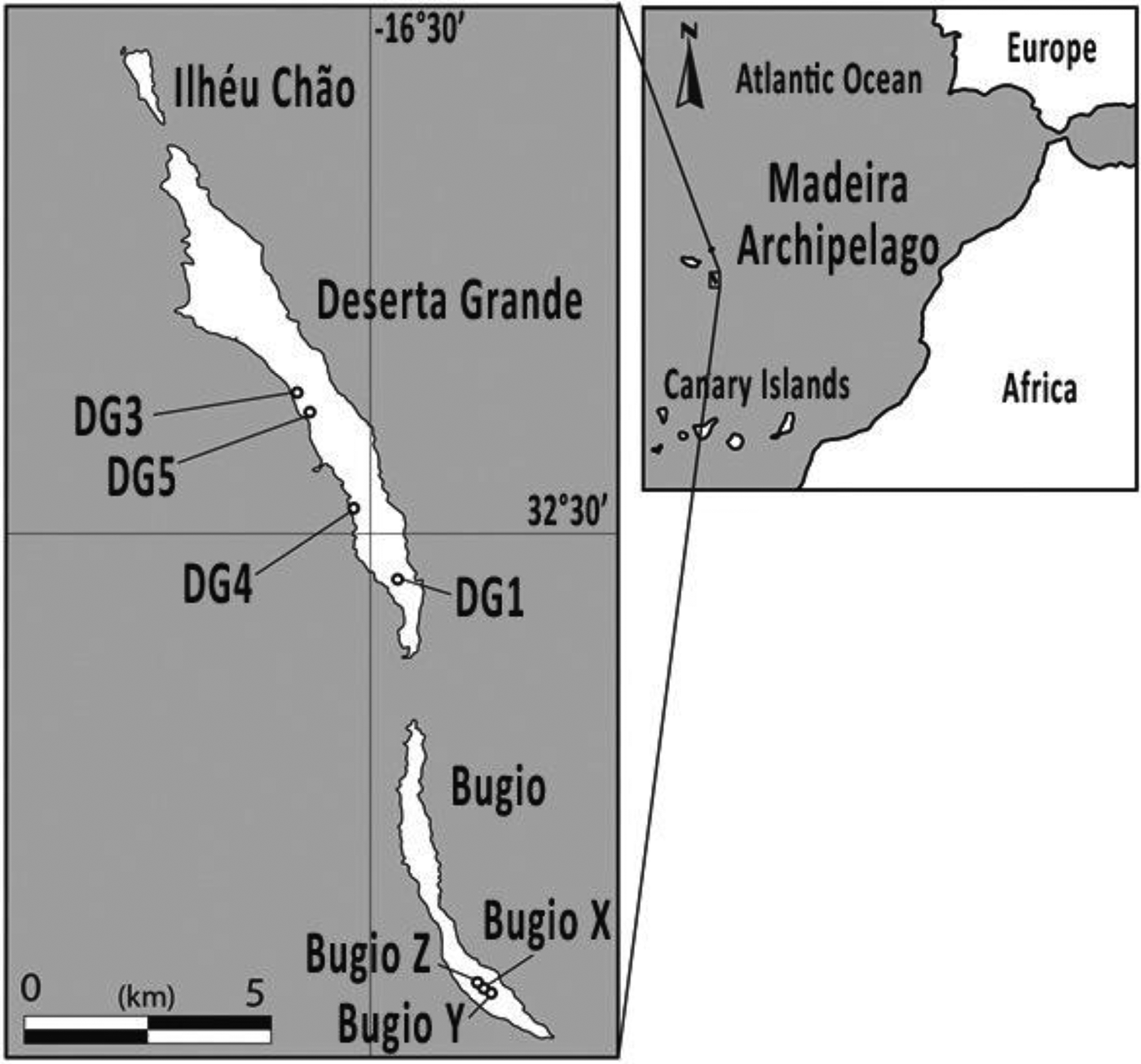
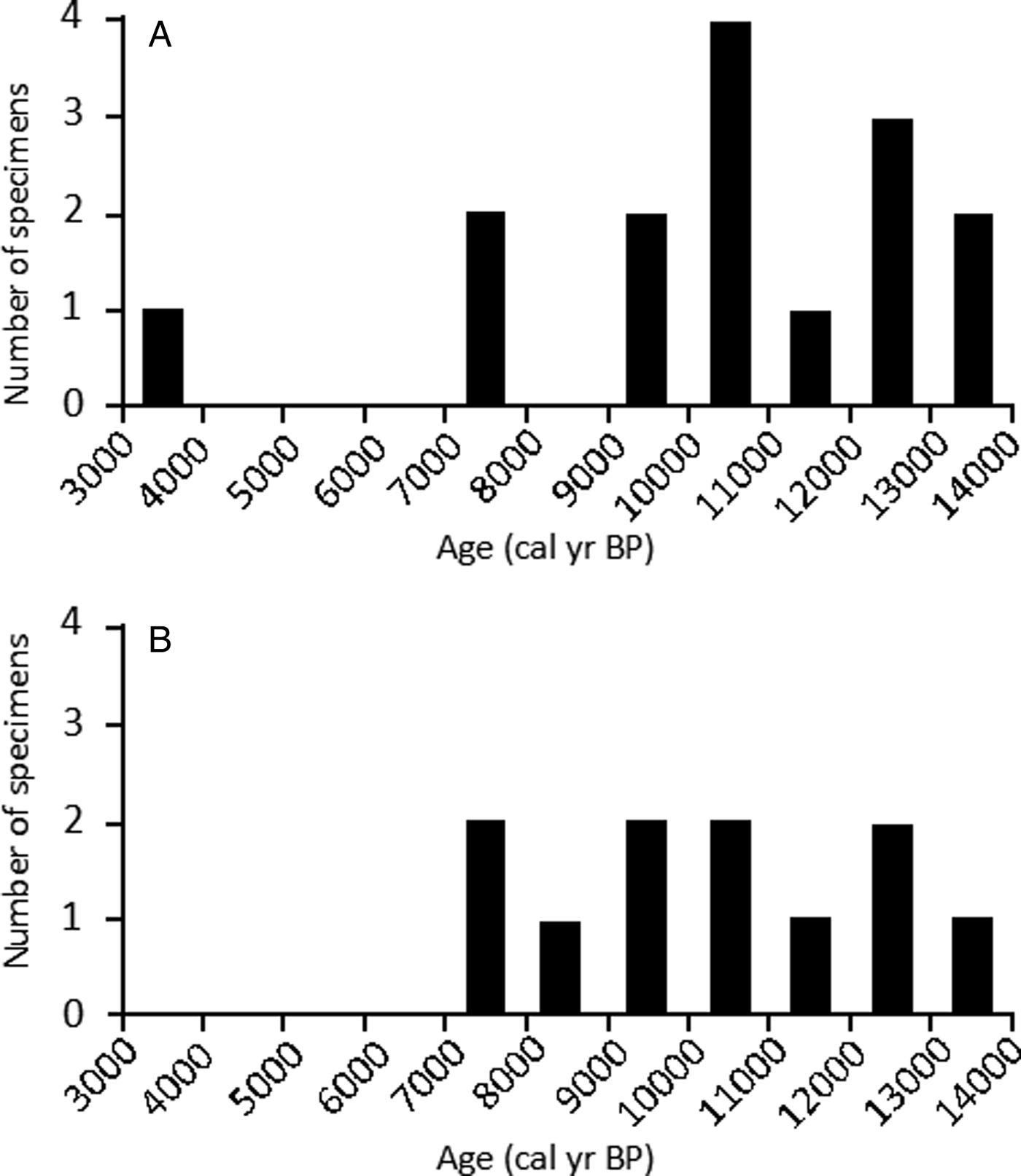
The Madeira Archipelago (32°39′4″N, 16°54′35″W) is located in the Atlantic Ocean 400 km north of the Canary Islands and 900 km southwest of Portugal (Fig. 1). The islands were formed by volcanic activity in a series of phases between 14.3 and 3.2 Ma (Geldmacher et al., Reference Geldmacher, van den Bogaard, Hoernle and Schmincke2000) and are divided into three island groups: Madeira, Porto Santo, and the Desertas Islands. While the climate of the Madeira archipelago is temperate across all the islands, the eastern islands (Fig. 1) from which samples were collected (Deserta Grande and Bugio) are semiarid.
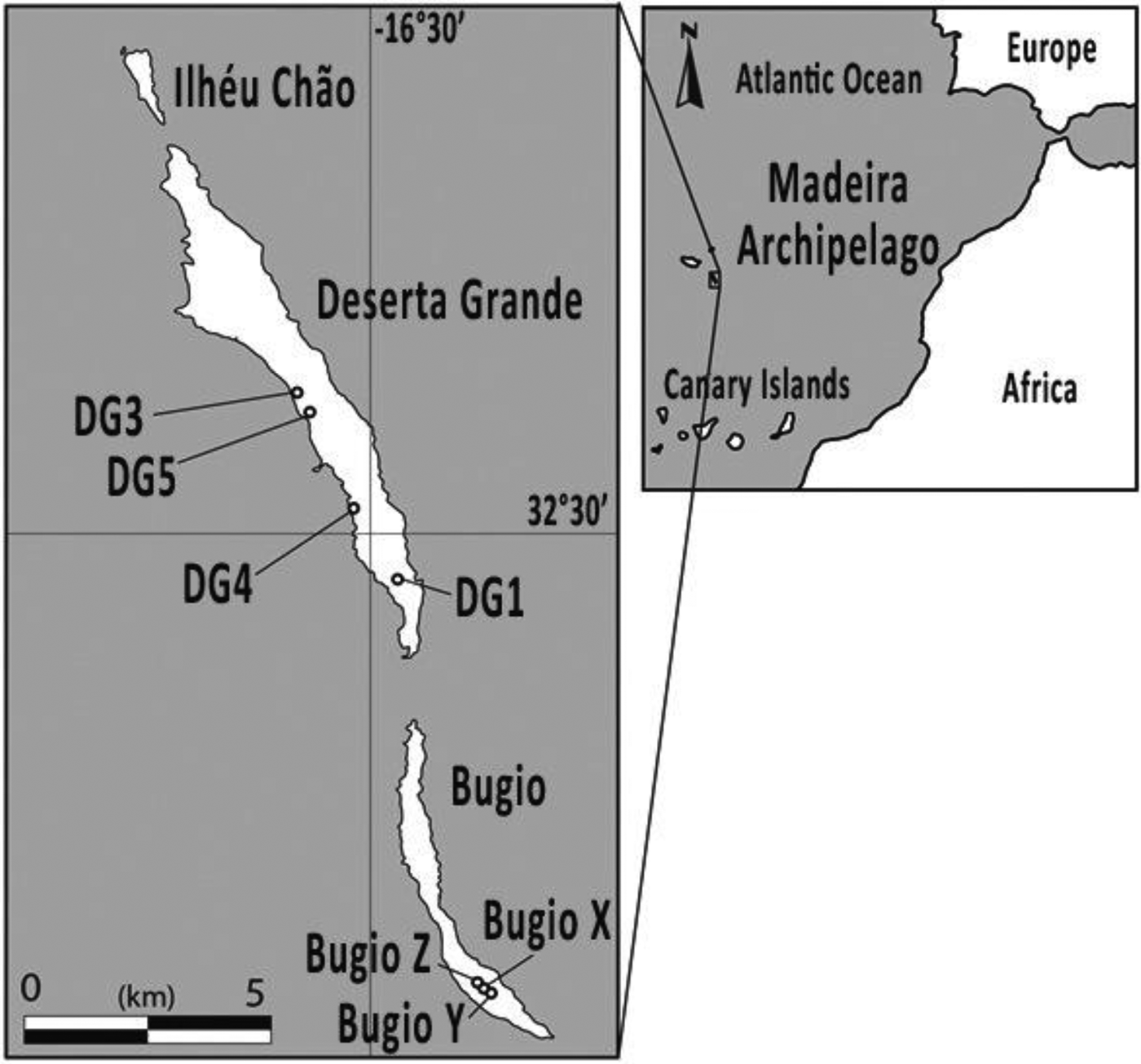
Figure 1. Location of the Desertas Islands chain, Madeira Archipelago. Circles depict colluvial sites from Deserta Grande and Bugio Islands dated in this study.
Air temperatures are mild year-round with a mean of 21°C in Funchal, Madeira, ranging from 16°C in January to 23°C in August. This tempered seasonality due to the oceanic influence is ideal for AAR studies, as racemization/epimerization rate is sensitive to changes in ambient temperature. Aridity is largely dependent on topography, with lower coastal areas being generally drier than those inland with greater elevation (Santos et al., Reference Santos, Valente, Miranda, Aguiar, Azevedo, Tome and Coelho2004). Evidence from dune deposits and plant remains indicates that the climate of Madeira has fluctuated over the last 150 ka, with evidence of increased aridity toward the present (Cameron and Cook, Reference Cameron and Cook1992). Parts of Madeira and the Desertas Islands show evidence of significantly increased aridity in the drier island environments due to fluctuations in climate and recent human impact via land clearance, fire, and grazing (Cameron and Cook, Reference Cameron and Cook1992).
The geology of the Desertas Islands is nearly entirely volcanic (basaltic) without presence of other rock types. Only small patches of colluvial silty-clay sediments with minor content of biogenic eolian carbonates (Goodfriend et al., Reference Goodfriend, Cameron, Cook, Courty, Fedoroff, Livett and Tallis1996) can be found scattered around these two islands. Due to the lack of extensive carbonate-rich sediments and limestone throughout the Desertas Islands, the potential incorporation of dead carbon into snail shells (Pigati et al., Reference Pigati, Quade, Shahanan and Haynes2004, Reference Pigati, Rech and Nekola2010; Quarta et al., Reference Quarta, Romaniello, D'Elia, Mastronuzzi and Calcagnile2007; Hill et al., Reference Hill, Reimer, Hunt, Prendergast and Barker2017) is likely limited in the study area.
Materials
Land snail shells were collected between 2012 and 2016 from seven different locations across two of the Desertas Islands: Bugio and Deserta Grande (Fig. 1). Whole snail shells were collected from deposits of colluvium (unconsolidated reworked sediment; Fig. 2). These deposits are all located on or at the base of exposures into erosive colluvial slopes and of varying texture, though predominantly loose unsorted sand and gravel. The colluvial sediments throughout The Desertas Islands are brown-reddish silty-clay sediments with some volcanic sand and lapilli (Fig. 2E and F), showing moderate weathering (Goodfriend et al., Reference Goodfriend, Cameron, Cook, Courty, Fedoroff, Livett and Tallis1996). To avoid racemization rate alterations due to daily and seasonal temperature fluctuations in surficial exposure, shells were collected from actively eroding slopes of unconsolidated sediments, buried under rocks or excavated by digging into looser sediments around them. Shells are likely to have reached the near surface only recently, as erosion carries surface material downslope. This should minimize the effects of seasonal temperature fluctuations on AAR rates. Each sample was collected from a single stratum of colluvium (Fig. 2).

Figure 2. (color online) Field photographs of Quaternary colluvial deposits from the Desertas Islands of the Madeira Archipelago, Portugal. (A) General view of the abrupt volcanic landscape of Deserta Grande Island. Black arrow denotes colluvial sediments from site DG4. (B) Close-up view of the DG4 site in Deserta Grande Island. Shell samples were retrieved from unconsolidated brown-reddish silt-clay sediments on the side base of the slope. (C) Escarpments formed into the flat-topped hill from Bugio Island. Black arrows indicate site Bugio Y. (D) General view of colluvial brown-reddish silt-clay sediments at Bugio Y site. (E) Close-up view of loose colluvial sediment with volcanic lapilli-sand and fossil (white) land snail shells at Bugio X site. (F) Detailed view of Quaternary Actinella nitidiuscula shell preserved in colluvial unconsolidated sediments at Bugio X site (shell diameter = ~10 mm).
We used only specimens of the abundant nominal species Actinella nitidiuscula (G.B. Sowerby I, 1824) (Gastropoda: Hygromiidae). While the nominate subspecies, A. nitidiuscula nitidiuscula (Fig. 3A) was found both alive and fossilized, the form A. nitidiuscula saxipotens (Wollaston, Reference Wollaston1878) (Fig. 3B), listed at subspecific rank, was found only as fossils. Evidence of coexistence in deposits indicates that these two forms should be regarded as distinct but closely related species, and they are, informally, treated so here. A. nitidiuscula (sensu lato) is ideal for geochronologic and paleoclimatic studies, as it is endemic to the Madeiran Archipelago, abundant, and ubiquitous throughout the Holocene and late Pleistocene (Cook et al., Reference Cook, Cameron and Lace1990). Moreover, A. nitidiuscula is a small and thin species (shell width ≤12 mm), which is preferable, considering that small snails appear to require less calcium input and are therefore less likely to display contamination from recycled carbonate (Ellis et al., Reference Ellis, Goodfriend, Abbott, Hare and von Endt1996; Pigati et al., Reference Pigati, Quade, Shahanan and Haynes2004, Reference Pigati, Rech and Nekola2010) as has been sometimes observed in larger land snail species living in carbonate-rich areas (e.g., Yanes et al., Reference Yanes, Kowalewski, Ortiz, Castillo, de Torres and de la Nuez2007; Hill et al., Reference Hill, Reimer, Hunt, Prendergast and Barker2017). This inferred relationship between land snail shell size and recycling of carbonate is still being examined but was taken into consideration when selecting Actinella over larger and similarly abundant land snail species in this locality. For this study, carbonate uptake by snails is of limited concern, because the bedrock geology of Deserta Grande and Bugio Islands is nearly entirely basaltic. Only some small areas of colluvial sediments include some biogenic eolian carbonates. Thus, overall, Desertas snails have limited carbonate accessibility.

Figure 3. (color online) Photographs of the two land snail subspecies selected for amino acid racemization and 14C accelerator mass spectrometry dating. (A) Modern individual of the extant Actinella nitidiuscula nitidiuscula. (B) Fossil individual of the extinct Actinella nitidiuscula saxipotens.
AAR dating
To test for the most informative amino acid in Actinella nitidiuscula shells from the Desertas Islands, we investigated racemization rates for seven amino acids: aspartic acid, glutamic acid, serine, alanine, valine, isoleucine, and leucine (Table 1). The extent of AAR was measured using reverse-phase liquid chromatography following Kaufman and Manley (Reference Kaufman and Manley1998). All sample preparation and analyses were performed in the Amino Acid Geochronology Laboratory at Northern Arizona University. Specimens were cleaned and prepared for analysis using methods described in Hearty and Kaufman (Reference Hearty and Kaufman2009). A small fragment of each shell was used for AAR analysis (n = 46), and other fragments of the same shells were separated for subsequent graphite-target (n = 6) and carbonate-target (n = 20) radiocarbon dating. Shell fragments were cleaned and weighed before being dissolved in microreaction vessels and hydrolyzed for 6 h at 110°C before being dried in a vacuum desiccator and rehydrated using an adjusted distilled and deionized water solution. Samples were then injected onto a high-performance liquid chromatograph unit for analysis. The method uses precolumn derivatization of samples with o-phthaldialdehyde and chiral thiol, N-isobutyryl-l-cysteine, to yield fluorescent diastereomeric derivatives of chiral primary amino acids (Kaufman and Manley, Reference Kaufman and Manley1998). The racemization rate of glutamic acid (Glu) had the best fit (highest R 2 value from an ordinary least-squares linear model) when calibrated with radiocarbon dates and was chosen for all subsequent analyses.
Table 1. d/l amino acid ratios of 20 land snail shells from Deserta Grande and Bugio. The same individual shells were also dated by 14C (Table 1), and all but three were used to calibrate the rate of amino acid racemization. Asterisks (*) denote modern (recently dead) shells.a
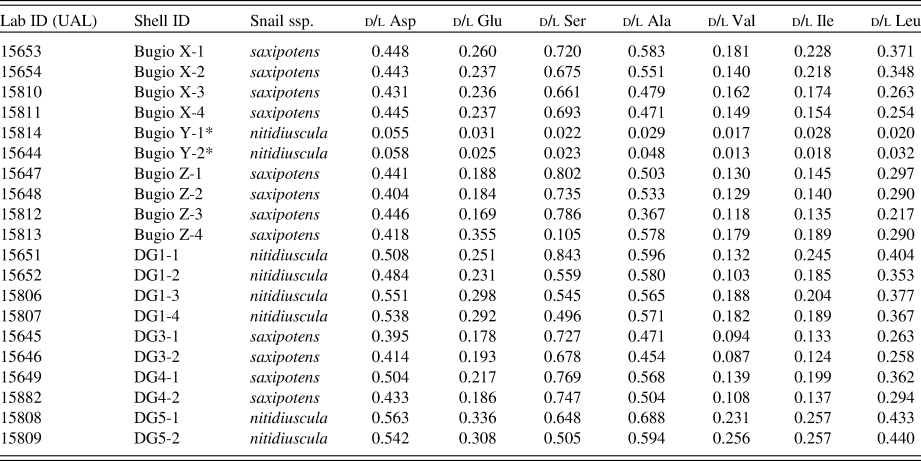
a Abbreviations: Lab ID, UAL # (carbonate-target); Snail ssp., Actinella nitidiuscula subspecies; d/l Asp, aspartic acid; d/l Glu, glutamic acid; d/l Ser, serine; d/l Ala, alanine; d/l Val, valine; d/l Ile, isoleucine; d/l Leu, leucine.
Radiocarbon dating
To radiocarbon date snail shells, we used both the novel carbonate-target AMS (Bush et al., Reference Bush, Santos, Xu, Southon, Thiagarajan, Hines and Adkins2013) and the standard graphite-target AMS. Carbonate-target AMS was developed for carbonate samples to provide a lower-cost, lower-precision alternative to standard graphite-target AMS radiocarbon dating (Bush et al., Reference Bush, Santos, Xu, Southon, Thiagarajan, Hines and Adkins2013). Samples are prepared differently for the two techniques. For carbonate-target AMS, specimen-derived, finely powdered CaCO3 is mixed with unbaked Sigma-Aldrich 400 mesh and 99.9% pure Fe, which is then poured into an aluminum cathode pressed for AMS measurement (see Bush et al. [2013] for additional details). In contrast, graphite-target AMS dating required filamentous graphite to be produced from carbonate samples. While carbonate-target reduces preparation time and lowers analytical costs, there is evidence that the method has difficulties dating specimens older than 10 ka. Carbonate samples that Bush et al. (Reference Bush, Santos, Xu, Southon, Thiagarajan, Hines and Adkins2013) dated to 10–12 14C ka BP showed ± 0.8–6.1% deviation, while those dated at 29–40 14C ka BP showed ± 27–108% deviation. As a result, subsequent studies using carbonate-target radiocarbon dating have limited analysis predominantly to Holocene samples (e.g., Hines et al., Reference Hines, Southon and Adkins2015; Grothe et al., Reference Grothe, Cobb, Bush, Cheng, Santos, Southon, Edwards, Deocampo and Sayani2016; Ritter et al., Reference Ritter, Erthal, Kosnik, Coimbra and Kaufman2017; Kowalewski et al., Reference Kowalewski, Casebolt, Hua, Whitacre, Kaufman and Kosnik2018). We also note that previous work was focused on marine invertebrates only. Here, we expand tests of carbonate-target radiocarbon dating to terrestrial land snails, which can be powerful paleoclimate proxies for Quaternary terrestrial and atmospheric systems (see review in Yanes et al., Reference Yanes, Al-Qattan, Rech, Pigati, Dodd and Nekola2019).
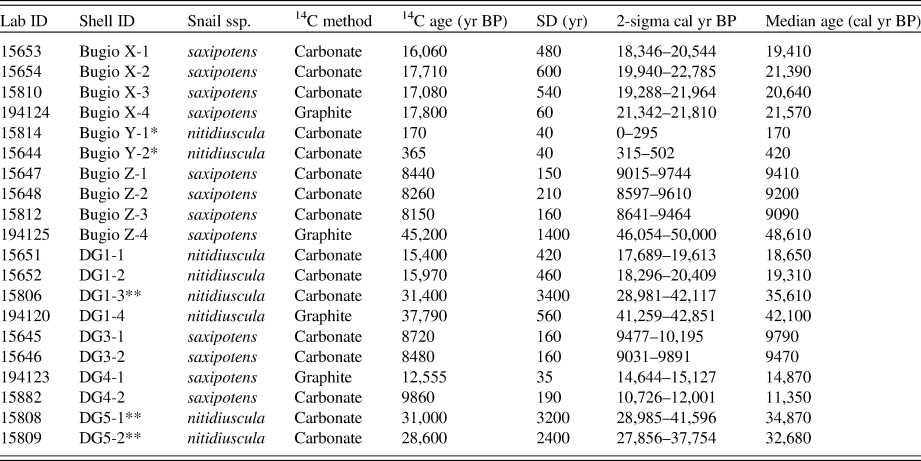
A total of 20 Actinella nitidiuscula land snail shell specimens retrieved from seven colluvial deposits across Deserta Grande and Bugio Islands (Fig. 1) were analyzed by both AAR analysis and carbonate-target AMS radiocarbon dating, including two recently dead specimens analyzed to examine any potential dead carbon anomaly. Of these, four Pleistocene shells and two Holocene shells (six total) were also selected for graphite-target radiocarbon dating to assess the accuracy of the carbonate-target radiocarbon method. All radiocarbon analyses were performed at the Keck-Carbon Cycle AMS Facility at the University of California, Irvine. All samples were pretreated via cleaning and dilute HCl treatment to remove potential environmental contaminants. Calibration of radiocarbon years to calendar years was performed for all samples using the Calib Radiocarbon Calibration (v. 7.1) (Stuiver and Reimer, Reference Stuiver and Reimer1993). Radiocarbon dates were calibrated using the IntCal13 Northern Hemisphere calibration curve (Reimer et al., Reference Reimer, Bard, Bayliss, Beck, Blackwell, Bronk Ramsey and Buck2013). All ages are presented in calendar (cal) years or thousands of years (ka) before present (assumed AD 1950).
AAR calibration
A total of 17 shells from seven colluvial sites were used to generate a calibration curve for estimating calendar year ages from amino acid d/l ratios. These included 13 carbonate-target-dated shells and 4 graphite-target-dated shells. All these shells also underwent AAR. For Holocene shells, the carbonate- and graphite-based ages overlap, and we chose not to pursue additional correction or manipulation of the ages. The amino acid calibration was limited to shells with graphite-target radiocarbon ages younger than ~22 14C ka BP due to increasing offset between graphite- and carbonate-target ages beyond that limit. Our resulting calibration was then used to estimate ages of 26 additional shells from two selected colluvial deposits from Deserta Grande Island (DG3 and DG4; Fig. 1), which were only analyzed using AAR.
We used nonlinear least squares to calculate the AAR–radiocarbon calibration. To obtain a 95% confidence estimate for the mean prediction of the calibration that incorporates our sampling of shell dates, we bootstrapped the calibration calculation 10,000 times by randomly resampling with replacement. After estimating ages of unknown shells in the two deposits on Deserta Grande Island (DG3 and DG4), the time averaging of the deposits was assessed using age-frequency distributions. Here, age refers to time of burial rather than ontogenetic age at death. These distributions depict an assemblages’ representation of species abundance through time and offer opportunities to consider changes in preservation and sorting mechanisms. Standard moments of these distributions (skewness and kurtosis, measuring the symmetry and “tailedness” of distributions, respectively) are used to describe the shape of recovered age distributions. To calculate uncertainty in skewness and kurtosis, we used a bootstrap algorithm (repeated 10,000 times) that incorporated the calculated confidence estimate of the overall calibration. For each AAR-dated shell, we iteratively sampled dates from a uniform distribution bounded by the calibration confidence interval at that age estimate. We then iteratively calculated kurtosis and skewness for each deposit. Finally, we calculated the resulting 95% confidence interval of these statistical moments for each deposit. A confidence interval of skewness that is entirely positive (>0.0) or negative (<0.0), provides evidence that the time averaging of a deposit is significantly skewed. A confidence interval for kurtosis that is entirely less than 3.0 (the kurtosis value for a normal distribution) indicates that the time averaging of the deposit has very broad tails (i.e., weakly peaked or not peaked at all). Distributions with very thin tails (more strongly peaked) would have confidence intervals that are entirely greater than 3.0. Based on depositional setting, there is some expectation that colluvial deposits could produce time-averaged assemblages that are approximately uniformly distributed with respect to age (low-peaked) or more normally distributed; but it is unlikely that they would produce highly peaked age distributions. All calculations were written in R v. 3.4.1 (R Core Team, 2017).
Artificial time averaging from dating uncertainties
To fully quantify time averaging, the degree of age mixing of individual shells needs to be evaluated relative to the expected age variation brought about by uncertainties related to the dating methods. Evaluation of the quality of radiocarbon/AAR calibration can determine the extent and significance of artificial time averaging in tested samples (e.g., Kowalewski et al., Reference Kowalewski, Goodfriend and Flessa1998; Yanes et al., Reference Yanes, Kowalewski, Ortiz, Castillo, de Torres and de la Nuez2007; Dominguez et al., Reference Dominguez, Kosnik, Allen, Hua, Jacob, Kaufman and Whitacre2016; Kosnik et al., Reference Kosnik, Hua, Kaufman, Kowalewski and Whitacre2017). Multiple sources of uncertainty contribute to error in age estimates, including: regression uncertainties, analytical error, conversion of 14C ages to calendar ages, reservoir age estimate, and effects of different postdepositional temperature history on the rate of AAR. We estimate an age uncertainly of around 1000 years.
RESULTS
Graphite- and carbonate-target radiocarbon results
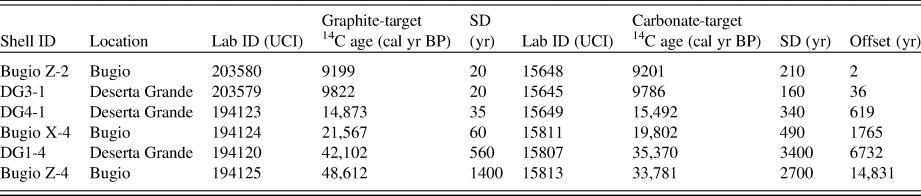
The 20 carbonate-target radiocarbon-dated land snail shells from the Desertas Islands ranged in age from modern (~0.1 cal ka BP) to ~48 cal ka BP (calibrated radiocarbon age) (Table 1). These results reveal that snail-rich colluvial deposits in these islands include the transition from the last glacial maximum to the early Holocene (interglacial) interval. Comparisons between graphite- and carbonate-target radiocarbon-dated shells show a significant overlap for Holocene shells, with an offset of 20 to 80 years only (Fig. 4; Table 3). Early Pleistocene shells show a slightly larger offset but still within the analytical error (0.4 to 1.4 ka), and finally, Pleistocene shells older than 22 cal ka BP showed a significant age offset between 6.3 and 15.6 ka (Fig. 4; Table 3).

Figure 4. Relationship between carbonate-target to graphite-target accelerator mass spectrometry radiocarbon results (median calibrated ages) of four Pleistocene and two early Holocene Actinella nitidiuscula shells (R 2 = 0.985). Dashed line depicts 1:1 line. Error bars represent 2-sigma calibrated age range. Data are listed in Table 3.
Table 2. Radiocarbon and calibrated ages of 20 Actinella land snail shells from Deserta Grande and Bugio analyzed via carbonate-target and graphite-target accelerator mass spectrometry radiocarbon dating. Asterisks (*) denote modern (recently dead) shells. Double asterisks (**) depict oldest shells (excluded from the calibration).a
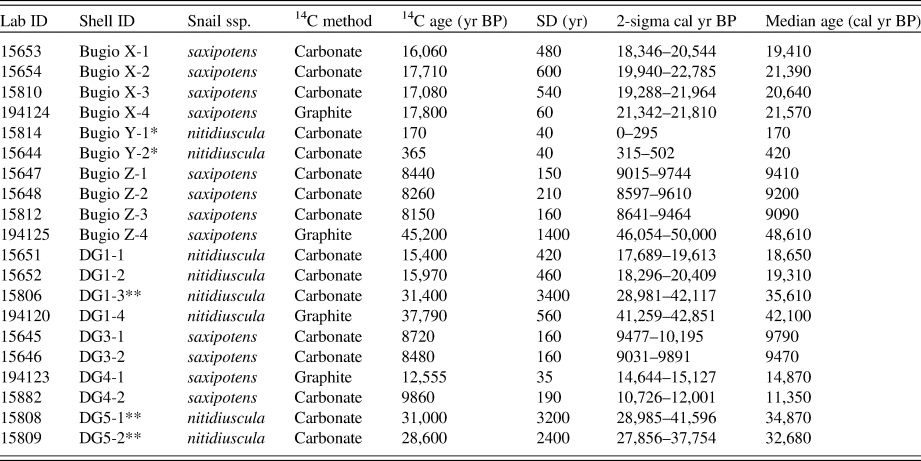
a Abbreviations: Lab ID, UCI # (graphite-target) or UAL # (carbonate-target); Snail spp., Actinella nitidiuscula subspecies; 14C age (yr BP); uncalibrated radiocarbon age.
Table 3. Comparison of calibrated radiocarbon age derived from graphite-target and carbonate-target accelerator mass spectrometry radiocarbon dating of Actinella nitidiuscula snail shells from the Desertas Islands.a

a Abbreviations: Lab ID, UCI # (graphite-target); SD, standard deviation; Offset, difference between graphite-target and carbonate-target calibrated ages.
Although the survival time of land snail shells on the ground surface or “taphonomic active zone” in The Desertas Islands is unclear, considering the semiarid conditions of these islands, it is hypothesized that the snails possibly died within the last several decades or last century. Two modern (recently dead) fresh-looking shells collected from the soil surface exhibit apparent 14C ages of one to three centuries (Table 2). As expected, recently dead snail shells showed d/l AAR ratios close to zero (Table 2).
Radiocarbon calibration of AAR values
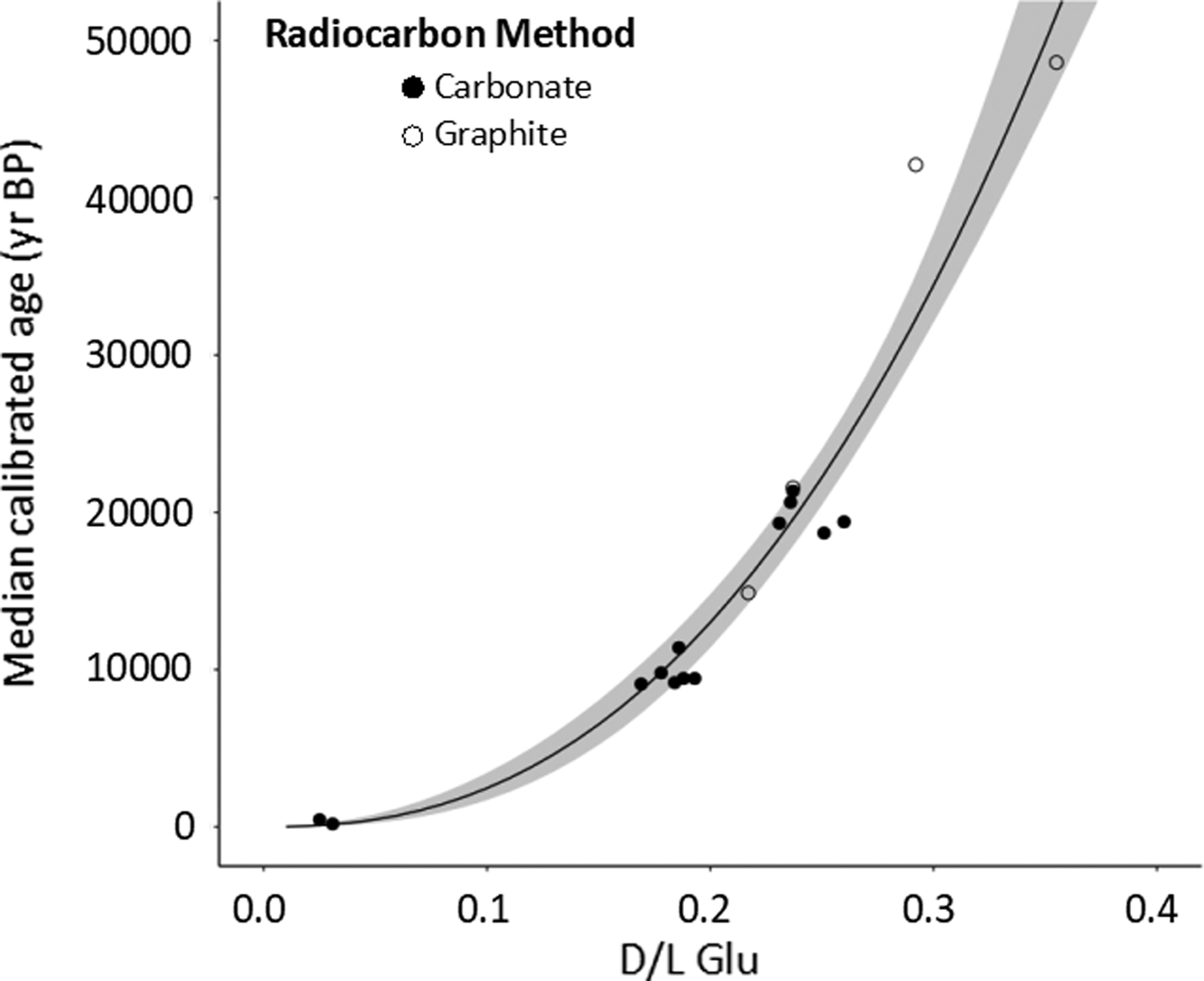
After the effectiveness and limits of carbonate-target radiocarbon dating had been established (Fig. 4), a calibration curve was created using calibrated 14C ages and AAR d/l ratios. For four samples older than 10 cal ka BP, graphite-target dates derived from the same shells were used rather than carbonate-target dates, due to greater reliability. The d/l ratios of glutamic acid (Glu) showed the highest correlation with calendar year calibrated 14C ages (Fig. 5). Therefore, the Glu d/l ratios were selected for age estimation of Madeira Islands shells. Calibrated AAR age is represented by the following equation, where age is in years BP (Fig. 5):

Figure 5. Relationship between glutamic acid d/l ratio and 14C ages of the 17 Actinella nitidiuscula shells used to calibrate the rate of amino acid racemization (AAR). Each dot depicts a single shell dated by both methods (AAR and 14C). Whiskers represent 2-sigma range of calibrated ages. Carbonate- and graphite-target 14C dating results were used in the regression analysis. Gray band represents 95% CI of the calibration curve. As an estimate of goodness of fit, ordinary least-squares regression of log-transformed data yields R 2 = 0.96. Data are listed in Tables 1 and 2.
The strong correlation between d/l Glu and 14C ages (Fig. 5) suggests that the influence of possible different temperature histories on the rate of AAR is limited.
Quantifying age mixing of snail-rich colluvial deposits
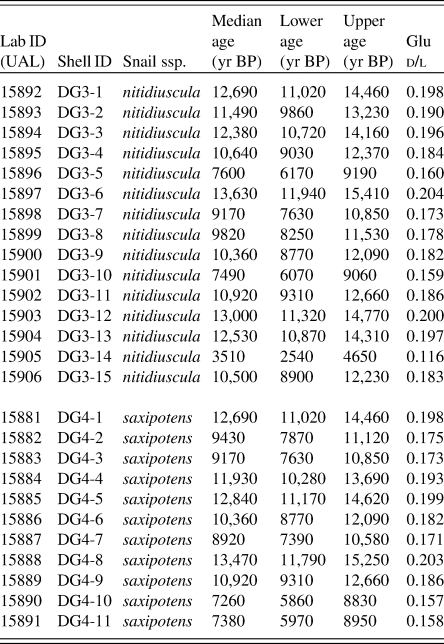
Two snail-rich colluvial deposits from Deserta Grande were selected to assess the scale and structure of time averaging in buried land snail assemblages, site DG3 and DG4 (Table 4). These two colluvial deposits were selected for their great abundance of pristine Actinella shells and other species of land snails that were accessible and easy to sample. The sites lacked clear soil structures or discernible stratigraphy. Shells were drawn from actively eroding slopes of unconsolidated silt-clay sediments at the base of unstable colluvial slopes.
Table 4. d/l glutamic acid (Glu) values and numerical ages for specimens from two colluvial deposits (DG4 and DG3) from Deserta Grande Island, Madeira, Portugal. Ages were derived from the calibration equation: Age = 622,247 · d/l Glu (2.404) as shown in Fig. 5.
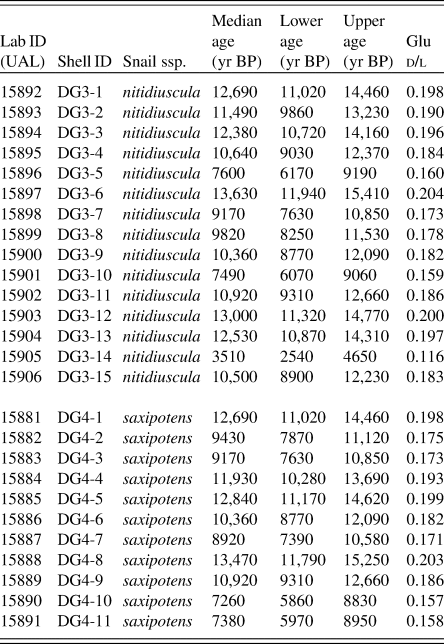
a Abbreviations: Lab ID, UAL # (carbonate-target); Snail ssp., Actinella nitidiuscula subspecies; Lower age, lower age limit derived from error range of AAR calibration expressed in years BP; Upper age, upper age limit derived from error range of AAR calibration expressed in years BP.
From site DG3, 15 analyzed shells ranged in age between ~3.5 and ~13.6 cal ka BP, with a median age of 10.8 cal ka BP. The frequency distribution is left-skewed (skewness 95% CI: −1.5, −0.4), that is, older specimens occur more frequently in the assemblage than younger specimens. Kurtosis estimates are wide, but bracket 3.0 (kurtosis 95% CI: 2.5, 5.4). Fourteen of the 15 dated shells ranged from ~7.4 to ~13.6 cal ka BP and appear normally distributed within this range. The remaining shell represents a substantially younger shell of ~3.5 cal ka BP (Fig. 6A).
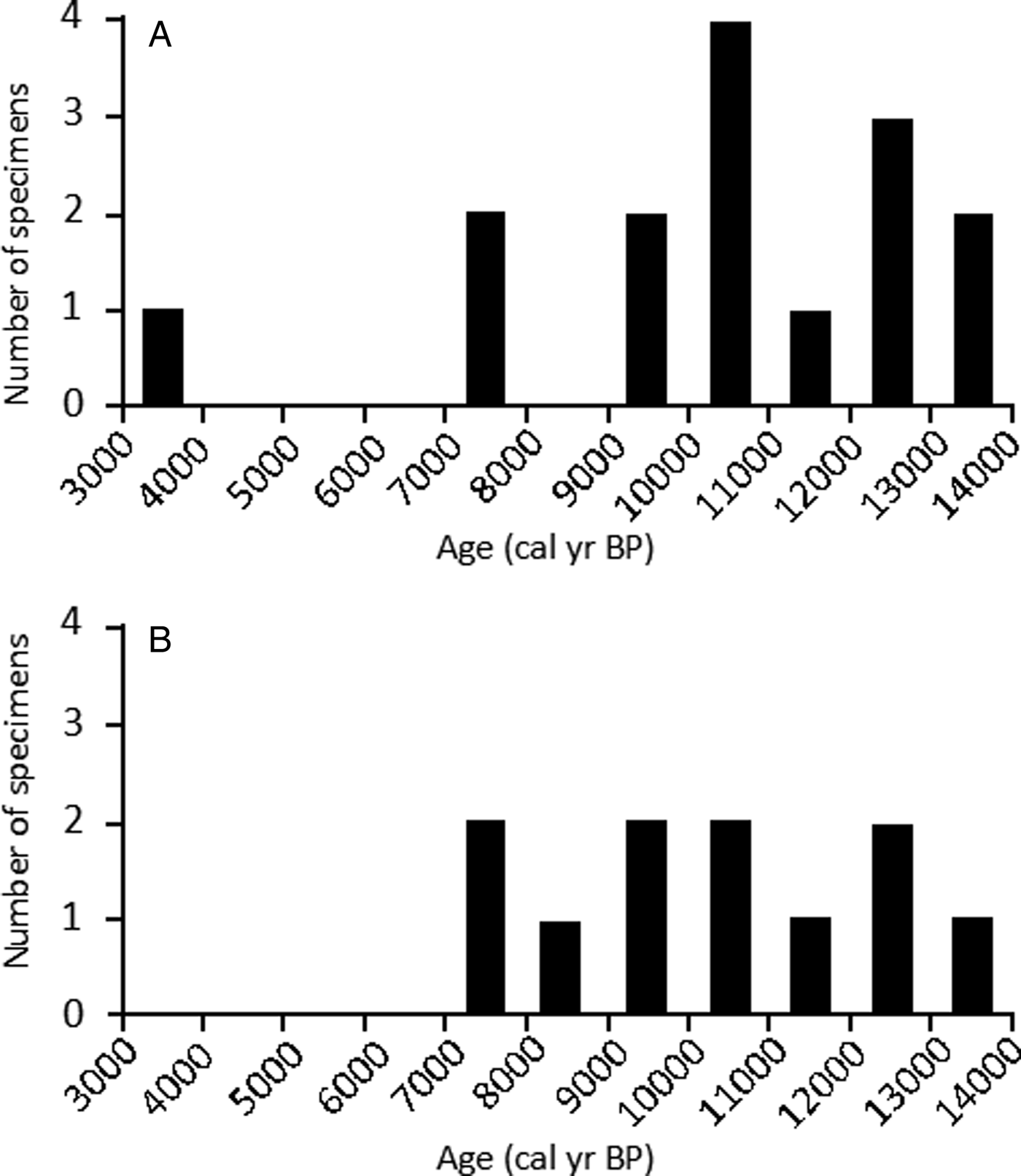
Figure 6. Age-frequency distributions of individual shell ages from colluvial sites in Deserta Grande Island, Madeira Archipelago, Portugal. (A) Structure of age mixing from site DG3 determined from dating 15 shells. (B) Structure of age mixing from site DG4 examined by dating 11 shells. The 1000-year bin size was chosen based on an age range that would eliminate the most gaps while also preserving structure of age distribution. Data are listed in Table 4.
From site DG4, the ages of 11 analyzed shells ranged from ~7.3 to ~13.5 cal ka BP, with median shell age of 10.6 cal ka BP. The frequency distribution is largely symmetric, with no clear relationship in the assemblage between age and frequency when examined at a 1 ka time resolution (skewness 95% CI: −0.6, 0.6; kurtosis 95% CI: 1.4, 2.8) (Fig. 6B).
DISCUSSION
Scale of time averaging in Quaternary colluvial deposits
The age ranges of dated shells within the two analyzed deposits in Deserta Grande Island point to variation in age (>6 ka) exceeding the error expected by various sources of uncertainties. Accordingly, the results support our hypothesis of real multimillennial age mixing of shells within colluvial deposits from The Desertas Islands. The scale of age mixing documented here was not significantly impacted by potential dead carbon intake by the snails (e.g., Hill et al., Reference Hill, Reimer, Hunt, Prendergast and Barker2017), because the studied islands are poor in carbonate sediments and recently dead (modern) shells yielded 14C ages younger than ~300 years that could potentially be explained by a post-bomb effect after the 1950s (Quarta et al., Reference Quarta, Romaniello, D'Elia, Mastronuzzi and Calcagnile2007). Even if snails’ carbonate intake is possible, the effects of carbonate ingestion (<300 years) cannot explain the large degree of age mixing within colluvial sediments (>6 ka). These facts support the idea that carbonate ingestion by Actinella was probably limited, more so than for snails living in carbonate-rich areas, and therefore, we argue the radiocarbon results should not be significantly distorted by dead carbon offsets.
Multimillennial-scale time averaging has been observed in several late Pleistocene and Holocene buried continental shelly assemblages. Similar studies conducted on Helicidae snail shells have found evidence of similar magnitude of age mixing in late Quaternary carbonate-rich paleosols, paleodunes, and cave sediments from other oceanic islands (Goodfriend, Reference Goodfriend1989; Goodfriend and Mitterer, Reference Goodfriend and Mitterer1993; Yanes et al., Reference Yanes, Kowalewski, Ortiz, Castillo, de Torres and de la Nuez2007), suggesting that multimillennial-scale time averaging may be pervasive in Quaternary land snail assemblages in colluvium from islands with semiarid to arid habitats. Given the potential of fossil land snail shells as paleoenvironmental and paleoecological proxies (e.g., Goodfriend et al., Reference Goodfriend, Cameron, Cook, Courty, Fedoroff, Livett and Tallis1996; Yanes et al., Reference Yanes, Al-Qattan, Rech, Pigati, Dodd and Nekola2019), these results reinforce the necessity for improving our understanding of time averaging in continental sedimentary records.
Most published research assessing the scale of time averaging has traditionally focused on marine bivalve assemblages retrieved from shallow-water environments raging in age from late Pleistocene to modern. Published work suggests that the extent of age mixing can be quite variable, ranging from multiannual to multimillennial (e.g., Meldahl et al., Reference Meldahl, Flessa and Cutler1997; Kowalewski et al. Reference Kowalewski, Goodfriend and Flessa1998, Reference Kowalewski, Casebolt, Hua, Whitacre, Kaufman and Kosnik2018; Kidwell et al., Reference Kidwell, Best and Kaufman2005; Barbour Wood et al., Reference Barbour Wood, Krause, Kowalewski, Wehmiller and Simões2006; Kosnik et al., Reference Kosnik, Hua, Kaufman and Wüst2009; Albano et al., Reference Albano, Filippova, Steger, Kaufman, Tomašových, Stachowitsch and Zuschin2016, Reference Albano, Gallmetzer, Haselmair, Tomašových, Stachowitsch and Zuschin2018; Ritter et al., Reference Ritter, Erthal, Kosnik, Coimbra and Kaufman2017; and numerous references therein). In contrast, the few land snail studies retrieved from paleosols and paleodunes (Yanes et al., Reference Yanes, Kowalewski, Ortiz, Castillo, de Torres and de la Nuez2007), cave sediments, and colluvial deposits (this study) indicate consistent multimillennial mixing. Deposits displaying extensive age mixing have also been reported in studies of non-molluscan fauna from Holocene and late Pleistocene settings, indicating that multimillennial time resolution is not limited to molluscan assemblages. This includes taphonomic and geochronological studies on other durable taxa like freshwater ostracods (Michelson and Park, Reference Michelson and Park2013), brachiopods (Barbour Wood et al., Reference Barbour Wood, Krause, Kowalewski, Wehmiller and Simões2006; Krause et al., Reference Krause, Barbour, Kowalewski, Kaufman, Romanek, Simoes and Wehmiller2010), corals (Edinger et al., Reference Edinger, Burr, Pandolfi and Ortiz2007), and terrestrial vertebrate assemblages (Hadly, Reference Hadly1999; Terry, Reference Terry2009, Reference Terry2010).
In addition to measuring the scale of time averaging by taxa and location, several studies have specifically examined how changes in burial depth could affect age mixing, either through direct comparison of age mixing at different depths or via examination of taphonomic processes. Buried specimens (those interred more than several centimeters beneath the surface) do not show significant differences in the scale of time averaging with increasing depth, as revealed through direct assessment of time averaging or examination of ecological fidelity in marine bivalves (e.g., Kosnik et al., Reference Kosnik, Kaufman and Hua2008, Reference Kosnik, Hua, Kaufman and Wüst2009), land snails (Goodfriend, Reference Goodfriend1989; Yanes et al., Reference Yanes, Aguirre, Alonso, Ibáñez and Delgado2011), and small mammals (Terry and Novak, Reference Terry and Novak2015).
Taphonomy may control the scale of time averaging. Studies of comparative taphonomy (e.g., Brett and Baird, Reference Brett and Baird1986) have shown that many factors, such as local sedimentation rates and environmental energy, can influence fossil preservation and, as a result, local time averaging. Similarly, a direct comparative study between marine mollusk and echinoid test preservation in a single site (Kowalewski et al., Reference Kowalewski, Casebolt, Hua, Whitacre, Kaufman and Kosnik2018) demonstrated that different taxa in the same deposit can exhibit different degrees of time averaging due to the intrinsic properties of those taxa, that is, more fragile echinoid tests display a smaller (yearly to decadal) scale of age mixing than more resilient (multimillennial) marine mollusks. While land snail shells from forested (humid) areas can experience high rates of shell destruction from enhanced shell dissolution in wet/acidic environments (e.g., Albano., Reference Albano2014), snail shells from semiarid habitats tend to be more resilient (e.g., Yanes et al., Reference Yanes, Aguirre, Alonso, Ibáñez and Delgado2011; Yanes, Reference Yanes2012).
All in all, the degree of age mixing can vary notably as a result of characteristics such as location, environment, ecology of taxa, burial conditions, and taphonomy (e.g., Tomašových et al., Reference Tomašových, Kidwell, Barber and Kaufman2014). In our snail assemblages from the Desertas Islands, the documented multimillennial resolution is feasibly explained by (1) the relatively brief life span (1 to 2 years) of Actinella nitidiuscula, which should result in high rates of shell production; (2) the relatively high durability of gastropod shells occupying semiarid habitats, that is, low rates of shell decay; and/or (3) the apparent slow burial/sedimentation rates in these volcanic (basaltic) islands.
Structure of time averaging in Quaternary colluvial deposits
“Structure of time averaging” refers to the frequency distribution of specimen ages through the observed age range of the assemblage (e.g., Meldahl et al., Reference Meldahl, Flessa and Cutler1997). The age distributions of the two sampled land snail assemblages from the DG3 and DG4 sites display different shapes (Fig. 6). Shell age distributions from DG3 display higher kurtosis and a more normal distribution, while shells from DG4 display a more platykurtic (less peaked) age distribution. Age-frequency distributions suggest that younger specimens are overall the least abundant in this depositional setting, pointing to high taphonomic durability of oldest specimens. Inconsistency in the shape of age-frequency distributions, including different samples in the same horizon, has been observed in Quaternary land snails (Yanes et al., Reference Yanes, Kowalewski, Ortiz, Castillo, de Torres and de la Nuez2007) and in marine bivalves in both buried (Kosnik et al., Reference Kosnik, Hua, Kaufman and Wüst2009) and surficial deposits (Ritter et al., Reference Ritter, Erthal, Kosnik, Coimbra and Kaufman2017; Kowalewski et al., Reference Kowalewski, Casebolt, Hua, Whitacre, Kaufman and Kosnik2018).
Taphonomy and sediment reworking play a large role in the structure of time averaging of faunal deposits (e.g., Flessa et al., Reference Flessa, Cutler and Meldahl1993; Olszewski, Reference Olszewski2004; Kosnik et al., Reference Kosnik, Hua, Kaufman and Wüst2009). In less sturdy skeletal remains such as brittle invertebrate shells and small mammal bones, older remains will inevitably experience a higher rate of destruction than younger examples. This can result in right-skewed distributions (indicating higher survivorship among younger fossil specimens) that are taphonomically driven, as is found in most studies of marine bivalves (Dexter et al., Reference Dexter, Kaufman, Krause, Barbour, Simões, Warren, Yanes, Romanek and Kowalewski2014; Ritter et al., Reference Ritter, Erthal, Kosnik, Coimbra and Kaufman2017) and terrestrial vertebrates (e.g., Hadly, Reference Hadly1999; Terry, Reference Terry2009; Terry and Novak, Reference Terry and Novak2015). However, in one study on Quaternary land snails by Yanes et al. (Reference Yanes, Kowalewski, Ortiz, Castillo, de Torres and de la Nuez2007), shell assemblages displayed a trend toward left-skewed distribution, suggesting a higher survivorship of older specimens.
The right-skewed trend continues even in nonsurficial assemblages, as deeper age-frequency distributions remain leptokurtic (Kosnik et al., Reference Kosnik, Hua, Kaufman and Wüst2009; Terry and Novak, Reference Terry and Novak2015). However, as before, a right-skewed distribution is most visible in shell ages pooled from multiple collection sites. Sediments that have undergone significant reworking, such as glacially reworked sediments, show less consistent age distribution (Briner et al., Reference Briner, Kaufman, Bennike and Kosnik2014). The structure of time averaging in colluvium, in particular, may be highly variable. This is likely because colluviation is a process of sediment reworking defined by erosion and transport of preexisting sediment driven by soil water saturation and physical disturbances (e.g., Leopold and Völkel, Reference Leopold and Völkel2007). As a result, the reworking of sediment to form colluvial deposits may incorporate multiple source units, including previously buried shells and other fossil materials (Lang and Hönscheidt, Reference Lang and Hönscheidt1999; Leopold and Völkel, Reference Leopold and Völkel2007). While the present study and the study by Yanes et al. (Reference Yanes, Kowalewski, Ortiz, Castillo, de Torres and de la Nuez2007) suggest that terrestrial mollusks do not appear to follow the same preservation and burial process as marine counterparts, future studies with higher numbers of dated shells will be used to further test the structure of age mixing in land snail assemblages across taxa, locations, and depositional settings.
Implications for shell geochronology
Mollusks and specifically land snail shells dated via AAR calibrated with graphite-target radiocarbon have been well established in developing local geochronologies (e.g., Goodfriend, Reference Goodfriend1989; Yanes et al., Reference Yanes, Kowalewski, Ortiz, Castillo, de Torres and de la Nuez2007; Hearty and Kaufman, Reference Hearty and Kaufman2009). The results presented here reinforce the use of land snail shells as excellent specimens for quantification of time averaging due to their ubiquity, abundance, and frequent shell durability in Quaternary settings. Graphite-target radiocarbon dating is limited by time and expense, while the carbonate-target method allows for faster and cheaper analysis of multiple localities and taxa. AAR analysis is applicable beyond the range of radiocarbon dating, but typically relies on an independent dating method to calibrate the rate of AAR to derive numerical ages. While initial carbonate-target results limited its effective dating range back to around 10 14C ka BP (e.g., Bush et al., Reference Bush, Santos, Xu, Southon, Thiagarajan, Hines and Adkins2013; Grothe et al., Reference Grothe, Cobb, Bush, Cheng, Santos, Southon, Edwards, Deocampo and Sayani2016; Ritter et al., Reference Ritter, Erthal, Kosnik, Coimbra and Kaufman2017), our results show a good correlation with amino acid d/l ages up to ~15–22 cal ka BP (Fig. 4), pushing its use into the late Pleistocene, at least for Actinella nitidiuscula land snail shells from the Desertas Islands and possibly other small (<12 mm) land snail taxa from semiarid regions. These findings are promising for paleoclimate and paleoecological studies using late Pleistocene and Holocene land snails, because a larger number of ancient shells retrieved from archaeological and paleontological settings can be dated for a given budget. With the methods and approach presented here, the temporal resolution of poorly stratified or reworked settings, like cave deposits, which are usually rich in continental faunas, can finally be resolved.
Implications for time span of colluvial deposition
Although the observed time range of the Deserta Grande Island sites DG3 and DG4 were relatively constrained (>6 ka), they could tentatively inform understanding about the timing of colluvial deposition. Colluvium formation is an irregular erosional process driven largely by gravity but influenced by climate and weathering of source sediments; it can occur in different climates and environments and in different orientations and under different conditions (Leopold and Völkel, Reference Leopold and Völkel2007). These properties make colluvial sediments very complex geoarchives to study; constraining the period of colluvial deposition is particularly difficult. Studies dating colluvial deposits (e.g., Kwaad and Mucher, Reference Kwaad and Mucher1979; Lang and Hönscheidt, Reference Lang and Hönscheidt1999) have shown that the period of colluvial deposition cannot be adequately expressed through dating of interred objects alone. However, the shell ages reported here, if corroborated by further sedimentological analysis, may indicate the time of sediment deposition when shells were transported and buried under colluvial sediments (Williams and Polach, Reference Williams and Polach1971). Youngest snail shell ages within colluvial units may indicate periods of recent colluvial sedimentation, whereas older ages may inform about earlier sediment deposition times. While dating of interred fossils may indicate time of colluvial deposition, further sedimentary and geochronologic studies are required to fully understand the timing of colluvial sediment accumulation.
CONCLUSIONS
Land snail shells from two colluvial deposits in the Desertas Islands of the Madeira Archipelago (Portugal) showed an ~6 ka-scale age mixing, exceeding the age variability caused by dating imprecision. This magnitude of time averaging should be considered and applied in future paleobiological and paleoclimatic research in the region. The majority of previous studies examining time averaging and age-frequency distribution have shown a typically right-skewed distribution inconsistent with results presented here. This may represent sampling deficiencies, or alternatively, a shell burial model of age distribution less dominated by taphonomic (destructive) processes.
The results reported here suggest that Quaternary land snails can be effectively dated using the carbonate-target radiocarbon dating method up to ~15–20 cal ka BP. This method allows for relatively inexpensive analysis of multiple individual specimens that makes large-scale dating of assemblages more feasible, particularly when used in conjunction with AAR analysis. This study illustrates that the carbonate-target method opens new opportunities to significantly improve the geochronological context of snail assemblages from unstratified and reworked deposits in archaeological and paleontological investigations.
ACKNOWLEDGMENTS
This research was funded by the 2017 and 2018 Geological Society of America (GSA) Graduate Student Research Grant and the 2017 Society for Sedimentary Geology (SEPM) Graduate Research Grant to EN. Additional funds were provided by the University of Cincinnati. Special thanks go to Katherine Whitacre for assistance with AAR analyses and John Southon (UCI) for AMS 14C analyses. The detailed and constructive reviews by associate editor Jeff Pigati and two anonymous reviewers greatly improved the quality and clarity of this article.