Introduction
It has been proposed (McGrath, Reference McGrath1999) that low prenatal vitamin D modifies the risk of schizophrenia, as it provides a logical explanation for epidemiological risk factors such as winter and spring birth, migration and urbanicity. There is some support in the literature for this hypothesis. Nevertheless, an association between low maternal and neonatal vitamin D and psychotic disorder has not been established conclusively. In a small pilot study, low maternal vitamin D levels during the third trimester of pregnancy were associated with risk of schizophrenia in offspring at trend-level significance, specifically in black mothers (McGrath et al., Reference McGrath, Eyles, Mowry, Yolken and Buka2003). Both low and high neonatal vitamin D concentrations have been associated with increased risk of schizophrenia (McGrath et al., Reference McGrath, Eyles, Pedersen, Anderson, Ko, Burne, Norgaard-Pedersen, Hougaard and Mortensen2010). A larger replication study confirmed only low neonatal vitamin D as a risk factor (Eyles et al., Reference Eyles, Trzaskowski, Vinkhuyzen, Mattheisen, Meier, Gooch, Anggono, Cui, Tan, Burne, Jang, Kvaskoff, Hougaard, Norgaard-Pedersen, Cohen, Agerbo, Pedersen, Borglum, Mors, Sah, Wray, Mortensen and McGrath2018). Another study showed that maternal vitamin D in a birth cohort was not associated with psychotic disorder in offspring at age 18 (Sullivan et al., Reference Sullivan, Wills, Lawlor, McGrath and Zammit2013). If a true association were to exist between maternal and/or neonatal vitamin D and psychotic disorder, this may be connected with known risk factors in early life, e.g. childhood urbanicity (Pedersen and Mortensen, Reference Pedersen and Mortensen2001).
Multiple observational and cross-sectional studies have been performed that have reported low vitamin D levels in individuals with psychotic disorder (Humble et al., Reference Humble, Gustafsson and Bejerot2010; Partti et al., Reference Partti, Heliovaara, Impivaara, Perala, Saarni, Lonnqvist and Suvisaari2010; Itzhaky et al., Reference Itzhaky, Amital, Gorden, Bogomolni, Arnson and Amital2012; Menkes et al., Reference Menkes, Lancaster, Grant, Marsh, Dean and du Toit2012; Boerman et al., Reference Boerman, Cohen, Schulte and Nugter2016); and specifically in dark-skinned immigrants to northern latitudes (Berg et al., Reference Berg, Melle, Torjesen, Lien, Hauff and Andreassen2010; Humble et al., Reference Humble, Gustafsson and Bejerot2010; Dealberto, Reference Dealberto2013). Two meta-analyses support these findings (Belvederi Murri et al., Reference Belvederi Murri, Respino, Masotti, Innamorati, Mondelli, Pariante and Amore2013; Valipour et al., Reference Valipour, Saneei and Esmaillzadeh2014). Additionally, a systematic review of 23 studies found a prevalence of hypovitaminosis D in psychotic patients of over 50% (Adamson et al., Reference Adamson, Lally, Gaughran, Krivoy, Allen and Stubbs2017).
Low vitamin D has been associated with more severe positive (Yuksel et al., Reference Yuksel, Altunsoy, Tikir, Cingi Kuluk, Unal, Goka, Aydemir and Goka2014; Graham et al., Reference Graham, Keefe, Lieberman, Calikoglu, Lansing and Perkins2015) and negative symptoms (Yuksel et al., Reference Yuksel, Altunsoy, Tikir, Cingi Kuluk, Unal, Goka, Aydemir and Goka2014; Graham et al., Reference Graham, Keefe, Lieberman, Calikoglu, Lansing and Perkins2015; Nerhus et al., Reference Nerhus, Berg, Kvitland, Dieset, Hope, Dahl, Weibell, Romm, Faerden, Andreassen and Melle2016; Yee et al., Reference Yee, See, Abdul Rashid, Neelamekam and Lee2016). The directionality of the association between (severity of) psychotic disorder and vitamin D concentration is uncertain. Low vitamin D may be the common denominator of the above mentioned risk factors (winter and spring birth, migration and urbanicity) (McGrath, Reference McGrath1999), suggesting an etiological role. However, low vitamin D may also well be the consequence of (prodromal) negative symptoms inherent to the disease, as patients exhibit withdrawal and inactivity and subsequently are less exposed to sunlight.
Urbanicity impacts vitamin D synthesis. Air pollution, shaded areas and more time spent indoors are examples of factors in urban settings that do not benefit vitamin D concentration (Mendes et al., Reference Mendes, Darling, Hart, Morse, Murphy and Lanham-New2019). An alternative explanation is that urbanicity is associated with environmental adversity. Childhood trauma has been associated with psychotic disorder, with incremental odds ratios as levels of childhood urbanicity increase (Frissen et al., Reference Frissen, Lieverse, Drukker, van Winkel and Delespaul2015). The same study finds evidence that childhood urbanicity and trauma are positively associated (Frissen et al., Reference Frissen, Lieverse, Drukker, van Winkel and Delespaul2015).
Studies conducted in populations presenting with first-episode psychosis (FEP) have found both low levels of vitamin D (Crews et al., Reference Crews, Lally, Gardner-Sood, Howes, Bonaccorso, Smith, Murray, Di Forti and Gaughran2013; Salavert et al., Reference Salavert, Grados, Ramiro, Carrion, Fadeuilhe, Palma, Lopez, Erra and Ramirez2017) and absence of association between vitamin D and FEP (Graham et al., Reference Graham, Keefe, Lieberman, Calikoglu, Lansing and Perkins2015; Nerhus et al., Reference Nerhus, Berg, Dahl, Holvik, Gardsjord, Weibell, Bjella, Andreassen and Melle2015). Interestingly, Crews et al. (Reference Crews, Lally, Gardner-Sood, Howes, Bonaccorso, Smith, Murray, Di Forti and Gaughran2013) did not find an association with negative symptoms in FEP, hereby perhaps providing indirect support for an aetiological mechanism. A study from Singapore revealed, for the first time, decreased levels of vitamin D in FEP at low latitude (Yee et al., Reference Yee, See, Abdul Rashid, Neelamekam and Lee2016). A meta-analysis (including the above mentioned negative findings) found that vitamin D was low in FEP (Firth et al., Reference Firth, Carney, Stubbs, Teasdale, Vancampfort, Ward, Berk and Sarris2017).
Given increased understanding of vitamin D's extraskeletal functions, specifically in the brain, the association between vitamin D and psychotic disorder may be biologically plausible. The effects of vitamin D in the brain are diverse, including the promotion of antioxidative and neurotrophic action, as well as regulation of various neurotransmitter systems (including dopamine) (Humble, Reference Humble2010). In animal studies, offspring of vitamin D deficient rodents exhibit structural brain alterations resembling those seen in schizophrenia, such as thinning of the cortex, enlarged lateral ventricles and increased brain size due to higher proliferation and lower elimination of neurons (Eyles et al., Reference Eyles, Burne and McGrath2011, Reference Eyles, Burne and McGrath2013). In psychotic disorder, vitamin D has been positively associated with peripheral gray matter volume, possibly indicative of a neuroprotective effect (Berg et al., Reference Berg, Jorgensen, Nerhus, Athanasiu, Popejoy, Bettella, Norbom, Gurholt, Dahl, Andreassen, Djurovic, Agartz and Melle2018).
Vitamin D3, also known as cholecalciferol, is synthesized in the skin following ultraviolet-B (UVB) exposure. Subsequently, two hydroxylations are required for vitamin D3 to attain a biologically active form. The first hydroxylation occurs in the liver by action of 25-hydroxylase, converting cholecalciferol to calcidiol [25-hydroxyvitamin D3, abbreviated as 25(OH)D3]. Hydroxylation of calcidiol by 1-α-hydroxylase (in the kidneys) results in calcitriol [1,25-dihydroxyvitamin D3, abbreviated as 1,25(OH)2D3] (Humble, Reference Humble2010; Eyles et al., Reference Eyles, Burne and McGrath2011; DeLuca et al., Reference DeLuca, Kimball, Kolasinski, Ramagopalan and Ebers2013). Vitamin D metabolites cross the blood-brain barrier for they are present in cerebrospinal fluid (Eyles et al., Reference Eyles, Burne and McGrath2011). Furthermore, there is evidence that conversion of calcidiol to calcitriol also occurs on site in the central nervous system, as enzymes responsible for both the synthetization and degradation of calcitriol have been detected in the brain (DeLuca et al., Reference DeLuca, Kimball, Kolasinski, Ramagopalan and Ebers2013; Eyles et al., Reference Eyles, Burne and McGrath2013). Although calcitriol is the active form, vitamin D status is assessed by measurement of calcidiol in blood. For clarification, vitamin D concentrations as discussed in this paper are in fact calcidiol [25(OH)D3] concentrations.
In this study of over 300 patients with psychotic disorder, we aim to investigate associations between vitamin D and disease status, symptomatology and other characteristics pertaining to psychotic disorder. To the best of our knowledge, the present study is the first to examine a potential relationship between vitamin D and urbanicity in individuals with psychotic disorder compared to controls. We expect to replicate the finding that vitamin D levels are reduced in patients with psychotic disorder. Furthermore, we anticipate a moderating effect of both birth and current urbanicity [suggestive of a role for (early life) environmental adversity]. We predict that vitamin D and symptom levels are inversely related and that vitamin D is lower in patients with longer illness duration.
Materials and methods
Participants
Data was collected in the context of a multicenter study Genetic Risk and Outcome of Psychosis (GROUP) in the Netherlands (Korver et al., Reference Korver, Quee, Boos, Simons and de Haan2012; van Mierlo et al., Reference van Mierlo, de Witte, Derksen and Otten2015), and representative parts of Belgium. Patients with a minimum age of 16 years with a diagnosis of non-affective psychosis were included. They were recruited through the mental health services where they were treated, either as outpatients (the majority of the sample) or inpatients. Diagnosis was based on DSM-IV criteria, assessed with the Comprehensive Assessment of Symptoms and History (CASH) interview (Andreasen et al., Reference Andreasen, Flaum and Arndt1992). Control subjects were recruited from the same area as described above, using random mailings in nearby municipalities and through advertisement in newspapers. The CASH was also used to confirm the absence of lifetime diagnosis of psychotic disorder in the control subjects. For the control subjects, the occurrence of any psychotic disorder in either the subject or any first-degree family member, assessed using the Family Interview for Genetic Studies, constituted an exclusion criterion. Sufficient command of the Dutch language was an additional criterion for inclusion.
Previous work using plasma samples from the GROUP study had 651 participants (van Mierlo et al., Reference van Mierlo, de Witte, Derksen and Otten2015). The present study analyzed 629 out of the original 651 samples, 22 samples were lost due to insufficient remaining plasma or laboratory error. No selection was made from the original sample.
The cohort consisted of 347 patients with a psychotic disorder and 282 controls. Of the patients, 277 were diagnosed with schizophrenia and 56 were diagnosed with schizoaffective disorder. Fourteen patients were diagnosed with schizophreniform disorder. The sample included 42 controls with a history of depressive disorder or dysthymia, as non-psychotic psychiatric morbidity was not an exclusion criterion for control subjects in the GROUP study.
Measures
Urbanicity
Both current urbanicity and urbanicity at birth were assessed. Urban exposure was defined at five levels according to the Dutch Central Bureau of Statistics rating: 1 ⩽ 500 inhabitants per square kilometer (km2), 2 = 500–1000/km2, 3 = 1000–1500/km2, 4 = 1500–2500/km2 and 5 ⩾ 2500/km2.
Positive and negative symptoms
Psychotic symptomatology was assessed with the Positive and Negative Syndrome Scale (PANSS) (Kay et al., Reference Kay, Fiszbein and Opler1987). The scores of the individual items of the positive and negative symptom dimensions were summed to obtain a total positive and negative score respectively.
Vitamin D status
Vitamin D status was classified as sufficient above 75 nanomol per liter (nmol/l).
Sampling season
The season in which blood was sampled was taken into account. Seasons were classified as follows: winter (December to February), spring (March to May), summer (June to August) and fall (September to November).
Plasma analysis
Blood was acquired by venipuncture. Samples were centrifuged and plasma was stored at −80 °C until analysis. 25-hydroxyvitamin D3 was isolated from blood plasma using solid phase extraction, followed by liquid chromatography (Spark Holland Symbiosis online SPE system). Detection of 25(OH)D3 was performed by isotope dilution tandem mass spectrometry (Waters Quattro Premier XE MS/MS) (ref: https://www.ncbi.nlm.nih.gov/pubmed/22247500).
Ethics statement
The study was approved by the standing ethics committee of Maastricht University Medical Center. All participants gave written informed consent in accordance with the committee's guidelines.
Statistical analyses
Multiple regression analyses were conducted in Stata (version 13.1), in which vitamin D concentration was the dependent variable and group was the independent variable. Within group analyses were performed specifically in patients to explore potential associations between vitamin D, symptom levels and other clinical variables. All analyses were adjusted for the a priori hypothesized confounders of sex, age, body mass index (BMI), smoking (number of cigarettes per day), ethnicity and season of sample acquisition.
Group × urbanicity interaction terms in the model of vitamin D concentration were investigated by Wald test (Clayton and Hills, Reference Clayton and Hills1993), both current urbanicity and urbanicity at birth were assessed. In order to even-out distribution while preserving a possible dose-response effect, the five urbanicity levels were converted into tertiles (low/medium/high) conform previous work in this sample (Frissen et al., Reference Frissen, van Os, Habets, Gronenschild and Marcelis2017). Interaction analyses were corrected for the same potential confounders as above. Additionally, the current urbanicity analysis was corrected for urbanicity at birth and vice versa. In case of significant interactions, analyses were stratified by group to further elucidate the association between urbanicity and vitamin D concentration.
Results
Descriptive analyses
There were more women in the control group, yet more male than female patients. Controls were older, by approximately 4 years. Significantly more non-Caucasian ethnicity was represented in the patient group. Patients had higher BMI and smoked more cigarettes per day (Table 1). Urbanicity at birth did not differ significantly between groups (Fig. 1), whereas current urbanicity was highest in patients (p = 0.007) (Fig. 2). There was no significant difference in sampling season between groups (p = 0.341).
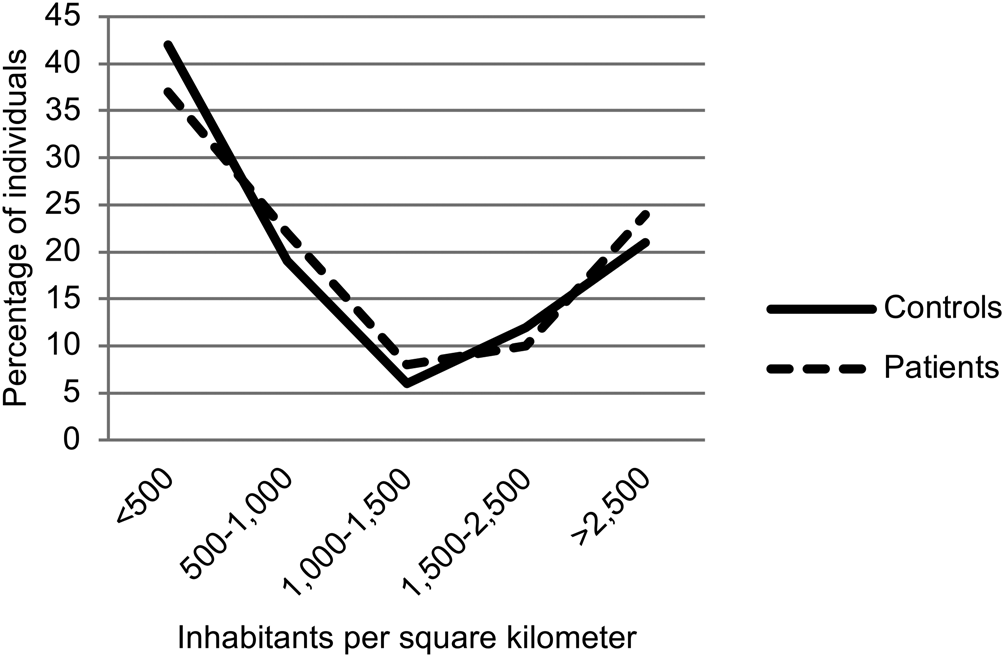
Fig. 1. Urbanicity at birth.

Fig. 2. Current Urbanicity.
Table 1. Demographic characteristics

Y/N/NA, yes/no/not available.
Means ± standard deviations reported.
Two hundred seventy-five out of 347 patients reported current use of antipsychotic (AP) medication. Seventy-two patients used clozapine, 62 used olanzapine, 43 used risperidone, 30 used aripiprazole, 21 used quetiapine, 13 used haloperidol, 6 used flupentixol, 5 zuclopenthixol, 5 pimozide, 4 penfluridol, 4 amisulpride, 1 bromperidol, 1 sulpiride, 1 sertindol, and 7 patients were unable to specify their AP use.
Associations between group and vitamin D concentration
Mean 25(OH)D3 concentrations (in nmol per liter) were 60.1 (±26.5) in controls and 47.0 (± 26.1) in patients. 37.5% of controls had sufficient vitamin D status compared to 17.2% of patients (Table 1). After corrections for potential confounders (sex, age, BMI, smoking, ethnicity and sampling season), vitamin D was significantly lower in patients than controls [B = −8.05; 95% confidence interval (CI) −13.68 to −2.42; p = 0.005].
Vitamin D and clinical variables in patients
In patients, a small but significant effect was detected: higher vitamin D concentration was associated with lower positive (B = −0.02; 95% CI −0.04 to 0.00; p = 0.049) and negative symptom levels (B = −0.03; 95% CI −0.05 to −0.01; p = 0.008) (Table 2). Neither illness duration nor the current use of antipsychotic medication (AP) was associated with vitamin D concentration in patients (Table 2).
Table 2. Associations between vitamin D and clinical variables in patients

B represents the regression coefficient from the multiple regression analyses.
Associations between vitamin D and urbanicity
The group × current urbanicity interaction was not significant (χ2 = 1.50 and p = 0.224). In contrast, the group × urbanicity at birth interaction was significant (χ2 = 6.76 and p = 0.001). Stratified analyses showed that higher urbanicity levels at birth were signicificantly associated with lower vitamin D concentration in patients (B = −5.11; 95% CI −9.41 to −0.81; p = 0.020). This negative association was absent in controls (B = 0.72; 95% CI −4.02 to 5.46; p = 0.765).
Discussion
Vitamin D levels in patients with psychotic disorder were lower than in controls. In the patient group, there was an inverse relationship between vitamin D and both positive and negative symptom levels. Illness duration and present use of AP medication were not associated with vitamin D level. There was a significant interaction between group and urbanicity at birth; in patients, high urbanicity at birth was associated with decreased vitamin D.
Findings
Our finding of lower vitamin D concentration in patients with psychotic disorder compared to controls is in accordance with previous original studies (Humble et al., Reference Humble, Gustafsson and Bejerot2010; Partti et al., Reference Partti, Heliovaara, Impivaara, Perala, Saarni, Lonnqvist and Suvisaari2010; Itzhaky et al., Reference Itzhaky, Amital, Gorden, Bogomolni, Arnson and Amital2012; Menkes et al., Reference Menkes, Lancaster, Grant, Marsh, Dean and du Toit2012; Boerman et al., Reference Boerman, Cohen, Schulte and Nugter2016) and two meta-analyses (Belvederi Murri et al., Reference Belvederi Murri, Respino, Masotti, Innamorati, Mondelli, Pariante and Amore2013; Valipour et al., Reference Valipour, Saneei and Esmaillzadeh2014). As such, this study contributes to the growing body of evidence of an association between vitamin D and psychotic disorder. Due to its cross-sectional design, the current study does not contribute to a better understanding of the directionality of the association between vitamin D and psychotic disorder. As argued by Crews and colleagues, there are several viable explanations for low vitamin D status at onset of psychotic disorder (Crews et al., Reference Crews, Lally, Gardner-Sood, Howes, Bonaccorso, Smith, Murray, Di Forti and Gaughran2013). Low vitamin D may be a true risk factor of psychotic disorder. Alternatively, it may the consequence of (prodromal) negative symptoms inherent to the disease, as patients exhibit behavior such as withdrawal and inactivity and subsequently are less exposed to sunlight (Bruins et al., Reference Bruins, Jorg, van den Heuvel, Bartels-Velthuis, Corpeleijn, Muskiet, Pijnenborg and Bruggeman2017).
An innovative study aiming to clarify causality in the relationship between vitamin D and schizophrenia used Mendelian randomization to examine bidirectional associations between genetic variants related to vitamin D and a schizophrenia polygenic risk score; no associations were uncovered (Taylor et al., Reference Taylor, Burgess, Ware, Gage, Richards, Davey Smith and Munafo2016). However, the study was designed upon the assumption that vitamin D related genetic variants are associated with biologically available vitamin D. The authors cannot rule out a threshold effect of vitamin D and that power may have been lacking to detect this (Taylor et al., Reference Taylor, Burgess, Ware, Gage, Richards, Davey Smith and Munafo2016).
Vitamin D concentration and both positive and negative symptoms of psychotic disorder were inversely related. Although the association was clearly significant, the effect sizes were small and therefore of dubious clinical relevance. Generally, our patient sample was clinically stable with an average illness duration of approximately seven years, possibly resulting in too little symptom variance to detect a meaningful effect. Nevertheless, the inverse relationship between vitamin D and symptoms of psychotic disorder is in line with prior work. Low vitamin D has been associated with more severe positive (Yuksel et al., Reference Yuksel, Altunsoy, Tikir, Cingi Kuluk, Unal, Goka, Aydemir and Goka2014; Graham et al., Reference Graham, Keefe, Lieberman, Calikoglu, Lansing and Perkins2015) and negative symptoms (Yuksel et al., Reference Yuksel, Altunsoy, Tikir, Cingi Kuluk, Unal, Goka, Aydemir and Goka2014; Graham et al., Reference Graham, Keefe, Lieberman, Calikoglu, Lansing and Perkins2015; Nerhus et al., Reference Nerhus, Berg, Kvitland, Dieset, Hope, Dahl, Weibell, Romm, Faerden, Andreassen and Melle2016; Yee et al., Reference Yee, See, Abdul Rashid, Neelamekam and Lee2016). In contrast, two studies found no association between vitamin D levels and symptomatology, in first-episode psychosis (Crews et al., Reference Crews, Lally, Gardner-Sood, Howes, Bonaccorso, Smith, Murray, Di Forti and Gaughran2013) and diagnosed schizophrenia (Itzhaky et al., Reference Itzhaky, Amital, Gorden, Bogomolni, Arnson and Amital2012).
Absence of associations between vitamin D status and the use of AP medication and illness duration have also been reported in earlier publications by Crews et al. (Reference Crews, Lally, Gardner-Sood, Howes, Bonaccorso, Smith, Murray, Di Forti and Gaughran2013) and Suetani et al. (Reference Suetani, Saha, Eyles, Scott and McGrath2017) respectively. In our sample, this is an indication that these two secondary disease factors, that are often a source of confounding, do not explain our findings.
To the best of our knowledge, this is the first time that the association between vitamin D and urbanicity has been investigated in individuals with psychotic disorder. High urbanicity at birth was associated with lower vitamin D in patients with psychotic disorder. This is an interesting finding as it links a known risk factor in early life, i.e. childhood urbanicity (Pedersen and Mortensen, Reference Pedersen and Mortensen2001), with low vitamin D as a factor associated with schizophrenia in later life, after the onset of disease.
Birth season has been shown to affect vitamin D concentration in adulthood, i.e. those born in winter have lower vitamin D in adulthood (Lippi et al., Reference Lippi, Bonelli, Buonocore and Aloe2015). It is unclear why a similar effect of urbanicity at birth (assuming this is related to neonatal vitamin D) is not related to vitamin D levels in controls in the present study. Possibly, there are other (protective) mechanisms or behaviors of influence in individuals without psychotic disorder.
Dealberto has argued that an interaction between vitamin D deficit and (childhood) adversity, leading to social defeat, culminates in increased risk of psychosis (Dealberto, Reference Dealberto2013). Urbanicity at birth and low vitamin D in later life may be yet another interaction influencing the etiology of schizophrenia. Low vitamin D status is clearly only a piece of the puzzle, as insufficient vitamin D concentration in controls was also highly prevalent (62.5%).
Urbanicity at birth may be a proxy for neonatal vitamin D status, alternatively it may be associated with maternal lifestyle. Duration of (childhood) urban exposure and associated lifestyle factors were not assessed in the present study, this may have provided more insight. Interestingly, a study conducted in the urban environment of The Hague in the Netherlands reported that migration from a non-Western country between zero and four years of age resulted in highest incidence rate ratios (Veling et al., Reference Veling, Hoek, Selten and Susser2011); the authors state low vitamin D as a possible mechanism.
To date, vitamin D still offers a ‘parsimonious explanation' for epidemiological risk factors of schizophrenia (McGrath, Reference McGrath1999). As summarized by Humble and colleagues (Humble, Reference Humble2010), it may explain excess winter or spring births (Davies et al., Reference Davies, Welham, Chant, Torrey and McGrath2003), higher risk at higher latitude (Saha et al., Reference Saha, Chant, Welham and McGrath2006; Kinney et al., Reference Kinney, Teixeira, Hsu, Napoleon, Crowley, Miller, Hyman and Huang2009) and higher risk in immigrants (especially dark-skinned individuals) (Cantor-Graae and Selten, Reference Cantor-Graae and Selten2005; Dealberto, Reference Dealberto2007, Reference Dealberto2010). Vitamin D deficiency potentially offers an alternative or complementary explanation to the social defeat hypothesis (McGrath, Reference McGrath1999; Eyles et al., Reference Eyles, Burne and McGrath2013), it (and other environmental influences) may underlie the risk related to migration and urbanicity (Kirkbride and Jones, Reference Kirkbride and Jones2011). If this is the case, there may be a possibility for prevention.
Randomized controlled trials (RCTs) investigating the effect of vitamin D supplementation as adjunctive therapy in schizophrenia are surprisingly lacking. One recent study investigated vitamin D supplementation in schizophrenia, specifically in chronic patients treated with clozapine (Krivoy et al., Reference Krivoy, Onn, Vilner, Hochman, Weizman, Paz, Hess, Sagy, Kimhi-Nesher, Kalter, Friedman, Friedman, Bormant, Trommer, Valevski and Weizman2017): this small RCT demonstrated a trend towards improved cognition after 8 weeks of supplementation. In a population-based study of ample size, high dietary intake of vitamin D was associated with lower psychotic-like symptoms in women (Hedelin et al., Reference Hedelin, Lof, Olsson, Lewander, Nilsson, Hultman and Weiderpass2010). Large RCTs with sufficient follow-up are required to examine the treatment potential of vitamin D supplementation in schizophrenia and other psychotic disorders. Besides amelioration of symptoms, vitamin D supplementation may also be beneficial in reducing physical comorbidity in psychotic disorder, such as decreased bone mineral density (Gomez et al., Reference Gomez, Stubbs, Shirazi, Vancampfort, Gaughran and Lally2016), inflammation (Zhu et al., Reference Zhu, Liu, Zhang, Chu, Wu, Li, Ge, Dong and Zhu2015), metabolic syndrome (Bruins et al., Reference Bruins, Jorg, van den Heuvel, Bartels-Velthuis, Corpeleijn, Muskiet, Pijnenborg and Bruggeman2017; Yoo et al., Reference Yoo, Choi, Hong, Lee, Kim, Shin, Yang, Amminger, Berk, Yoon and Kim2018) and cardiovascular risk (Lally et al., Reference Lally, Gardner-Sood, Firdosi, Iyegbe, Stubbs, Greenwood, Murray, Smith, Howes and Gaughran2016). These are important issues as the degree of excess natural-cause mortality in psychotic disorder is striking (Reininghaus et al., Reference Reininghaus, Dutta, Dazzan, Doody, Fearon, Lappin, Heslin, Onyejiaka, Donoghue, Lomas, Kirkbride, Murray, Croudace, Morgan and Jones2015). A simple and safe intervention such as vitamin D supplementation may be able to make a meaningful difference in the lives of individuals with psychotic disorder. The need for intervention studies aside, adjunctive treatment with vitamin D is already a clinically sound option. To illustrate: in this large and heterogeneous sample, generalizable to other populations, vitamin D insufficiency was rampant at 82.8%.
Methodological considerations
There are several limitations in the present study that deserve mention. The present study contributes to the literature by corroborating previous findings of group differences in vitamin D and associations with symptomatology, in a large sample of patients with psychotic disorder and non-psychotic controls. However, due to its cross-sectional design no conclusions can be drawn regarding the directionality of the association between vitamin D concentration and psychotic disorder (as discussed above).
Although analyses were corrected for ethnicity, this remains an important possible confounder as there were significantly more non-Caucasian individuals in the patient group. Skin with more melanin needs more UVB to produce vitamin D. Furthermore, non-Caucasian individuals may avoid the sun and/or wear more concealing clothing due to cultural and religious background (Mendes et al., Reference Mendes, Darling, Hart, Morse, Murphy and Lanham-New2019). In the present study, we did not account for these factors. Matching for ethnicity and certain cultural/religious behavior is a way to avoid this issue in future work.
Controls with a history of depression (n = 42) were a part of the study sample. We did not exclude these individuals in order to preserve the methodology of the GROUP study (Korver et al., Reference Korver, Quee, Boos, Simons and de Haan2012). It may be argued that their inclusion leads to more correct representation of the general population, as opposed to completely healthy controls. An additional motive is of course to maintain statistical power. If findings were influenced, we expect to have diluted the group difference as depression is also associated with decreased vitamin D concentration (Ju et al., Reference Ju, Lee and Jeong2013).
As stated in the Introduction, the GROUP study recruited cases and controls from similar areas. Although current urbanicity was significantly different between cases and controls, there remains a possibility that the absence of an interaction between group and current urbanicity is caused by recruitment in similar areas of residence. Replication studies are required.
It should be noted that the optimal range of vitamin D concentration for extraskeletal health is undetermined. This study applied the minimum of 75 nanomol per liter, above which vitamin D concentration was considered sufficient. This is in line with the majority of work in this field. The American Geriatric association also employs a minimum level of 75 nanomol per liter for adequate skeletal health and to minimize fracture risk (American Geriatric Society, 2014). However, regulation of CYP27B1 action in non-renal tissue (e.g. the brain) differs from that in the kidney, therefore serum levels for extraskeletal effects may need to be higher (Berg et al., Reference Berg, Melle, Torjesen, Lien, Hauff and Andreassen2010). The minimum threshold to promote mental health is currently still unknown.
Conclusions
Lower vitamin D levels in patients with psychotic disorder, dependent on the level of urbanicity at birth, possibly indicate accumulation of risk due to factors both in early and later life. The within patient group associations between symptoms and vitamin D levels suggest that lower vitamin D may contribute to symptom formation or that the clinical phenotype impacts vitamin D status.
Acknowledgements
The authors thank Truda Driesen and Inge Crolla for their coordinating roles in the data collection, as well as the G.R.O.U.P. investigators: Richard Bruggeman, Wiepke Cahn, Lieuwe de Haan, René S. Kahn, Carin Meijer, Inez Myin-Germeys, Jim van Os and Durk Wiersma.
Financial support
This work was supported by the Dutch organization for scientific research NWO [Genetic Risk and Outcom of Psychosis (G.R.O.U.P.)], and the European Community's Seventh Framework Programme under grant agreement No. HEALTH-F2-2009-241909 (European Network of National Schizophrenia Networks Studying Gene-Environment Interactions Consortium). Both funding sources had no further role in the study design; in the collection, analysis and interpretation of data; in the writing of the report, nor in the decision to submit the paper for publication.
Conflict of interest
Jim van Os is or has been, in the last 3 years, an unrestricted research grant holder with, or has received financial compensation as an independent symposium speaker from: Lundbeck and Janssen. Machteld Marcelis has received, in the last 3 years, financial compensation as an independent symposium speaker from Janssen. All other authors report no biomedical financial interests or potential conflicts of interest.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.






