Introduction
Electroconvulsive therapy (ECT) is a highly effective treatment for severe depression (UK ECT Review Group, 2003) but questions remain regarding the extent of the effect of the treatment on cognitive function. It is generally accepted that after remission of the acute disorientation phase following ECT, both retrograde and anterograde memory impairments are common (Rami-Gonzajez et al. Reference Rami-Gonzajez, Bernardo, Boget, Gil-Verona, Salamero and Junque2001).
A number of studies have examined the cognitive consequences of ECT. However, few of these have comprehensively focused on cognitive functioning during or following treatment. This gap in evidence was raised in the UK ECT Review Group (2003) systematic review of randomized controlled trials of data focusing on the efficacy and safety of ECT. The review concluded that current evidence does not provide a clear quantitative estimate of the degree of cognitive impairment that persists after symptomatic recovery and little evidence on the possible long-term cognitive effects of ECT is available.
At present there is no single standardized method for measuring the cognitive consequences of ECT. ECT clinics across the world vary in their selection of which test or tests they employ to measure potential memory loss during or after a course of ECT. For example, many use simple neuropsychological tests which give gross measures of mental function such as the Mini-Mental State Examination (Folstein et al. Reference Folstein, Folstein and McHugh1975). Other tests used such as the Rey Auditory Verbal Learning test (Ryan et al. Reference Ryan, Geisser, Randall and Georgemiller1986), the Rey Complex Figure Test (Spreen & Strauss, Reference Spreen and Strauss1991), or the subtests of the Wechsler Memory Scale (Wechsler, Reference Wechsler1987) may not adequately reflect the levels of impairment experienced by individuals. Furthermore, Robertson & Pryor (Reference Robertson and Pryor2006) have reviewed the limited worth of very simple, brief measures generally used by researchers – typically, highly structured tests of verbal learning involving familiar material with very short intervals within testing to detect cognitive deficits (Robertson & Pryor, Reference Robertson and Pryor2006).
Some studies have adopted more extensive neuropsychological batteries that include a range of neuropsychological tests which can include both objective and subjective measures. Nevertheless, in most studies the tests used are inadequate for detecting memory changes. This is because most of the tests employed have not been specifically developed to assess the nature of memory deficits as a consequence of ECT. Tests of memory and cognitive ability should assess a range of functions because ECT impairment may vary not only between individuals but also within individuals when they undergo more than one course of treatment (Goldstein et al. Reference Goldstein, Filskov and Weaver1977).
This paucity of research means that patients' questions about safety and the impact of ECT cannot be comprehensively answered. More data are required on the cognitive consequences of ECT in order to, if appropriate, increase the acceptability of this treatment to patients and perhaps to some clinicians.
The aim of the present study was to evaluate the utility of using the Cambridge Neuropsychological Test Automated Battery (CANTAB) in measuring the cognitive impact of a course of ECT on visual and visuospatial memory on a clinical sample of severely depressed patients aged from 26 to 83 years who met the International Classification of Diseases (ICD)-10 diagnostic criteria. To our knowledge this is the first time the CANTAB has been used to examine cognitive and neurological functioning of patients during and following a course of ECT. The CANTAB (Fray et al. Reference Fray, Robbins and Sahakian1996) has been extensively validated for assessing brain–behaviour relationships in adult populations (Robbins et al. Reference Robbins, James, Owen, Sahakian, McInnes and Rabbitt1994) and has shown to be sensitive to detecting brain dysfunctions in the frontal, temporal and amygdalo-hippocampal regions (Owen et al. Reference Owen, Sahakian, Semple, Polkey and Robbins1995). Furthermore the CANTAB has been supported by other studies of patients with neurodegenerative diseases (Sahakian et al. Reference Sahakian, Downes, Eagger, Evenden, Levy, Philpot, Roberts and Robbins1990; Sahgal et al. Reference Sahgal, Sahakian, Robbins, Wray, Lloyd, Cook, McKeith, Disley, Eagger, Boddington and Edwardson1991; Lange et al. Reference Lange, Sahakian, Quinn, Marsden and Robbins1995; Fowler et al. Reference Fowler, Saling, Conway, Semple and Louis1997; Owen et al. Reference Owen, Iddon, Hodges, Summers and Robbins1997; Rahman et al. Reference Rahman, Robbins and Sahakian1999) and psychiatric disorders such as depression (Elliott et al. Reference Elliott, McKenna, Robbins and Sahakian1996; Porter et al. Reference Porter, Gallagher, Thompson and Young2003). If the CANTAB does prove to be an effective measure of memory problems associated with ECT it could provide a standardized method of assessment.
Methods
Subjects
The patient sample consisted of 17 women and seven men (see Fig. 1); the average age of the participants was 52 years (s.d.=16.54, range 26–83 years). They were in-patients or out-patients at Royal Cornhill Hospital (Aberdeen, North-East Scotland, UK), referred for ECT with a clinical diagnosis of a depressive disorder. All participants satisfied ICD-10 criteria for major depression (single episode or recurrent). Patients were excluded from the study if they had gross brain damage, learning disability, a major psychiatric disorder which was not a depressive disorder (e.g. schizophrenia or another functional psychosis) or co-morbid substance misuse. Patients were at least 18 years old and had not received ECT in the past 6 months. All patients were receiving antidepressant medication, and had previously failed to respond to at least one course of chemical treatment. All participating patients gave written, informed consent to take part in the study and for their data to be published anonymously. Ethics approval was granted from the Grampian Research Ethics Committee.
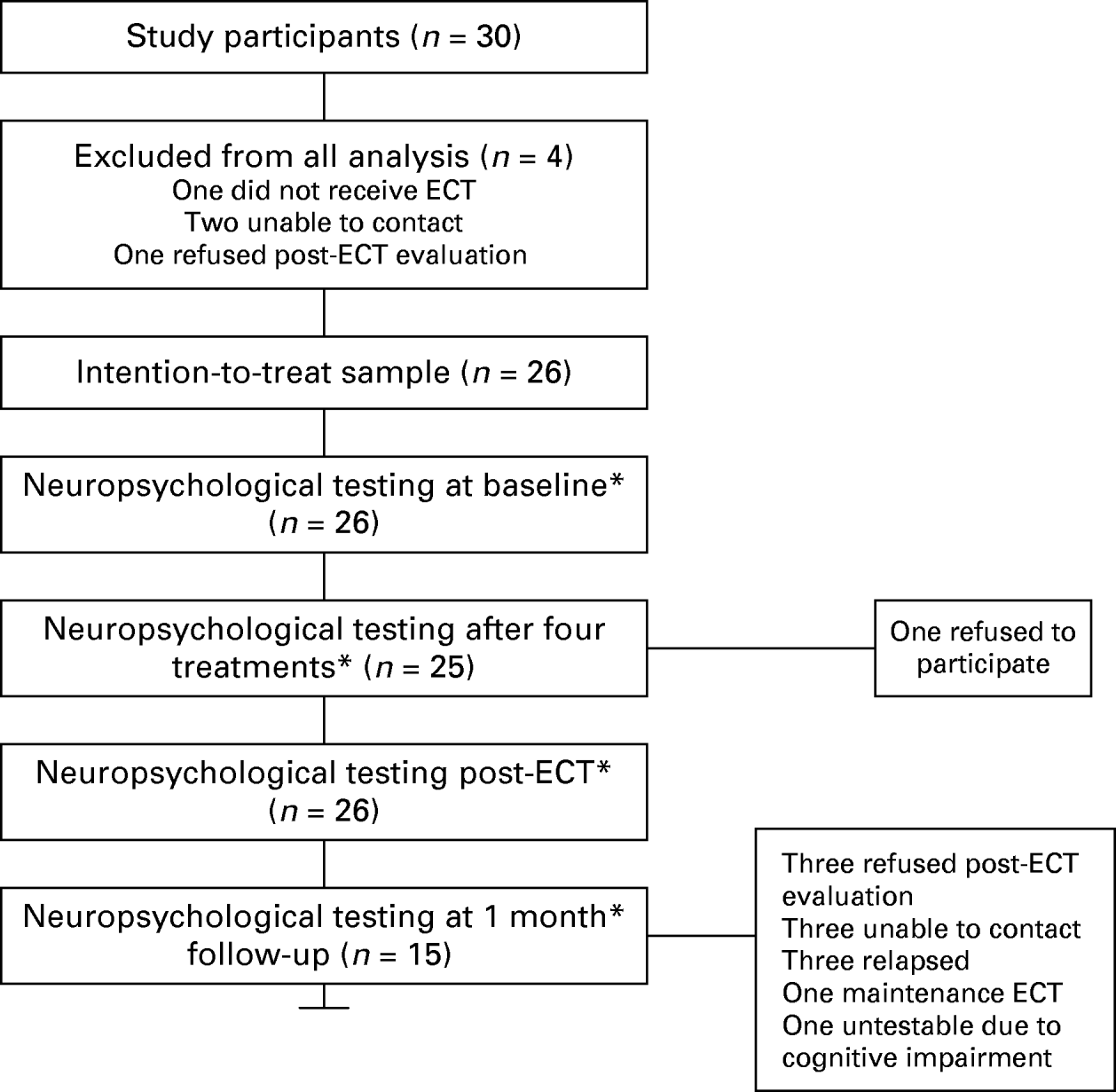
Fig. 1. Participant flow chart. ECT, Electroconvulsive therapy. * Includes at least one cognitive test from the battery.
Clinical ratings and neuropsychological testing
The clinical effects of ECT were assessed using the Montgomery–Åsberg Depression Rating Scale (Montgomery & Åsberg, Reference Montgomery and Åsberg1979) and the Clinical Global Impressions scale. Admission ratings were made 1 day before the first treatment and discharge ratings were made 1–7 days after the final treatment as part of the clinical routine.
The neuropsychological computerized tests were administered the day before the start of treatment, 1–3 days after four treatments, within the week after the final treatment and 1 month after the final treatment. The tests were taken from the visual memory battery within the CANTAB. The CANTAB was selected for this study because of its advantages of efficiency, the achievement of highly standardized administrations, and automated response recording that would be difficult to accomplish by hand. For example, response times can be recorded with millisecond precision, which can be important for scoring purposes (Luciana, Reference Luciana2003). CANTAB subtests are also very simple to administer and complete, staff training is minimal and the tests are acceptable to severely depressed or elderly patients who lack motivation and/or find instructions hard to follow (Fraser & Glass, Reference Fraser and Glass1980). In addition, the CANTAB has the added availability of parallel forms for retest. This was important to facilitate the interpretation of findings from repeated-measures designs by excluding the possibility of re-learning from the original visual subtests.
Not all participants completed the full battery of tests at all time points (see Table 1). Patients were included in the analysis if pre-treatment and at least one of the post-treatment tests was completed. Tests were administered according to CANTAB manual protocols, on a colour, touch-sensitive screen. Descriptions of the tests are summarized below. For more detailed descriptions, see Owen et al. (Reference Owen, Sahakian, Semple, Polkey and Robbins1995).
Tests of visuospatial memory and learning
Pattern recognition memory (PRM)
Subjects were required to learn a series of 12 simple, but abstract, coloured visual patterns located in the centre of the screen. Each pattern was presented for 3 s. In the recognition phase, 12 pairs of coloured patterns were then presented on the screen one at a time and the subject had to identify the target pattern from a ‘distractor’ pattern. This procedure was repeated with 12 new patterns, and the subject was given a total score (maximum=24) expressed as a percentage.
Spatial recognition memory
Subjects were required to learn the on-screen spatial location of five unfilled, white squares, appearing one at a time in different locations on the screen for 3 s. In the recognition phase, two squares appeared simultaneously on the screen and the subject had to target the familiar one. The procedure was repeated three more times using new locations for the target. The subject was given a total correct score (maximum=20) expressed as a percentage.
Paired-associates learning (PAL)
The CANTAB PAL task (Sahakian et al. Reference Sahakian, Morris, Evenden, Heald, Levy, Philpot and Robbins1988) requires subjects to learn and then replicate the matching of two complex pattern–location associations from six white boxes within 10 attempts. The number of pattern–location pairs then increase to three, to six and finally eight. In total there were two sets with two patterns, one set with three patterns, then six and lastly eight. Performance was assessed according to three measures: (i) ‘memory score’ represents the total number of patterns correctly located after the first presentation, summed over the entire five trials of the task; (ii) ‘trials’ represents the total number of presentation required to locate all the patterns correctly over the entire task; and (iii) ‘errors’ represents the total number of errors summed across the entire task.
Simultaneous and delayed matching to sample
Delayed matching to sample presents the subject with a complex visual pattern (the sample) and then, after a brief delay of either 0, 4 or 12 s, it disappears and the subject is then presented with four patterns from which she or he must choose the sample pattern. Each pattern is made up of four sub-elements, each of a different colour. One of the choice patterns is identical to the sample, one is a novel distracter pattern, one has the shape of the sample and the colours of the distracter, and the fourth has the colours of the sample and the shape of the distracter. To discourage strategies based on encoding single quadrants, all four choice patterns have a quadrant in common with the sample. In the simultaneous matching condition the four choice patterns appear beneath the sample pattern while it remains on the screen simultaneously.
ECT
All subjects received ECT two times per week via electrodes placed in the standard bifrontotemporal (bilateral) position. ECT was administered in accordance with the guidelines set out by the Royal College of Psychiatrists (The ECT Handbook; Scott, 2005), with a brief pulse, constant current apparatus (Thymatron DGx, Somatics Inc., USA). Intramuscular glycopyrrolate (0.2 mg/kg) was given approximately 30 min before ECT. All treatments were given under anaesthesia with either propofol (1–2 mg/kg), thiopentone sodium (3–5 mg/kg) or etomidate (0.3 mg/kg). The muscle relaxant of choice was suxamethonium (0.5 mg/kg). All patients received positive pressure ventilation with 100% oxygen until resumption of spontaneous respiration. Seizure duration was monitored clinically in a cuffed upper limb and by the electroencephalogram, included in the ECT device. The first ECT stimulus was titrated to threshold, defined as the lowest stimulus intensity (millicoulombs) which induced a seizure exceeding 15 s in duration. Subsequent treatments were administered at an intensity of two times above threshold. Medication was not changed during treatment.
Statistical analysis
The pattern of change for patients' depression rating scores was analysed using paired t tests contrasting scores post-ECT with pre-ECT scores. The changes in CANTAB neuropsychological scores within patients over time were analysed using a linear mixed model adjusted for sex and age. An overall test of the fixed effect for ECT group was obtained along with the pairwise comparisons of interest. These were comparing the scores for before treatment with each of ‘during’, ‘after four ECTs’ and ‘after 1 month’. A mixed model was selected for analysis as it uses all available data, can properly account for correlation between repeated measurements on the same subject, has greater flexibility to model time effects, and can handle missing data more appropriately. The flexibility of mixed models makes them the preferred choice for the analysis of repeated measures (Gueorguieva & Krystal, Reference Gueorguieva and Krystal2004). Initially all cognitive datasets were examined for normality using the Kolmogorov–Smirnov test to meet the assumptions of the mixed model. In the matching task, data were analysed separately for the simultaneous and delayed matching conditions. Given that performance was measured in terms of proportion correct, an arcsine transformation [2×arcsine (√x)] was applied to data prior to statistical analysis (see Howell, Reference Howell1997). To reduce skewness in distribution, all measures of response latency were log10 transformed prior to analysis.
Results
CANTAB test of visual and visuospatial memory
Pattern and spatial recognition
The mixed model found that there was a significant difference in pattern recognition scores across the ECT groups (p=0.01). Pattern recognition test scores fell between baseline (80.2%) and after four ECT treatments (76.6%) with borderline significance (p=0.054) and fell significantly (p=0.028) after the final treatment (74.5%) compared with baseline scores (Table 1). At 1-month follow-up there was no significant difference (p=0.32) in the proportion correct (81.7%) relative to baseline (80.2%). In terms of response latency, there was no statistical difference (p>0.05) found between baseline and all subsequent tests. Spatial recognition test scores were 75.2% at baseline, 60.4% during treatment, 58.3% after treatment and 64.7% at 1 month after the final treatment (Table 1). The overall test for the fixed effect of ECT group was significant (p<0.001), suggesting that the spatial recognition scores differed across the period of treatment. The changes from baseline were significant at all three time points, with p values as follows: during (p<0.001), after the final treatment (p<0.001) and 1 month after treatment (p=0.042). No differences in latency measures were found between baseline and all subsequent tests.
Table 1. Summary of results from individual CANTAB tests

Values are given as mean (standard error).
CANTAB, Cambridge Neuropsychological Test Automated Battery; ECT, electroconvulsive therapy.
Mean value was significantly different from that at baseline:
* p<0.05, ** p<0.01.
PAL
Across all ECT groups there was a significant difference in the successful trials (p=0.003). Patients completed significantly fewer successful trials on their first presentation after four ECT treatments (p<0.001) and after the final treatment (p<0.021) compared with their baseline attempt (Table 1). At 1 month there was no significant difference in scores relative to baseline (p=0.85). The patients' total error scores differed significantly across the four ECT groups (p=0.006). With specific comparison to before treatment, patients' total error scores increased significantly (p=0.014) after four ECT treatments. There was no significant difference in error scores after the final treatment (p=0.073) or after 1 month (p=0.084) compared with baseline scores. However, error scores did improve after 1 month compared with pre-treatment levels but did not reach significance (p=0.084). Table 1 shows the memory scores did not differ significantly between initial baseline scores and subsequent tests after four ECT treatments (p=0.23), after the final treatment (p=0.78) and at 1-month follow-up (p=0.45).
Simultaneous and delayed matching to sample
ECT patients did not differ in simultaneous matching scores between baseline and after four ECT treatments (p=0.25), after the final treatment (p=0.50) and at 1-month follow-up (p=0.57) (Table 1). No effect was found between baseline and all subsequent tests on latency measures. Across delayed trials there was no significant difference between initial baseline scores and subsequent tests after four ECT treatments, after the final treatment and at 1-month follow-up (Table 1; p=0.169, p=0.822 and p=0.968, respectively). However, there was one significant difference found between baseline scores and after four ECT treatments at the 12 s delay with a reduction in scores from 0.69 (s.e.m.=0.04) to 0.61 (s.e.m.=0.05). This difference of 0.08 (95% confidence interval 0.09–0.20) was significant (p=0.033). No effect was found between baseline and all subsequent tests on latency measures.
Discussion
Anterograde memory loss following ECT is often assessed with tests of visual or verbal memory. However, simple, brief measures – typically, highly structured tests of verbal learning involving familiar material with very short retention intervals have limited worth (Robertson & Pryor, Reference Robertson and Pryor2006). Furthermore, even when researchers have used extensive neuropsychological batteries, anterograde amnesia for newly learned visual or verbal material is rarely apparent more than 10 days after ECT (Squire et al. Reference Squire and Miller1974; Squire, Reference Squire1986; Sackeim, Reference Sackeim1992; Sackeim et al. Reference Sackeim, Prudic, Devanand, Kiersky, Fitzsimons, Moody, McElhiney, Coleman and Settembrino1993, Reference Sackeim, Prudic, Devanand, Nobler, Lisanby, Peyser, Fitzsimons, Moody and Clark2000). Only three studies have found anterograde deficits beyond this point. Ng et al. (Reference Ng, Schweitzer, Alexopoulos, Celi, Wong, Tuckwell, Sergejew and Tiller2000) found impairment of immediate memory in a picture recognition task at 1 month post-ECT, Halliday et al. (Reference Halliday, Davidson, Browne and Kreeger1968) found deficits in verbal and non-verbal learning 3 months post-ECT, and a study by Sackeim et al. (Reference Sackeim, Prudic, Fuller, Keilp, Lavori and Olfson2007) found a pronounced slowing of reaction time at 6 months post-ECT.
Robertson & Pryor (Reference Robertson and Pryor2006) suggested that ECT should be evaluated with neuropsychological batteries that would be used on patients with a suspected history of brain injury or disease. The CANTAB has been used successfully to assess cognitive dysfunction in patients with dementia (Lee et al. Reference Lee, Rahman, Hodges, Sahakian and Graham2003), Huntington's disease (Lawrence et al. Reference Lawrence, Watkins, Sahakian, Hodges and Robbins2000) and major depressive disorder (Porter et al. Reference Porter, Gallagher, Thompson and Young2003). It has also been used successfully to detect deficits in visuospatial short-term memory in neurosurgical patients with temporal or frontal lobe excision (Owen et al. Reference Owen, Sahakian, Semple, Polkey and Robbins1995). These studies indicate that the CANTAB is sensitive to the presence of brain dysfunction in adults and that discrimination among subtypes of brain disorder is possible using profile interpretation. For example, patients with frontal versus temporal lobe pathology differ in their performance on CANTAB measures of recognition and spatial memory (Owen et al. Reference Owen, Sahakian, Semple, Polkey and Robbins1995). This is important where frontal or temporal lobe function may be affected by the passage of the electrical current by means of bilateral electrodes.
Following Robertson & Pryor (Reference Robertson and Pryor2006), in this study we assessed the CANTAB's sensitivity in detecting cognitive impairments following a course of ECT. Within the CANTAB four subtests were chosen for their capacity to detect visual memory disturbances that may occur when an electrical current is passed through the right hemisphere of the brain. This is a feature of both bilateral and right unilateral electrode placement which may cause some disturbances to the right temporal lobe responsible for visual cognitive functioning (Squire & Slater, Reference Squire and Slater1978). However, the current may also affect the frontal lobe, which has been implicated in visual memory functioning (Owen et al. Reference Owen, Sahakian, Semple, Polkey and Robbins1995). Bilateral and right unilateral treatment, particularly bilateral ECT, is mainly used to treat patients in the USA and the UK (Sackeim et al. Reference Sackeim, Prudic, Devanand, Nobler, Lisanby, Peyser, Fitzsimons, Moody and Clark2000); therefore, it is vital that visual memory impairments are measured, though, of course, the seizures induced are bilateral whatever the electrode placement.
Clinical efficacy of ECT
Bilateral ECT was shown to be an effective treatment for severe depression with a 66% reduction in the average depression rating scores recorded within a few days following treatment. These findings are comparable with antidepressant efficacy of bilateral ECT found in other trials (Lerer et al. Reference Lerer, Shapira, Calev, Tubi, Drexler, Kindler, Lidsky and Schwartz1995; Sackeim et al. Reference Sackeim, Prudic, Devanand, Nobler, Lisanby, Peyser, Fitzsimons, Moody and Clark2000; McCall et al. Reference McCall, Dunn and Rosenquist2004).
Effects on memory
In this study, recognition memory for visually presented patterns and spatial locations was impaired both during a course of ECT treatment and in the days following ECT. These deficits were also recorded in the visuospatial associative learning task. Cognitive impairments were resolved at 1 month post-ECT for both pattern recognition memory and visuospatial associative learning; however, significant impairments remained in spatial memory. These deficits point to an associative and general visuospatial recognition memory deficit in ECT patients. It is generally accepted that the anatomical location of recognition and associative memory for visuospatial stimuli is the right medial temporal lobe (Milner, Reference Milner1975; Barr, Reference Barr1997). Neuroimaging studies in healthy volunteers have demonstrated increased activation in the right parahippocampal gyrus during tasks involving associating objects and locations (Maguire et al. Reference Maguire, Frackowiak and Frith1996; Owen et al. Reference Owen, Morris, Sahakian, Polkey and Robbins1996). Furthermore, it is known that the hippocampus is vital to the integrity of both associative (Eichenbaum, Reference Eichenbaum1999) and recognition memory (Manns & Squire, Reference Manns and Squire1999). Therefore, cognitive impairments in both recognition and associative memory in ECT patients would seem to indicate impairments in the function of the medial temporal lobe, perhaps more specifically the hippocampus. Deficits in performance during treatment on the delayed matching to sample task after a 12 s delay also points to temporal lobe or amygdalo-hippocampectonmy dysfunction, where similar significant deficits in this task were found by Owen et al. (Reference Owen, Sahakian, Semple, Polkey and Robbins1995) in patients who had these brain regions excised. In terms of the deficits in the spatial memory task this could also point to impairments in frontal lobe structures given that Owen et al. (Reference Owen, Sahakian, Semple, Polkey and Robbins1995) found that impairments in spatial recognition memory were more affected in patients with frontal lobe damage than by patients with lesions of the temporal lobe structures. It could be that frontal lobe dysfunction causes impairments in patients on tasks that require manipulation of spatial rather than visual or verbal material. However, the temporal lobes may also be involved given that spatial learning deficits have been demonstrated in right temporal lobectomy patients (Milner, Reference Milner1965; Smith & Milner, Reference Smith and Milner1981). Furthermore, spatial learning ability in the rat is severely compromised by electroconvulsive stimulation (Reid & Stewart, Reference Reid and Stewart1997), which mirrors deficits seen following hippocampal damage or dysfunction (Morris, Reference Morris1989). Cognitive impairments at 1 month post-ECT are resolved in all the test domains except for the spatial recognition task. However, the impairments in the spatial memory task at 1 month post-ECT do appear to improve in comparison with previous test occasions. This reflects the findings of animal studies. The generalized seizure induced by ECT is known to cause a deleterious effect on synaptic plasticity required for memory formation: when rats were given electroconvulsive seizures the effect on synaptic function gradually resolved over a period of around 40 days (Reid & Stewart, Reference Reid and Stewart1997).
Clinical implications
Previous studies have indicated that using highly structured tests of verbal learning with brief delays may not provide a reliable measure of the cognitive impact of ECT. Even when extensive neuropsychological batteries have been employed by researchers with longer delays, anterograde amnesia for newly learned visual or verbal material is rarely apparent, in group data, more than a few weeks after ECT. This is further highlighted by the evidence found in this study indicating that ECT causes deficits in visual memory functioning which resolve by 1 month post-treatment. However, it was also evident that ECT significantly impairs memory for spatial location at 1 month post-ECT. This is one of only a few studies that have detected anterograde memory loss more than 2 weeks after treatment, suggesting that the CANTAB is a sensitive instrument for measuring the cognitive impact of ECT on aspects of both visual and visuospatial memory. If future studies are carried out using this methodology, subtests providing the greatest sensitivity to cognitive decline could be used routinely as a clinical bedside test. This will allow clinicians to rapidly monitor cognitive functioning of the patient at any time point during the course of ECT and the risks associated with the continuation of treatment.
However, due to the small sample size in this study, larger studies with longer follow-up periods are required to fully validate the benefits of this technology. It is logistically difficult to manage such investigations. When patients were discharged from the hospital, some refused to continue to participate in the study, while others relapsed and required additional ECT, thereby excluding them from further participation. In addition, tests such as the paired associate learning task and the delayed matching task were more difficult to complete than the other tests. This has implications for future work: it may be that paired associative learning tests are sensitive but disliked by participants, which questions their suitability for inclusion into routine clinical practice. Another limitation of the study was that data on duration of illness, treatment resistance, medication at every stage of assessment, general cognitive impairment and estimated intelligence of each patient were not included in the study. Therefore, the representativeness of the sample may be in question and future researchers should bear this in mind.
In summary, our results found that patients receiving a course of bilateral ECT showed deficits in associative and general visuospatial recognition memory during ECT and within the week after the end of treatment. ECT also significantly impairs recognition memory for spatial locations at 1 month post-ECT. The CANTAB appears to be a sensitive instrument for detecting aspects of both visual and visuospatial memory both during and following a course of ECT.
Acknowledgements
We thank all the patients who participated in this study. We also thank the staff at Royal Cornhill Hospital for their assistance in patient recruitment, especially the ECT nurses, Kate Ferries and Ellen Robertson.
Declaration of Interest
None.




