Introduction
Many studies show that elderly people with higher scores on self-rating depression questionnaires perform less well on cognitive tests (e.g. Blazer & Williams, Reference Blazer and Williams1980; Griffiths et al. Reference Griffiths, Good, Watson, O'Donnell, Fell and Shakespear1987; Austin et al. Reference Austin, Mitchell and Goodwin2001; Norman et al. Reference Norman, Troester, Fields and Brooks2002). Nearly all have compared normal elderly against psychiatric patients or individuals whose self-report scores indicate risk of clinically significant depression. However, as Blazer & Williams (Reference Blazer and Williams1980) point out, clinically significant depression represents only the upper end of a continuum of states of unhappiness found in randomly selected older samples of older people. Further, even within ranges well below levels of clinical concern, scores on the Beck Depression Inventory (Beck et al. Reference Beck, Ward, Mendelson, Mock and Erbaugh1961) correlate negatively with performance on laboratory tests of fluid intelligence and memory (Rabbitt et al. Reference Rabbitt, Donlan, Watson, McInnes and Bent1995). This raises the question of why mental abilities are impaired by even mild discontent.
One possibility is that discontent is due to factors that also impair cognitive performance. For example, both contentment and cognitive performance are reduced by poor health (McInnes & Rabbitt, Reference McInnes, Rabbitt, Vellas, Albarede and Garry1997; Anstey et al. Reference Anstey, Luszcz, Giles and Andrews2001), and by unfavourable demographics and socio-economic disadvantage (Rabbitt et al. Reference Rabbitt, Diggle, Holland and McInnes2004). To interpret associations between discontent and cognition these and other similar factors must be taken into consideration. Data from the University of Manchester Longitudinal Study made this possible.
A further issue is whether discontent not only reduces levels of cognitive performance but also accelerates rates of age-related decline. Large epidemiological studies suggest that relatively severe depression increases age-related cognitive loss over periods of 2–5 years (e.g. Dufouil et al. Reference Dufouil, Fuhrer, Dartigues and Alperovitch1996; Bassuk et al. Reference Bassuk, Berkman and Wypij1998; Comijs et al. Reference Comijs, Jonker, Beekman and Deeg2001). However, these studies included individuals with clinically significant depression, so it is not yet known whether even mild discontent accelerates age-related cognitive decline. To test this we need longitudinal data that can take into account two further methodological issues.
One is that even elderly volunteers show marked practice gains when the same tests are repeated, albeit at intervals of many months (Mitrushina & Satz, Reference Mitrushina and Satz1991; Paola et al. Reference Paola, Troster and Ryan1997; Dikmen et al. Reference Dikmen, Heaton, Grant and Temkin1999; Unger et al. Reference Unger, van Belle and Heyman1999; Rabbitt et al. Reference Rabbitt, Diggle, Holland and McInnes2004) or even of 7 or more years (Rabbitt et al. Reference Rabbitt, Diggle, Holland and McInnes2004). However, because more-able participants experience greater gains (Rabbitt et al. Reference Rabbitt, Diggle, Smith, Holland and McInnes2001, Reference Rabbitt, Diggle, Holland and McInnes2004), practice effects during longitudinal studies may selectively mask declines in individuals who are relatively more contented, and so more able, giving the misleading impression that less-able depressed individuals decline more rapidly.
A second issue is that analyses that neglect deaths and drop-outs miscalculate true trajectories of age-related cognitive decline (Rabbitt et al. Reference Rabbitt, Lunn and Wong2005). Rabbitt et al. (Reference Rabbitt, Lunn and Wongin press) found that accelerations of cognitive decline were equally marked with approach to drop-out and death. Depression is associated with poor health and with earlier mortality (McInnes & Rabbitt, Reference McInnes, Rabbitt, Vellas, Albarede and Garry1997; Anstey et al. Reference Anstey, Luszcz, Giles and Andrews2001) and with higher risk of drop-out. Thus neglect of drop-out and death confound the effects of depression and pathology on rates of age-related cognitive change.
An unexplored question is whether, irrespective of their associations with rates of age-related cognitive changes over time, scores on depression inventories predict overall levels of performance over periods of 11–16 years even after the effects of practice and sample attrition have been taken into consideration. A further question is whether these relationships are found even within ranges of scores below the median for an atypically healthy and cheerful volunteer population. Data from the University of Manchester Longitudinal Study of cognitive performance in healthy old age (Rabbitt et al. Reference Rabbitt, Diggle, Holland and McInnes2004) allowed analyses of the effects of differences, even within very low ranges of Beck Depression Inventory scores, on rates of age-related cognitive change after differences in general health indexed by consumption of prescribed medicines and by approach to death and drop-out, sex, socio-economic advantage, geographical location of residence and sample recruitment have also been taken into account.
Method
Participants, demographics and Beck scores
Schedules of recruitment and details of all tests used have been described in detail by Rabbitt et al. (Reference Rabbitt, Diggle, Holland and McInnes2004). Volunteers were 5070 independent, community residents of Greater Manchester (n=2201) and of Newcastle upon Tyne, UK (n=2879). They were 1507 men aged from 49 to 93 years (mean age 65.6, s.d.=7.7) and 3563 women aged from 49 to 92 years (mean age 64.4, s.d.=7.8) who had attended two or more of four quadrennial assessments on the same battery of five cognitive tests. These were atypically healthy, able and highly motivated members of their age groups who had volunteered in response to media advertisements. On recruitment, 87% had scores on the Beck Depression Inventory (Beck et al. Reference Beck, Ward, Mendelson, Mock and Erbaugh1961) that were lower than 14, which is taken as a threshold value for clinical concern. This allowed comparisons between substantial subgroups with Beck scores below and just above average within this unusually cheerful population to check whether even low Beck scores on entry can predict overall performance and rates of decline on five different cognitive tests quadrennially administered over the next 4–16 years.
To take effects of sample attrition into account, dates of each of 2342 deaths between 1983 and July 2004 were obtained from the General Register Office (Southport, UK). There were 3204 drop-outs, 1208 of whom also died before the July 2004 cut-off date. Because many drop-outs did not give advance notice of withdrawal, all are dated from the last testing session attended. While it is known that individuals withdrawing because of illness or frailty perform worse that those who leave from boredom or other life demands, this differentiation was not possible because reasons for all drop-outs were not given. However, since Rabbitt et al. (Reference Rabbitt, Lunn and Wongin press) found that, in this population, amounts and time courses of changes preceding deaths and all unattributed drop-outs are almost identical, deaths and drop-outs are combined into a single category. Because Rabbitt et al. (Reference Rabbitt, Lunn and Wong2005) had found that cognitive performance is affected by the timing, as well as by the incidence of death and drop-out, participants were further divided into four groups according to their histories of survival or drop-out and death (Drop-out) with respect to the four assessment time points, T1, T2, T3 and T4. Note that logging deaths and drop-outs also provides a particularly robust proxy index of general health status. Because McInnes & Rabbitt (Reference McInnes, Rabbitt, Vellas, Albarede and Garry1997) had found that both cognitive performance and contentment vary with a second, weaker index of general health status, consumption of prescribed drugs, participants were also divided into groups who reported taking no, one, or two or more prescribed drugs on entry to the study.
Earlier analyses had found that both depression and cognitive performance vary with levels of socio-economic advantage (Rabbitt et al. Reference Rabbitt, Donlan, Watson, McInnes and Bent1995). This was taken into consideration by grouping participants by reference to the UK Office of Population Censuses and Surveys classification of occupational categories (1980). These are: SEA C1, professionals such as doctors, lawyers, senior managers and academics; SEA C2, professionals such as schoolteachers, pharmacists and junior managers; SEA C3N, skilled non-manual workers such as secretaries; SEA C3M, skilled manual workers such as craftsmen, joiners and machinists; SEA C4, non-skilled non-manual workers such as security guards and watchmen; SEA C5, non-skilled manual workers such as cleaners and janitors.
Because Rabbitt et al. (Reference Rabbitt, Donlan, Watson, McInnes and Bent1995) had also found that Beck scores and cognitive performance also vary with cities of residence (Newcastle upon Tyne or Manchester), sex and recruitment cohorts, analyses also included these factors.
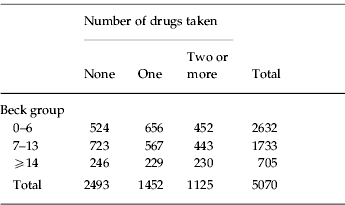
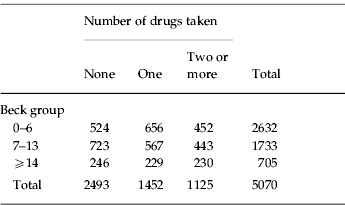
A Beck score of 14 is considered a lower threshold for clinical concern. The average Beck score for this sample was 7.2 and studies of other large UK samples aged from 18 to 90 years have reported population averages between 7.9 and 8.5. To compare the effects on cognitive performance of greater than average contentment, mild depression and severe depression participants were grouped into those with Beck scores from 0 to 6, who were more contented than the sample average, with scores of 7–13 who were averagely contented or mildly discontented and with scores of ⩾14 who were judged to be depressed. Table 1 cross-tabulates Beck scores against drug consumption.
Table 1. Beck group versus number of drugs taken
Simple regressions using Beck scores as a continuous variable found that depression scores increase with number of drugs taken and so with poorer health (Spearman's ρ=0.21, r 2=0.043, p<0.001), replicating earlier findings by McInnes & Rabbitt (1997).
Beck scores are higher for participants who completed the study in 2003 and survived until July 2004 than for those who died before July 2004 or who dropped out during the study before 2003 (F=46, p=0.00001). Since drop-out and death are strong proxy indices of poor health this is unsurprising evidence that depression is related to general health. Simple regressions also showed that residents of Newcastle upon Tyne have higher Beck scores than residents of Manchester (R=0.087, F=37.2, p=0.000), women have higher Beck scores than men (R=0.079, F=29.3, p=0.000) and Beck scores decline with occupational advantage (R=0.19, F=45.3, p=0.000). Beck scores also significantly differ between cohorts recruited in different years (R=0.69, F=0.19, p=0.000), but not with age on recruitment.
Procedure and cognitive tests
Participants travelled independently to the Universities of Manchester and Newcastle upon Tyne and were tested in groups of 5–25 by experienced assessors. Testing sessions lasted for 60 to 90 min. Volunteers were each paid £5 (UK) per session to cover travelling expenses. The tests were the Alice Heim (AH) 4-1 and -2 intelligence tests (Heim, Reference Heim1970), the Raven (Reference Raven1965) Mill Hill A vocabulary test, Free Recall of 30 words and cumulative learning of 15 words. Volunteers completed the Beck Inventory and the battery of five tests on entry to the study and took the cognitive tests again, from one to three times, at 4-year intervals.
The AH4-1 intelligence test includes verbally presented logic problems, arithmetic and completion of number series and verbal comparisons. The AH4-2 is a non-verbal test including correct completions of logical series defined by progressive mental rotation, or by addition and subtraction, or by other comparisons between line-drawn shapes. Both AH4 tests begin with a set of five unscored practice examples followed by 65 scored problems with a 10 min limit deadline. Scores analysed are percentage correct responses. The Mill Hill A vocabulary test requires selection of the most exact synonym for each of 33 words from among six alternatives without any time limit. For the Verbal Free Recall (VFR) test, volunteers are shown 30 six-letter nouns matched for frequency (1/10 000; Kucera & Francis, Reference Kucera and Francis1967) and for concreteness projected, one at a time, at a rate of one per 1.5 s in Times Roman Bold on a screen 5 m from the most distant participant. Scores are percentages of words correctly recalled in any order. For the California Verbal Learning task 15 six-letter nouns, selected as for the VFR task, are presented four times in the same way and at the same rate as in the VFR task. The order of appearance is randomized between presentations. After each presentation volunteers write down as many nouns as they can remember, in any order without sight of previous attempts. Scores are percentages of correct recalls summed over the four presentations.
Analytical model
A random-effects model first estimated the mean response over time after considering the effects of a set of explanatory variables and then estimating the random variation about the mean response using random intercepts and differences in age between individuals. The model has two levels, one at individual level including random intercept and random age effect, and the other a residual error. The methodological issues and notation of this model are discussed in detail elsewhere (Rabbitt et al. Reference Rabbitt, Diggle, Holland and McInnes2004).
This model examines how population average scores for each cognitive test vary with the effects of age, sex, occupational category, city of residence, year of entry (recruitment cohort) and practice (i.e. whether the score is obtained on the first, second, third or fourth testing session). Because Rabbitt et al. (Reference Rabbitt, Lunn and Wong2005) had found that declines in test scores accelerate as death or drop-out approaches, a drop-out covariate was included, participants being grouped according to whether they died or dropped out after the second, third or fourth test session following their initial assessments. Participants who entered the study later, and so were tested fewer than four times, but did not die or drop out, were classed as non-drop-outs. The same approach was used to analyse data separately for each of the five cognitive tests.
Age was coded in years over the minimum age at entry of 44 years and the relationship between age and cognitive decline was modelled as a linear or a quadratic trend. Thus increases in individuals' ages at successive test sessions track the progress of age-related changes in test scores. Practice effects were modelled as a succession of step-increases at each testing occasion. Years of entry from 1983 to 1992 were entered to take differences between recruitment cohorts into consideration.
Results
AH4-1 scores
Table 2 shows model estimates for AH4-1 mean percentage test scores. The intercept estimates represent scores for a male participant aged 44 years, from Greater Manchester, who entered the study in 1982, in the C1 and C2 (professional) occupational category, taking the test for the first time, with a Beck score between 0 and 6, who is taking no prescribed drugs and completed the study without dying or dropping out before 2003.
Table 2. Model estimates for response AH4-1 percentage correct, with random effects and residual error

AH, Alice Heim; s.e., standard error; CI, confidence interval; s.d., standard deviation.
Under random effects the s.d. (intercept) and s.d. (age) are both estimates at the level of the individual.
After all other factors are considered, AH4-1 scores averaged over the entire study significantly decline as Beck scores increase. Significant linear and quadratic age-terms confirm accelerating declines in scores with increasing age. However, there is no evidence that Beck groups differ in rates of cognitive decline during the study because Beck scores do not interact either with age or with numbers of testing sessions.
Though women score significantly lower than men, the significant age×sex interaction shows that their scores decline less rapidly as they grow older. City is not significant but a significant age×city interaction indicates that subjects from Newcastle upon Tyne decline faster than subjects from Manchester. Occupational category is highly significant but does not interact with age so there is no evidence that occupational advantage slows age-related decline.
Practice effects are significant overall but reduce with increasing experience of the test. Because the practice effects adequately capture the age×practice interaction (see Rabbitt et al. Reference Rabbitt, Diggle, Holland and McInnes2004) this also means that practice gains are smaller for older than for younger participants, confirming a similar finding by Rabbitt et al. (Reference Rabbitt, Diggle, Smith, Holland and McInnes2001, Reference Rabbitt, Diggle, Holland and McInnes2004) using a different analytical model. However there is no interaction between Beck scores and practice and so no evidence that Beck scores affect amounts of practice gains.
There is no significant main effect of prescribed drugs and no Beck score group×drugs interactions. There is a significant main effect of deaths and drop-outs, which are much stronger proxies for the presence of pathologies than is drug intake, but no interaction between the effects of these factors and Beck scores. There are also significant interactions between the effects of drop-out and of age, with older drop-outs showing greater declines. There are significant differences between recruitment cohorts. Participants recruited in 1990 score markedly higher than those recruited in 1982. There are no significant interactions between cohort and other effects.
The remaining four tests
These tests are AH4-2, Mill Hill A, Cumulative Learning and Free Recall. Table 3 shows the effects of Beck scores and number of drugs taken after all other variables have been taken into account using the model above as for AH4-1. Full analyses are available from the authors.
Table 3. Model estimates for other tests, showing Beck score and number of drugs taken

s.e., Standard error; AH, Alice Heim.
Because Beck scores correlate with number of drugs, we show both here. Clearly the Beck score has a significant effect, with the effect of number of drugs taken being significant only in the cumulative learning test. The strongest effects of Beck score are seen in AH4-2 and Free Recall. The AH4-2 result is entirely consistent with the result for AH4-1.
Significant linear and quadratic age effects are present in all models and show that decline accelerates as age increases (except that the linear age effect is not significant in the AH4-2 model).
Men perform significantly better than women on all tests except on Cumulative Learning, on which women score higher.
For Cumulative Learning and Free Recall there were no significant interactions between any of the demographic variables or health in the final models.
Random effects
The maximum-likelihood estimates of the standard deviations and correlation for the random effects, random intercept: σ (intercept) and slope: σ (age-44) (random age effect), are available for all models from the authors. The random intercept displayed the deviation in the average response value between individuals and the random slope measured the deviation in individuals' rates of decline.
The standard deviation estimates for the intelligence tests, AH4-1 and AH4-2, were similar. The random slope: σ (age-44) standard deviation estimate can also be used to assess whether variability in test scores between individuals increases with age. This estimate is amplified by the multiplication with age (in years over 44) in the model (Rabbitt et al. Reference Rabbitt, Diggle, Holland and McInnes2004). For example, in the AH4-1 test a difference of 0.43 in the slopes for two individuals with equal scores on entry to the study would result in a difference of 4.3% and 8.6% after 10 and 20 years, respectively. Variability between individuals increased with sample age in tests where the σ (age-44 years) standard deviation estimate was larger. This increase in variability between individuals as the study continued was evident in all tests.
Discussion
The analysis of random effects shows that variance in cognitive performance between individuals significantly increases as the sample ages because individuals decline at very different rates. This, no doubt, is partly due to individual differences in exposure to factors that accelerate or retard cognitive decline (Rabbitt et al. Reference Rabbitt, Diggle, Holland and McInnes2004). For example, sex affects rate of decline, with women declining more slowly and approach to death and drop-out accelerate decline. The finding that occupational category neither affects mortality nor rate of cognitive decline is unexpected because convincing previous studies find that socio-economic advantage reduces mortality and pathology (e.g. Nagi & Stockwell, Reference Nagi and Stockwell1973; Snowden et al. Reference Snowden, Ostwald, Kane and Keenan1989).
Previous studies have found that clinically significant depression reduces cognitive performance (e.g. see review by Austin et al. Reference Austin, Mitchell and Goodwin2001 and empirical studies by Blazer & Williams, Reference Blazer and Williams1980; Griffiths et al. Reference Griffiths, Good, Watson, O'Donnell, Fell and Shakespear1987; Norman et al. Reference Norman, Troester, Fields and Brooks2002). The present analyses add the new finding of significant differences in intelligence, vocabulary and verbal memory between groups with Beck scores in the ranges of 0–6 and 7–13, indicating differences between more than average contentment and mild discontent. However, in spite of significant effects of Beck scores on verbal learning and memory there is no evidence that they affect the amount by which individuals improve with practice during the study. These associations are independent of other factors such as age, sex, socio-economic advantage, city of residence and recruitment cohort that are known to affect both depression and cognitive performance. They are also independent of general health, indexed both by consumption of prescribed drugs (McInnes & Rabbitt, Reference McInnes, Rabbitt, Vellas, Albarede and Garry1997) and by a much stronger proxy marker, the occurrence of death or drop-out. The new finding is that Beck scores on entry to the study significantly affect overall levels of performance over the next 4–16 years.
However, in spite of this, there is no evidence that, within the range indicating differences in contentment rather than depression, Beck scores affect rates of age-related cognitive declines.
These analyses find no evidence that Beck scores affect rates of age-related cognitive decline. At first sight this seems discrepant with earlier findings (e.g. Dufouil et al. Reference Dufouil, Fuhrer, Dartigues and Alperovitch1996; Bassuk et al. Reference Bassuk, Berkman and Wypij1998; Comijs et al. Reference Comijs, Jonker, Beekman and Deeg2001). One possible explanation is that earlier studies did not take into account practice gains that might selectively benefit more-able individuals (see Rabbitt et al. Reference Rabbitt, Diggle, Smith, Holland and McInnes2001, Reference Rabbitt, Diggle, Holland and McInnes2004) who were also among the less depressed, and so give a misleading impression that the more depressed and less able decline faster. However the present analyses find no interactions between Beck scores and practice gains. Earlier studies also did not include deaths and drop-outs that are robust markers for depression and also for pathology and for accelerated cognitive decline (Rabbitt et al. Reference Rabbitt, Diggle, Smith, Holland and McInnes2001, Reference Rabbitt, Diggle, Holland and McInnes2004, Reference Rabbitt, Lunn and Wong2005). However, again, while the present study confirmed the effects of impending death or drop-out on cognitive change it found no interaction between the effects of death and drop-out and Beck scores on cognitive performance. The remaining explanation for the difference between studies is that earlier studies included many severely depressed individuals while the present study was on an atypically cheerful population. Acceptance of this explanation implies the possibility that the effects on cognition of levels of depression of clinical concern are causally different from those of levels of depression, or discontent, indicated by lower ranges of Beck scores.
A main finding of these analyses is the replication and extension of earlier work showing significant associations between cognitive performance and Beck scores, even within ranges below the median for this unusually cheerful population. The associations found in the present study cannot be entirely attributed to associations between depression and pathology, such as those found by McInnes & Rabbitt (Reference McInnes, Rabbitt, Vellas, Albarede and Garry1997) and Anstey et al. (Reference Anstey, Luszcz, Giles and Andrews2001) because they found no interaction between the effects of Beck scores and of death and drop-out, or between Beck scores and drug consumption. The finding that participants with Beck scores of 0-6 perform significantly better than those with scores of 7–14 must be explained in other ways.
One plausible explanation is that differences in Beck scores within the 0–14 range are strongly determined by factors that affect extents of people's satisfaction with their everyday lives and also lead to general well-being and so maintenance of cognitive function. Individuals who are more than averagely content with their lives are likely to be so because of favourable personal circumstances, including care, support and social stimulation from relatives and friends, which enable them to thrive and so maintain their abilities. The relationship between thriving and cognitive performance can also be interpreted in a positive way as evidence that greater happiness is associated with longer maintenance of cognitive ability in later life. In this frame of reference it is unsurprising, but methodologically informative, to find a significant relationship between Beck scores and cognitive performance even after other factors that are associated with both contentment and cognitive ability, such as general health status, occupational status and city of residence, have been taken into account. The lesson is that the available indices for these particular aspects of general well-being do not adequately capture other strong determinants of quality of life, such as social support, that markedly contribute to a more benign and cheerful old age.
In choosing directions for further research it is worth noting that this framework of interpretation does not exclude a provocative converse possibility. It has been widely, if tacitly, taken for granted that examinations of relationships between depression and cognitive performance must reveal functional mechanisms by which unhappiness or depression acts to impair mental abilities. This assumption may well be justified when depression is sufficiently severe to entail neurophysiological changes that also affect cognitive abilities (e.g. Austin et al. Reference Austin, Mitchell and Goodwin2001). However, for the milder levels of discontent examined in the present study it is equally plausible that higher levels of lifelong cognitive ability increase the quality of everyday life and so promote happiness and thriving in old age. It has long been established that individuals' intelligence test scores robustly predict their educational and occupational attainments and socio-economic success (e.g. Terman & Oden, Reference Terman and Oden1947, Reference Terman and Oden1959). Recent analyses by Whalley & Deary (Reference Whalley and Deary2001) show that higher intelligence in childhood is associated with longer survival in old age and, indeed, with reduced risk of death or of drop-out within the same sample from which these data were collected (Rabbitt et al. Reference Rabbitt, Lunn and Wongin press). Thus in addition to the effects of demographic factors associated with intelligence, such as socio-economic advantage, it seems that high intelligence, per se, may also be a significant resource for successful ageing.
Acknowledgements
The authors are grateful for support for data collection over 20 years from the Economic and Social Research Council and the Medical Research Council, and for support for data analysis by Unilever PLC.
Declaration of Interest
None.