Suicide is a leading cause of death worldwide and presents a significant public health concern. Indeed, approximately 800 000 die by suicide each year, and suicide is consistently ranked in the top 15 causes of death globally (World Health Organization, 2014). Given the challenges of studying suicide as an outcome, previous studies have focused on suicidal ideation (SI) and suicidal behaviors such as a non-lethal suicide attempt (SA) as proxies for suicide. SI and SA are promising research targets as they are strongly related to suicide and occur more frequently than suicide with lifetime prevalence rates of 9.2% and 2.7% for SI and a non-lethal SA, respectively (Nock et al., Reference Nock, Borges, Bromet, Alonso, Angermeyer, Beautrais and Williams2008). SI and SA also represent points on a continuum of suicide risk as 29% of individuals with lifetime SI attempt suicide (Nock et al., Reference Nock, Borges, Bromet, Alonso, Angermeyer, Beautrais and Williams2008). Hence, progress toward addressing the high suicide rate will require the identification of novel and modifiable contributors to both SI and SA.
Sleep disturbance including short sleep duration, long sleep duration, and insomnia has been consistently linked to suicide risk including a higher likelihood of reporting SI or a prior SA (Bernert & Joiner, Reference Bernert and Joiner2007; Bernert & Nadorff, Reference Bernert and Nadorff2015; Liu et al., Reference Liu, Tu, Lai, Lee, Tsai, Chen and Chiu2019). Insomnia is characterized by difficulties initiating and maintaining sleep, and insomnia symptoms have been associated with greater SI and prior SAs despite differing definitions of insomnia across studies (Brower et al., Reference Brower, McCammon, Wojnar, Ilgen, Wojnar and Valenstein2011; Chakravorty et al., Reference Chakravorty, Siu, Lalley-Chareczko, Brown, Findley, Perlis and Grandner2015; Li, Lam, Yu, Zhang, & Wing, Reference Li, Lam, Yu, Zhang and Wing2010; Pompili et al., Reference Pompili, Innamorati, Forte, Longo, Mazzetta, Erbuto and Girardi2013). Evidence from large-scale epidemiological studies has provided evidence that short sleep duration is associated with SI and SA (Chakravorty et al., Reference Chakravorty, Siu, Lalley-Chareczko, Brown, Findley, Perlis and Grandner2015; Goodwin & Marusic, Reference Goodwin and Marusic2008; Kim et al., Reference Kim, Park, Cho, Park, Park, Choi and Chang2013). Highlighting the non-linear association between sleep duration and suicide risk, researchers also have reported that long sleep duration was related to higher odds of SI and SA (Guo et al., Reference Guo, Xu, Deng, Huang, Huang, Gao and Lu2017; Kim et al., Reference Kim, Park, Cho, Park, Park, Choi and Chang2013; Michaels, Balthrop, Nadorff, & Joiner, Reference Michaels, Balthrop, Nadorff and Joiner2017). The preceding evidence indicates that sleep disturbance such as insomnia, short sleep duration, and long sleep duration contribute to a higher likelihood of reporting SI and prior SAs. In addition to identifying modifiable risk factors for suicide such as sleep disturbance, there is also a need to better understand how sleep disturbance may confer risk for suicidal thoughts and behaviors.
Inflammation is one possible biological mechanism that may be involved in the association between sleep disturbance and suicide risk. Inflammation is a non-specific immune system response to a foreign pathogen or tissue damage, which is coordinated locally and systemically by multiple immune system mediators such as cytokines (Dantzer, O'Connor, Freund, Johnson, & Kelley, Reference Dantzer, O'Connor, Freund, Johnson and Kelley2008; Irwin, Reference Irwin2015; Szelényi, Reference Szelényi2001). Inflammation is typically resolved quickly, but chronic systemic inflammation may also persist with the ongoing stimulus or ineffective regulation. Among the most important cytokines involved in both acute and chronic inflammation are interleukin (IL)-6 and tumor necrosis factor (TNF)-α (Arai et al., Reference Arai, Lee, Miyajima, Miyatake, Arai and Yokota1990). Additionally, IL-6 triggers the production of acute-phase proteins like C-reactive protein (CRP) in the liver (Wium-Andersen et al., Reference Wium-Andersen, Ørsted, Nielsen, Nordestgaard, Kim Wium-Andersen, Dynnes Ørsted and Grønne Nordestgaard2013). Chronic, low-grade inflammation has been linked to psychosocial stress and the onset and maintenance of mental disorders including depression (Dantzer et al., Reference Dantzer, O'Connor, Freund, Johnson and Kelley2008; Irwin & Miller, Reference Irwin and Miller2007; Prather, Rabinovitz, Pollock, & Lotrich, Reference Prather, Rabinovitz, Pollock and Lotrich2009; Raison, Capuron, & Miller, Reference Raison, Capuron and Miller2006) and anxiety disorders (Salim, Chugh, & Asghar, Reference Salim, Chugh, Asghar and Donev2012; Vogelzangs, Beekman, de Jonge, & Penninx, Reference Vogelzangs, Beekman, de Jonge and Penninx2013). Assessing the levels of IL-6, TNF-α, and CRP has become increasingly utilized in behavioral and psychosocial research given their key role in orchestrating the inflammatory response, association with mental and physical health outcomes, and the availability of reliable, high sensitivity assays to measure these cytokines (Dantzer et al., Reference Dantzer, O'Connor, Freund, Johnson and Kelley2008; Irwin, Reference Irwin2015; Szelényi, Reference Szelényi2001).
Evidence from human and animal studies indicates that the immune system has a reciprocal association with sleep regulation (Bryant, Trinder, & Curtis, Reference Bryant, Trinder and Curtis2004; Irwin, Olmstead, & Carroll, Reference Irwin, Olmstead and Carroll2015b; Mullington, Simpson, Meier-Ewert, & Haack, Reference Mullington, Simpson, Meier-Ewert and Haack2010). A meta-analysis of 72 studies reported that sleep disturbance including short sleep duration, long sleep duration, and insomnia may be related to higher levels of inflammatory cytokines (Irwin et al., Reference Irwin, Olmstead and Carroll2015b). Individual studies have reported that self-reported sleep disturbance and insomnia were related to higher IL-6 (Burgos et al., Reference Burgos, Richter, Klein, Fiebich, Feige, Lieb and Riemann2006; Motivala, Sarfatti, Olmos, & Irwin, Reference Motivala, Sarfatti, Olmos and Irwin2005; Okun et al., Reference Okun, Reynolds, Buysse, Monk, Mazumdar, Begley and Hall2011), although findings have been mixed (Prather, Vogelzangs, & Penninx, Reference Prather, Vogelzangs and Penninx2015). A non-linear association between sleep duration and inflammation also has been observed such that both long and short sleep duration have been linked to higher inflammation (Irwin et al., Reference Irwin, Olmstead and Carroll2015b). Several large epidemiological studies have provided evidence that both short and long sleep duration are associated with higher levels of IL-6 and CRP (Dowd, Goldman, & Weinstein, Reference Dowd, Goldman and Weinstein2011; Miller et al., Reference Miller, Kandala, Kivimaki, Kumari, Brunner, Lowe and Cappuccio2009). Excessive sleep duration may be especially relevant to understanding chronic inflammation as each additional hour of sleep duration was associated with a 7% increase in IL-6 and 8% increase in CRP in the Cleveland Family Study (Patel et al., Reference Patel, Zhu, Storfer-Isser, Mehra, Jenny, Tracy and Redline2009). Together, these studies demonstrate that multiple types of sleep disturbance contribute to higher levels of inflammation.
Inflammation has been identified as one putative contributor to the pathophysiology of suicidality including SI, SA, and death by suicide (Black & Miller, Reference Black and Miller2015; Brundin, Bryleva, & Thirtamara Rajamani, Reference Brundin, Bryleva and Thirtamara Rajamani2017; Ganança et al., Reference Ganança, Oquendo, Tyrka, Cisneros-Trujillo, Mann and Sublette2016). Across 18 studies included in a meta-analysis, elevated inflammatory mediators such as IL-6 and IL-1β were observed in blood and postmortem brain samples of patients who died by suicide (Black & Miller, Reference Black and Miller2015). Previous studies have also reported that TNF-α may be associated with a non-lethal SA in patients with depression (Janelidze, Mattei, Westrin, Träskman-Bendz, & Brundin, Reference Janelidze, Mattei, Westrin, Träskman-Bendz and Brundin2011), although meta-analyses do not provide strong support for this association (Black & Miller, Reference Black and Miller2015; Ducasse, Olié, Guillaume, Artéro, & Courtet, Reference Ducasse, Olié, Guillaume, Artéro and Courtet2015). A large prospective cohort study in the general population also found that individuals with higher levels of CRP were 4.2 times more likely to die by suicide compared to those with lower levels of CRP (Batty, Bell, Stamatakis, & Kivimäki, Reference Batty, Bell, Stamatakis and Kivimäki2016). Theories of inflammation and suicide propose that acute and chronic stressors (e.g. sleep disturbance) increase pro-inflammatory cytokine activity, which contribute to greater tryptophan catabolism via the kynurenine pathway, altered glutamatergic transmission, and changes to monoamine metabolism (Brundin et al., Reference Brundin, Bryleva and Thirtamara Rajamani2017; Ganança et al., Reference Ganança, Oquendo, Tyrka, Cisneros-Trujillo, Mann and Sublette2016). Dysregulation of these neurobiological processes influences emotional, cognitive, and behavioral changes, which are hypothesized to increase suicide risk in vulnerable individuals (Brundin et al., Reference Brundin, Bryleva and Thirtamara Rajamani2017; Ganança et al., Reference Ganança, Oquendo, Tyrka, Cisneros-Trujillo, Mann and Sublette2016).
The preceding evidence suggests that sleep disturbance including insomnia, short sleep duration, and long sleep duration are contributors to elevated levels of inflammation and risk for SI and SAs. Inflammation also appears to be implicated in the risk for SI and SAs. Previous studies have not yet evaluated whether the negative consequences of sleep disturbance for suicide risk may operate through inflammation. The goal of the present study was to make progress toward identifying the predictors and mechanisms of SI and SA in adults diagnosed with current or remitted depressive and/or anxiety disorders enrolled in the Netherlands Study of Depression and Anxiety (NESDA). The first aim was to examine how sleep duration or insomnia symptoms were related to SI during the past week and a lifetime history of an SA. It was hypothesized that both short and long sleep duration, as compared to normal sleep duration, and greater insomnia symptoms would be associated with more SI and a prior SA. The second aim was to evaluate how inflammation was associated with SI and a prior SA. It was hypothesized that higher levels of IL-6, CRP, and TNF-α would be associated with more SI and a prior SA. The third aim was to test the indirect effects of sleep duration and insomnia on SI or a prior SA through inflammation. It was hypothesized that the association between sleep duration and/or insomnia symptoms and SI or a prior SA would be explained, at least partly, via the levels of inflammation.
Methods
Sample
Data come from the NESDA, which is an ongoing cohort study designed to investigate the long-term course and consequences of depressive and anxiety disorders. A detailed description of the NESDA study design can be found elsewhere (Penninx et al., Reference Penninx, Beekman, Smit, Zitman, Nolen and Spinhoven2008). Participants (n = 2981) were adults aged 18–65 recruited from the community (19%), general practice (54%), and secondary mental health (27%) facilities. Participants were assessed at baseline between 2004 and 2007. Exclusion criteria for the NESDA study were not speaking the Dutch language and a known clinical diagnosis of bipolar disorder, obsessive–compulsive disorder, severe addiction disorder, psychotic disorder, or organic psychiatric disorder. The baseline assessment was comprised of an in-person interview, including a standardized diagnostic psychiatric interview, a medical assessment, computer tasks, written questionnaires, and biological measurement (among which was a blood draw in a fasting state). The research protocol was approved by the Ethical Committee of participating universities, and after complete description of the study, all respondents provided written informed consent.
A subset (n = 2329) of NESDA participants were included in this study. Participants were included in this sample if they had a current (6-month recency) anxiety disorder (n = 543), current depressive disorder (n = 396), current comorbid depressive and anxiety disorder (n = 762), or remitted depressive and/or anxiety disorder (n = 628) at baseline. Individuals without a psychiatric diagnosis (n = 652) were not included in this sample given the low occurrence of SI (n = 5) and SA (n = 9) in this group and to reduce the impact of psychiatric status (i.e. absence v. presence) on findings. The presence of psychiatric disorders was determined with the Composite International Diagnostic Interview assessed at baseline (CIDI, versions 2.1). The CIDI is a standardized psychiatric diagnostic interview that follows the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria to establish diagnoses. The CIDI is a highly reliable and valid instrument for assessing depressive and anxiety disorders (Wittchen, Reference Wittchen1994) and was administered by specially trained research staff.
Participants had missing data concerning sleep duration (n = 358), insomnia (n = 318), inflammatory markers (CRP: n = 37; IL-6: n = 37; TNF-α: n = 47), and SI (n = 8), or SA (n = 9). Compared to those without missing data, participants with any missing data were more likely to be younger [t(2300) = −4.70, p < 0.001, Cohen's d = −4.63], to have less education [t(2327) = −5.75, p < 0.001, Cohen's d = −4.70], to have higher depression symptoms [t(2291) = 4.50, p < 0.001, Cohen's d = 2.58], to have higher anxiety symptoms [t(2295) = 4.79, p < 0.001, Cohen's d = 1.79], to have a higher BMI [t(2325) = 6.22, p < 0.001, Cohen's d = 6.89], to have never smoked [χ2(3) = 18.25, p < 0.001, Cramer's V = 0.12], and to be occupationally disabled/on sickness benefit or unemployed [χ2(2) = 32.03, p < 0.001, Cramer's V = 0.09]. Participants with missing data did not differ on the measures of SI during the past week [χ2(1) = 0.01, p = 0.913, Cramer's V = 0.004] or SA [χ2(1) = 1.57, p = 0.210, Cramer's V = 0.03].
Measures
Suicidal ideation and suicide attempts
A lifetime history of an SA was assessed in the face-to-face baseline interview. The question was derived from the World Health Organization (WHO)/Euro multicenter study on parasuicide. The following single retrospective question was asked: ‘Have you ever made a serious attempt to end your life, for instance by harming or poisoning yourself or by getting into an accident? No/yes.’ This item was used to categorize SA (0 = no SA, 1 = prior SA). SI during the past week was assessed at baseline with the first five items of the Scale for Suicidal Ideation (SSI; Beck, Kovacs, & Weissman, Reference Beck, Kovacs and Weissman1979). Each item had three answer categories in the ascending order of severity (0–2). The first five items from the SSI were used because the NESDA sample included participants with and without depression and/or anxiety and varying levels of severity, which meant there would likely be many participants without SI. Thus, the first five items from the SSI were used to balance the need to collect high-quality data in an epidemiological interview with participant burden. These five items had good internal consistency (α = 0.80). A response >0 on one or more SSI items was used to categorize SI (0 = no SI, 1 = presence of SI). Given that prior studies have been limited by combining individuals with SI and an SA (May & Klonsky, Reference May and Klonsky2016), analyses for SI included participants with past week SI and no prior SA.
Sleep duration and insomnia symptoms
Sleep duration was assessed at baseline by asking participants ‘How many hours per night did you sleep on average during the last 4 weeks?’ Responses included ‘10 or more hours’, ‘9 h’, ‘8 h’, ‘7 h’, ‘6 h’, ‘5 or less h’. Sleep duration was categorized as short sleep (⩽6 h per night; n = 636), normal sleep (7–9 h per night; n = 1280), and long sleep (⩾10 h per night; n = 97), as used previously (Prather et al., Reference Prather, Vogelzangs and Penninx2015). This decision was guided by the categorical nature of the sleep duration question as well as evidence demonstrating non-linear effects of sleep duration on SI, SA, and inflammatory markers (Chakravorty et al., Reference Chakravorty, Siu, Lalley-Chareczko, Brown, Findley, Perlis and Grandner2015; Dowd et al., Reference Dowd, Goldman and Weinstein2011; Goodwin & Marusic, Reference Goodwin and Marusic2008; Guo et al., Reference Guo, Xu, Deng, Huang, Huang, Gao and Lu2017; Irwin et al., Reference Irwin, Olmstead and Carroll2015b; Kim et al., Reference Kim, Park, Cho, Park, Park, Choi and Chang2013; Miller et al., Reference Miller, Kandala, Kivimaki, Kumari, Brunner, Lowe and Cappuccio2009; Patel et al., Reference Patel, Zhu, Storfer-Isser, Mehra, Jenny, Tracy and Redline2009). Insomnia symptoms were assessed at baseline using the Women's Health Initiative Insomnia Rating Scale (IRS; Levine et al., Reference Levine, Kaplan, Kripke, Bowen, Naughton and Shumaker2003a). This scale consists of five questions rated 0–4 that address core symptoms of insomnia including difficulty falling asleep, waking up during the night, early morning awakenings, trouble getting back to sleep after waking up, and sleep quality in the past month. The IRS has good test-retest reliability and is strongly associated with other actigraphy-related sleep measures (Levine et al., Reference Levine, Lewis, Bowen, Kripke, Kaplan, Naughton and Shumaker2003b).
Inflammatory markers
Fasting blood samples were obtained at the baseline measurement to assess the markers of systemic inflammation, including CRP, IL-6, and TNF-α. Samples were obtained in the morning between 8:00 and 9:00 h and kept frozen at −80 °C until assay. CRP and IL-6 were assayed at the Clinical Chemistry department of the VU University Medical Center. High-sensitivity plasma levels of CRP were measured in duplicate by an in-house enzyme-linked immunosorbent assay (ELISA) based on purified protein and polyclonal anti-CRP antibodies (Dako, Glostrup, Denmark). Intra- and inter-assay coefficients of variation were 5% and 10%, respectively. Plasma IL-6 levels were measured in duplicate by high-sensitivity ELISA (PeliKine Compact ELISA, Sanquin, Amsterdam, The Netherlands). Intra- and inter-assay coefficients of variation were 8% and 12%, respectively. Plasma TNF-α levels were assayed in duplicate at Good Biomarker Science, Leiden, The Netherlands, using high-sensitivity solid-phase ELISA (Quantikine HS Human TNF-α Immunoassay, R&D Systems, Minneapolis, MN, USA). Intra- and inter-assay coefficients of variation were 10% and 15%, respectively. IL-6, TNF-α, and CRP were log transformed for analyses due to skewness. Participants with CRP levels >10 mg/L (n = 125) were not included in the analyses as these values likely reflect acute infection rather than chronic inflammation (Pearson et al., Reference Pearson, Mensah, Alexander, Anderson, Cannon, Criqui and Vinicor2003).
Covariates
Selection of covariates was guided by previous research examining multivariate predictors of suicide risk (Eikelenboom, Beekman, Penninx, & Smit, Reference Eikelenboom, Beekman, Penninx and Smit2018) as well as potential confounders correlated with study variables (see online Supplementary Table S1). Age (in years), gender, education (in years), employment status (employed; occupational disabled/on sickness benefit; unemployed; other), and smoking status (current smoker; former smoker; never smoker) were assessed during the baseline interview. The Inventory of Depressive Symptomatology, Self-Report (IDS-SR) assessed depression symptoms at baseline (Rush, Gullion, Basco, Jarrett, & Trivedi, Reference Rush, Gullion, Basco, Jarrett and Trivedi1996). The IDS-SR has demonstrated good reliability and validity (Rush et al., Reference Rush, Gullion, Basco, Jarrett and Trivedi1996). The sleep-related questions (items 1–4) from the IDS-SR were removed from the sum score to prevent overlap with sleep measures. The Beck Anxiety Inventory (BAI) assessed anxiety severity at baseline and has demonstrated good reliability and validity (Beck, Epstein, Brown, & Steer, Reference Beck, Epstein, Brown and Steer1988). Antidepressant medication was classified according to the Anatomical Therapeutic Chemical (ATC) classification system and included tricyclic antidepressants (ATC-code N06AA), selective serotonin re-uptake inhibitors (ATC-code N06AB), and other antidepressants (ATC-code N06AF/N06AX). Body mass index (BMI; kg/m2) and number of chronic diseases were included as covariates in the analyses involving inflammatory markers given previously identified associations with inflammation (Scrivo, Vasile, Bartosiewicz, & Valesini, Reference Scrivo, Vasile, Bartosiewicz and Valesini2011). Number of chronic diseases was calculated as the sum of the following categories for each participant: cardiometabolic, respiratory, endocrine, neurological, musculoskeletal, digestive disorders, and cancer (Gerrits, van Oppen, van Marwijk, van der Horst, & Penninx, Reference Gerrits, van Oppen, van Marwijk, van der Horst and Penninx2012).
Data analysis
The first aim of this study was addressed with logistic regression using the glm function in R (R Development Core Team, 2018), and included the main effect of covariates (age, sex, education, employment status, BMI, number of chronic diseases, smoking status, psychiatric diagnosis status, depression symptoms, anxiety symptoms, and antidepressant medication), the main effect of sleep duration (⩽6 h per night or ⩾10 h per night with 7–9 h per night as the reference), and the main effect of insomnia symptoms as the predictors and SI or SA as the outcome. The second aim also used logistic regression and included the main effect of the covariates from aim one and inflammatory markers (CRP, IL-6, and TNF-α) as the predictors of SI or SA. Variance inflammation factor (VIF) scores were used to evaluate multicollinearity between the predictors described in aims one and two. Multicollinearity becomes a concern when the VIF is >10 and all VIF scores were acceptable (range = 1.04–2.99, mean = 1.41, standard deviation = 0.40).
Path analysis was used to evaluate the third aim with the lavaan package in R (Rosseel, Reference Rosseel2012). Path analysis is a multivariate statistical method that tests both direct and indirect effects between observed variables. An advantage of path analysis over multiple regression is that path analysis tests all specified relationships simultaneously and each coefficient is adjusted for the associations between other variables included in the model. Two separate path analysis models were specified for SI and SA. The path analysis included (1) direct effects of age, sex, education, employment status, BMI, number of chronic diseases, smoking status, psychiatric diagnosis status, depression symptoms, anxiety symptoms, and antidepressant medication on SI or SA; (2) direct effects of short sleep duration, long sleep duration, and insomnia symptoms on SI or SA; (3) direct effects of short sleep duration, long sleep duration, and insomnia symptoms on CRP, IL-6, and TNF-α; (4) direct effects of CRP, IL-6, and TNF-α on SI or SA; and (5) indirect effects of short sleep duration, long sleep duration, and insomnia symptoms on SI or SA via CRP, IL-6, and TNF-α. The proportion mediated was calculated as the indirect effect divided by the total effect, ab/(ab + c′), based on recommendations for continuous mediators in logistic regression (Rijnhart, Twisk, Eekhout, & Heymans, Reference Rijnhart, Twisk, Eekhout and Heymans2019).
Results
Participant characteristics
Demographic, clinical, sleep, and inflammation variables for participants with and without SI or a prior SA are presented in Table 1. Participants with SI during the past week were more likely to report a prior SA (n = 99, 28.9%) than participants without SI during the past week [(n = 239, 12.1%), χ2(1) = 61.10, p < 0.001, Cramer's V = 0.12]. Likewise, participants who reported a prior SA were more likely to report SI during the past week (n = 97, 28.9%) than participants without a prior SA [(n = 244, 12.3%), χ2(1) = 61.10, p < 0.001, Cramer's V = 0.12].
Table 1. Means, standard deviations, and percentages for demographic, clinical, sleep, and inflammation variables by suicidal ideation or suicide attempt

CRP, C-reactive protein; IL-6, interleukin-6; TNF-α, tumor necrosis factor α; s.d., standard deviation.
Note: p values for categorical variables with more than two levels (employment status, diagnostic status, and sleep duration) were derived from χ2 tests based on 2 by n tables such that n was the number of levels of the categorical variable. p values for each level within the categorical variable were derived from χ2 tests based on 2 by 2 tables using effect coding comparing the indicated level to all other levels. p values for continuous variables were derived from t tests. The effect size for continuous variables was Cohen's d. The effect size for categorical variables was Cramer's V. CRP, IL-6, and TNF-α were log-transformed. Sleep-related questions (items 1–4) from the Inventory of Depressive Symptomatology were removed.
Sleep disturbance and suicidal ideation or suicide attempt
The association between sleep duration and insomnia symptoms on SI or a prior SA is presented in Table 2. Participants with short sleep duration compared to normal sleep duration were more likely to report a prior SA [adjusted odds ratio (AOR) 1.68, 95% CI 1.13–2.51]. Long sleep duration compared to normal sleep duration was associated with a higher likelihood of reporting SI in the past week (AOR 2.22, 95% CI 1.02–4.82). More severe insomnia symptoms were associated with higher odds of reporting SI (AOR 1.44, 95% CI 1.14–1.83). Long sleep duration and insomnia symptoms were not significantly related to a prior SA (Table 2). Short sleep duration was not significantly associated with SI (Table 2).
Table 2. The association between sleep duration, insomnia symptoms, and inflammation on suicidal ideation or a prior suicide attempt

CRP, C-reactive protein; IL-6, interleukin-6; TNF-α, tumor necrosis factor α; AOR, adjusted odds ratio.
Note: Statistical models were adjusted for age, sex, education, employment status, body mass index, number of chronic diseases, smoking status, psychiatric diagnosis status, depression symptoms, anxiety symptoms, and antidepressant medication.
Inflammation and suicidal ideation or suicide attempt
The association between inflammatory markers and past week SI or a prior SA was evaluated next (Table 2). Higher levels of IL-6 were associated with a greater likelihood of reporting SI in the past week (AOR 1.31, 95% CI 1.02–1.68). TNF-α and CRP were not significantly associated with SI. Inflammatory markers were not significantly associated with a prior SA.
Indirect effect of sleep disturbance on suicidal ideation or suicide attempt via inflammation
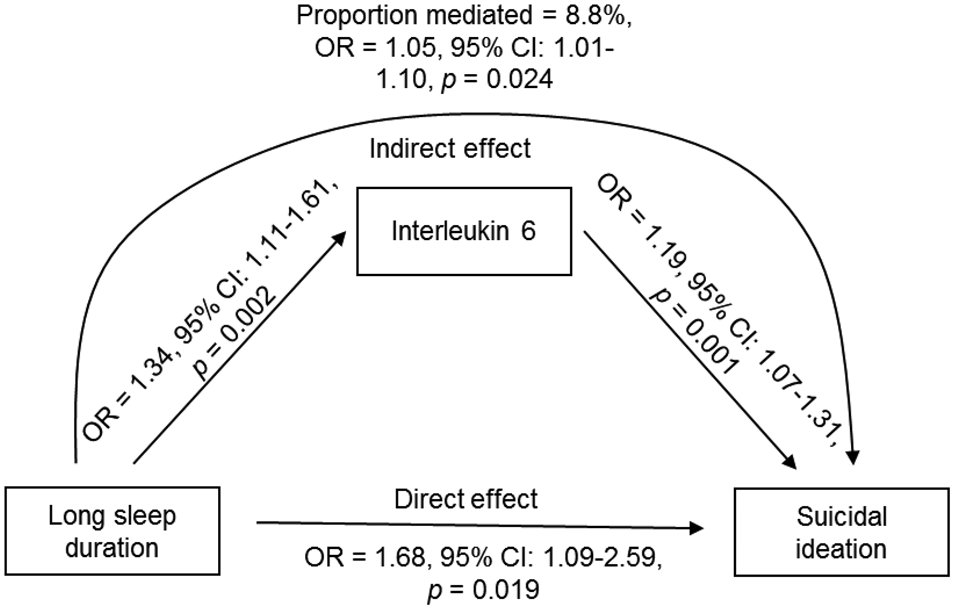
Indirect effects of sleep duration and insomnia on SI or a prior SA through inflammation were tested with path analysis (Table 3). There was a significant indirect effect of long compared to normal sleep duration on SI via IL-6 (Fig. 1; B = 0.050, s.e. = 0.022, z = 2.252, p = 0.024, AOR 1.05, 95% CI 1.01–1.10). The strength of the association was attenuated by 8.8% after accounting for the indirect effect of IL-6 in the model. The direct effect of long compared to normal sleep duration on SI remained significant (B = 0.518, s.e. = 0.221, z = 2.342, p = 0.019, AOR 1.68, 95% CI 1.09–2.59). The rates of SI by hours of sleep duration and quartiles of IL-6 are depicted in Fig. 2. The indirect effect of short sleep duration or insomnia symptoms on SI or a prior SA via inflammation was non-significant.

Fig. 1. Indirect effect of long compared to normal sleep duration on SI via IL-6 derived from path analysis.

Fig. 2. Raw rates of suicidal ideation during the past week by hours of sleep duration and interleukin-6 quartiles. Note: The denominator was the sum of participants with and without past week suicidal ideation by hour of sleep and quartile of IL-6. IL-6, interleukin-6.
Table 3. Indirect effects of short sleep duration, long sleep duration, and insomnia on suicidal ideation or a prior suicide attempt via inflammation derived from path analysis

CRP, C-reactive protein; IL-6, interleukin-6; TNF-α, tumor necrosis factor α.
Note: Path analysis models were adjusted for age, sex, education, employment status, body mass index, number of chronic diseases, smoking status, psychiatric diagnosis status, depression symptoms, anxiety symptoms, and antidepressant medication. The proportion mediated was calculated as ab/(ab + c′). Note that the proportion mediated is not reported for instances in which the sign of the indirect effect differed from the direct effect or total effect.
Discussion
The present study examined the associations between sleep duration, insomnia symptoms, and inflammation with recent SI and a prior SA in adults diagnosed with current or remitted depressive and/or anxiety disorders. Short sleep duration was associated with a higher likelihood of a prior SA but was not associated with SI. Long sleep duration was associated with a higher likelihood of past week SI and not associated with a prior SA. One possible explanation for the differential findings for short and long sleep duration may be that short sleep duration, in contrast to long sleep duration, typically results in more nocturnal wakefulness, which may contribute to suicidal behavior at night (Perlis et al., Reference Perlis, Grandner, Chakravorty, Bernert, Brown and Thase2015). Furthermore, previous studies have indicated that insomnia with objective short sleep duration may be an especially severe insomnia phenotype linked to the risk for hypertension, diabetes, and neurocognitive impairment (Vgontzas, Fernandez-Mendoza, Liao, & Bixler, Reference Vgontzas, Fernandez-Mendoza, Liao and Bixler2013). Future research should be conducted to examine whether insomnia with objective short sleep duration confers the risk for suicide.
The present findings also suggested that insomnia symptoms were significantly associated with a higher likelihood of SI during the past week. Notably, these associations were independent of anxiety and depression symptoms. Previous research on insomnia and suicide risk has been limited by not controlling for the presence of depression (Bernert & Joiner, Reference Bernert and Joiner2007; Bernert & Nadorff, Reference Bernert and Nadorff2015). Insomnia symptoms were not significantly associated with a prior SA, which was surprising given that previous research has indicated that insomnia symptoms are associated with suicidal behavior and death by suicide (Bernert & Joiner, Reference Bernert and Joiner2007; Bernert & Nadorff, Reference Bernert and Nadorff2015; Liu et al., Reference Liu, Tu, Lai, Lee, Tsai, Chen and Chiu2019). Identifying the factors that differentially predict SI and SA is an important priority to understand who may be at imminent risk for suicide (May & Klonsky, Reference May and Klonsky2016). Future research is needed to evaluate whether insomnia is primarily a general suicide risk factor or whether insomnia may help differentiate who is at risk for progressing from SI to a SA.
Our second hypothesis was partially supported. We found evidence that IL-6 was related to a higher likelihood of SI but not a prior SA, controlling for covariates. IL-6 has both pro-inflammatory and anti-inflammatory properties (Scheller, Chalaris, Schmidt-Arras, & Rose-John, Reference Scheller, Chalaris, Schmidt-Arras and Rose-John2011), and IL-6 may continue to signal a pro-inflammatory state during chronic inflammation (Fuster & Walsh, Reference Fuster and Walsh2014; Gabay, Reference Gabay2006). This chronic pro-inflammatory state may contribute to higher suicide risk via various downstream biological pathways including alterations to the HPA axis, dysregulated glutamate transmission, or tryptophan metabolism into kynurenine (Brundin et al., Reference Brundin, Bryleva and Thirtamara Rajamani2017; Ganança et al., Reference Ganança, Oquendo, Tyrka, Cisneros-Trujillo, Mann and Sublette2016). We did not find evidence that CRP or TNF-α were significantly associated with SI or a prior SA. Although previous studies have reported that these markers may be related to suicide (Batty et al., Reference Batty, Bell, Stamatakis and Kivimäki2016; Janelidze et al., Reference Janelidze, Mattei, Westrin, Träskman-Bendz and Brundin2011), meta-analyses do not strongly indicate that CRP and TNF-α are associated with suicide risk (Black & Miller, Reference Black and Miller2015; Ducasse et al., Reference Ducasse, Olié, Guillaume, Artéro and Courtet2015). Additionally, a systematic review of 22 studies from 1980 to 2015 concluded that IL-6 and IL-1β were most robustly linked to suicide risk (Ganança et al., Reference Ganança, Oquendo, Tyrka, Cisneros-Trujillo, Mann and Sublette2016). Nevertheless, additional research is warranted given that IL-6 stimulates CRP production and IL-6 functions synergistically with TNF-α and IL-1β to coordinate inflammation (Brebner, Hayley, Zacharko, Merali, & Anisman, Reference Brebner, Hayley, Zacharko, Merali and Anisman2000).
To better understand how sleep disturbance may be related to SI or a prior SA, we also conducted path analyses that included inflammatory markers as mediators. Path analysis indicated that the association between long sleep duration and SI may operate, at least partly, via IL-6. Epidemiological studies have consistently shown that long sleep duration is associated with greater all-cause mortality (Jike, Itani, Watanabe, Buysse, & Kaneita, Reference Jike, Itani, Watanabe, Buysse and Kaneita2018), and inflammation has been identified as a putative mediator of this association (Dowd et al., Reference Dowd, Goldman and Weinstein2011; Grandner & Drummond, Reference Grandner and Drummond2007; Hall et al., Reference Hall, Smagula, Boudreau, Ayonayon, Goldman, Harris and Newman2015). One possible explanation for the current findings is that an underlying medical condition or psychiatric disorder may be contributing to both long sleep duration and elevated IL-6. Although this study controlled for these potential confounders, it remains possible that a subclinical disease may be involved in this association. Alternatively, evidence from human and animal studies suggest that cytokines such as IL-6 are somnogenic (Bryant et al., Reference Bryant, Trinder and Curtis2004), and thus may contribute to excessive sleep duration. Replication with experimental and prospective studies is warranted given the cross-sectional nature of these data. For example, administration of endotoxin can temporarily and safely induce inflammation, and has been used as an experimental model of depression (DellaGioia & Hannestad, Reference DellaGioia and Hannestad2010). Similar experimental paradigms could be utilized to further examine how inflammation may be involved in the association between sleep duration and suicide risk, particularly given that experimental sleep deprivation or restriction does not reliably affect inflammation (Irwin et al., Reference Irwin, Olmstead and Carroll2015b).
The present findings, if replicated, may also provide insight to guide future research on treatment and prevention of suicide. Psychosocial and pharmacological treatments are available that improve sleep and target inflammation. Cognitive-behavioral therapy for insomnia (CBT-I) is among the first-line treatments for insomnia disorder and has demonstrated efficacy in reducing SI (Trockel, Karlin, Taylor, Brown, & Manber, Reference Trockel, Karlin, Taylor, Brown and Manber2015) and inflammation (Irwin et al., Reference Irwin, Olmstead, Breen, Witarama, Carrillo, Sadeghi and Cole2015a). The combination of zolpidem and a selective serotonin reuptake inhibitor improved insomnia and reduced SI among adults with major depressive disorder, insomnia, and SI (McCall et al., Reference McCall, Benca, Rosenquist, Youssef, McCloud, Newman and Krystal2019). Anti-inflammatory medication may also have therapeutic potential for targeting inflammation and suicide risk. A meta-analysis of RCTs found that non-steroidal anti-inflammatory drugs (NSAIDs), cytokine inhibitors, statins, and other anti-inflammatory agents improved depression as an adjunct to other treatments or as a monotherapy (Köhler-Forsberg et al., Reference Köhler-Forsberg, Lydholm, Hjorthøj, Nordentoft, Mors and Benros2019). Modafinil, a wake-promoting medication used to treat narcolepsy and excessive daytime sleepiness, has been shown to reduce inflammation in the animal models of atherosclerosis (Han, Chen, Liu, & Zhu, Reference Han, Chen, Liu and Zhu2018). The extent to which these psychosocial and pharmacological interventions may be effective in targeting suicide via improved sleep and/or reduced inflammation remains to be evaluated in well-designed clinical trials.
Although the findings from the present study provide evidence for associations between sleep duration, insomnia symptoms, and inflammation on SI or SA, there are limitations to this study. First, this study was cross-sectional, and it is not possible to determine the directionality of these effects. For instance, inflammation has been linked to the onset of depression (Prather et al., Reference Prather, Rabinovitz, Pollock and Lotrich2009), which may lead to sleep disturbance and SI. Second, this study used self-reported sleep duration and insomnia symptoms. Although a strength of the present study was the use of biological measures of inflammation, objective measures of sleep duration and diagnosis of insomnia should be a priority in future research, particularly given evidence that insomnia with objective short sleep duration has been associated with immune-mediated health problems (Vgontzas et al., Reference Vgontzas, Fernandez-Mendoza, Liao and Bixler2013). Third, this study included participants with current or remitted depression and/or anxiety disorders, and it is possible that the current results may differ by psychiatric diagnosis. Moderator analyses were not conducted due to power concerns. Although previous studies with this sample have suggested that the association between sleep and inflammation does not differ by diagnostic status (Prather et al., Reference Prather, Vogelzangs and Penninx2015), future studies should further examine how associations between sleep, inflammation, and suicide risk may be impacted by psychiatric diagnosis. Fourth, covariate selection was guided by previous research on the predictors of suicide risk (Eikelenboom et al., Reference Eikelenboom, Beekman, Penninx and Smit2018). The covariates were selected with the goal of adequately controlling for potential confounders while retaining a parsimonious model; however, it is possible that other variables may influence the findings reported in this study. Finally, although SI and SA often precede death by suicide, the findings from the present study may not generalize to those at imminent risk for suicide.
In conclusion, these findings provide additional support for associations between sleep duration, insomnia, and measures of suicidality. Specifically, this study showed that short sleep duration was associated with a prior SA. Long sleep duration, more severe insomnia symptoms, and higher IL-6 were related to higher SI. Additionally, there was evidence that IL-6 may be involved in the association between long sleep duration and SI. These findings provide preliminary evidence that sleep disturbance and inflammation may be involved in the pathophysiology of suicide-related thoughts and behaviors. Although replication and additional research will be needed to understand the clinical implications of the current findings, psychosocial treatments such as CBT-I and medications including zolpidem, NSAIDs, and modafinil have promising therapeutic potential for targeting sleep disturbance, inflammation, and suicidality. Further research on the therapeutic potential of these treatments may be important future research and clinical targets for understanding and preventing suicide. Given the need to identify the mechanisms that increase vulnerability to suicide, the present study represents an important initial step in identifying the novel and modifiable mechanisms associated with SI or a lifetime history of a SA.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291720000860.
Acknowledgements
The infrastructure for the NESDA study (http://www.nesda.nl) is funded through the Geestkracht program of the Netherlands Organization for Health Research and Development (ZonMW, grant number 10-000-1002) and from participating universities and organizations (VU University Medical Center, GGZ inGeest, Leiden University Medical Center, Leiden University, GGZ Rivierduinen, University Medical Center Groningen, University of Groningen, Lentis, GGZFriesland, GGZ Drenthe, Rob Giel Onderzoekcentrum).
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interest
Dr Penninx has received research funding from Jansen Research and Boehringer Ingelheim. Dolsen has received research support from the National Institute of Mental Health (grant number T32 MH020006). All other authors reported no financial interests or potential conflicts of interest.