Introduction
Anorexia nervosa (AN) is characterized by extreme restriction of caloric intake despite severe bodily emaciation. This asceticism is not limited to food, but also expressed in denial of other primary needs and desires, such as intimate and personal relationships (Schmidt & Treasure, Reference Schmidt and Treasure2006). In search for neurobiological mechanisms underlying these puzzling behaviors, researchers have begun to focus on the reward system (O'Hara et al. Reference O'Hara, Campbell and Schmidt2015). The reward system, encompassing cortical as well as subcortical structures including striatum, amygdala, hypothalamus, insula, and orbitofrontal cortex (OFC), processes (incentive) salience, (dis)pleasure, and positive reinforcement (Berridge & Kringelbach, Reference Berridge and Kringelbach2015). However, when investigating the processing of reward-related stimuli, the reward system should not be considered in isolation. Several studies using an array of tasks suggest that cognitive processes can modulate (enhance or inhibit) the experience of reward-related stimuli (Goschke, Reference Goschke2014; Botvinick & Braver, Reference Botvinick and Braver2015). Brain regions that have been associated with such processes including attention and inhibition are the lateral prefrontal cortex (lPFC) and the parietal cortex (Botvinick & Cohen, Reference Botvinick and Cohen2014; Cole et al. Reference Cole, Repovš and Anticevic2014). When choosing between healthy and tasty food, the neural integration of competing goal values in OFC has been shown to be modulated by activity in the lPFC (Hare et al. Reference Hare, Camerer and Rangel2009); a brain region broadly implicated in top–down biasing (Miller & Cohen, Reference Miller and Cohen2001) and self-control (Heatherton & Wagner, Reference Heatherton and Wagner2011). Its role as a key regulator in the control of food choice is also underlined by a transcranial magnetic stimulation study, which demonstrated changed preference ratings for high-caloric food items after inhibitory stimulation to the lPFC (Camus et al. Reference Camus, Halelamien, Plassmann, Shimojo, O'Doherty, Camerer and Rangel2009).
Previous studies on reward processing in AN have reported alterations in reward-related brain regions (Wagner et al. Reference Wagner, Aizenstein, Venkatraman, Fudge, May, Mazurkewicz, Frank, Bailer, Fischer and Nguyen2007b ; Wierenga et al. Reference Wierenga, Bischoff-Grethe, Melrose, Irvine, Torres, Bailer, Simmons, Fudge, McClure, Ely and Kaye2015) but also in the cognitive control system (Ehrlich et al. Reference Ehrlich, Geisler, Ritschel, King, Seidel, Boehm, Breier, Clas, Weiss, Marxen, Smolka, Roessner and Kroemer2015; King et al. Reference King, Geisler, Bernardoni, Ritschel, Böhm, Seidel, Mennigen, Ripke, Smolka, Roessner and Ehrlich2016). While some of these studies have focused on monetary rewards, neuroimaging studies applying taste- or food-related stimuli in AN confirmed alterations in both networks, albeit as increased and decreased activation (Joos et al. Reference Joos, Saum, van Elst, Perlov, Glauche, Hartmann, Freyer, Tüscher and Zeeck2011; Kim et al. Reference Kim, Ku, Lee, Lee and Jung2012; Frank et al. Reference Frank, Shott, Hagman and Mittal2013; Sanders et al. Reference Sanders, Smeets, van Elburg, Danner, van Meer, Hoek and Adan2015). A recent study (Foerde et al. Reference Foerde, Steinglass, Shohamy and Walsh2015) suggested that alterations in the circuit between lPFC and dorsal striatum may underpin maladaptive food choice in AN. Although processing of social reward in AN has received relatively less attention, two recent functional imaging studies reported reduced blood oxygenation level-dependent (BOLD) responses in the prefrontal (Via et al. Reference Via, Soriano-Mas, Sánchez, Forcano, Harrison, Davey, Pujol, Martínez-Zalacaín, Menchón, Fernández-Aranda and Cardoner2015) and parietal (McAdams et al. Reference McAdams, Lohrenz and Montague2015) regions when processing positive social feedback. In summary, these findings remain inconclusive and we lack a clear understanding of reward-related processes in AN.
Despite the relative lack of substantiated findings, different etiological models have been developed to create a comprehensive framework describing interactions between the reward system and the cognitive control system and their contribution to the pathogenesis of AN. A recent model by O'Hara et al. (Reference O'Hara, Campbell and Schmidt2015) proposes increased reward responsiveness in the striatal dopamine system toward AN-related cues and behaviors. As in other models (Walsh, Reference Walsh2013; Steinglass & Walsh, Reference Steinglass and Walsh2016), it is postulated that a hyper-responsive striatal dopamine system promotes a learning process of pathological behavior. Over time, these learned behaviors may turn into robust habits and impede the treatment of the illness. In contrast, others (Kaye et al. Reference Kaye, Fudge and Paulus2009; Brooks et al. Reference Brooks, Owen, Uher, Friederich, Giampietro, Brammer, Williams, Schiöth, Treasure and Campbell2011a ) have emphasized the role of an overactive prefrontal control system in AN and hypothesize that this system inhibits motivational drives or compensates for primary deficits in limbic regions.
To gain a deeper understanding of reward and cognitive control processes in AN, it would be helpful to examine the role of the reward system uncoupled from modulation by the cognitive control system during reward processing. One opportunity that may offer a certain degree of decoupling is subliminal stimulation (Zedelius et al. Reference Zedelius, Veling and Aarts2011; Mudrik et al. Reference Mudrik, Faivre and Koch2014). By definition, subliminal processing is a condition where bottom–up stimulation is less likely to provoke robust and outlasting fronto-parietal responses (Dehaene et al. Reference Dehaene, Changeux, Naccache, Sackur and Sergent2006). In line with that, research using masked priming paradigms suggests that primarily supraliminal stimuli gain access to a ‘global workspace’, which can be used as input for a variety of higher cognitive processes such as inhibition (Baars, Reference Baars2002; Dehaene et al. Reference Dehaene, Changeux, Naccache, Sackur and Sergent2006). Meta-analytic data of healthy and clinical samples (Brooks et al. Reference Brooks, Savov, Allzén, Benedict, Fredriksson and Schiöth2012b ) indicate that subliminal stimulation with arousing stimuli provokes activation in subcortical regions including the amygdala and thalamus independently from higher order cortical regions, but also the cingulo-insular network thought to integrate autonomic and sensory information to determine their relevance (Seeley et al. Reference Seeley, Menon, Schatzberg, Keller, Glover, Kenna, Reiss and Greicius2007). However, another meta-analysis unexpectedly did not find subcortical regions to be associated with subliminal processing (Meneguzzo et al. Reference Meneguzzo, Tsakiris, Schioth, Stein and Brooks2014). Subliminal stimulation nevertheless remains a potentially powerful tool for investigating the early response of the reward system without strong modulating influence of the fronto-parietal control system in health and disease (Childress et al. Reference Childress, Ehrman, Wang, Li, Sciortino, Hakun, Jens, Suh, Listerud, Marquez, Franklin, Langleben, Detre and O'Brien2008; Brooks et al. Reference Brooks, Savov, Allzén, Benedict, Fredriksson and Schiöth2012b ). Accordingly, previous work in AN showed that supraliminal but not subliminal distractor stimuli interfered with working memory task performance (Dickson et al. Reference Dickson, Brooks, Uher, Tchanturia, Treasure and Campbell2008) as well as performance in a Stroop task (Sackville et al. Reference Sackville, Schotte, Touyz, Griffiths and Beumont1998). In a new paradigm, we present subliminal and supraliminal reward-related stimuli to differentiate early bottom–up subcortical responses from later, top–down cortical responses. If AN patients were predominantly characterized by abnormalities in the reward system, one would expect alterations in the corresponding brain regions during subliminal stimulation, while alterations in consequence of a regulation mechanism of the cognitive control system would only be evident under the supraliminal stimulation. In the latter case, modulating influence of the cognitive control system, which is hypothesized to be hyperactive during the supraliminal processing of reward-related stimuli in AN, would become visible by increased functional connectivity between brain areas of the cognitive control and reward system.
Methods
Participants
The sample of the current study consisted of a total of 70 female volunteers: 35 patients with acute AN according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V) (12–29 years old) and 35 female healthy controls (HC) (12–29 years old). Within the AN group, 33 were of the restrictive and two of the binge/purging subtype; three had comorbid psychiatric disorders (one patient with depressive disorders, one with anxiety disorder, and one with obsessive compulsive disorder). All patients were admitted to eating disorder programs of a university child and adolescent psychiatry and psychosomatic medicine department and were assessed within 96 h after beginning a behaviorally oriented nutritional rehabilitation program. HC participants had to be of normal weight and eumenorrheic and without any history of psychiatric illness. HC were recruited through advertisement among pupils and university students.
We applied several additional exclusion criteria for each group (see online Supplementary Material 1.1) – most importantly psychotropic medication within 4 weeks prior to the study, binge eating, or diagnosis of bulimia nervosa, substance abuse, neurologic or medical conditions.
This study was approved by the local Institutional Review Board, and all participants (and if underage their guardians) gave written informed consent.
Clinical measures
For all participants, current diagnoses of eating disorders were ascertained by evaluation of the expert form of the Structured Interview of AN and Bulimia Nervosa (Fichter & Quadflieg, Reference Fichter and Quadflieg1999). Eating disorder-specific psychopathology was assessed with the short version of the Eating Disorders Inventory (EDI-2; Paul & Thiel, Reference Paul and Thiel2005).
Self-reported appetite, including the subscale ‘hunger’, was measured with the use of a visual analog scale (Blundell et al. Reference Blundell, De Graaf, Hulshof, Jebb, Livingstone, Lluch, Mela, Salah, Schuring, Van Der Knaap and Westerterp2010; see online Supplementary Material 1.2).
Task
During functional magnetic resonance imaging (fMRI), participants passively viewed streams of food stimuli, social stimuli, and neutral stimuli presented either subliminally or supraliminally (referred to as ‘condition’) with an Acer projector P5290DLP capable of presenting stimuli for 17 ms. The paradigm was divided into four equally long blocks in which the first two involved subliminal stimulation and the last two supraliminal stimulation. Subliminal blocks were shown first as familiarity of the stimuli may promote conscious processing even under subliminal stimulation condition (Mudrik et al. Reference Mudrik, Faivre and Koch2014). Every block consisted of nine mini-blocks (three of each stimulus category in pseudo-randomized order), each composed of 10 trials. In the subliminal trials, the stimuli were presented for 17 ms, followed by a mask for 150 ms, and a cross-hair (fixation) for 1309 ms. In the supraliminal trials, stimuli were presented for 500 ms, followed by a cross-hair presented for 973 ms (see online Supplementary Material 1.3).
Stimuli
Thirty neutral and 30 social stimuli were selected from the International Affective Picture System (Lang et al. Reference Lang, Bradley and Cuthbert1999) and the database EmoPics (Wessa et al. Reference Wessa, Kanske, Neumeister, Bode, Heissler and Schönfelder2010). Neutral stimuli showed, e.g. plants or household items, while social stimuli showed happy social sceneries (e.g. playing children). The 30 food stimuli employed in this study originated from a dataset by Kroemer et al. (Reference Kroemer, Krebs, Kobiella, Grimm, Vollstädt-Klein, Wolfensteller, Kling, Bidlingmaier, Zimmermann and Smolka2013). The picture selection was based on the following criteria: (1) social stimuli needed to be free of eating disorder-relevant content (e.g. pictures showing women in bikini were excluded), (2) only neutral and social stimuli with valence and arousal ratings that were similar in AN patients and HC (established in an independent pilot study) were selected, (3) the stimuli of all three conditions had to be similar regarding entropy of intensity distribution and brightness to exclude that the perception is biased by differing stimulus properties. The mask used in the subliminal trials was created by scrambling fractioned pieces of the original stimuli and were similar with respect to entropy and brightness to the original stimuli.
Visibility test
After a short break, a second task was carried out in the scanner during structural scanning (to simulate similar conditions to those during the main task) to measure the participant's ability to recognize subliminal stimuli. This visibility test adapts the structure of the above described subliminal trial, but was followed by a forced choice, where the participants had to identify by button press the preceding subliminally presented stimulus (target) out of three distractor stimuli, which were displayed at the same time for 3000 ms.
Structural and functional image acquisition
Data were acquired between 8:00 and 9:00 after an overnight fast using a standard 3 T Siemens Trio, equipped with a standard 12-channel head coil. T1-weighted structural brain scans were acquired with rapid acquisition gradient echo sequence with the following parameters: number of slices = 176; repetition time = 1900 ms; echo time = 2.26 ms; flip angle = 9°; slice thickness = 1 mm; voxel size = 1 mm × 1 mm × 1 mm; field-of-view (FoV) = 256 mm × 224 mm; bandwidth = 2004 Hz/pixel.
Functional images were acquired by using a gradient-echo T2*-weighted echo planar imaging with the following parameters: tilted 30° toward AC–PC line (to reduce signal dropout in orbitofrontal regions); number of volumes = 190; number of slices = 40; repetition time = 2410 ms; echo time = 25 ms; flip angle of 80°; 3.4 mm in-plane resolution; slice thickness of 2 mm (1 mm gap resulting in a voxel size of 3 × 3 × 2 mm); FoV = 192 × 192 mm; bandwidth of 2112 Hz/pixel.
Image data preprocessing
Functional and structural images were processed using SPM8 toolbox (http://www.fil.ion.ucl.ac.uk/spm/) within the Nipype framework (http://nipy.sourceforge.net/nipype/). The slice time corrected functional data were realigned and registered to their mean. The realigned files were coregistered to the subject's structural brain image. A DARTEL template was created using structural images from all subjects (Ashburner, Reference Ashburner2007). The echo planar image (EPI) volumes were then normalized to Montreal Neurological Institute (MNI) space using the DARTEL template and corresponding flow field. The resulting data were smoothed with an isotropic 8 mm full-width at half maximum (FWHM) Gaussian kernel.
We evaluated the quality of the fMRI data by manual inspection and using artifact detection tools (Whitfield-Gabrieli et al. Reference Whitfield-Gabrieli, Thermenos, Milanovic, Tsuang, Faraone, McCarley, Shenton, Green, Nieto-Castanon and LaViolette2009). Volumes that exceed an intensity threshold of three standard deviations or a threshold of 2 mm normalized movement in any direction were classified as outliers [motion outlier: AN patients = 3.21 (6.96), HC = 8.35 (19.85); intensity outlier: AN patients = 6.73 (3.90), HC = 8.35 (4.89)]. The two groups did not differ regarding numbers of motion and intensity outliers [motion outlier: T(65) = 1.41, p = 0.17; intensity outlier: T(65) = 1.50, p = 0.14].
Statistical analyses
On the first level, a general linear model was fitted to the hemodynamic response to each of the six combinations of stimulation conditions and stimulus types: supraliminal food stimuli (foodsupra), subliminal food stimuli (foodsublim), supraliminal social stimuli (socialsupra), subliminal social stimuli (socialsublim), supraliminal neutral stimuli (neutralsupra), and subliminal neutral stimuli (neutralsublim) using boxcar functions with duration of 15 s (epoch-related design). Additionally, six realignment parameters and outlier volumes identified by quality control as described above were included as nuisance regressors of no interest. On the second level, a linear mixed model including a two-level within-subject variable (stimulation condition: supraliminal and subliminal), a three-level within-subject variable (stimulus type: food, neutral, and social), and a binary between-subject variable (group: AN patients, HC) were estimated using the GLMflex toolbox (http://mrtools.mgh.harvard.edu/index.php/GLM_Flex). To confirm that the task worked as intended, we first examined stimulus type-specific activation patterns within both the supraliminal and the subliminal stimulation conditions by calculating the contrasts socialsublim > neutralsublim, foodsublim > neutralsublim as well as socialsupra > neutralsupra and foodsupra > neutralsupra in HC. To address our main research question regarding differences between the groups when processing the different stimulus types under the two stimulation conditions, we examined the group × stimulation condition × stimulus type interaction effect. If AN patients were predominantly characterized by abnormalities in the reward system, we would predict alterations in those brain regions during subliminal stimulation condition, while alterations in consequence of top–down regulation mechanisms would be expected to be mirrored by alterations in cognitive control areas under the supraliminal stimulation condition.
To guard against type I errors, results were corrected based on permutations using the updated version of 3DClustSim, released August 2016 (http://afni.nimh.nih.gov/pub/dist/doc/program_help/3dClustSim.html). This tool runs Monte Carlo simulations to estimate the cluster size at which the false-positive probability is below an α level of 0.05 for a voxelwise p value level, which was set at p = 0.005. The simulations resulted in a threshold for minimal cluster size of κ = 468 (whole-brain).
Region-of-interest (ROI) analyses
To further specify group differences revealed by the whole-brain analysis of the group × stimulation condition × stimulus type interaction, we extracted condition-specific β values for each participant from all voxels belonging to significant clusters using MarsBaR toolbox for SPM (Brett, Anton, Valabregue, & Poline, Reference Brett, Anton, Valabregue and Poline2002). To explore what factors contribute to the group differences revealed by the whole-brain analysis of the group × stimulation condition × stimulus type interaction effect, extracted β values were subjected to a linear mixed model using SPSS. Predictors in the model included the fixed-effects group, stimulation condition, stimulus type and their interaction, and a by-subject random adjustment for the intercept. Bonferroni correction for multiple comparisons was used for post hoc tests.
The individual sensitivity toward subliminal stimuli in the visibility test was estimated using d′, a measure of the ability to discriminate between target items and distractor items in a four-alternative forced choice task (Hacker & Ratcliff, Reference Hacker and Ratcliff1979).
Associations between BOLD activity and clinical variables, as well as the individual sensitivity (d′) to detect subliminal targets were investigated using Pearson's r separately in each group. The latter association is of interest, as one might assume that the neural response during subliminal stimulation might be modulated by the individual ability to perceive subliminal targets.
Connectivity analysis
To follow-up on results revealed by the analysis described above, we investigated changes in functional connectivity between a brain region identified as a putative source of top–down control signals, the inferior frontal junction (IFJ; see results), using the generalized psychophysiological interaction approach (gPPI; McLaren et al. Reference McLaren, Ries, Xu and Johnson2012). This approach allowed us to assess whether the effect of stimulus type (psychological factors: foodsupra, neutralsupra, socialsupra) is modulated by the activity of a source region (physiological factor). We chose to investigate the supraliminal condition only since potential modulating effects of prefrontal brain regions were only evident during this condition. The investigation of alterations in functional connectivity comprised two steps: First, based on existing hypothesis of hyperactive cognitive control over limbic brain regions in AN (Kaye et al. Reference Kaye, Fudge and Paulus2009), we explored changes in functional connectivity between the source region IFJ and limbic target regions: ventral striatum, amygdala, OFC, and insula. Second, an exploratory approach with no a priori defined target regions of the top–down control signal (see online Supplementary Material 1.4).
Results
Sample characteristics
As shown in Table 1, there were no group differences for age, IQ, and d′ (parameter of the visibility test). As expected, AN patients had a lower body mass index, higher EDI-2 total scores, and reported less hunger at the time of the scanning session than HC.
Table 1. Sociodemographic and clinical variables of the two groups

d′ = index of sensitivity toward subliminal stimuli (a value of zero indicates a performance at chance level; d′ values of HC are not different from zero [T(34) = 1.451; p = 0.153] but d′ values of AN patients [T(34) = 3.242; p = 0.003)], Hunger was assessed with the use of a visual analogue scale ranging from ‘not at all’ (value of 0) to ‘extremely’ (value of 10). Group differences were tested using Student's t tests. Displayed are means ± standard deviations; AN, anorexia nervosa; HC, healthy controls; BMI, body mass index; BMI-SDS, body mass index standard deviation score; IQ, intelligence quotient; EDI-2, Eating Disorder Inventory 2.
Imaging results
Stimulus-specific activation patterns were found for both, the supraliminal as well as the subliminal stimulation condition, i.e. increased BOLD responses in reward-related brain regions, such as the ventral striatum for social v. neutral stimuli types in HC (see online Supplementary Material 2.1).
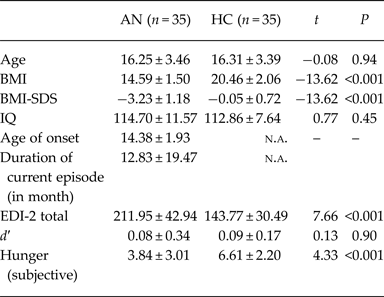
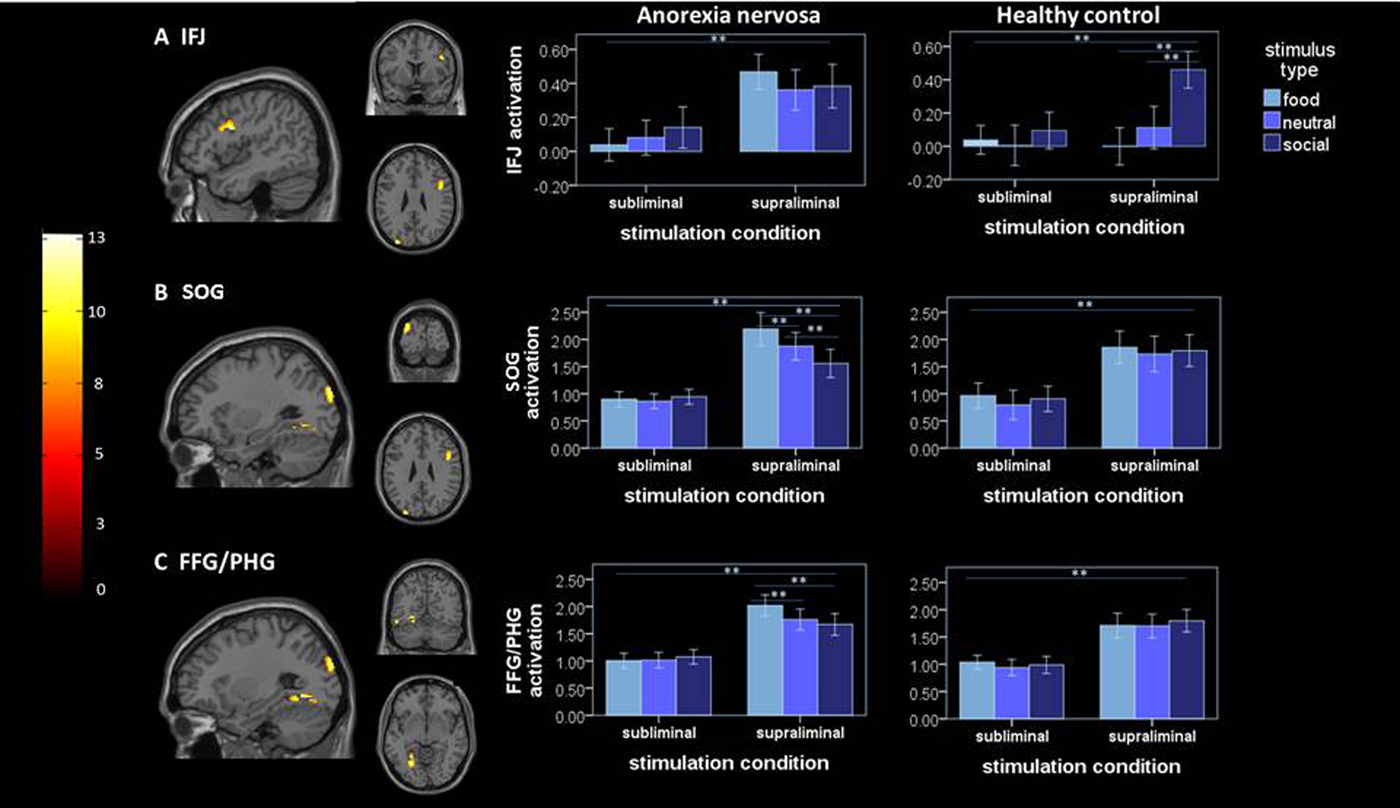
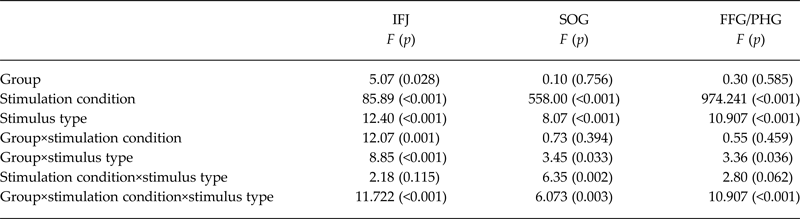
Analysis of the critical group × stimulation condition × stimulus type interaction showed significant clusters in a region of lPFC centered at the IFJ (Fig. 1a ), the superior occipital gyrus (SOG; Fig. 1b ) and the fusiform gyrus/parahippocampal gyrus (FFG/PG; Fig. 1c ). To explore what factors contribute to the group differences, parameter estimates (β values) were extracted and subjected to a linear mixed model. Analysis of activation within these clusters revealed that the three-way interaction was driven by distinct factors in the respective ROIs. In the IFJ, while a two-way group × stimulation condition interaction (see Table 2) driven by increased activity in AN v. HC in the supraliminal condition was evident [T(96.37) = −3.14; p = 0.001], the three-way interaction effect was driven by a differential influence of stimulus type in HC. Specifically, HC showed an increased activity of the IFJ for socialsupra in comparison to neutralsupra stimuli [T(340) = 6.27; p < 0.001] and foodsupra [T(340) = 8.93; p < 0.001]. In sum, this indicates that, within the given task context, in HC the IFJ responds stronger to social stimuli, while this brain region is strongly active irrespectively of the stimulus type in patients with AN.
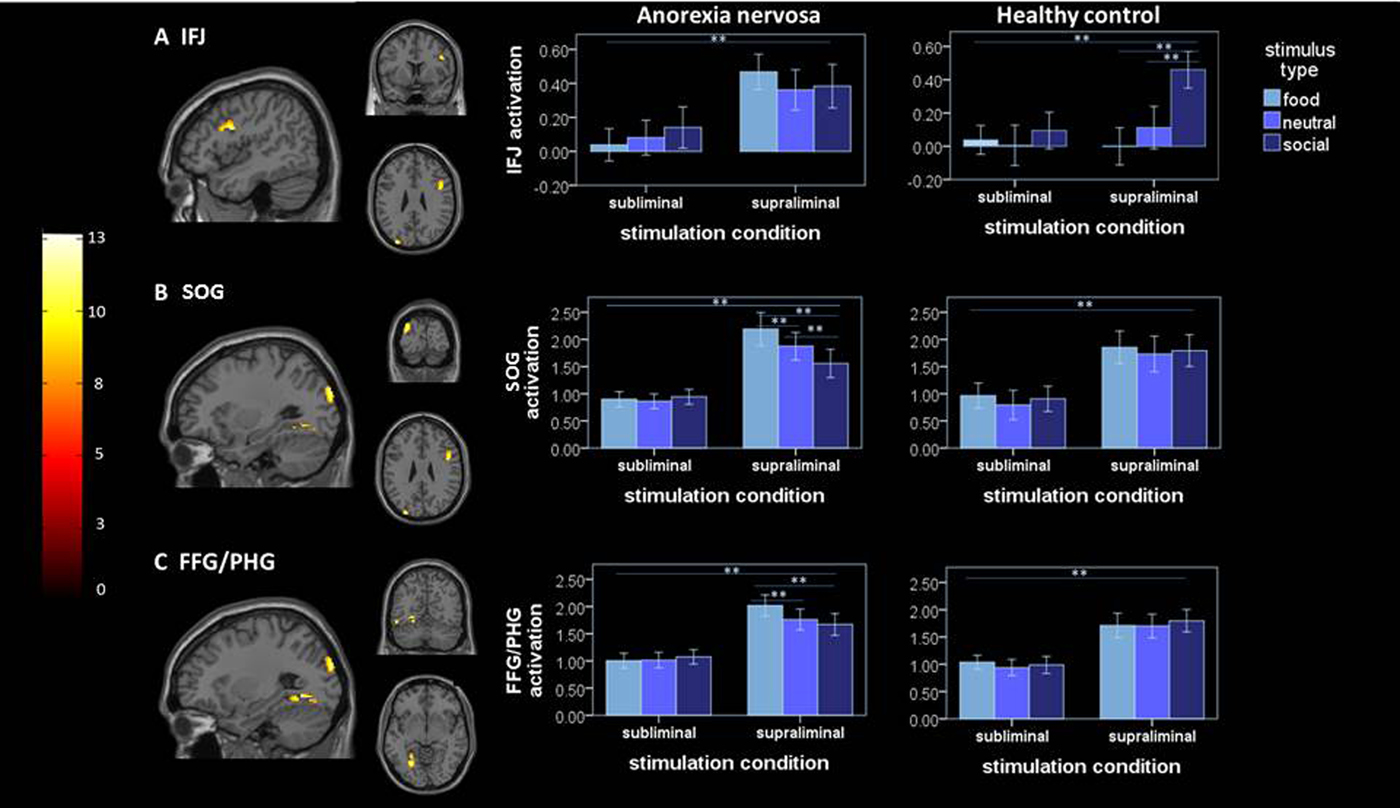
Fig. 1. Left, significant (family-wise error-corrected) clusters revealed by the whole-brain three-way group × stimulation condition × stimulus type interaction analysis: (a) cluster at IFJ [46, 6, 26]; (b) cluster at SOG [−30, −90, 28]; (c) cluster at FFG/PHG [−26, −90, 28]; right mean activation (β-estimates ± s.e.m.) extracted from the respective significant clusters identified by the whole-brain analysis are plotted for both groups under each stimulation condition and for each stimulus type; ** = significant difference of extracted β estimates at p = 0.001; IFJ, inferior frontal junction; SOG, superior occipital gyrus; FFG/PHG, fusiform gyrus/parahippocampal gyrus.
Table 2. Main effects and interaction effect of the linear mixed model (based on extracted ß-values for each relevant cluster)
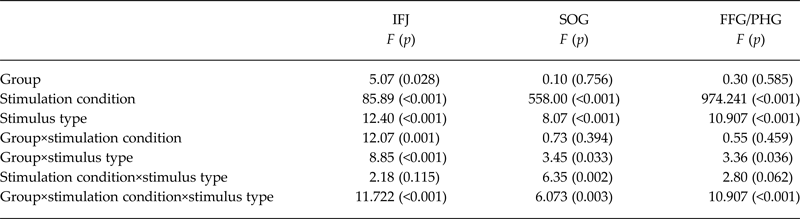
IFJ, inferior frontal junction; SOG, superior occipital gyrus; FFG/PHG, fusiform gyrus/parahippocampal gyrus.
In the SOG, the three-way interaction was driven by a differential influence of the stimulus type under supraliminal stimulation within AN, while no differential effect of stimulus type under supraliminal stimulation was evident in HC. In detail, AN patients showed an increased response to foodsupra in comparison to neutralsupra [T(340) = 3.27; p = 0.004] and socialsupra [T(340) = 6.75; p < 0.001] as well as to socialsupra in comparison to neutralsupra [T(340) =−4.02; p = 0.004]. A similar pattern was found for the FFG/PHG, the three-way interaction was modulated by the type of stimulus under supraliminal stimulation in AN, while HC showed no differential response to stimulus type under supraliminal stimulation condition. Within the AN group, the FFG/PHG showed increased activity for foodsupra in comparison to neutralsupra [T(340) = 4.98; p < 0.001] and socialsupra [T(340) = 5.52; p < 0.001] but not for socialsupra in comparison to neutralsupra. Control analyses accounting for (1) the relatively broad age range (online Supplementary Material 2.2) and (2) patients comorbidities (n = 3; online Supplementary Material 2.3) and (3) AN binge/purge subtype (n = 2; online Supplementary Material 2.4), revealed statistically identical results.
Association of hemodynamic activity, clinical variables, and sensitivity index (d′)
Given the broad role of the IFJ in executive functions (Brass et al. Reference Brass, Derrfuss, Forstmann and von Cramon2005; Derrfuss et al. Reference Derrfuss, Brass, Neumann and von Cramon2005) and the hypothesis of elevated cognitive control in AN (Kaye et al. Reference Kaye, Fudge and Paulus2009), we next inspected relationships between neural responses in this region and eating disorder symptoms as gauged by the EDI-2. Since we established a group difference in hemodynamic activity during supraliminal stimulation condition irrespectively of stimulus type, we explored the association between the average BOLD activity and EDI-2 in patients with AN, which was found to be significant (r = 0.402, p = 0.017).
Next, we tested for a potential association between d′ and the IFJ response during each stimulus type under subliminal stimulation condition. Interestingly, IFJ activation during subliminal stimulation condition of social stimuli was associated with the probability to detect subliminal targets (r = 0.496, p = 0.002) in HC. This indicates that with increasing individual visibility under subliminal stimulation condition neural pattern indicative for supraliminal processing emerge.
Connectivity analyses
To explore whether the IFJ serves as a putative source region of top-down control during the supraliminal stimulation condition, we conducted a gPPI analysis using the IFJ as seed region and foodsupra, socialsupra, and neutralsupra as psychological factors. The exploratory approach with no a priori defined target regions, as well as the hypothesis-based approach with the four limbic target regions revealed no significant group differences in functional connectivity.
Discussion
The present study analyzed neural responses to subliminally and supraliminally presented reward-related food and social stimuli in AN. The objective of our experimental design and analysis strategy was to determine whether differences in brain response to these stimuli are predominantly present in subcortical (bottom-up) or cortical (top-down) networks. As a first step, we confirmed that the subliminal and supraliminal processing of rewarding stimuli elicited the expected reward-related neural activation pattern in HC (Dehaene et al. Reference Dehaene, Changeux, Naccache, Sackur and Sergent2006; Brooks et al. Reference Brooks, Prince, Stahl, Campbell and Treasure2011b ; Zedelius et al. Reference Zedelius, Veling and Aarts2011; Mudrik et al. Reference Mudrik, Faivre and Koch2014; Wang et al. Reference Wang, Smith and Delgado2016).
Contrasting the two groups, we found no difference under the subliminal stimulation condition, suggesting that adolescent (non-chronic) AN patients may not have particularly strong alterations in the bottom–up neural response to rewarding stimuli. In response to the supraliminal stimulation condition, we found activation to be elevated in the AN group in a region of lPFC associated with cognitive control, the IFJ (Brass et al. Reference Brass, Derrfuss, Forstmann and von Cramon2005; Derrfuss et al. Reference Derrfuss, Brass, Neumann and von Cramon2005). IFJ activation was increased in the AN group irrespective of stimulus type – suggestive of a sustained response – which was associated with the level of eating disorder symptoms. HC, however, showed comparably elevated activity in this region only to social stimuli. These findings are in accordance with studies reporting no interference with working memory or performance monitoring when distracting food stimuli are presented subliminally (Brooks et al. Reference Brooks, O'Daly, Uher, Schiöth, Treasure and Campbell2012a ), while supraliminal distractors, irrespective of salience, seem to interfere with working memory performance in patients with AN (Dickson et al. Reference Dickson, Brooks, Uher, Tchanturia, Treasure and Campbell2008). The authors of the latter study argued that this might indicate that AN patients are more easily distracted due to their extreme attention to details – an interpretation which dovetails with our finding of a sustained elevated IFJ activation.
In contrast to the notion of altered reward processing in AN (O'Hara et al. Reference O'Hara, Campbell and Schmidt2015), no group differences were evident in the reward system, neither during subliminal nor supraliminal stimulation. This seems surprising given that previous studies using food stimuli or monetary rewards in AN demonstrate alterations in those brain areas (Wagner et al. Reference Wagner, Aizenstein, Mazurkewicz, Fudge, Frank, Putnam, Bailer, Fischer and Kaye2007a ; Cowdrey et al. Reference Cowdrey, Park, Harmer and McCabe2011). A potential explanation for this discrepancy might be that alterations in the reward system evolve with the chronic manifestation of the disorder and potential alterations in this system have not (yet) developed in our primarily young non-chronic sample (85% younger than 18 years; 74% with a duration of illness shorter than 1 year). Indeed, other studies investigating adolescent AN reported no difference in the reward system during processing of monetary rewards (Bischoff-Grethe et al. Reference Bischoff-Grethe, McCurdy, Grenesko-Stevens, Irvine, Wagner, Wendy Yau, Fennema-Notestine, Wierenga, Fudge and Delgado2013; Ehrlich et al. Reference Ehrlich, Geisler, Ritschel, King, Seidel, Boehm, Breier, Clas, Weiss, Marxen, Smolka, Roessner and Kroemer2015; King et al. Reference King, Geisler, Bernardoni, Ritschel, Böhm, Seidel, Mennigen, Ripke, Smolka, Roessner and Ehrlich2016). This resonates with the argument postulated by the reward-centered model of AN that patients do not suffer from a generalized inability to experience reward, but that processes within this system change with the progress of the disorder (O'Hara et al. Reference O'Hara, Campbell and Schmidt2015). However, to investigate this notion in more detail, future studies contrasting chronic and non-chronic patients are needed.
As mentioned above, we found a neural pattern suggestive of sustained increased IFJ responsivity in AN during supraliminal processing, which was associated with eating disorder psychopathology. The IFJ, a region located at the junction of the inferior frontal sulcus and the inferior precentral sulcus (Derrfuss et al. Reference Derrfuss, Brass, Neumann and von Cramon2005), plays a pivotal role in a variety of cognitive control functions, including selective attention (Baldauf & Desimone, Reference Baldauf and Desimone2014) and updating task representations (Derrfuss et al. Reference Derrfuss, Brass, Neumann and von Cramon2005). This finding provides support for the notion of AN as model disorder for excessive cognitive control (Kaye et al. Reference Kaye, Fudge and Paulus2009). However, according to the framework of Kaye et al. (Reference Kaye, Fudge and Paulus2009) and Brooks et al. (Reference Brooks, Owen, Uher, Friederich, Giampietro, Brammer, Williams, Schiöth, Treasure and Campbell2011a ), one could have expected that an overactive cognitive control system in AN has a top–down modulatory effect on limbic brain regions implicated in motivational drives. In this study, we found: (1) no alteration in functional connectivity between the IFJ and the reward system and (2) the elevated neural response of the IFJ in patients with AN was not limited to reward-related stimuli. The unspecific response of the IFJ may indicate an attempt of sustained control in AN patients once a meaningful picture is presented, while in HC such a cognitive control reaction is only evident in response to emotionally salient stimuli. One might also speculate that AN patients are less able to disengage cognitive control reactions.
The IFJ has also been associated with attentional allocation. Studies have demonstrated neural synchrony between the IFJ and posterior sensory brain areas (Zanto et al. Reference Zanto, Rubens, Bollinger and Gazzaley2010; Sundermann & Pfleiderer, Reference Sundermann and Pfleiderer2012). Within this network, the IFJ is proposed as the source of attentional modulation, facilitated by long-distance projections, linking the frontal cortex with the visual association areas. By this means, top–down modulation facilitates neural excitability toward selected stimuli, thus forming perceptual biases (Baldauf & Desimone, Reference Baldauf and Desimone2014; Xu, Reference Xu2014). Interestingly, we found increased activity in brain areas associated with visual processing, namely the SOG (dorsal visual stream) and the FFA/PHG (ventral visual stream), during supraliminal processing of food stimuli in AN patients. These findings can be interpreted in light of an attentional bias to food stimuli that has been argued to be a maintenance factor for AN (Shafran et al. Reference Shafran, Lee, Cooper, Palmer and Fairburn2007). Nevertheless, we found no evidence for altered coupling between the IFJ and these brain areas when processing food stimuli. Second, although we discovered increased activation in visual areas in AN when processing supraliminally presented food stimuli, BOLD activity in the IFJ was elevated when processing all stimulus types.
Limitations
Our study has to be interpreted in light of the following limitations. First, it is plausible that the lack of group differences in the subliminal stimulation condition may be due to a relative insufficiency of our task to evoke reward-related neural processes. This possibility seems unlikely, given that subliminal stimulation did elicit an expected pattern of stimulus-specific activation in HC (see online Supplementary Material 2.1). Second, when studying AN patients, we cannot distinguish between predisposing factors and consequences of the acute undernutrition. Future studies will need to investigate recovered patients or apply longitudinal study designs. Third, HC were scanned at different phases of their menstrual cycle. Variance in sex hormone levels could have influenced our ability to uncover potential group differences in reward-related brain regions (Toffoletto et al. Reference Toffoletto, Lanzenberger, Gingnell, Sundström-Poromaa and Comasco2014).
A strength of our study, however, is the large homogenous sample of young acute AN patients, who have had a short duration of illness.
Conclusions
In summary, this study suggests that adolescent AN patients may not (yet) show alterations in the brain reward system, while we found a hyperactivity of a brain region implicated in cognitive control during the supraliminal processing of all stimulus types. The latter finding is remarkable, since the passive viewing task does not require any control functions per se. These findings thus lend further support for the hypothesis of sustained and elevated (self-) control in AN (Kaye et al. Reference Kaye, Fudge and Paulus2009; Brooks et al. Reference Brooks, Owen, Uher, Friederich, Giampietro, Brammer, Williams, Schiöth, Treasure and Campbell2011a ). Since we also found increased neural response in visual brain areas to food stimuli in patients, our findings may also be interpreted in light of biased attention toward food stimuli or increased attention to details (weak central coherence; Lopez et al. Reference Lopez, Tchanturia, Stahl, Booth, Holliday and Treasure2008). These findings open up new avenues for cognitive behavioral or neuromodulatory therapeutic approaches which try to target the tendency of AN patients to ‘stay in control’ even when it is not necessary (Pop-Jordanova, Reference Pop-Jordanova2000; Wildes & Marcus, Reference Wildes and Marcus2011; McClelland et al. Reference McClelland, Bozhilova, Nestler, Campbell, Jacob, Johnson-Sabine and Schmidt2013).
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291717002161.
Acknowledgements
This work was supported by the Deutsche Forschungsgemeinschaft (EH 367/5-1 & SFB 940) and the Technische Universität Dresden. The authors thank the Center for Information Services and High Performance Computing (ZIH) at TU Dresden for generous allocations of computer time.
Declaration of Interest
In the last two years, Dr Roessner has received lecture fees from Eli Lilly, Janssen-Cilag, Medice, and Novartis and was a member of advisory boards of Eli Lilly, Novartis. All other authors declare that there are no conflicts of interest in relation to the subject of this study.