Introduction
Common mental disorders (CMD) represent one of the largest burdens of disease in western countries with a point prevalence of 20% (Ohayon, Reference Ohayon2002; Kessler et al. Reference Kessler, Berglund, Demler, Jin, Merikangas and Walters2005; Fernandez et al. Reference Fernandez, Mendive, Salvador-Carulla, Rubio-Valera, Luciano, Pinto-Meza, Haro, Palao, Bellon and Serrano-Blanco2012). CMD lead to a substantial reduction of functioning and quality of life (Wells et al. Reference Wells, Stewart, Hays, Burnam, Rogers, Daniels, Berry, Greenfield and Ware1989; Comer et al. Reference Comer, Blanco, Hasin, Liu, Grant, Turner and Olfson2011), and cause most long-term sick-leave of all medical conditions (Henderson et al. Reference Henderson, Harvey, Overland, Mykletun and Hotopf2011). Depression and anxiety are the most prevalent mental disorders (Kessler et al. Reference Kessler, Berglund, Demler, Jin, Merikangas and Walters2005), but insomnia, adjustment disorder and exhaustion disorder have also been found to be highly prevalent (Ohayon, Reference Ohayon2002; Carta et al. Reference Carta, Balestrieri, Murru and Hardoy2009) and important causes of sick-leave (Koopmans et al. Reference Koopmans, Bultmann, Roelen, Hoedeman, Van Der Klink and Groothoff2011; The Swedish Social Insurance Agency, 2014).
There is strong empirical support for cognitive behavioural therapy (CBT) in the treatment of depression, anxiety and insomnia (Öst, Reference Öst2008; Morin & Benca, Reference Morin and Benca2012; Cuijpers et al. Reference Cuijpers, Berking, Andersson, Quigley, Kleiboer and Dobson2013). For adjustment disorder and exhaustion disorder there is some support for CBT (Hofmann et al. Reference Hofmann, Asnaani, Vonk, Sawyer and Fang2012), but there is a large need of further evaluations (e.g. Carta et al. Reference Carta, Balestrieri, Murru and Hardoy2009). The vast majority of patients with CMD are treated in primary care (Fernandez et al. Reference Fernandez, Mendive, Salvador-Carulla, Rubio-Valera, Luciano, Pinto-Meza, Haro, Palao, Bellon and Serrano-Blanco2012). However, accessibility to treatment is low (OECD, 2012). Of those who do receive treatment, a minority obtain interventions that are evidence-based (Wolitzky-Taylor et al. Reference Wolitzky-Taylor, Zimmermann, Arch, De Guzman and Lagomasino2015). Lack of resources and lack of qualified therapists have been pointed out as important explanations for this situation (Shafran et al. Reference Shafran, Clark, Fairburn, Arntz, Barlow, Ehlers, Freeston, Garety, Hollon, Ost, Salkovskis, Williams and Wilson2009; Layard & Clark, Reference Layard and Clark2014).
Guided self-help (GSH) refers to treatment delivered via, for example, a book or the internet through which the patient learns about the disorder and how to apply treatment techniques to their own problems while receiving limited support by a clinician. CBT delivered as GSH has been shown to have positive effects on symptoms of anxiety and depression, comparable with traditional face-to-face CBT (Cuijpers et al. Reference Cuijpers, Donker, Van Straten, Li and Andersson2010; Andersson et al. Reference Andersson, Cuijpers, Carlbring, Riper and Hedman2014), though the effects in regular clinical settings have not been fully tested. The use of GSH within a stepped care model has been suggested as a viable solution to improve accessibility to evidence-based psychological treatments of mental disorders (Andrews & Titov, Reference Andrews and Titov2006; National Collaborating Centre for Mental Health, 2009). Stepped care including GSH has also been implemented in the English initiative Improving Access to Psychological Treatments (IAPT) (Clark, Reference Clark2011). The core idea of a stepped care model is that patients should be treated at the lowest appropriate service level and stepped up to more advanced care only when clinically indicated. Some recent studies have tested stepped care models for anxiety and depression with mixed results (Seekles et al. Reference Seekles, Van Straten, Beekman, Van Marwijk and Cuijpers2011; Tolin et al. Reference Tolin, Diefenbach and Gilliam2011; Van Straten et al. Reference Van Straten, Hill, Richards and Cuijpers2015; Nordgreen et al. Reference Nordgreen, Haug, Öst, Andersson, Carlbring, Kvale, Tangen, Heiervang and Havik2016). A small study of patients with obsessive compulsive disorder (OCD) showed comparable and good results of stepped care CBT compared with face-to-face CBT (Tolin et al. Reference Tolin, Diefenbach and Gilliam2011) as did a study of patients with social anxiety disorder and panic disorder (Nordgreen et al. Reference Nordgreen, Haug, Öst, Andersson, Carlbring, Kvale, Tangen, Heiervang and Havik2016). In another study, 120 patients with anxiety or depression received stepped care including four steps of watchful waiting, GSH, problem solving therapy and medication. This stepped care showed no difference from care as usual (Seekles et al. Reference Seekles, Van Straten, Beekman, Van Marwijk and Cuijpers2011). A meta-analysis of stepped care for depression analysed 10 studies with 4580 patients. The interventions varied in content, number of steps and length of treatment and had an overall moderate effect (Cohens’ d = 0.34) compared with care as usual (Van Straten et al. Reference Van Straten, Hill, Richards and Cuijpers2015). In sum, results have been mixed, possibly related to methodological problems, at least for depression (Van Straten et al. Reference Van Straten, Hill, Richards and Cuijpers2015). To our knowledge, there has been no previous study of stepped care CBT that has included the whole range of patients with anxiety, depression, insomnia, adjustment disorder and exhaustion disorder. This is important since this is what constitutes the broad range of CMD in primary care. Moreover, to our knowledge, there is no previous study evaluating whether non-responders to GSH-CBT benefit from being stepped up to face-to-face CBT.
The aim of the present study was to evaluate a stepped care model with GSH-CBT and face-to-face CBT, for patients in primary care with symptoms of CMD. In Step I the aim was to estimate baseline to post-treatment symptom changes after GSH for CMD. We expected that approximately 50% of the patients would be in remission after Step I. In Step II the aim was to evaluate the additive effect of face-to-face CBT v. continued GSH-CBT for patients who were not in remission after Step I. We hypothesized that face-to-face treatment would yield a significant additive effect compared with GSH.
Methods
Design
This multi-site-study tested a stepped care model in the treatment of consecutively recruited primary care patients with symptoms of CMD. In Step I, all patients (N = 396) received GSH-CBT in a pretest–posttest effectiveness trial. In Step II, patients with remaining clinically relevant psychiatric symptoms (N = 214) were offered to participate in a randomized controlled trial (RCT). Of these, 161 (75%) accepted and were randomized to face-to-face CBT (n = 80) or continued GSH-CBT (n = 81). The regional ethics review board in Stockholm approved the study and the trial was registered at Clinicaltrials.gov (Identifier NCT01667822). Unique randomization sequences were generated for each participating primary care centre, using a random number generator, Research Randomizer (https://www.randomizer.org/). Study personnel at the centres were blind to the allocation sequence. All patients provided written informed consent and outcome assessors were blind to allocation status.
Recruitment
The study was conducted at four primary care centres in Stockholm County, Sweden. Prior to the start of the study, general practitioners at the clinics attended a 1-h informational meeting about the study. They were instructed to refer all patients with mild to moderate mental disorders, interested in receiving psychological treatment, to the study. Patients were recruited consecutively from routine primary care and treated at these clinics from 1 September 2012, until 31 October 2014. There were no self-referrals or media advertisements. Potential patients underwent a structured psychiatric assessment conducted by licensed psychologists using the Mini International Neuropsychiatric Interview (MINI) (Sheehan et al. Reference Sheehan, Lecrubier, Sheehan, Amorim, Janavs, Weiller, Hergueta, Baker and Dunbar1998) with additional criteria for exhaustion disorder. Exhaustion disorder is equivalent to clinical burnout (Grossi et al. Reference Grossi, Perski, Osika and Savic2015), and requires stressors to be present for at least 6 months. The symptoms comprise severe cognitive dysfunctions as well as physical and emotional exhaustion. Exhaustion disorder is listed in the Swedish ICD-10 (ICD-code 43.8) (The National Board of Health & Welfare, 2003) as a reaction to severe stress.
Inclusion criteria were: (a) mild to moderate symptoms of major depression, social anxiety disorder (SAD), generalized anxiety disorder (GAD), panic disorder (PD; with or without agoraphobia), OCD, insomnia, adjustment disorder or exhaustion disorder, (b) a score of 2–6 on the clinician's severity rating (CSR) (scale range 0–8) (Di Nardo et al. Reference Di Nardo, Moras, Barlow, Rapee and Brown1993), (c) age of 18–65 years, (d) if on medication for CMD, the dosage had to be stable since at least 12 weeks and kept constant throughout the treatment period, (e) low risk of suicide, (f) no current psychosis, bipolar disorder, dementia, self-harm or eating disorder, (g) no current substance abuse and (h) ability to read Swedish. Figure 1 presents the flow of patients throughout the study.
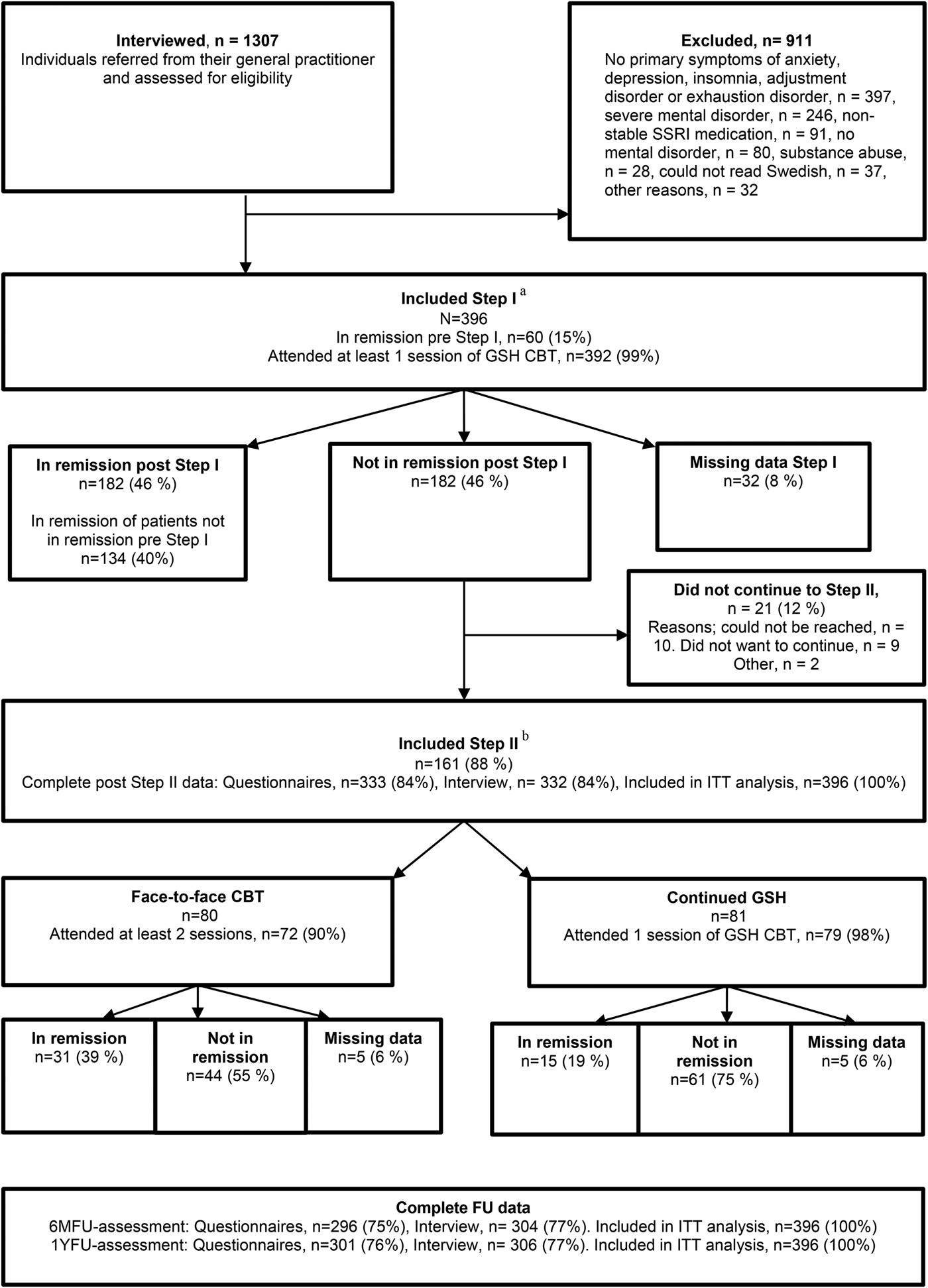
Fig. 1. Participant flow, number of patients in remission at each step, attrition, and reasons for dropping out throughout the trial. CBT, cognitive behavioural therapy; GSH, guided self help; 6MFU, 6 months follow-up; 1YFU, 1 year follow-up; ITT, intention to treat. aStep I, an open trial with 9 weeks of GSH-CBT. bStep II, a randomized controlled trial with GSH-CBT or Face-to-Face CBT.
Patients
The sample (N = 396) consisted of 286 women (72%) and 110 men (28%). The mean age was 37.2 years (s.d. = 11.4). In this sample, 206 patients (52%) had at least one comorbid disorder. Table 1 presents pre-treatment characteristics of patients for each condition.
Table 1. Characteristics of patients at baseline
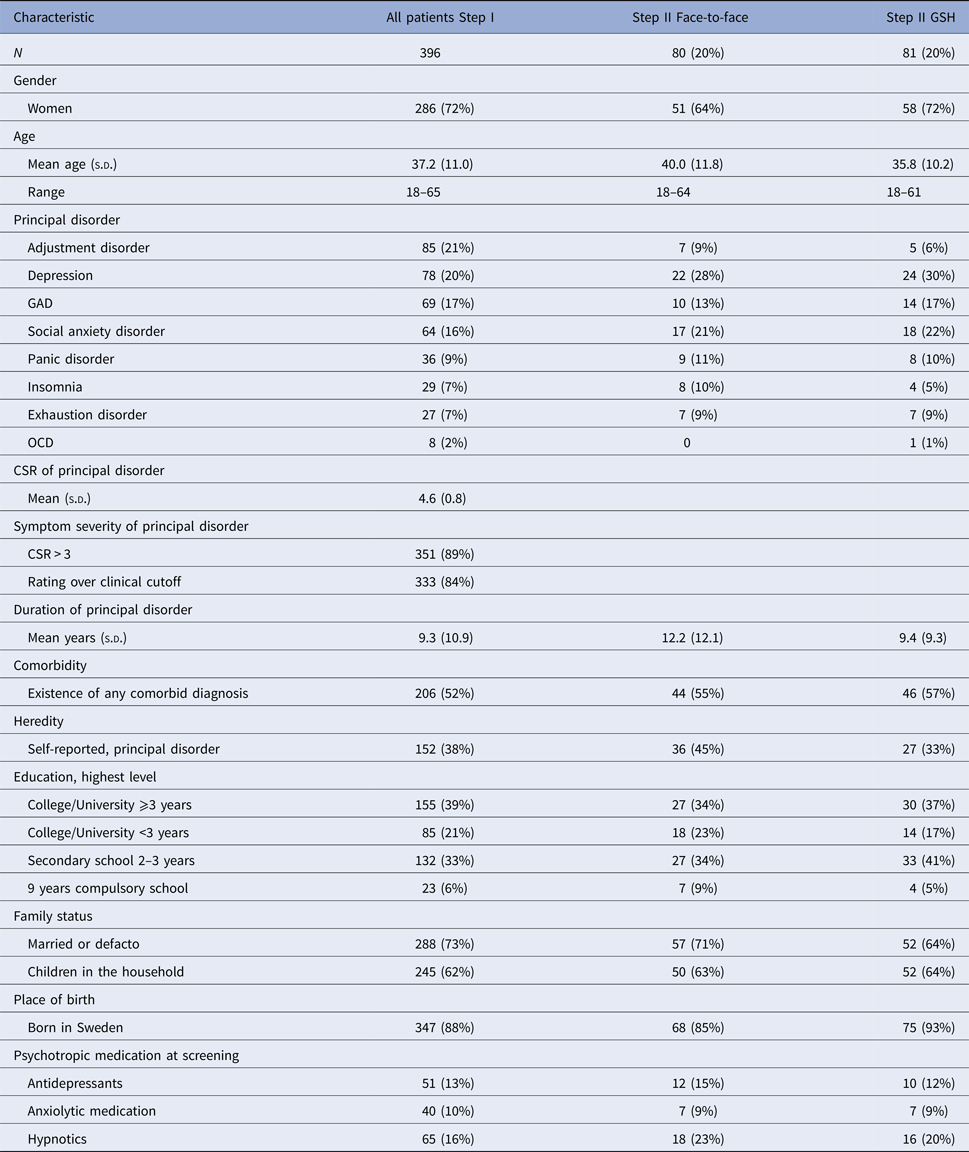
GSH, guided self-help; GAD, generalized anxiety disorder; OCD, obsessive compulsive disorder; CSR, clinician's severity rating.
Treatments and therapists
GSH-CBT
GSH was delivered via disorder-specific self-help books and face-to-face guidance sessions with a therapist. The books contained week-by-week programs with psychoeducation, illustration of the maintenance of symptoms and weekly exercises to register thoughts, feelings, and changing behaviours assumed to maintain the disorder. In Step I, treatments lasted 9 weeks and therapists saw patients for two sessions, 30–45 min each. There was no other support online or via telephone. In the first session, patients received the disorder-specific self-help book and received instructions on how to work with the program. Therapists encouraged patients to schedule their therapy at home with weekly sessions of reading and planning, as well as daily registrations and experiments. After 4 weeks patients came back for a second guidance session. Step II lasted for 11 weeks and patients in continued GSH received one additional guidance session of 30–45 min.
Table 2 presents the GSH treatment protocols used in the trial. The books used in this trial to treat depression, insomnia, panic disorder, and social anxiety disorder were based on internet programs of GSH-CBT that have been tested with strong effects in several RCTs and in routine practice with more than 1500 patients (Hedman et al. Reference Hedman, Andersson, Ljótsson, Andersson, Rück, Mörtberg and Lindefors2011, Reference Hedman, Ljótsson, Rück, Bergstrom, Andersson, Kaldo, Jansson, Andersson, Andersson, Blom, El Alaoui, Falk, Ivarsson, Nasri, Rydh and Lindefors2013, Reference Hedman, Ljótsson, Kaldo, Hesser, El Alaoui, Kraepelien, Andersson, Rück, Svanborg, Andersson and Lindefors2014; Kaldo et al. Reference Kaldo, Jernelöv, Blom, Ljótsson, Brodin, Jorgensen, Kraepelien, Rück and Lindefors2015). The GSH treatments for OCD and GAD were also based on evidence-based treatments, i.e. exposure with response prevention (Foa et al. Reference Foa, Liebowitz, Kozak, Davies, Campeas, Franklin, Huppert, Kjernisted, Rowan, Schmidt, Simpson and Tu2005) and applied relaxation (Borkovec & Costello, Reference Borkovec and Costello1993), respectively, but the self-help books had not previously been tested. For adjustment and exhaustion disorder, a manual developed by the research group was used in a self-help format as described below.
Table 2. Treatment protocols used in study

GAD, generalized anxiety disorder; OCD, obsessive compulsive disorder; CBT, cognitive behavioural therapy.
Face-to-face CBT
Table 2 presents the face-to-face treatment protocols used in the trial. Treatments were based on available evidence-based CBT protocols for each specific disorder. If more than one protocol was available for a disorder, we selected the one having less patient drop-outs or demanding lesser therapist resources. The standard lengths of protocols were 10 weekly sessions, with exception for insomnia with five sessions and social anxiety disorder with 12 recommended sessions. Treatments were individualized, therapist and patient summarized lessons learned during GSH and treatment continued according to the patient's needs.
As no evidence-based treatments are established for adjustment or exhaustion disorder, a CBT protocol for these disorders that has been developed by the research group and tested in clinical practice since 2007 was used. Adjustment and exhaustion disorder share the common aetiology of prolonged exposure to non-life-threatening stressors. The treatment was based on a model assuming that the disorders are maintained by a deficit of recuperation time (Åkerstedt et al. Reference Åkerstedt, Kecklund and Axelsson2007; Söderstrom et al. Reference Soderstrom, Jeding, Ekstedt, Perski and Akerstedt2012). Similar to depression and anxiety disorders, afflicted patients show increased emotional reactivity and instead of adaptive strategies to deal with stressful emotions, patients engage in behaviours to alter, avoid, or control emotional responding (Cloninger et al. Reference Cloninger, Svrakic and Przybeck2006; Farchione et al. Reference Farchione, Fairholme, Ellard, Boisseau, Thompson-Hollands, Carl, Gallagher and Barlow2012). These maintaining mechanisms were targeted in treatment where the main components were psychoeducation of stress, practicing relaxation, scheduled recuperation, behavioural activation, and exposure to break fear-avoidance patterns. These are the same components that have been used in the few existing previous studies of CBT for stress management (Murphy, Reference Murphy1996) that have been shown to be effective (Van Der Klink et al. Reference Van Der Klink, Blonk, Schene and Van Dijk2001).
Therapists
Therapists were 14 licensed psychologists, with 1–8 years of experience of working with CBT. The therapists received 2 days training in each protocol and supervision every other week by supervisors specialized in CBT and were trained in the treatment protocols used in the study. Manuals and session checklists were used to aid adherence to the protocols and therapists were instructed on how to deliver guidance of self-help.
Outcome measures
Primary outcome
The primary outcome was remission, defined as the patient rating below a pre-established cutoff on a disorder-specific scale measuring symptoms of the patient's principal disorder. The cutoff was constructed by calculating if a patient was closer to the normal population than the clinical population or, if normal population data was lacking, if a patient was at least two s.d.s from the clinical population in accordance with criteria from Jacobson and Truax (Reference Jacobson and Truax1991). Cutoff for each disorder-specific outcome measure is presented in online Supplement A. The disorder-specific scales were Montgomery Åsberg Depression Rating Scale-Self Rated (MADRS-S) (Svanborg & Åsberg, Reference Svanborg and Asberg1994), The Penn State Worry Questionnaire (Meyer et al. Reference Meyer, Miller, Metzger and Borkovec1990), Liebowitz Social Anxiety Scale-Self report (Fresco et al. Reference Fresco, Coles, Heimberg, Liebowitz, Hami, Stein and Goetz2001), Obsessive–Compulsive Inventory-Revised (Foa et al. Reference Foa, Huppert, Leiberg, Langner, Kichic, Hajcak and Salkovskis2002), The Panic Disorder Severity Scale-Self-report (Houck et al. Reference Houck, Spiegel, Shear and Rucci2002), Insomnia Severity Index (Bastien et al. Reference Bastien, Vallieres and Morin2001), Perceived Stress Scale (PSS) (Cohen & Williamson, Reference Cohen and Williamson1988), and Shirom-Melamed Burnout Questionnaire (Melamed et al. Reference Melamed, Kushnir and Shirom1992). Patient ratings were obtained pre-treatment, weekly during treatment, post-Step I, post-Step II and at follow-up 6 months (6MFU) and 1 year (1YFU) after post-Step II. Pre-treatment, 333 patients (84%) rated over the clinical cutoff on their principal disorder.
Secondary outcomes
All patients filled out the following secondary outcome measures: the Hospital Anxiety and Depression Rating Scale (HADS) (Zigmond & Snaith, Reference Zigmond and Snaith1983), MADRS-S, PSS, Quality of Life Inventory (QOLI) (Frisch et al. Reference Frisch, Clark, Rouse, Rudd, Paweleck, Greenstone and Kopplin1992) and Work Ability Index (WAI) (de Zwart et al. Reference De Zwart, Frings-Dresen and Van Duivenbooden2002). These scales were filled out pre-Step I, post-Step I, post-Step II, at 6MFU, and at 1YFU. Psychologists also conducted diagnostic interviews using the MINI and assessed the severity of the clinical disorder with the CSR (Brown et al. Reference Brown, Di Nardo, Lehman and Campbell2001) pre-Step I, post-Step II at 6MFU, and at 1YFU. The CSR is a clinician-administered measure where the psychiatric disorder is rated according to a 0–8 severity scale. The CSR has demonstrated good test-retest and inter-rater reliability (Brown et al. Reference Brown, Di Nardo, Lehman and Campbell2001). At post-Step II and follow-up assessments, the interview was conducted by a psychologist blind to the patient's principal disorder and allocation status. Additional measures of, for example, mediators and health economic impact were also used but will be published elsewhere.
Treatment satisfaction and adverse events
Post-Step II, treatment satisfaction was measured by the Client Satisfaction Questionnaire (CSQ) (Attkison & Greenfield, Reference Attkison, Greenfield, Sederer and Dickey1996). Post-Step I and post-Step II patients were asked to report any adverse events that they associated with their participation in treatment. The questions regarding adverse events were as follows: ‘During treatment, have you experienced any unwanted event that you think is due to treatment or any unwanted effect of treatment?’, ‘Describe the unwanted event or unwanted effect.’ If patients reported an adverse event, they were also asked to rate the effects on a four graded scale from ‘no impact at all’ to ‘affected me very negatively’. The questions were: ‘How negative impact do you consider that these unwanted events or unwanted effects had on your well-being when they occurred?’, ‘How negative impact do you consider that these unwanted events or unwanted effects have on your well-being today?’
Statistical analyses
Data analysis was conducted using SPSS 20.0 (IBM, Chicago) based on the intention to treat principle. Continuous data were analysed using mixed effects models or t tests, dichotomous data using χ2 tests. In mixed effects model analysis of between-group differences the interaction effect of group and time was the central estimate.
We also calculated effect sizes using Cohen's d based on pooled standard deviations. Weekly ratings will be presented elsewhere. However, if a patient did not complete ratings post-Step I or post-Step II but had a weekly rating from week 4, i.e. mid-treatment, or later, their last rating was used as post-rating. This occurred in 20 cases (5%) in Step I and nine cases (2%) in Step II. Participants with missing data, or where data were only available for the first 3 weeks, were considered as not in remission in the main analysis.
As a complement to the primary outcome, remission status, we also calculated reliable change (RC) and clinically significant change (CSC) according to the Jacobson and Truax (Reference Jacobson and Truax1991) criteria. RC is a dichotomous indicator of whether a patient has improved beyond what can be expected from measurement error. A patient is considered to have made a CSC if they meet criteria for both RC and remission status. RC can be applied also for patients with subclinical symptoms, whereas remission and CSC only apply if patients rate over the clinical cutoff pre-treatment. RC for each disorder-specific outcome measure is presented in online Supplement A.
The power analysis was based on the assumption that 50% of the sample would achieve remission after Step I and 10% of eligible patients would decline participation in Step II. Thus, 180 patients were expected to continue to Step II. With 90 patients in each group, an alpha-level of 0.05, and an expected effect difference of 25 percentage points in proportion of remission (50% in face-to-face CBT v. 25% in GSH-CBT), the power was slightly lower than 95%. With 80 patients in each group, i.e. the actually obtained sample size in Step II, the power was just above 90% to detect a 25% difference between groups. As it was not a requirement that patients had symptom levels above the clinical cutoff at baseline, 16% of participants had subthreshold symptoms. Results are reported both for patients who had symptoms above the cutoff at baseline and for the sample as a whole.
Results
Figure 1 shows patient flow throughout the study with number of patients that were in remission at each step and missing data on questionnaires and interviews. On average, patients in Step I completed 1.9 (s.d. = 0.4) of the planned two GSH sessions. In Step II, patients in face-to-face CBT completed on average 6.2 sessions (s.d. = 2.9) which corresponds to 76% of the planned treatment sessions. In continued GSH, patients attended on average 1.1 sessions (s.d. = 0.4), which means that a few patients received an additional session apart from the one planned guidance session. For the stepped care model, this means that if all patients that did not respond to GSH were stepped up to face-to-face treatment, an average 5.7 sessions per patient would be required.
Step I
Primary outcome
After 9 weeks of GSH-CBT, 134 patients (40%) of the 333 patients that rated over cutoff pre-Step I, rated under cutoff for clinical symptoms and were considered in remission. Of these 333 patients, 111 (33%) made a clinically significant change. Of all 396 patients, including patients with subthreshold symptoms, 182 (46%) were in remission post-Step I and 193 (49%) made a reliable change on their primary disorder.
Secondary outcomes
Means, s.d.s, effect sizes (d), and statistics are presented in Table 3. The mixed models analysis showed a significant difference (pre-post-Step I) on all secondary outcome measures, and effect sizes indicated large improvements of psychiatric symptoms after nine weeks of GSH-CBT and moderate improvements on quality of life and work ability. In online Supplement B and C data from assessments conducted post-Step II, at 6MFU and 1YFU are presented for patients who were in remission post-Step I. Patients who were not in remission post-Step I were offered treatment in Step II. Six out of eight of the diagnostic groups made large improvements post Step I, i.e. Cohen's d > 0.80. Patients with SAD and PD had moderately large effect sizes (Cohen's d = 0.63–0.70). Among patients that were not in remission post Step I, the effect size was d = 0.60 [confidence interval (CI) 0.43–0.76] for depressive symptoms, d = 0.38 (CI 0.23–0.53) for symptoms of stress, and d = 0.43 (CI 0.28–0.59) for symptoms of anxiety.
Table 3. Means and effect sizes on secondary outcomes for all patients in Step I

MADRS-S, Montgomery Åsberg Depression Rating Scale-Self Rated; HADS, hospital anxiety and depression scale anxiety subscale; PSS, perceived stress scale; QOLI, quality of life inventory; WAI, work ability index; CI, confidence interval; effect size (d), effect size Cohen's d.
Note: Estimates are based on observed and imputed data.
Step II
Primary outcome
In Table 4, observed numbers and percentages of patients in remission after Step II are reported as well as patients with RC and CSC. For all three outcomes, face-to-face treatment was significantly more effective than continued GSH in Step II (see Table 4). At 6MFU and 1YFU, the observed rates of patients in remission, with RC and CSC were higher in the face-to-face group compared to GSH, but differences no longer reached statistical significance.
Table 4. Number of patients in remission, with reliable change and clinically significant change after Step II

GSH, guided self-help; RC, reliable change; CSC, clinically significant change.
*Significant difference, p < 0.05, Pearson's χ2.
Secondary outcomes
Means, s.d.s, and effect sizes (d) are presented in Table 5. The mixed models analysis showed a significant group × time interaction (pre-Step II–post-Step II) on all secondary outcome measures indicating that face-to-face CBT was significantly more effective than continued GSH-CBT for patients in Step II concerning depression, anxiety, stress, quality of life, and work ability (F = 4.7–9.8, df = 147–154, p = 0.002–0.031). Mixed models analysis showed no significant group × time interaction on these measures from post-Step II to 6MFU (F = 0.8–3.0, df = 126–138, p = 0.088–0.378). At 1YFU, patients that had received continued GSH had improved on almost all measures to the level of patients that had received face-to-face CBT, and changes from post-Step II to 1YFU were significantly larger in the GSH group compared with the face-to-face group regarding symptoms of depression, anxiety, stress, and quality of life (F = 4.5–9.3, df = 130–140, p = 0.003–0.036). For work ability, the difference between groups was non-significant (F = 3.7, df = 131, p = 0.055).
Table 5. Patients in Step II; means and effect sizes Post-Step II, 6MFU and 1YFU

MADRS-S, Montgomery Åsberg Depression Rating Scale-Self Rated; HADS, hospital anxiety and depression scale; PSS, perceived stress scale; QOLI, quality of life inventory; WAI, work ability index; CSR; clinician's severity rating; GSH, guided self-help.
a Negative effect size between groups indicates favour of GSH. Note: Estimates are based on observed and imputed data.
Treatment satisfaction and adverse events
The mean score on CSQ (scale range 8–32) after Step II was 25.4 (s.d. = 4.6) for patients who received face-to-face CBT and 21.8 (s.d. = 4.9) for patients who received continued GSH. Patients who received face-to-face CBT in Step II were significantly more satisfied with treatment than patients who received continued GSH-CBT (t 143 = 4.44, p < 0.0001).
Adverse events during treatment were reported by 47 patients (12%) after Step I. The reported adverse events were categorized as follows: Treatment or questionnaires increased stress (n = 19), increased symptoms (n = 15), increased awareness of symptoms (n = 6), were perceived as negative (n = 3). For those who received face-to-face treatment in Step II, 11 patients (14%) reported adverse events and 15 patients (19%) did so after continued GSH. In face-to-face CBT the reported adverse events were categorized as: Treatment or questionnaires increased stress (n = 6), increased symptoms (n = 2), increased awareness of symptoms (n = 2), and too little interaction with psychologist (n = 1). In continued GSH the reported adverse events were categorized as: Treatment or questionnaires increased stress (n = 3), increased symptoms (n = 8), increased awareness of symptoms (n = 3), and too little interaction with psychologist (n = 1). There was no significant difference between patients after GSH or face-to-face in reporting adverse events (χ2 = 0.20, df = 1, p = 0.658). After both Step I and Step II, patients rated the effect of the adverse event as having little negative impact on their wellbeing after treatment.
Treatment received outside of the study
At assessment post-Step II, five patients (6%) in the continued GSH group reported additional psychological or medical treatment compared with none of the patients in the face-to-face group (χ2 = 5.03, df = 1, p = 0.025). At 1YFU, 21 participants (26%) in the face-to-face group and 23 (28%) in the continued GSH group reported having received additional treatment since post-Step II assessment, which was not a significant difference (χ2 = 0.09, df = 1, p = 0.760). Among patients in the face-to-face group who received additional treatment after Step II, 14% were in remission at 1YFU compared with 55% among those who had not received additional treatment in the face-to-face CBT group. In the continued GSH group, the remission rate among those who had received additional treatment since post-Step II assessment was 23% compared with 44% among those in the same treatment condition who had not received additional treatment. Taken together, this suggests that the superior effects of the face-to-face group at post-Step II assessment were, not due to other treatments and that effect estimates at 1YFU were not biased by treatments received outside of the study.
Discussion
Main findings
This is to our knowledge the first study to employ a rigorous method to investigate the additional effect of face-to-face CBT after GSH-CBT. The results showed a substantial decrease of symptoms after GSH for CMD and an additional effect of face-to-face treatment compared with GSH for non-responders. After the initial step with 9 weeks of GSH, 40% of patients with clinical baseline ratings were in remission. Of all patients, 49% made a reliable change post-Step I and within-group effect sizes (d) were large concerning depression, anxiety, and stress and moderately large on measures of quality of life and work ability. In Step II, patients who were not in remission after Step I were randomized to face-to-face CBT or continued GSH-CBT. In the face-to-face group, 39% achieved remission compared with 19% in the continued GSH group, a statistically significant difference. This indicates that stepping up patients to face-to-face CBT is of additional clinical value for those who do not respond to GSH-CBT. Further, using the stepped care model, patients received on average 5.7 sessions, compared with the usual face-to-face treatments of 10–12 sessions. Thus, using the stepped-care model tested in this trial is indicated to be a resource efficient way of achieving large improvements for primary care patients with CMD.
Effects at follow-ups
Among patients who continued to Step II, i.e. patients with remaining clinical symptoms after Step I, the proportion of participants in remission in both treatment groups was equally high or higher at 6MFU and 1YFU compared with baseline. At these longer term follow-ups there were no longer significant differences between groups in terms of number of patients in remission, reflecting slightly larger improvements post-Step II to 6MFU and 1YFU in the GSH group compared with patients who received face-to-face in Step II. All in all, the results indicate that both GSH and face-to-face treatments were long-term effective but face-to-face led to faster remission.
Stepped care CBT – an estimation of effects and resources required
An important aspect of using a stepped care model is that it has the potential to yield large treatment effects while using limited therapist resources. Assuming that the effects and resource utilization of the stepped care model used in this study is generalizable to primary care in general, our results suggest that if 100 patients were treated, 40 patients (40%) would be in remission after GSH and an additional 23 (39% of the remaining 60 patients) after face-to-face treatment in Step II. In total, 63% would be in remission after treatment with this stepped care model. Because a large proportion of patients would remit after Step I, and not need face-to-face treatment, the average number of sessions required for each patient to achieve this remission rate would be 5.7. In other words, using this stepped-care model would lead to remission for nearly two thirds of the patients, and this would be achieved with approximately 50% of the therapist resources required in conventional face-to-face CBT.
Comparison with previous studies
There is no consensus regarding the definition of remission or recovery in treatment of CMD (Hiller et al. Reference Hiller, Schindler and Lambert2012; Loerinc et al. Reference Loerinc, Meuret, Twohig, Rosenfield, Bluett and Craske2015). In the English IAPT initiative (Clark, Reference Clark2011), recovery has been defined as a patient rating below clinical cutoff on both the Generalised Anxiety Disorder 7-item scale (Danhof-Pont et al. Reference Danhof-Pont, Van Veen and Zitman2011) and the Patient Health Questionnaire-9 (Kroenke et al. Reference Kroenke, Spitzer and Williams2001). In an article presenting IAPT outcomes for more than 19.000 patients with anxiety and depression receiving psychological treatments within a stepped care model, it was reported that 42.4% of patients were considered recovered after treatment (Gyani et al. Reference Gyani, Shafran, Layard and Clark2013). In a systematic review of stepped care for depression, the overall recovery rates varied between 40 and 60% (Firth et al. Reference Firth, Barkham and Kellett2015). For anxiety disorders, a recent systematic review of CBT including studies of individual therapy, group therapy, and GSH (Loerinc et al. Reference Loerinc, Meuret, Twohig, Rosenfield, Bluett and Craske2015) found an average recovery rate of 49.5%. Recovery rates of these mentioned studies ranging from 40 to 60% are to be compared with the estimated 63% of patients in remission after stepped care with GSH and face-to-face CBT as demonstrated in the present study. If including the patients who did not want to continue to Step II in the present study and assuming they would be non-remitters, the overall remission rate would be 50%. Remission rates of the present study are thus similar to those of previously published studies. And of course, caution is warranted when comparing effects between studies due to differences in inclusion criteria, settings and outcome measures.
Clinical implications
The present study supports that stepped care CBT is an effective and resource efficient model to treat CMD in primary care. An estimated remission rate of 63% for the stepped care model is well in line with previous research of effective treatments. Using stepped care reduced the sessions needed for treatment in the present patient population to an average of 5.7, which can be compared with the standard recommendations of usually around 12 sessions in the treatment of CMD (National Institute for Health & Clinical Excellence [NICE], 2011; Pilling et al. Reference Pilling, Mayo-Wilson, Mavranezouli, Kew, Taylor and Clark2013). A previous study of predictors of outcome found no differences between stepped care and face-to-face CBT for panic disorder and social anxiety disorder (Haug et al. Reference Haug, Nordgreen, Öst, Kvale, Tangen, Andersson, Carlbring, Heiervang and Havik2015), suggesting that a stepped care model can be as suitable as face-to-face CBT for most patients. Looking at previous research one could question if it is necessary to implement stepped care when GSH has strong support in itself (Cuijpers et al. Reference Cuijpers, Donker, Van Straten, Li and Andersson2010). The remission rate in the present study of 40% for GSH in Step I, supports that GSH is in the range of effective treatments in previous studies. However, when adding face-to-face CBT, the remission rate increases to 63%, which is more than a 50% increase compared with GSH in Step I. Considering that these are very disabling disorders, this reflects a clinically relevant improvement. Differences between GSH and face-to-face treatment in Step II were not significant at follow-ups, however all observed remission and response rates favoured face-to-face treatment and on the blinded CSR, there was a significant between group difference at 1YFU. Even though long-term effects are important, achieving remission faster, is also of high significance. Regarding costs, face-to-face CBT had an estimated cost of £750 in 2007 (Layard et al. Reference Layard, Clark, Knapp and Mayraz2007). In the same article, the remission rate of face-to-face CBT was expected to be 50% and the costs calculated to be recovered within 2 years – and certainly within 5. In sum, it is suggested that investments to speed up and increase recovery for patients with mental disorders is likely to be a cost saving strategy.
Another important issue is whether it is justifiable to implement stepped care with initial GSH instead of face-to-face treatment straight away. A previous study by Nordgreen et al. (Reference Nordgreen, Haug, Öst, Andersson, Carlbring, Kvale, Tangen, Heiervang and Havik2016) showed that face-to-face CBT and stepped care yield similar outcomes for patients with PD and SAD, indicating that the response rates may not necessarily be reduced if using stepped care compared with using only face-to-face CBT. In the present study, GSH had a moderate to strong effect in Step I. For patients that continued to Step II, there was a moderate effect of GSH in Step I. This suggests that also for patients that were not in remission after GSH, Step I was an active, helpful part of treatment.
Strengths and limitations
Strengths of the present study were the large, consecutively included primary care sample comprising all of the highly prevalent CMD, the use of reliable and valid instruments, relatively low data attrition, 1YFU after treatment, and the randomized controlled design in Step II. Limitations were that the treatment in Step I was not compared with a control group, that not all non-remitted patients after Step I continued to Step II, and that adherence to protocols and competence of therapists was not measured. It would also have been valuable with a short-term follow-up to obtain a more precise estimate of how much faster the speed of remission was in face-to-face CBT compared with GSH.
Suggestions for future research
Further studies need to replicate the findings that stepped care is effective in treatment of CMD in primary care and that the use of face-to-face CBT for patients who do not respond to GSH-CBT yields additional treatment effects. It is of high importance to compare stepped care models to face-to-face treatment or GSH and to study whether type of psychiatric disorder moderates the treatment effect. Future research should investigate predictors of outcome to further understand whether all patients should start with GSH or if it is possible to identify patients who will not respond to GSH and who should be directly allocated to face-to-face CBT. Proper investigation of cost-effectiveness, in addition to numbers of sessions included in each treatment format, is also required. Another important area would be to investigate treatments that would be effective for patients that do not respond to CBT.
Conclusions
The current study suggests that stepped care CBT can be implemented in primary care and that patients who do not benefit from initial GSH can be offered individual CBT and show symptomatic improvement. This relies on patients’ willingness to initiate individual CBT after a course of non-successful GSH. If confirmed, using this treatment model in primary care can lead to a high overall proportion of treatment success at an average cost of less than six treatment sessions.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291717003129.
Acknowledgements
This research was funded by Karolinska Institutet and Stockholm County Council, none of which had any role in the design, execution or publication of the study.
Declarations of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.








