Introduction
Anxiety disorders are among the most prevalent psychopathologies (Kessler et al. Reference Kessler, Berglund, Demler, Jin, Merikangas and Walters2005) and include panic disorder (PD), specific phobia (SP), social anxiety disorder (SAD), generalized anxiety disorder (GAD) and post-traumatic stress disorder (PTSD) (Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, DSM-IV; American Psychiatric Association, 2000). Current changes in research transferred PTSD into a new group of trauma- and stressor-related disorders, which distinguishes PTSD from the other anxiety disorders (DSM-5; American Psychiatric Association, 2013). There are still controversies about this new classification (Zoellner et al. Reference Zoellner, Rothbaum and Feeny2011, Reference Zoellner, Bedard-Gilligan, Jun, Marks and Garcia2013), mainly criticizing the insufficient evidence for PTSD being distinct from anxiety disorders.
One key region in the development, maintenance, and expression of pathological fear and anxiety is the amygdala (Janak & Tye, Reference Janak and Tye2015; Marsh, Reference Marsh2016), which comprises the basolateral (BLA), centromedial (CeA), and superficial (SF) nucleus (Amunts et al. Reference Amunts, Kedo, Kindler, Pieperhoff, Mohlberg and Shah2005; Ball et al. Reference Ball, Rahm, Eickhoff, Schulze-Bonhage, Speck and Mutschler2007; Janak & Tye, Reference Janak and Tye2015). Especially the BLA and CeA hold special importance in healthy as well as pathological fear processing. The BLA is known as the main entrance center for information about the external environment, and therefore plays an important role in detecting relevant threat stimuli. Due to its strong connections to the CeA, which acts as an essential part in behavioral output processes, the BLA is able to potentiate fear responses via these projections (Janak & Tye, Reference Janak and Tye2015). Thus, given the amygdala's critical role during threat processing (Öhman, Reference Öhman2005), its function in detecting relevant stimuli in the environment and initiation of subsequent behavioral reactions is pronounced concerning anxiety or anxiety-related disorders (Etkin & Wager, Reference Etkin and Wager2007; Patel et al. Reference Patel, Spreng, Shin and Girard2012; Duval et al. Reference Duval, Javanbakht and Liberzon2015). There are several studies suggesting abnormal amygdala hyperactivity in single patient groups, even during unconscious threat processing (Bryant et al. Reference Bryant, Kemp, Felmingham, Liddell, Olivieri and Peduto2008; Kemp et al. Reference Kemp, Felmingham, Falconer, Liddell, Bryant and Williams2009; Lipka et al. Reference Lipka, Miltner and Straube2011; Ottaviani et al. Reference Ottaviani, Cevolani, Nucifora, Borlimi, Agati and Leonardi2012; Rabellino et al. Reference Rabellino, Densmore, Frewen, Théberge and Lanius2016).
For investigation of conscious and unconscious amygdala responses to general fear-related stimuli, fearful facial expressions have proved to be a reliable probe in healthy controls (HC) and patients (Fusar-Poli et al. Reference Fusar-Poli, Placentino, Carletti, Landi, Allen and Surguladze2009; Mendez-Bertolo et al. Reference Mendez-Bertolo, Moratti, Toledano, Lopez-Sosa, Martinez-Alvarez and Mah2016). Several single-disorder studies suggest abnormal amygdala responses to fearful faces in different disorders (Domschke et al. Reference Domschke, Ohrmann, Braun, Suslow, Bauer and Hohoff2008; Fonzo et al. Reference Fonzo, Simmons, Thorp, Norman, Paulus and Stein2010). However, to gain a better understanding of common and distinct processing mechanisms in different anxiety-related disorders, it is necessary to use the same experimental procedures and analytical methods for between-group comparisons within one study. To our knowledge, until now only two studies investigated fearful face processing across different anxiety-related disorders. One study demonstrated higher amygdala activity across all anxiety disorders (GAD, SAD, PD) compared with controls during conscious processing of fearful v. happy faces (Fonzo et al. Reference Fonzo, Ramsawh, Flagan, Sullivan, Letamendi and Simmons2015). Another study investigated unconscious affective face processing across different anxiety-related disorders (PD, SP, PTSD) using a backward masking design (Killgore et al. Reference Killgore, Britton, Schwab, Price, Weiner and Gold2014). They showed differences during unconscious affective face processing between a combined patient group (PD, SP, PTSD) and HC and additionally between PTSD and HC but not between anxiety disorders, which was attributed to small samples sizes (PTSD and PD each n = 14, SP n = 15).
These studies made important first steps in the understanding of shared and distinct amygdala involvement during fearful face processing in anxiety-related disorders. Nevertheless, besides the issue of sample sizes, some methodological and interpretative aspects may be critical in previous single-disorder and disorder-comparison (backward masking) studies (for discussion see Pessoa et al. Reference Pessoa, Japee, Sturman and Ungerleider2006; Pessoa & Adolphs, Reference Pessoa and Adolphs2010; Straube et al. Reference Straube, Dietrich, Mothes-Lasch, Mentzel and Miltner2010a, Reference Straube, Mothes-Lasch and Miltner2011). For example, both former disorder-comparison studies used block designs, which do not allow for the investigation of immediate trial-based responses, regardless of expectation and regulation aspects, or to control for visibility on a trial-by-trial basis. Furthermore, one study used a neutral face as mask for backward masking (Killgore et al. Reference Killgore, Britton, Schwab, Price, Weiner and Gold2014), which clearly influences amygdala activity in backward masking studies due to target-mask effects (Kim et al. Reference Kim, Loucks, Neta, Davis, Oler and Mazzulla2010; Straube et al. Reference Straube, Dietrich, Mothes-Lasch, Mentzel and Miltner2010a).
In the present study, we aimed to investigate amygdala activation during conscious and unconscious processing of fearful faces in different anxiety-related disorders (PD, GAD, PTSD, combined sample: n = 60) and a large sample of HC (n = 60) using event-related functional magnetic resonance imaging (fMRI). We used trial-by-trial responses of face awareness for a signal detection theory (SDT)-based analysis of face detection performance. To manipulate conscious visibility, we varied the inter-stimulus-interval (ISI) between target and mask, but kept all stimulus durations constant to avoid confounding conscious visibility and presentation duration. By means of probabilistic activation tracking, we also aimed to better characterize the anatomical amygdala subregion showing significant differential activation to fearful v. neutral faces.
Methods and materials
Subjects
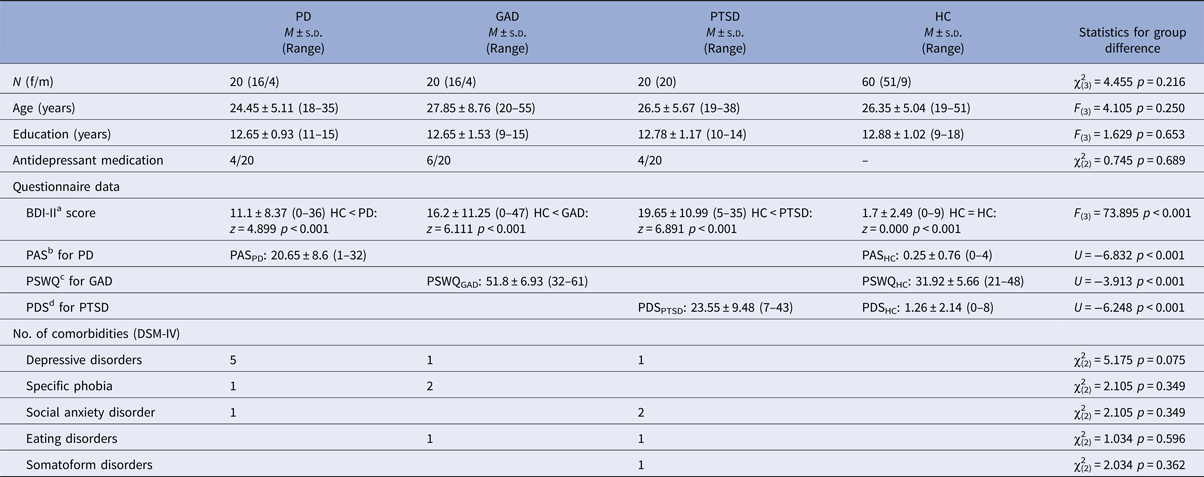
One hundred and twenty right-handed (assessed by the Edinburgh Handedness Inventory; Oldfield, Reference Oldfield1971) subjects participated in the study (group characteristics in Table 1). They were recruited via public announcements in newspapers and were paid for their participation. Since the study started in 2012, all subjects were screened with the German version of the Structured Clinical Interview for DSM-IV (SCID; Wittchen et al. Reference Wittchen, Zaudig and Fydrich1997). As primary diagnosis, 20 subjects fulfilled the diagnostic criteria for PD with or without agoraphobia [16 female (f)], 20 patients for GAD (16 f) and 20 patients for PTSD after interpersonal violence (all f). The three single anxiety patient groups were matched according to age, gender, years of education, antidepressant medication (selective serotonin reuptake inhibitor, SSRI), and comorbid diagnosis. An exclusion criterion for patients was the comorbid diagnosis of at least one of the other two investigated disorders. Afterwards, 60 HC (51 f, 9 m) were matched for gender, age, and education (number of years in school) to the anxiety disorder group. HC were free of any psychiatric diagnosis. Common exclusion criteria for patients (PAT) and HC were presence or history of neurological, psychotic, or bipolar disorders, drug dependence or abuse within the last 10 years, suicidal ideations, psychotropic medication (except SSRI), and fMRI contraindications. All participants had normal or corrected-to-normal vision. Written informed consent was obtained from each subject. The study conformed to the Declaration of Helsinki and was approved by and conducted in accordance with guidelines of the Ethics Committee of Muenster (Germany).
Table 1. Demographic and clinical characterization
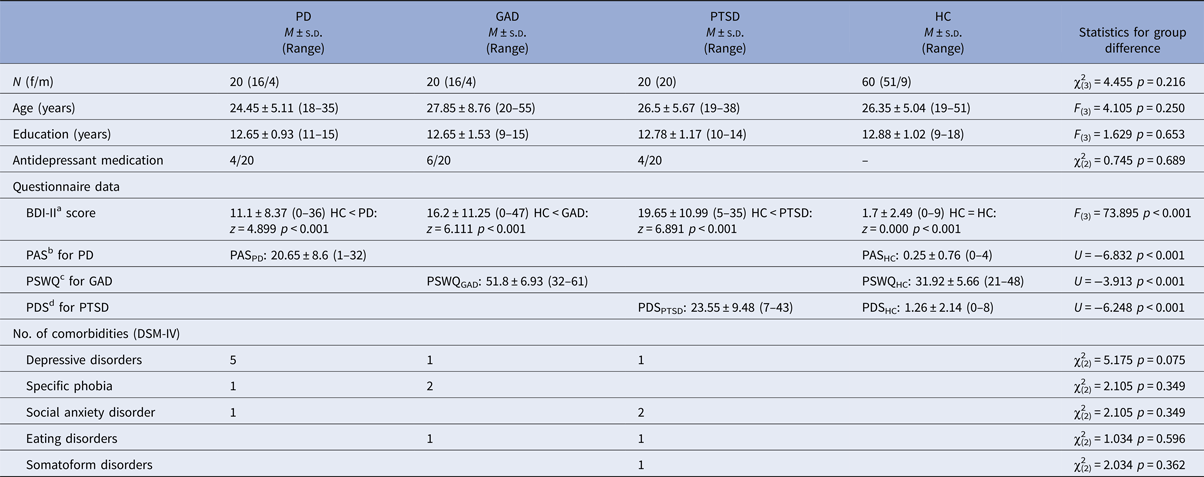
a German version (Hautzinger et al. Reference Hautzinger, Keller and Kühner2009) of the Beck Depression Inventory (Beck et al. Reference Beck, Steer and Brown1996).
b German version of the Panic and Agoraphobia Scale (Bandelow, Reference Bandelow1997).
c German version of the Penn State Worry Questionnaire (Meyer et al. Reference Meyer, Miller, Metzger and Borkovec1990).
d German version by Anke Ehlers (unpublished) of the Posttraumatic Diagnostic Scale (Foa et al. Reference Foa, Cashman, Jaycox and Perry1997).
Paradigm and stimuli
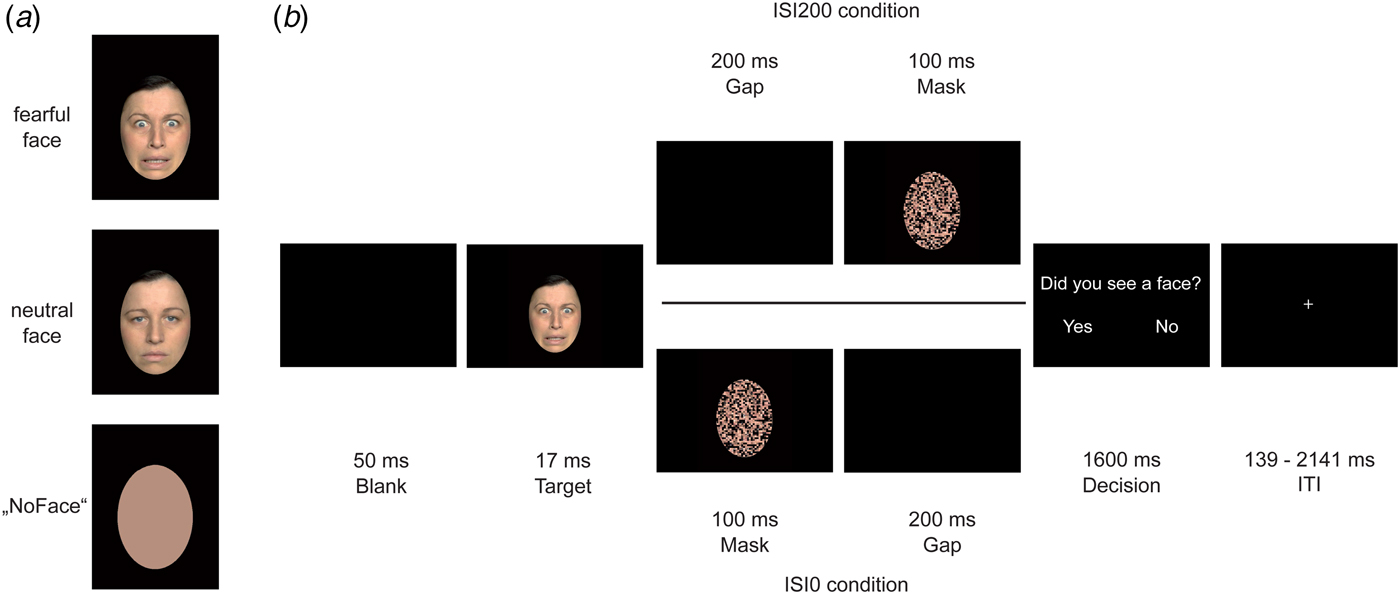
During the event-related functional run (13 min 8 s), participants underwent a backward masking design. Each trial began with a 50 ms blank screen after which a target face (fearful or neutral) was presented for 17 ms. As target faces, we used 40 female and 40 male different individuals, target faces’ age ranged approximately between 18 and 40 years. The stimuli were taken from different face sets: FACES database, which was provided by the Max-Planck Institute for Biological Cybernetics in Tuebingen, Germany (Ebner et al. Reference Ebner, Riediger and Lindenberger2010), the NimStim set (Tottenham et al. Reference Tottenham, Borscheid, Ellertsen, Marcus and Nelson2002), and the Radbound Faces Database (Langner et al. Reference Langner, Dotsch, Bijlstra, Wigboldus, Hawk and van Knippenberg2010). Furthermore, artificially generated ‘NoFaces’ were presented as targets as well. These ‘NoFaces’ were originally neutral or fearful faces, which were converted into a skin-colored oval using a soft drawing filter of Adobe Photoshop CS6 (version 13.0.1, Adobe Systems Inc., San Jose, California, USA). After a variable ISI, a scrambled face mask (see below) was presented for 100 ms. In order to vary the degree of conscious visibility, we manipulated the ISI between the target face and the mask: in the ISI0 condition the masks onset coincided with target offset, whereas in the ISI200 condition target offset and mask onset were separated by a blank screen lasting for 200 ms. We expected the majority of target faces to be unconscious in the ISI0 condition and to be conscious in the ISI200 condition. As a mask, we used the same face of the target, either the same or other emotion distributed equally, in a scrambled shape (scramble filter) using Adobe Photoshop CS6. Afterwards, a decision screen (‘Did you see a face?’) was presented for 1600 ms. Participants were asked to press the ‘yes’ button (when seeing a face stimuli) or ‘no’ button (when not seeing a face stimuli or when seeing a ‘NoFace’). We varied the inter-trial-interval by using a jitter that ranged between 139 ms and 2141 ms. Stimuli were presented using Presentation® software (Version 17.2, Neurobehavioral Systems, Inc., Berkeley, CA, www.neurobs.com) and rear-projected via an LCoS (Liquid Crystal on Silicon) projector (DLA-RSxx, JVCKenwood USA Corporation, Los Angeles, California, USA) onto a screen that the participants viewed through a mirror on the MRI head coil. Before fMRI scanning, participants were trained to perform the task in a 5-min training session outside the scanner. Figure 1 illustrates trial sequences for both ISI200 and ISI0 trials.
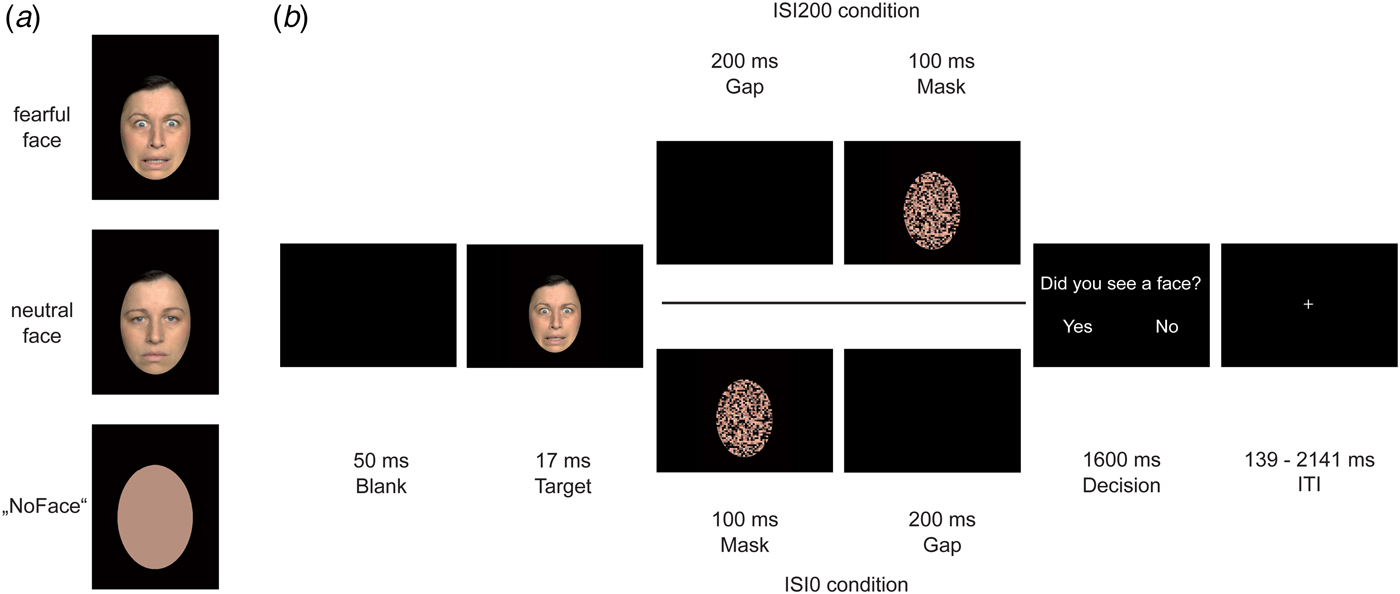
Fig. 1. Experimental trial procedure. In (a), exemplary target faces are displayed: fearful face, neutral face, and ‘NoFace’. In (b), an exemplary trial procedure for the ISI200 and ISI0 condition is shown. Each trial began with a 50 ms blank, followed by a target face lasting for 17 ms. The ISI200 condition (upper path) differed from the ISI0 condition (lower path) due to a 200 ms gap that was either presented between target offset and mask onset (ISI200 condition) or after mask offset (ISI0 condition). In both trial variants, a decision screen followed lasting for 1600 ms.
Behavioral data analysis
Behavioral data analysis was based on SDT methods (Green & Swets, Reference Green and Swets1966). Each participants’ detection rate (d′) was calculated based on the relative frequencies of hits and false alarms, separate for the ISI0 and ISI200 condition and for fearful and neutral faces, resulting in four d′ values for each participant. To compare emotion processing between anxiety patients and HC in general, we performed, per ISI condition, a 2 (within; fearful/neutral) × 2 (between; PAT/HC) repeated measures analysis of variance (rANOVA), henceforth referred to as overall rANOVA. In order to compare anxiety disorders within the group of patients regarding emotion processing, we performed a 2 (within; fearful/neutral) × 3 (between; PD/GAD/PTSD) rANOVA in parallel, henceforth referred to as differential rANOVA. For reaction times, the same procedure was conducted after applying a log transformation to compensate for the skewed distribution of reaction times.
Image acquisition and analysis
FMRI scanning was conducted on a 3-Tesla scanner (Magnetom Prisma; Siemens, Erlangen, Germany). First a T1-weighted anatomical scan was acquired. The functional run consisted of 375 volumes (36 axial slices per volume, thickness = 3 mm, gap = 0.3 mm, in-plane resolution = 2.26 mm × 2.26 mm, orientated at a tilted angle to the anterior–posterior commissural plane) using a T2*-weighted echo planar sequence (repetition time = 2080 ms, echo time = 30 ms, flip angle = 90°, field of view = 208 mm, matrix = 92 × 92 voxel). The first 10 volumes of the run were discarded to ensure steady-state tissue magnetization. Image preprocessing and analyses were performed using BrainVoyager QX (Version 2.8, Brain Innovation, Maastricht, The Netherlands). All volumes were realigned to the first volume, were corrected for slice time errors, and spatially (6-mm full-width half-maximum isotropic Gaussian kernel) and temporally (high-pass filter, 10 cycles per run; low-pass filter, 2.8 s) smoothed. Anatomical and functional images were coregistered and normalized to Talairach space (Talairach & Tournoux, Reference Talairach and Tournoux1988). Statistical analysis was performed using multiple linear regression of the signal time course at each voxel. The blood oxygen level-dependent signal change was modeled with a two γ hemodynamic response function for each event type. Based on each participants’ behavioral data, predictors of interest for unconscious face processing were obtained from trials in the ISI0 condition where fearful or neutral faces were ‘unseen’ (button press ‘no face’), and for conscious face processing by trials in the ISI200 condition where fearful or neutral faces were ‘seen’ (button press ‘face’). The other events were treated as predictors of no interest. For second-level analysis, we conducted a small volume correction analysis for the a priori defined amygdala region of interest (ROI). The left and right amygdala ROIs were created according to the Automated Anatomical Labeling (AAL) atlas (and 1 mm dilating factor according to AAL; Tzourio-Mazoyer et al. Reference Tzourio-Mazoyer, Landeau, Papathanassiou, Crivello, Etard and Delcroix2002; Maldjian et al. Reference Maldjian, Laurienti, Kraft and Burdette2003; Etkin et al. Reference Etkin, Klemenhagen, Dudman, Rogan, Hen and Kandel2004) and were transformed into Talairach space (according to Lancaster et al. Reference Lancaster, Tordesillas-Gutiérrez, Martinez, Salinas, Evans and Zilles2007) using ICBM2TAL in Matlab (MATLAB 2012, The MathWorks, Inc., Natick, Massachusetts, USA). The anatomical assignment of the observed activation patterns to an amygdala subregion was verified using anatomical probability maps of the Anatomy toolbox (Eickhoff et al. Reference Eickhoff, Stephan, Mohlberg, Grefkes, Fink and Amunts2004; Amunts et al. Reference Amunts, Kedo, Kindler, Pieperhoff, Mohlberg and Shah2005). Second-level analyses were performed for unconscious and conscious conditions separately. Based on a regression model for each patient, we calculated an overall 2 (within; fearful/neutral face) × 2 (between; PAT/HC) rANOVA for main effect of emotion and interaction effects of emotion by group (overall PAT v. HC). Furthermore, a differential 2 (within; fearful/neutral face) × 3 (between; PD/GAD/PTSD) rANOVA was calculated to further investigate interaction effects of emotion by anxiety-related group (PD, GAD v. PTSD). To further resolve interaction effects resulting from the differential rANOVA, average percent signal change across all voxels within each significant cluster was extracted for each regressor per subject. Afterwards, a 2 (within; fearful/neutral face) × 2 (within; conscious/unconscious) × 3 (between; PD, GAD v. PTSD) rANOVA was conducted in SPSS (Version 24; IBM, Armonk, New York, USA).
The issue of detectable amygdala effects to unconscious threat was further taken into account by statistical analyses based on estimated effect sizes. With regard to effect size estimates in a backward masking study (d = 0.58) (Hoffmann et al. Reference Hoffmann, Mothes-Lasch, Miltner and Straube2015), we performed a statistical power analysis of our design. This calculation yielded an unsatisfactorily voxel-level Power of 1–β = 0.74 at an initial voxel threshold of p < 0.001, but not with voxel threshold of p < 0.005 (Power of 1–β = 0.88) (see also Lieberman & Cunningham, Reference Lieberman and Cunningham2009). Using the amygdala ROI, thresholded maps were submitted to an ROI-based correction criterion for multiple comparisons based on the estimate of the map's spatial smoothness and on an iterative procedure (Monte Carlo simulation as implemented in BrainVoyager), which do not use the Gaussian random-field approach for cluster-size thresholding. After 1000 iterations, the minimum cluster size threshold that yielded a cluster-level false-positive rate of 5% was applied to the statistical maps. Post hoc group comparisons of the differential rANOVA were calculated by (1) extraction of the β values of the significant effect and (2) statistical analysis using SPSS (Version 22.0, IBM Corp, 2013). Post hoc group comparisons for the differential rANOVA were calculated by Duncan (homogeneity of variance) or Games-Howell (inhomogeneity of variance) tests. To further check whether group differences differ from a healthy population, we calculated a Dunnett-t test to compare each anxiety group against the HC group. To further explore significant differential effects across patients, we calculated additional analyses of covariance (ANCOVAs), to test whether results were maintained after inclusion of Beck Depression Inventor (BDI)-II score and antidepressant medication intake as covariates.
In this report, we only investigated activation of the amygdala. However, for the sake of completeness, whole brain analyses are included as online Supplementary material (method description Section S1 and S2; results online Supplementary Tables S2 and S3).
Results
Behavioral data – detection rate (d′)
ISI200
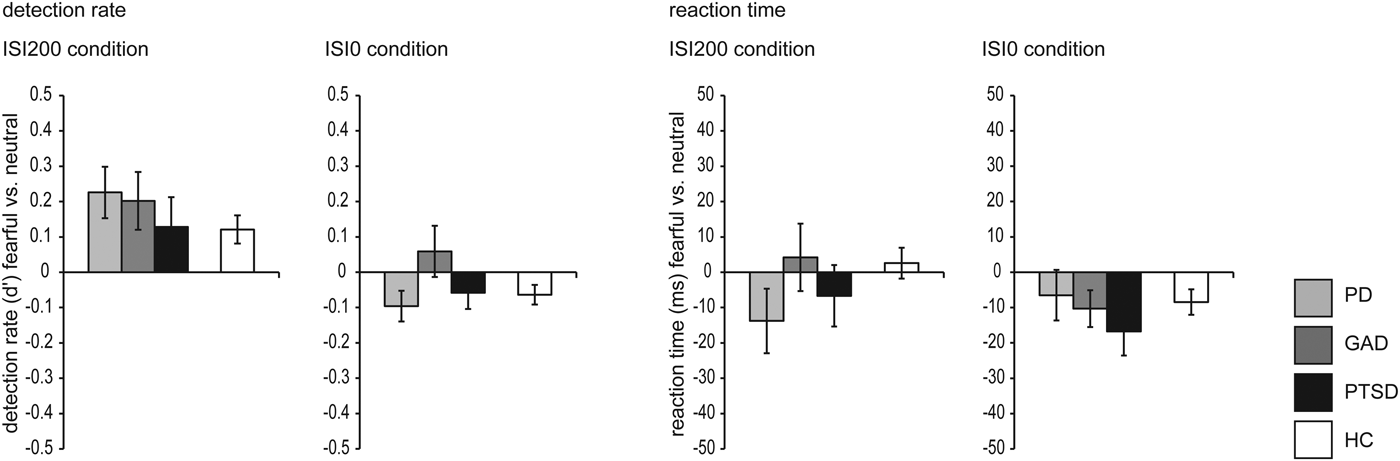
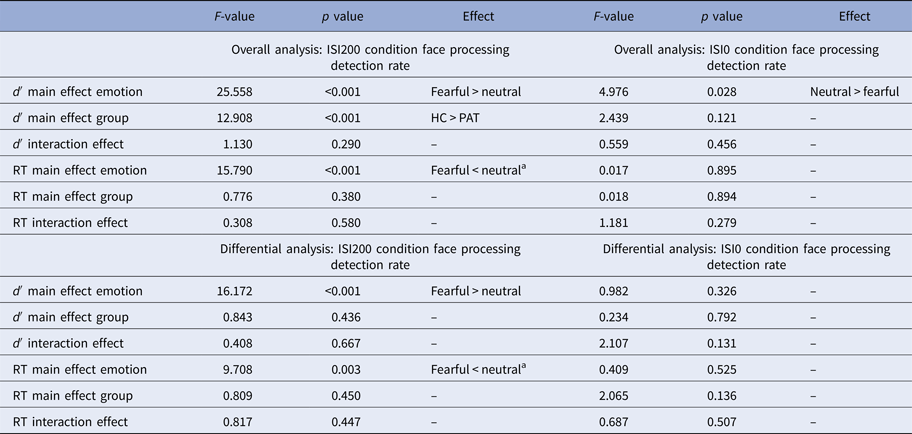
In the ISI200 condition, the overall rANOVA revealed a significant main effect of emotion [F (1,118) = 25.558, p < 0.001], resulting from a higher detection rate within trials with ISI200 for fearful compared with neutral faces (d′fearful = 3.82, d′neutral = 3.67). Furthermore, we found a main effect of group [F (1,118) = 12.908, p < 0.001], showing that HC were more likely to detect faces than PAT, independent of emotional expression (d′HC = 3.98, d′PAT = 3.51). We did not find any interaction effect (p > 0.290).
In the ISI200 condition, the differential rANOVA revealed a main effect of emotion [F (1,57) = 16.172, p < 0.001], resulting from a higher detection rate within trials with ISI200 for fearful compared with neutral faces (d′fearful = 3.60, d′neutral = 3.42). We did not find any main effect of group or an interaction effect between emotion and group (all p > 0.436).
ISI0
Overall rANOVA for ISI0 during face processing revealed a significant main effect of emotion [F (1,118) = 4.976, p = 0.028]. Although d′neutral = 0.082 (0.51 of 40 trials 1.28%) compared with d′fearful = 0.034 (0.33 of 40 trials = 0.83%) revealed to be significant, absolute numbers of trials for ISI0 but ‘seen’ neutral faces within participants ranged between 0 and 7 of 40 trials. For face detection during the ISI0 condition, d′ of just three participants were slightly above chance level (0.85, 0.54, 0.54), while the d′ confidence interval of chance performance is (−0.4774; 0.4795) as calculated by bootstrapping (Micheyl et al. Reference Micheyl, Keaernbach and Demany2008). We did not find any main effect of group (PAT/HC) or interaction effect emotion × group (all p > 0.121).
Concerning the ISI0 condition, in the differential rANOVA, we did not find any main or interaction effect (all p > 0.131). For a presentation of the results, see also Table 2 and Fig. 2.
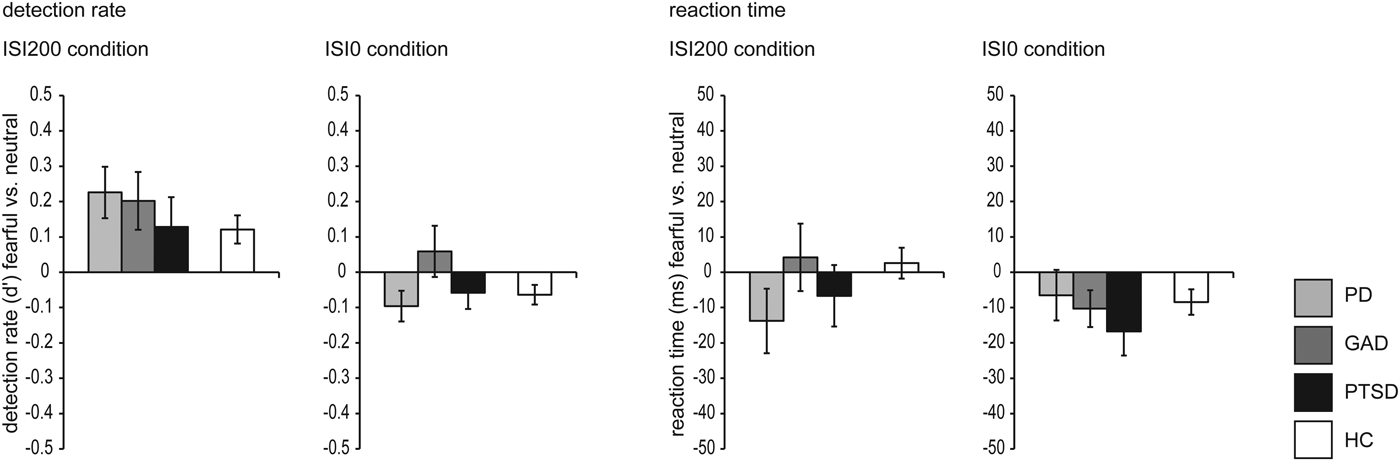
Fig. 2. Detection rate (left side) and reaction time (right side), respectively, for the ISI200 and ISI0 condition. Concerning detection rate in the ISI200 condition, the overall and differential ANOVA revealed main effects of emotion (fearful > neutral faces). Additionally, the overall ANOVA revealed a main effect of group (healthy controls > anxiety-related disorders). Concerning detection rate in the ISI0 condition, the overall ANOVA revealed a main effect of emotion (neutral > fearful faces). Analysis of reaction time in the ISI200 condition revealed main effects of emotion for the overall and differential ANOVA (faster reaction time for fearful than neutral faces). Concerning the ISI0 condition, there were no significant results.
Table 2. Results for behavioral data (detection rate and reaction time)
d′, d prime detection rate; RT, reaction time; HC, healthy controls; PAT, patients.
a Faster reaction time for fearful compared with neutral faces.
Behavioral data – reaction time (RT)
ISI200
In the ISI200 condition, the overall rANOVA revealed a main effect of emotion [F (1,118) = 15.790, p < 0.001], indicating faster reactions within trials with ISI200 for fearful than neutral faces across all participants (RTfearful = 453.75, RTneutral = 463.57). There was no main effect of group or an interaction effect (all p > 0.380).
In the ISI200 condition, the differential rANOVA revealed a main effect of emotion [F (1,57) = 9.708, p = 0.003], indicating faster reactions to fearful than neutral faces (RTfearful = 458.77, RTneutral = 469.95) in the ISI200 condition. There was no main effect of group or an interaction effect (all p > 0.450).
ISI0
For the ISI0 condition, the overall rANOVA revealed neither main effects nor an interaction effect (all p > 0.279). Concerning the ISI0 condition, in the differential rANOVA, no main effect or interaction effect was found (all p > 0.136).
FMRI data
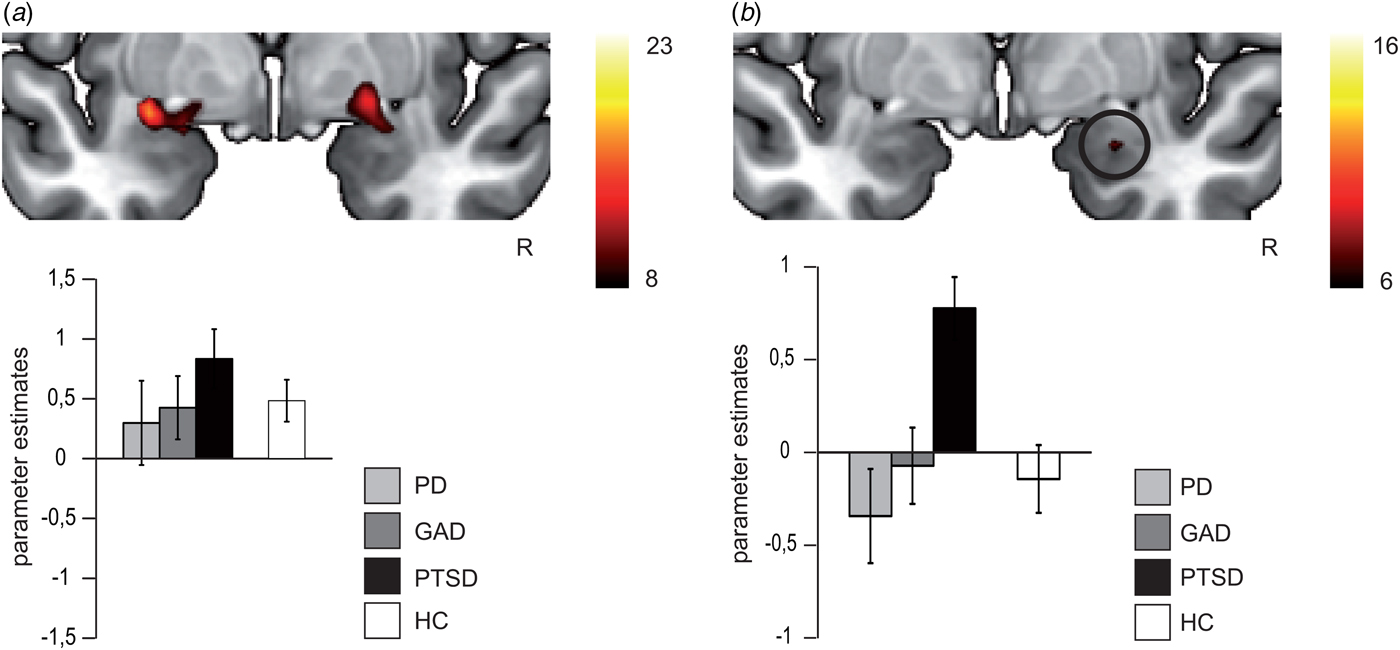
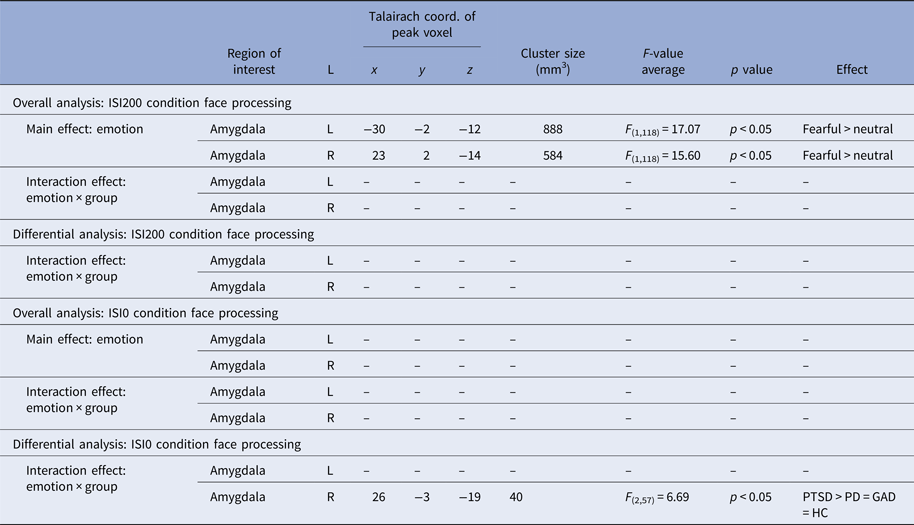
ISI200: For the overall rANOVA, we found a main effect of emotion in bilateral amygdala [right amygdala: average F (1,118) = 15.60, k = 73, p < 0.05 corrected, peak voxel: x = 23, y = 2, z = −14; left amygdala: average F (1,118) = 17.07, p < 0.05 corrected, k = 111, peak voxel: x = −30, y = −2, z = −12), indicating more activation during fearful v. neutral face processing across all participants (see Fig. 3a). Both activation clusters comprise the BLA and SF subregion of the amygdala. The probability of the anatomical assignment of the respective maximal voxel suggests an activation of the SF amygdala subregion with p > 0.3 (0.3–0.6) for the right activation cluster and of the SF amygdala subregion with p > 0.2 (0–0.5) for the left activation cluster. There was no effect of or interaction with group during conscious face processing in the overall or differential rANOVA.
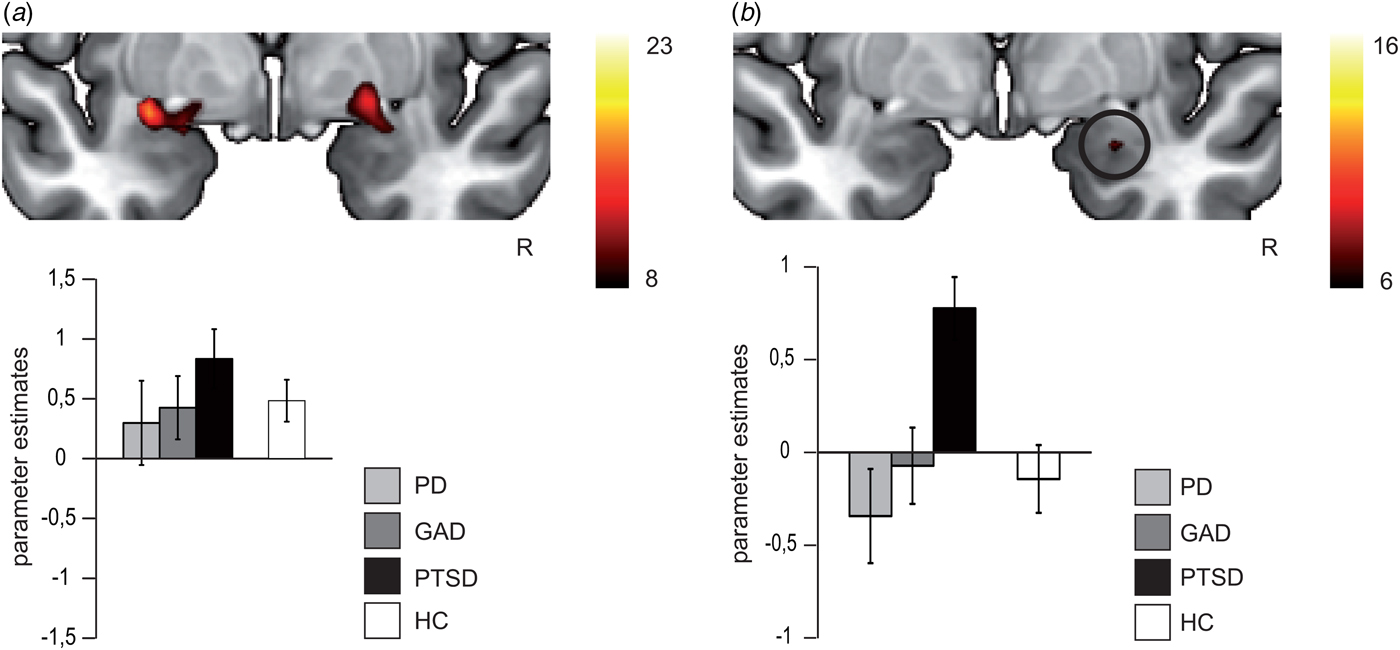
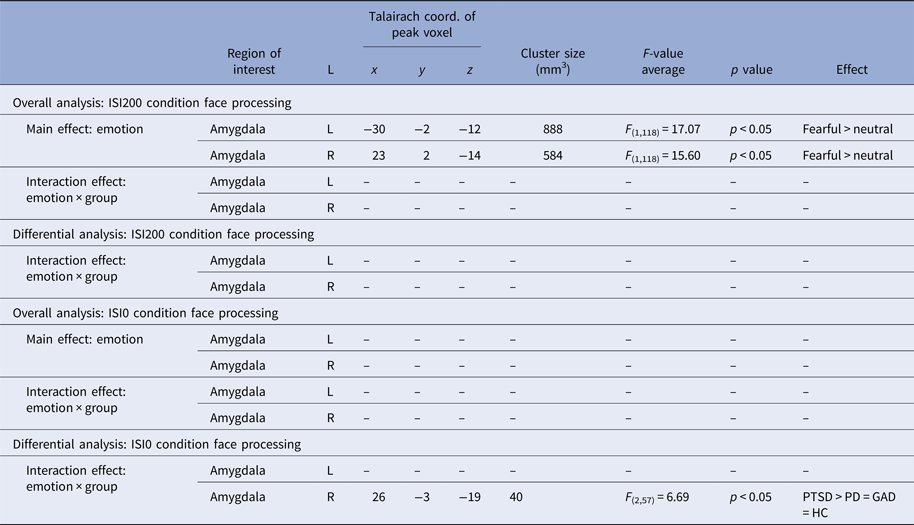
Fig. 3. In (a), differential brain activation for fearful v. neutral faces in anxiety-related disorder patients and healthy controls in the bilateral amygdala (p < 0.05 corrected). All participants show increased bilateral amygdala activation for fearful > neutral faces (main effect of emotion). In (b), differential brain activation for fearful v. neutral faces in anxiety-related disorder patients in the right amygdala (p < 0.05 corrected). Post-traumatic stress disorder (PTSD) patients relative to panic disorder (PD) and generalized anxiety disorder (GAD) patients show increased right amygdala activation for fearful > neutral faces (interaction effect). Compared with healthy controls (HC), PTSD patients show increased right amygdala activation, whereas right amygdala activation of PD and GAD did not differ from HC. Note that in (a) and (b) scales for parameter estimates are different.
ISI0: There was neither a main effect of emotion nor a group or interaction effect in the overall rANOVA. For the differential rANOVA, a significant effect indicating differences between the disorders was found in the right amygdala [average F (2,57) = 6.69, p < 0.05 corrected, k = 5, peak voxel: x = 26, y = −3, z = −19; see Fig. 3b and Table 3). Post hoc test Games–Howell revealed significant stronger activation in PTSD compared with PD (average difference M PTSD–M PD = 0.94, p = 0.026) and GAD patients (M PTSD–M GAD = 0.73, p = 0.018). Additional comparison with the HC group with post hoc test Dunnett-t showed that HC significantly differ from PTSD patients (M HC–M PTSD = 0.81, p = 0.035), but not from PD (M HC–M PD = −0.13, p = 0.962) and GAD patients (M HC–M GAD = 0.08, p = 0.992). Finally, the probability of the anatomical assignment of the observed cluster suggests an activation of the BLA amygdala subregion with p > 0.7 (0.6–0.8).
Table 3. Results for amygdala activation in the overall and differential analysis
Furthermore, to investigate whether the effect in the amygdala represents differences between conscious and unconscious conditions, we conducted an additional 2 (within; fearful/neutral face) × 2 (within; conscious/unconscious) × 3 (between; PD, GAD v. PTSD) rANOVA for the average percent signal change across all voxels within the significant amygdala cluster. Results revealed a significant emotion by group by condition interaction [F (1,57) = 5.999, p = 0.004] for this amygdala cluster.
Although only ‘unseen’ faces in the ISI0 condition were classified as unconscious, residual visibility of incompletely masked faces may still have contributed to the BLA effect (e.g. in trials where faces were actually seen but the subjects confused the response keys). Therefore, we checked whether the BLA interaction effect was modulated by the degree of visibility during unconscious processing by correlating individual BLA effects and d′ values. There was no significant correlation (r = −0.147, p = 0.264), and thus no evidence for a contribution of residual visibility to the BLA effect in the unconscious condition. The influences of depressive symptoms (using BDI-II scores) and antidepressant medication intake were tested by means of ANCOVAs and revealed no significant effects of the covariates (all p > 0.473) and maintained group effects (all F-values ⩾7.58, all p < 0.001) for the significant amygdala cluster. An overview of antidepressant medication intake is found in the online Supplementary Table S1.
Discussion
The present study is the first that allows for direct comparisons between three anxiety-related disorders during conscious and unconscious general threat processing. By meeting strong methodological requirements, we found increased BLA activation to unconscious, but not conscious, fearful faces in PTSD compared with PD and GAD patients as well as with HC.
In the present study, all participants showed increased responses in several amygdala subregions, to fearful relative to neutral faces during conscious processing. This finding underlines the amygdala's key role in processing fear-related facial stimuli in both normal as well as pathological fear (Domschke et al. Reference Domschke, Ohrmann, Braun, Suslow, Bauer and Hohoff2008; Fusar-Poli et al. Reference Fusar-Poli, Placentino, Carletti, Landi, Allen and Surguladze2009; Gamer & Büchel, Reference Gamer and Büchel2009; Straube et al. Reference Straube, Langohr, Schmidt, Mentzel and Miltner2010b; van den Bulk et al. Reference van den Bulk, Meens, van Lang, de Voogd, van der Wee and Rombouts2014; Marsh, Reference Marsh2016; Mendez-Bertolo et al. Reference Mendez-Bertolo, Moratti, Toledano, Lopez-Sosa, Martinez-Alvarez and Mah2016). The BLA is an important interface by sending information about potential threat to the CeA, which, e.g. modulates behavior reactions like startle response, freezing (Phelps & LeDoux, Reference Phelps and LeDoux2005; Janak & Tye, Reference Janak and Tye2015; Perusini et al. Reference Perusini, Meyer, Long, Rau, Nocera and Avershal2016), or orienting responses (Gamer & Büchel, Reference Gamer and Büchel2009; Straube et al. Reference Straube, Langohr, Schmidt, Mentzel and Miltner2010b).
However, we found no evidence that patients are characterized by a hyper-responsivity to clearly seen stimuli relative to HC. This finding is in contrast to other studies (Domschke et al. Reference Domschke, Ohrmann, Braun, Suslow, Bauer and Hohoff2008; Fonzo et al. Reference Fonzo, Simmons, Thorp, Norman, Paulus and Stein2010). It should be noted that in previous studies, target presentation time ranged between 500 and 5000 ms, while in the present work target faces were presented for 17 ms. Furthermore, previous studies used block designs, which on the one hand might have more power to detect potential effects. On the other hand, block designs are associated with expectation and regulation effects. Finally, our study did not require patients to attend to emotional information of faces at all. Thus, our study investigated very rapid and automatic responses to fearful faces. Under such conditions, we show comparable immediate amygdala responsivity to fearful faces in anxiety patients and controls.
Interestingly, when comparing different anxiety-related disorders during the unconscious condition, only patients suffering from PTSD exhibited exaggerated amygdala responsivity compared with other anxiety disorders and with HC. This finding is in line with previous PTSD studies, reporting heightened amygdala responsivity to general emotional stimuli during unconscious processing of threat stimuli (Bryant et al. Reference Bryant, Kemp, Felmingham, Liddell, Olivieri and Peduto2008). In addition to these findings, the current study suggests a specificity of this response in PTSD compared with other anxiety disorders. In the current study, the specificity of this response in PTSD points to different neurobiological mechanisms in the processing of fear-related stimuli between anxiety-related disorders. Given the BLA's important role in detecting threat stimuli, this activation might be associated with increased hypervigilance to very subtle threat cues in PTSD relative to other anxiety disorders. Interestingly, behavioral data did not show any differential effects between the investigated anxiety-related disorders. Thus, PTSD patients might show an automatic reaction to emotionally relevant stimuli on the neural level, without altered behavioral reactions during the task. This dissociation could be interpreted by the irrelevance of facial expression (whether it is fearful or neutral) for solving the task (whether there was a face or not). It might also indicate that the automatic amygdala response represents a reduced neural threshold for the elicitation of a variety of threat-related responses, which might contribute to uncontrollable and automatic hyper-responsivity to threat cues in PTSD (Bryant et al. Reference Bryant, Kemp, Felmingham, Liddell, Olivieri and Peduto2008; Kemp et al. Reference Kemp, Felmingham, Falconer, Liddell, Bryant and Williams2009). Our findings suggest a difference between PTSD and other disorders at least for this kind of general fear-related stimulus. However, the implementation of an unconscious condition is necessary to differentiate between anxiety-related disorders (here in terms of amygdala responsivity).
Some limitations of this study need to be mentioned. First, with the present sample size of n = 20 per anxiety-related group, we were able to discover effects of d > 0.53 with a statistical power of 1–β = 0.8. More subtle differences (e.g. d = 0.3) would require sample sizes of n = 60 per anxiety-related group. Thus, future studies should implement larger samples within each patient group. Additionally, these studies should include more male patients than the current study. At present, we cannot draw any conclusions regarding gender influences on general threat processing due to the absence of male participants in the PTSD group and low number of male participants in the PD and GAD patient groups (0⩽n male < 5).
To conclude, we found evidence for a PTSD-specific hyper-responsivity in the BLA during unconscious, but not conscious, fearful face processing. This finding provides evidence for PTSD being distinct from anxiety-related disorders based on a specific automatic BLA response to general fear-related cues, which might be associated with pronounced hypervigilance in these patients. Furthermore, the current study illustrates the importance of including automatic processing designs to investigate differences in neural responses between anxiety-related disorders.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291717002513.
Acknowledgements
This work was supported by grants awarded to Professor Dr Thomas Straube by the German Research Society (Deutsche Forschungsgemeinschaft, DFG; SFB/ TRR 58: C06, C07). The authors report no financial relationships with commercial interests.
Declaration of Interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.