Introduction
Major depressive disorder (MDD) is a common psychiatric disorder and leading cause of physical and mental disability (Kessler et al. Reference Kessler, Chiu, Demler, Merikangas and Walters2005). Although effective pharmacological and psychosocial interventions exist, there is a considerable lag before clinically relevant efficacy, which further increases suicide risk and illness burden (Trivedi et al. Reference Trivedi, Rush, Wisniewski, Nierenberg, Warden, Ritz, Norquist, Howland, Lebowitz, McGrath, Shores-Wilson, Biggs, Balasubramani and Fava2006), particularly during the first days after starting antidepressants (Jick et al. Reference Jick, Kaye and Jick2004). Electroconvulsive therapy (ECT) has more rapid antidepressant effects than standard pharmacotherapy (Husain et al. Reference Husain, Rush, Fink, Knapp, Petrides, Rummans, Biggs, O'Connor, Rasmussen, Litle, Zhao, Bernstein, Smith, Mueller, McClintock, Bailine and Kellner2004), but its invasive nature and adverse cognitive effects make it usually a last treatment choice for MDD (Pagnin et al. Reference Pagnin, de Queiroz, Pini and Cassano2004).
Several options have been explored to expedite antidepressant action. In the past decade, attention has focused on the glutamatergic system's role in the pathophysiology of MDD and the mechanism of antidepressant action (O'Connor et al. Reference O'Connor, Finger, Flor and Cryan2010). Several N-methyl-d-aspartate (NMDA) antagonists, such as ketamine, MK-801 (dizocilpine) and Ro25-6981, have been found to have antidepressant effects; however, compared to ketamine, the antidepressant effects MK-801 and Ro25-6981 at subanaesthetic doses were not sustained as long as those of ketamine (Maeng et al. Reference Maeng, Zarate, Du, Schloesser, McCammon, Chen and Manji2008). Ketamine, a high-affinity non-competitive antagonist at the NMDA receptor, is an anesthetic used for surgical procedures (Lanning & Harmel, Reference Lanning and Harmel1975). Since the first report of ketamine's antidepressant efficacy (Berman et al. Reference Berman, Cappiello, Anand, Oren, Heninger, Charney and Krystal2000). several case reports and controlled studies have been published (Aan Het Rot et al. Reference Aan Het Rot, Zarate, Charney and Mathew2012; Caddy et al. Reference Caddy, Giaroli, White, Shergill and Tracy2014; Fond et al. Reference Fond, Loundou, Rabu, Macgregor, Lancon, Brittner, Micoulaud-Franchi, Richieri, Courtet, Abbar, Roger, Leboyer and Boyer2014; McGirr et al. Reference McGirr, Berlim, Bond, Fleck, Yatham and Lam2015), concluding that a single infusion of low-dose intravenous (i.v.) ketamine (0.5 mg/kg over 40 min) rapidly improves depressive symptoms with efficacy onset within 1-h post-infusion, peak effect sizes at 24 h, and lasting effects for depression symptom ratings of up until 5–8 days (Zarate et al. Reference Zarate, Singh, Carlson, Brutsche, Ameli, Luckenbaugh, Charney and Manji2006; Skolnick et al. Reference Skolnick, Popik and Trullas2009; Ibrahim et al. Reference Ibrahim, Diazgranados, Franco-Chaves, Brutsche, Henter, Kronstein, Moaddel, Wainer, Luckenbaugh, Manji and Zarate2012; Mathew et al. Reference Mathew, Shah, Lapidus, Clark, Jarun, Ostermeyer and Murrough2012; Katalinic et al. Reference Katalinic, Lai, Somogyi, Mitchell, Glue and Loo2013). Despite the rapid and marked efficacy followed by a gradual loss of the therapeutic benefit, repeated ketamine infusions seem to be able to extend ketamine's efficacy, but may be less effective compared to single infusions (Naughton et al. Reference Naughton, Clarke, O'Leary, Cryan and Dinan2014). Ketamine's antidepressant action may be explained by enhanced neuroplasticity through improved prefrontal glutamate homeostasis and sustained attenuations in default mode network connectivity and activity (Salvadore et al. Reference Salvadore, Cornwell, Colon-Rosario, Coppola, Grillon, Zarate and Manji2009; Scheidegger et al. Reference Scheidegger, Walter, Lehmann, Metzger, Grimm, Boeker, Boesiger, Henning and Seifritz2012). In addition, ketamine could rapidly activate the mammalian target of rapamycin (mTOR) pathway, leading to increased synaptic signaling proteins and then increased number and function of new spine synapses in the prefrontal cortex. Furthermore, blockade of mTOR signaling blocked ketamine induction of synaptogenesis and behavioral responses in animal models of depression (Li et al. Reference Li, Lee, Liu, Banasr, Dwyer, Iwata, Li, Aghajanian and Duman2010).
Studies that examined ketamine's role in the treatment of MDD have several limitations. Most trials were cross-over studies and targeted treatment-resistant depression, limiting the generalizability of the findings. Moreover, the effects of add-on ketamine to currently available antidepressants have not been examined, and it is unknown if concurrent initiation or oral antidepressant treatment with a single dose of i.v. ketamine could speed up antidepressant efficacy and bridge the gap of the first few weeks until clinically relevant antidepressant effects are seen with oral antidepressants.
Therefore, we aimed to determine the antidepressant and antisuicidal effects and safety of low-dose single i.v. ketamine infusion (0.5 mg/kg over 40 min) combined with escitalopram initiation in MDD. We hypothesized that compared to placebo (0.9% i.v. saline), ketamine augmentation of escitalopram would be associated with significantly shorter time to antidepressant response and remission, faster and clinically significant improvements in depressive symptoms and suicidal thoughts, and acceptable tolerability.
Method
Patients and study settings
This was a randomized, double-blind, parallel-group trial conducted between September 2013 and December 2014 in the Outpatient Unit of Psychological Medicine at Beijing Chao-Yang Hospital, a university-affiliated teaching hospital in China.
To maximize the generalizability of the findings, only patients seeking psychiatric treatment (as opposed to those recruited by advertisements) were enrolled. Inclusion criteria were: (1) age 18–60 years; (2) both genders; (3) diagnosis of non-psychotic MDD established by treating psychiatrists and confirmed by a checklist based on DSM-IV criteria at study entry (Trivedi et al. Reference Trivedi, Rush, Wisniewski, Nierenberg, Warden, Ritz, Norquist, Howland, Lebowitz, McGrath, Shores-Wilson, Biggs, Balasubramani and Fava2006); (4) severe MDD, defined as a total score of ⩾24 on the 17-item Hamilton Rating Scale for Depression (HAMD) – Chinese version (Hamilton, Reference Hamilton1960; Xie & Shen, Reference Xie and Shen1984) and a score of ⩾1 on item 3, suicide risk (Zhu & Zhang, Reference Zhu, Zhang and Zhang1998). We focused on severe MDD, as the deleterious effects of delayed response and remission are highest; (5) ability to communicate and provide written consent. Exclusion criteria included (1) lifetime history of drug/alcohol dependence, psychotic, bipolar or obsessive-compulsive disorders; (2) Axis I disorder other than MDD judged to be the primary presenting problem; (3) history of inefficacy or intolerance to escitalopram; (4) pregnant or breast-feeding; (5) suicide attempt in the current episode; (6) major medical conditions contraindicating the use of ketamine and/or escitalopram; or (7) ECT or NMDA antagonist medications administered within the past 6 months.
The study protocol was approved by the Human Research and Ethics Committee of Beijing Chao-Yang Hospital. All patients provided written informed consent.
Study design
Patients meeting entry criteria entered a 2-week wash-out phase of previously taken psychotropic medications (fluoxetine = 4 weeks). After wash-out, patients were randomized according to a table of random numbers to 4 weeks of fixed-dose escitalopram 10 mg/day plus a single saline solution infusion (placebo) or fixed dose escitalopram 10 mg/day plus a single subanaesthetic dose of i.v. ketamine hydrochloride (total dose of 0.5 mg/kg) administered over 40 min.
The solutions were provided in identical 50-ml syringes. Ketamine forms a clear solution when dissolved in 0.9% saline. Following overnight fasting, infusions were administered by an anesthesiologist in the Department of Anesthesiology over 40 min via an infusion pump with standard telemetry monitoring. The anesthesiologist was blind to the group membership of patients.
Concurrently, both groups were started on 10 mg/day fixed-dose escitalopram, a dose within the therapeutic range recommended by the Guidelines for the Prevention and Treatment of MDD in China (Chinese Medical Association, 2003). Escitalopram was chosen because it is one of the most commonly prescribed antidepressants in China. Besides escitalopram, only zolpidem was allowed sparingly as needed for insomnia. Other medications not affecting the central nervous system were allowed.
Outcomes and assessments
Patients' demographic and clinical characteristics were collected by medical record review using a data-collection form designed for this study. Treatment-resistant MDD was defined as lack of/insufficient response to ⩾2 adequate antidepressant treatment trials in the current episode (Price et al. Reference Price, Nock, Charney and Mathew2009; aan het Rot et al. Reference aan het Rot, Collins, Murrough, Perez, Reich, Charney and Mathew2010).
The primary outcome was time until response, defined as a ⩾50% reduction from the baseline 10-item Montgomery–Asberg Depression Rating Scale (MADRS) – Chinese version (Montgomery & Asberg, Reference Montgomery and Asberg1979; Zhong et al. Reference Zhong, Wang, Chen and Wang2011) total score. Remission, defined as MADRS total score ⩽10 (Zimmerman et al. Reference Zimmerman, Posternak and Chelminski2004), was also assessed. Secondary outcomes were the proportion of responders and remitters in each group, severity of investigator-rated depressive symptoms (MADRS), self-rated depressive symptoms using the validated Quick Inventory of Depressive Symptomatology – Self-Report (QIDS-SR) – Chinese version (Rush et al. Reference Rush, Trivedi, Ibrahim, Carmody, Arnow, Klein, Markowitz, Ninan, Kornstein, Manber, Thase, Kocsis and Keller2003; Liu et al. Reference Liu, Xiang, Wang, Zhu, Ungvari, Kilbourne, Lai, Zhong, Zhang, Zhang, Zou, Xiao, Zhao, Li, Wu, Zhang and Chiu2013), suicidal ideation (QIDS-SR item 12), as well as reported side-effects and severity of manic, psychotic and dissociative symptoms. As in earlier studies (Larkin & Beautrais, Reference Larkin and Beautrais2011; Murrough et al. Reference Murrough, Iosifescu, Chang, Al Jurdi, Green, Perez, Iqbal, Pillemer, Foulkes, Shah, Charney and Mathew2013a ), psychotic and manic side-effects were measured by the four items (suspiciousness, unusual thought content, hallucinations, conceptual disorganization) of the Brief Psychiatric Rating Scale (BPRS; Overall & Gorham, Reference Overall and Gorham1962; Zhang et al. Reference Zhang, Zhou, Tang, Chi, Xia and Wang1983) and the 11-item Young Mania Rating Scale (YMRS; Young et al. Reference Young, Biggs, Ziegler and Meyer1978), respectively. Dissociative symptoms were measured by the Clinician Administered Dissociative States Scale (CADSS; Bremner et al. Reference Bremner, Krystal, Putnam, Southwick, Marmar, Charney and Mazure1998). A checklist with the following common somatic side-effects was used to measure side-effects at each treatment visit: dry mouth, diarrhoea, dizziness, drowsiness, loss of appetite or nausea, headache, fatigue, nightmares, restlessness, palpitation, blurred vision and increasing salivation.
Assessment schedule
Patients were assessed with the MADRS, QIDS-SR, QIDS-SR – suicide, BPRS, YMRS and CADSS at baseline, and 1, 2, 4, 24, 48 and 72 h, and 7, 14, 21 and 28 days after the end of ketamine or placebo infusion. Digital pulse oximetry, heart and respiratory rate, blood pressure and ECG were recorded every 5 min for 1 h beginning 5 min prior to the infusion. Two raters with >5 years of clinical experience and blind to the study protocol and treatment assignment independently assessed patients using the above measurements. Prior to the study, the two raters conducted a reliability exercise of the use of the MADRS, BPRS, YMRS and CADSS. Inter-rater reliability (inter-class correlation coefficients for scaled ratings and kappa values for categorical measures) was >0.9 for all measurements.
Patients were removed from the study if they had a manic or hypomanic episode, suicide attempt, severe medical condition or suffered from newly emerging side-effects that they found intolerable. Patients removed from the study received antidepressant treatment as part of their ongoing clinical care.
Statistical analysis
Data were analyzed using SPSS for Windows v. 20.0 (SPSS Inc., USA). The analyses were conducted in the modified intent-to-treat sample, i.e. including patients with a baseline and ⩾1 follow-up assessment. Continuous and categorical outcomes were analyzed as last-observation-carried-forward data. Estimated time to response, the primary outcome, and remission was analyzed using Kaplan–Meier survival analyses. Cox proportional-hazards regression models were used to compare the estimated time to response and to remission between the two groups, controlling for number of depressive episodes and length of current depressive episode at baseline. Baseline socio-demographic, clinical characteristics and response and remission rate were compared between the two groups using independent sample t test, Mann–Whitney U test, χ2 test and Fisher's exact test, as appropriate. Continuous outcomes, i.e. MADRS, QIDS-SR and its suicide item, four pooled BPRS items, YMRS and CADSS, were compared between ketamine and placebo at each assessment time point with analysis of covariance (ANCOVA) adjusting for baseline score, number of depressive episodes and length of current episode. Alpha was set at 0.05 (two-tailed).
Results
Of 33 screened patients, 30 (90.1%) were randomized to escitalopram + i.v. ketamine (n = 15) or escitalopram 10 mg/day + placebo (n= 15) (Fig. 1).
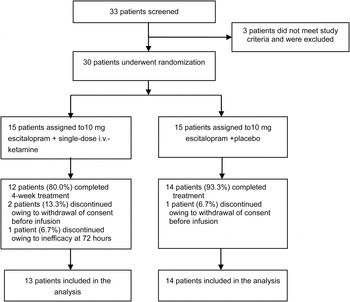
Fig. 1. Flow chart of the patient disposition.
Socio-demographic and clinical characteristics
There were no significant demographic or clinical differences between the two groups, except that patients in the escitalopram + ketamine group had more depressive episodes and shorter length of the current episode (Table 1). Altogether, 55.6% patients had treatment-resistant depression and the mean total MADRS score was 34.3 ± 7.3 points.
Table 1. Baseline demographic and clinical characteristics of the study sample

BPRS – 4 items, Brief Psychiatric Rating Scale items: suspiciousness, unusual thought content, hallucinations and conceptual disorganization; CADSS, Clinician Administered Dissociative States Scale; MADRS, Montgomery–Asberg Depression Rating Scale; QIDS-SR, Quick Inventory of Depressive Symptomatology – Self-Report; YMRS, Young Mania Rating Scale.
a Fisher's exact test.
b Mann–Whitney U test; bold values are p < 0.05.
Treatment response
At week 4, the cumulative response rate was 57.1% v. 92.3% in the escitalopram + placebo and escitalopram + i.v. ketamine groups, respectively (χ2 = 4.3, df = 1, p = 0.04), and the average time to response was 26.5 ± 4.0 v. 6.4 ± 9.5 days, respectively (t = 7.1, df = 25, p < 0.001). For patients with treatment-resistant depression, the response rate was 33.3% (2/6) in the escitalopram + placebo group and 88.9% (8/9) in the escitalopram + ketamine group (χ2 = 5.0, df = 1, p = 0.02), and the average time to response was 28.0 ± 0.0 v. 8.9 ± 10.6 days, respectively (t = 4.3, df = 13, p = 0.001).
At week 4, the cumulative remission rate was 14.3% v. 76.9% in the escitalopram + placebo and escitalopram + i.v. ketamine groups, respectively (χ2 = 10.7, df = 1, p = 0.001), and the average time to remission was 27.0 ± 3.7 v. 14.0 ± 12.0 days, respectively (t = −3.8, df = 25, p = 0.001). In the subgroup with treatment-resistant depression, the cumulative remission rate was 0% v. 66.7% in the escitalopram + placebo and escitalopram + i.v. ketamine groups, respectively (χ2 = 6.6, df = 1, p = 0.01).
The raw response and remission rates at each study time point are presented in the Supplementary Table S1. By week 4, there were no longer any significant differences between the escitalopram + placebo and escitalopram + i.v. ketamine groups regarding response (50.0% v. 61.5%; χ2 = 0.3, df = 1, p = 0.54) and remission (7.1% v. 38.5%; χ2 = 3.8, df = 1, p = 0.0504).
The Kaplan–Meier analysis-derived, estimated time until response and remission in the two treatment groups was significantly longer with escitalopram + placebo than escitalopram + i.v. ketamine [response 26.5 (95% confidence interval (CI) 24.3–28.6) v. 6.4 (95% CI 1.4–11.4) days; log rank 16.7, p < 0.001; remission 27.0 (95% CI 24.3–29.6) v. 14.0 (95% CI 7.3–20.6) days; log rank 12.2, p < 0.001] (Fig 2a, b ).
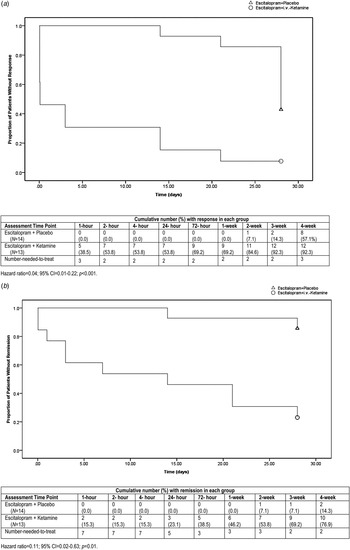
Fig. 2. (a) Estimated mean time to treatment response by Kaplan–Meier analysis. (b) Estimated mean time to treatment remission by Kaplan-Meier analysis.
After controlling for the potentially confounding effects of number of depressive episodes and duration of current episode, the estimated time to response and remission was significantly shorter [response: hazard ratio (HR) 0.04, 95% CI 0.01–0.22, p < 0.001; remission: HR 0.11, 95% CI 0.02–0.63, p = 0.01) with escitalopram + i.v. ketamine than escitalopram + placebo.
Study discontinuation
The escitalopram + placebo and escitalopram + i.v. ketamine groups did not differ significantly regarding all-cause discontinuation (6.7% v. 20.0%, p = 0.28) (Fig. 1), or discontinuation due to inefficacy (0% v. 7.6%, p = 0.29) or intolerability (0.0% v. 0.0%, p = 1.0).
Symptom ratings
Compared with escitalopram + placebo, escitalopram + i.v. ketamine infusion was associated with significantly reduced MADRS scores from 2 h to 2 weeks [peak = 3 days to 2 weeks; effect size (ES) = 1.08–1.18] (Table 2, Fig. 3). Similarly, the QIDS-SR scores were significantly lower with escitalopram + i.v. ketamine than escitalopram + placebo from 2 h to 2 weeks (maximum ES = 1.27), with significantly lower QIDS-SR suicidality item ratings from 2 to 72 h (maximum ES = 2.24) (Table 2).

Fig. 3. Total scores of the Montgomery-Asberg Depression Rating Scale (MADRS) over time. * p < 0.05, ** p < 0.01, *** p < 0.001.
Table 2. Mean scores of the rating scales measuring efficacy and selected tolerability outcomes

BPRS – 4 items, Brief Psychiatric Rating Scale items: suspiciousness, unusual thought content, hallucinations and conceptual disorganization; CADSS, Clinician Administered Dissociative States Scale; MADRS, Montgomery–Asberg Depression Rating Scale; QIDS-SR, Quick Inventory of Depressive Symptomatology – Self-Report; YMRS, Young Mania Rating Scale.
Bold values are p < 0.05.
a Positive effect sizes indicate lower values for ketamine, negative effect sizes indicate lower values for placebo.
Adverse events
Escitalopram + i.v. ketamine was not associated with significantly higher scores of the four BPRS-positive symptom items or higher dissociative symptom scores compared to escitalopram + placebo at any time point (Table 2). Only YMRS scores increased significantly with escitalopram + i.v. ketamine, but only at 1 and 2 h (Table 2).
Patients in the escitalopram + i.v. ketamine group experienced a number of mild, transient side-effects at the peak of infusion: dissociative symptoms measured by the CADSS (n = 2; CASSS score: 2 and 3, respectively), nightmares (n = 1), restlessness (n = 2), dizziness, nausea, headache and increasing salivation (n = 5 each), which each disappeared within 40 min post-infusion. Other mild side-effects present within 4 h post-infusion included nausea (n = 1), dizziness (n = 6), palpitation (n = 1), headache (n = 2) and blurred vision (n = 1), which disappeared by 24 h post-infusion. In the placebo group, one patient complained of dry mouth at the peak of infusion, but remitted after infusion. Other mild side-effects within 1 h post-infusion included dry mouth (n = 1), dizziness (n = 1), palpitation (n = 1) and fatigue (n = 1), which disappeared within 2 h. There were significantly more patients with at least one adverse event in the escitalopram + i.v. ketamine group (10/13, 76.9%) than that in the escitalopram + placebo group (4/14, 28.6%) (χ2 = 6.3, df = 1, p = 0.01, number-needed-to-harm = 3), but none of the patients discontinued the 4-week treatment due to intolerability.
Concomitant psychotropic medications
10 mg zolpidem was prescribed sparingly to 57.1% (8/14) and 76.9% (10/13) of patients in the escitalopram + placebo and escitalopram + i.v. ketamine groups, respectively (χ2 = 0.4, df = 1, p = 0.42).
Discussion
While at least seven previos randomized controlled studies examined single ketamine infusion v. placebo for unipolar or bipolar depression, all but one that used midazolam as an active placebo (Murrough et al. Reference Murrough, Iosifescu, Chang, Al Jurdi, Green, Perez, Iqbal, Pillemer, Foulkes, Shah, Charney and Mathew2013a ) were cross-over studies (Caddy et al. Reference Caddy, Giaroli, White, Shergill and Tracy2014; Fond et al. Reference Fond, Loundou, Rabu, Macgregor, Lancon, Brittner, Micoulaud-Franchi, Richieri, Courtet, Abbar, Roger, Leboyer and Boyer2014; McGirr et al. Reference McGirr, Berlim, Bond, Fleck, Yatham and Lam2015). Thus, to the best of our knowledge, this was the first randomized, parallel-group double-blind, controlled trial comparing single ketamine infusion with inactive placebo and the first one to systematically investigate the effect of a single intravenous infusion of ketamine used as an adjunct to newly initiated treatment in patients with MDD, aiming to speed up antidepressant efficacy and to bridge the gap of the first few weeks until clinically relevant antidepressant effects are seen with oral antidepressants.
In our study, compared to placebo, i.v. ketamine augmentation of escitalopram was associated with significantly greater response and remission, and, importantly, shorter time to response and remission. In fact, single-dose i.v. ketamine augmentation of escitalopram reduced the time to response and remission by 20 days and 12 days, respectively. Furthermore, augmentation of newly initiated escitalopram with a single i.v. ketamine infusion yielded symptomatic benefits over placebo augmentation of excitalopram in the first 2 weeks of escitalopram treatment after which time point the escitalopram monotherapy started to catch up. In particular, escitalopram + i.v. ketamine was associated with lower MADRS scores from 2 h to 2 weeks post-infusion, peaking between days 3 and 14 with large effect sizes >1.0 (peak = 3 days to 2 weeks, ES = 1.08–1.18). Similarly, QIDS-SR scores were significantly lower than with placebo augmentation from 2 h to 2 weeks post-infusion (maximum ES = 1.27), and the QIDS-SR suicidality item was also significantly lower with ketamine v. placebo augmentation from 2 to 72 h post-infusion (maximum ES = 2.24). Although the superiority of a single-dose i.v. ketamine augmentation against the background of concurrently initiated 10 mg oral escitalopram treatment in both groups were not sustained on the investigator-rated MADRS and self-rated QIDS at the week 3 and week 4 rating time point, at the 4-week study endpoint considerably more ketamine-treated patients were remitted (38.5% v. 7.1%), even though the gap between the response rates with escitalopram + i.v. ketamine v. escitaloptram + placebo had narrowed (61.5% v. 50.0%). Importantly, these benefits of single-dose i.v. ketamine augmentation were not offset by greater all-cause or specific-cause discontinuation, co-medication use, or adverse effects. In particular, there were no significant group differences in psychotic or dissociative symptoms at any time point, and only total YMRS scores were significantly higher with i.v. ketamine, but this was only the case at the 1- and 2-h assessment time point.
The significant reduction in depressive symptoms within 1–2 h is consistent with previous single-infusion ketamine studies (Caddy et al. Reference Caddy, Giaroli, White, Shergill and Tracy2014; Fond et al. Reference Fond, Loundou, Rabu, Macgregor, Lancon, Brittner, Micoulaud-Franchi, Richieri, Courtet, Abbar, Roger, Leboyer and Boyer2014; McGirr et al. Reference McGirr, Berlim, Bond, Fleck, Yatham and Lam2015). However, our study adds to the literature by being the first to give the ketamine infusion on day 1 of a newly initiated antidepressant, which was done to accelerate/boost symptom reduction, response and remission status, and to potentially extend the duration of the beneficial effects of ketamine. In our study, both goals were achieved. Single-dose i.v. ketamine augmentation resulted in the acceleration and enhancement of escitalopram's efficacy, and the combination of a single dose of i.v. ketamine with escitalopram also extended the duration of significant ketamine effects, at least somewhat.
In general, antidepressants act relatively slowly until robust improvement can be observed. For example, SSRIs achieve statistically significant symptomatic improvement, which is not necessarily clinically relevant yet, by the end of 1 week at best (Taylor et al. Reference Taylor, Freemantle, Geddes and Bhagwagar2006). The delayed antidepressant efficacy leads to the prolongation of severe morbidity suicide risk. Treatments with rapid onset of antidepressant effects that could be maintained have great clinical implications (Price et al. Reference Price, Nock, Charney and Mathew2009). In this study, depressive symptoms significantly improved in both groups by the end of 4 weeks, but the improvement in the escitalopram + i.v. ketamine group was observed as early as 2-h post-infusion, much faster than in the escitalopram + placebo group. In addition, this rapid onset of antidepressant action with ketamine was evident across both interviewer-rated (MADRS) and self-reported (QIDS) measures of depression that was only lost by the 3-week rating time point. Thus, the combination of single ketamine infusion with initiation of escitalopram was able to double, if not triple the duration of significant depression symptom advantages v. placebo infusion found in previous studies (Caddy et al. Reference Caddy, Giaroli, White, Shergill and Tracy2014; Fond et al. Reference Fond, Loundou, Rabu, Macgregor, Lancon, Brittner, Micoulaud-Franchi, Richieri, Courtet, Abbar, Roger, Leboyer and Boyer2014; McGirr et al. Reference McGirr, Berlim, Bond, Fleck, Yatham and Lam2015). However, our sample consisted to only 55.6% of patients with treatment-resistant depression and patients with co-morbid DSM-IV Axis I diagnoses and prior non-response or intolerability to escitalopram were excluded, whereas all previous studies included only patients with treatment-resistant depression without restricting co-morbidities or prior non-response to escitalopram, which complicates the direct comparison with these previous studies. Nevertheless, consistent with earlier findings of response rates of 25–85% at 24 h and 14–70% at 72 h (aan Het Rot et al. Reference Aan Het Rot, Zarate, Charney and Mathew2012), response rates in our study were 38.5% at 24-h and 53.8% at 72-h post-infusion.
In a study involving 14 depressed patients in an emergency unit, single-dose i.v. ketamine (0.2 mg/kg) was administered over 1–2 min, while psychopharmacological and psychosocial interventions continued. Suicidal ideation improved significantly, which was sustained for up to 10 days. Similarly, in our study, add-on i.v. ketamine use had rapid antisuicidal effects, starting at 2 h and lasting 3 days. Interestingly, the effect sizes v. placebo infusion for the reduction of suicidal thinking were almost double that of the antidepressant effect, starting also at 2 h post-infusion, but this superiority v. placebo injection lasted only 3 days, not at least 2 weeks like the antidepressant effect. The discordance in duration between ketamine's antidepressant and antisuicidal effects may be due to different neurobiological mechanisms involved in these mental phenomena and therapeutic actions. It appears that a single ketamine infusion can improve suicidal ideation, which is likely related to, but not entirely driven by, the improvement in depression (Ballard et al. Reference Ballard, Ionescu, Vande Voort, Niciu, Richards, Luckenbaugh, Brutsche, Ameli, Furey and Zarate2014).
Different from most (Zarate et al. Reference Zarate, Singh, Carlson, Brutsche, Ameli, Luckenbaugh, Charney and Manji2006; aan het Rot et al. Reference aan het Rot, Collins, Murrough, Perez, Reich, Charney and Mathew2010; Murrough et al. Reference Murrough, Iosifescu, Chang, Al Jurdi, Green, Perez, Iqbal, Pillemer, Foulkes, Shah, Charney and Mathew2013a ) but not all previous studies (Lapidus et al. Reference Lapidus, Levitch, Perez, Brallier, Parides, Soleimani, Feder, Iosifescu, Charney and Murrough2014), in this study patients receiving ketamine did not experience significantly greater, mild, transient elevations in measures of dissociative, and psychotomimetic side-effects; solely mania-like symptoms were significantly more pronounced than with placebo infusions, but this effect was only apparent at the 1- and 2 h post-infusion time point. The rate of dissociative symptoms here (2/13 = 15.4%) was basically similar to the figure in an earlier study (8/47 = 17.0%) (Murrough et al. Reference Murrough, Iosifescu, Chang, Al Jurdi, Green, Perez, Iqbal, Pillemer, Foulkes, Shah, Charney and Mathew2013a ). In addition, the very limited increase in dissociative and psychotomimetic effects in our study may result from ethnic differences in pharmacokinetics and bioavailability of ketamine between Chinese and Western patients, but this needs to be examined further. However, more patients in the ketamine augmentation group experienced at least one somatic adverse effect, but these side-effects occurred mostly during the infusion period, were transient and mild, did not require medical or psychiatric interventions, and did not lead to treatment discontinuation. While these symptoms could have led to functional unblinding in patients and raters, the lasting nature of the group differences up to week 2 argue against functional unblinding as a relevant issue driving the treatment differences. Moreover, a different study that used midazolam, another anesthetic, as an active placebo demonstrated similar advantages of ketamine as demonstrated in true placebo-controlled trials. (Murrough et al. Reference Murrough, Iosifescu, Chang, Al Jurdi, Green, Perez, Iqbal, Pillemer, Foulkes, Shah, Charney and Mathew2013a )
While it has been well-established that single ketamine infusion has rapid-acting antidepressant (Caddy et al. Reference Caddy, Giaroli, White, Shergill and Tracy2014; Fond et al. Reference Fond, Loundou, Rabu, Macgregor, Lancon, Brittner, Micoulaud-Franchi, Richieri, Courtet, Abbar, Roger, Leboyer and Boyer2014; McGirr et al. Reference McGirr, Berlim, Bond, Fleck, Yatham and Lam2015) and antisuicidal effects (Thakurta et al. Reference Thakurta, Das, Bhattacharya, Saha, Sen, Singh and Bisui2012; Zarate et al. Reference Zarate, Brutsche, Ibrahim, Franco-Chaves, Diazgranados, Cravchik, Selter, Marquardt, Liberty and Luckenbaugh2012), efforts to sustain its antidepressant response have been rather disappointing (aan het Rot et al. Reference aan het Rot, Collins, Murrough, Perez, Reich, Charney and Mathew2010). Strategies to maintain ketamine's rapid and robust antidepressant effect have largely depended on repeated infusions. However, although ketamine's effect has been extended by repeated infusions, the results in relapse prevention have been less than promising (aan het Rot et al. Reference aan het Rot, Collins, Murrough, Perez, Reich, Charney and Mathew2010; Murrough et al. Reference Murrough, Perez, Pillemer, Stern, Parides, aan het Rot, Collins, Mathew, Charney and Iosifescu2013b ; Naughton et al. Reference Naughton, Clarke, O'Leary, Cryan and Dinan2014). In addition, ketamine is a ‘club drug’ with significant potential for abuse; thus repeated administrations warrant caution. Further, prolonged ketamine use might lead to neurotoxicity (Liao et al. Reference Liao, Tang, Ma, Wu, Yang, Wang, Liu, Chen, Fletcher and Hao2010; Liao et al. Reference Liao, Tang, Corlett, Wang, Yang, Chen, Liu, Chen, Hao and Fletcher2011). By contrast, administration of a single dose i.v. ketamine augmenting standard antidepressant initiation is a safer attempt to maintain ketamine's rapid antidepressant and antisuicidal effects. Nevertheless, despite starting 10 mg escitalopram concurrently with the ketamine infusion and treating patients with this antidepressant for the next 4 weeks, antidepressant effects of escitalopram were no longer different from placebo augmentation of escitalopram at the 3-week time point, and response and remission rates did not differ statistically significantly any longer at week 4. This finding suggests that other treatment options are needed to sustain ketamine's significant antidepressant benefits in those patients in whom standard antidepressant treatment is ineffective. However, our study indicates that the ketamine effect did not simply diminish; rather, the escitalopram monotherapy effect increased, catching up with the rapid and robust ketamine effect that was most pronounced in the first 2 weeks of treatment.
Several limitations of this study should be noted. First, the results were obtained by restricting the antidepressant to escitalopram and may not be applicable to other antidepressants. Moreover, the dose was fixed at 10 mg/day and the long-term effect of ketamine cannot be evaluated in this study. Second, similar to most studies (Zarate et al. Reference Zarate, Singh, Carlson, Brutsche, Ameli, Luckenbaugh, Charney and Manji2006; Price et al. Reference Price, Nock, Charney and Mathew2009; aan het Rot et al. Reference aan het Rot, Collins, Murrough, Perez, Reich, Charney and Mathew2010; Diazgranados et al. Reference Diazgranados, Ibrahim, Brutsche, Newberg, Kronstein, Khalife, Kammerer, Quezado, Luckenbaugh, Salvadore, Machado-Vieira, Manji and Zarate2010; Ibrahim et al. Reference Ibrahim, Diazgranados, Luckenbaugh, Machado-Vieira, Baumann, Mallinger and Zarate2011), the sample size (n = 30) was relatively small in this study. Therefore our results are preliminary and need to be replicated in future studies with a large sample size. In addition, this was a single-site study, which allowed us to have the same two raters do all the assessments, but the sample size was and the generalizability of the results may be somewhat reduced. Third, similar to all previous studies (Berman et al. Reference Berman, Cappiello, Anand, Oren, Heninger, Charney and Krystal2000; aan het Rot et al. Reference aan het Rot, Collins, Murrough, Perez, Reich, Charney and Mathew2010; Katalinic et al. Reference Katalinic, Lai, Somogyi, Mitchell, Glue and Loo2013) except for one that used midazolam (Murrough et al. Reference Murrough, Iosifescu, Chang, Al Jurdi, Green, Perez, Iqbal, Pillemer, Foulkes, Shah, Charney and Mathew2013a ), saline was used as the inactive placebo. The transient mania-like side-effects in the ketamine group may have compromised the blinding. However, the very low level of side-effects in the ketamine group should have mitigated against this limitation. In addition, similar effect sizes to ours were obtained when midazolam was used as an active placebo (Murrough et al. Reference Murrough, Iosifescu, Chang, Al Jurdi, Green, Perez, Iqbal, Pillemer, Foulkes, Shah, Charney and Mathew2013a ), suggesting that the observed effect sizes in our study are unlikely majorly biased by functional unblinding. Fourth, different from previous studies, we included 44.4% of patients without treatment-resistant depression. However, for patients without treatment-resistant depression, prolonged suicidality and longer time to response and remission are also serious. Moreover, only one dose of i.v. ketamine was given, which has been shown to be safe in previous studies and which was also safe in this study. Therefore, we do not consider our including non-resistant patients as raising any ethical concern. Fifth, it would have been useful to include an additional study group receiving pill placebo + single-dose i.v. ketamine (0.5 mg/40 min) in order to be able to establish the additional benefit of the combination of escitalopram+i.v. ketamine over i.v. ketamine alone. However, since all previous single-dose i.v. ketamine studies showed an attenuation of the antidepressive effects by the end of week 1 and return to baseline in most patients thereafter, we did not consider this arm crucial for testing our hypothesis. Finally, similar to the STAR*D project (Trivedi et al. Reference Trivedi, Rush, Wisniewski, Nierenberg, Warden, Ritz, Norquist, Howland, Lebowitz, McGrath, Shores-Wilson, Biggs, Balasubramani and Fava2006), no structured diagnostic interview for major depression was administered. The clinical diagnosis of major depression was confirmed by a checklist based on DSM-IV criteria at baseline.
Conclusions
Single-dose i.v. ketamine (0.5 mg/kg over 40 min) added to newly initiated 10 mg/day escitalopram treatment was safe, resulting in rapid and robust antidepressant and antisuicidal effects in severe major depression lasting up to 2 weeks. Although response rates were impressive at 4 weeks, antidepressant symptom rating benefits ceased being significant v. placebo after 2 weeks, calling for additional research into ways to optimize ketamine dosing, administration, and duration in order to sustain antidepressant benefits from ketamine infusions.
Supplementary material
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0033291715002159.
Acknowledgements
This study was funded by the National Science and Technology Major Projects for ‘Major New Drugs Innovation and Development’ (No.: 2012ZX09303014-002) and the Key Medical Specialties Development Project of Beijing Municipal Administration of Hospitals (No.: ZY201403). [Trial Registration: ChiCTR-TRC Identifier: 14004936.]
Declaration of Interest
Dr Correll has been a consultant and/or advisor to or has received honoraria from AbbVie, Acadia, Actavis, Alkermes, Bristol–Myers Squibb, Eli Lilly, Forum, Genentech, Gerson Lehrman Group, IntraCellular Therapies, Janssen/J&J, Lundbeck, Medavante, Medscape, Otsuka, Pfizer, ProPhase, Reviva, Roche, Sunovion, Supernus, Takeda, and Teva. He has received grant support from Bristol–Myers Squibb, Janssen/J&J, Otsuka and Takeda. The remaining authors report no biomedical financial interests or potential conflicts of interest.







