Introduction
Major depressive disorder (MDD) has been associated with reduced neural activity in the prefrontal cortical regions implicated in emotional regulation, such as the dorsal prefrontal cortex, accompanied by increased activity in the limbic regions involved in emotional processing, such as the amygdala, the insular and the thalamus (Mayberg et al. Reference Mayberg, Liotti, Brannan, McGinnis, Mahurin, Jerabek, Silva, Tekell, Martin, Lancaster and Fox1999; Drevets, Reference Drevets2000; Kennedy et al. Reference Kennedy, Evans, Kruger, Mayberg, Meyer, McCann, Arifuzzman, Houle and Vaccarino2001; Anand et al. Reference Anand, Li, Wang, Wu, Gao, Bukhari, Mathews, Kalnin and Lowe2005; Fales et al. Reference Fales, Barch, Rundle, Mintun, Snyder, Cohen, Mathews and Sheline2008). These findings have led to a proposed model of fronto-limbic dysregulation to explain the negative affective bias that characterizes MDD (Mayberg, Reference Mayberg1997; Mayberg et al. Reference Mayberg, Liotti, Brannan, McGinnis, Mahurin, Jerabek, Silva, Tekell, Martin, Lancaster and Fox1999; Seminowicz et al. Reference Seminowicz, Mayberg, McIntosh, Goldapple, Kennedy, Segal and Rafi-Tari2004; Disner et al. Reference Disner, Beevers, Haigh and Beck2011). Other brain regions, such as the temporal, parietal and occipital cortices and the cerebellum, have also been implicated in MDD (Guo et al. Reference Guo, Liu, Xue, Yu, Ma, Tan, Sun, Chen, Liu, Xiao, Chen and Zhao2011a ,Reference Guo, Sun, Liu, Xu, Wu, Liu, Tan, Chen and Zhao b , Reference Guo, Liu, Xue, Xu, Wu, Ma, Wooderson, Tan, Sun, Chen, Liu, Xiao, Chen and Zhao2012; Wu et al. Reference Wu, Li, Kuang, Zhang, Lui, Huang, Chan, Kemp and Gong2011).
Antidepressant drugs are commonly used to treat MDD. It seems that most antidepressant drugs act by inhibiting the uptake of monoamines, thereby upregulating their synaptic availability. However, the neurophysiological correlates of these neurochemical effects remain poorly understood. According to cognitive models of MDD, the main beneficial effect of antidepressant drugs is to reverse or eliminate the negative affective bias observed in MDD (Pringle et al. Reference Pringle, Browning, Cowen and Harmer2011; Roiser et al. Reference Roiser, Elliott and Sahakian2012). Congruent with the behavioral alterations produced by antidepressants, studies using positron emission tomography (PET) or functional magnetic resonance imaging (fMRI) have demonstrated that short-term (2–8 weeks) treatment of MDD patients with antidepressants could reverse the increased activity in the limbic regions such as the amygdala, the thalamus and the insula, coupled with decreased activity in the prefrontal regions both in the rest (Mayberg et al. Reference Mayberg, Brannan, Tekell, Silva, Mahurin, McGinnis and Jerabek2000; Kennedy et al. Reference Kennedy, Evans, Kruger, Mayberg, Meyer, McCann, Arifuzzman, Houle and Vaccarino2001) and in response to negative stimuli (Kalin et al. Reference Kalin, Davidson, Irwin, Warner, Orendi, Sutton, Mock, Sorenson, Lowe and Turski1997; Fu et al. Reference Fu, Williams, Cleare, Brammer, Walsh, Kim, Andrew, Pich, Williams, Reed, Mitterschiffthaler, Suckling and Bullmore2004). Such effects have been observed without a simultaneous impact on mood or behavior in healthy volunteers receiving acute (Takahashi et al. Reference Takahashi, Yahata, Koeda, Takano, Asai, Suhara and Okubo2005; Bruhl et al. Reference Bruhl, Kaffenberger and Herwig2010) or 7-day (Harmer et al. Reference Harmer, Mackay, Reid, Cowen and Goodwin2006) antidepressant treatment. These findings suggest that brain areas within the fronto-limbic circuit may be target sites for antidepressant drugs. This hypothesis is of particular interest with respect to treatment with selective serotonin reuptake inhibitors (SSRIs), as these drugs are known to exert their action through effects on the raphe nuclei, which have strong connections with limbic regions, such as the amygdala, the insula and the thalamus, and neocortical sites, such as the prefrontal cortex (Lanzenberger et al. Reference Lanzenberger, Kranz, Haeusler, Akimova, Savli, Hahn, Mitterhauser, Spindelegger, Philippe, Fink, Wadsak, Karanikas and Kasper2012).
Despite advances in the treatment of MDD, some patients fail to respond to antidepressants. Investigating the neurological basis underlying the varied clinical responses will contribute to the development of more effective treatment strategies. A recent systematic review (Fu et al. Reference Fu, Steiner and Costafreda2013) summarized the neural predictors of the clinical responses to the most commonly used treatments in MDD, including pharmacological and psychological therapies. The review concluded that increased activity in the anterior cingulate cortex can predict a higher likelihood of improvement whereas increased activity in the insula and the striatum is associated with a higher likelihood of a poor response to treatment.
Resting-state fMRI (R-fMRI), a promising neuroimaging technique that can measure intrinsic or spontaneous neural activity (Fox & Raichle, Reference Fox and Raichle2007), has been increasingly used in studies of healthy and patient groups. Compared with task-based experimental paradigms, R-fMRI is easy to implement and avoids the confounds presented by differences in task designs. R-fMRI could also be useful for mapping the neural effects of antidepressant drugs, although few such studies have been conducted. One R-fMRI study demonstrated that 6 weeks of sertraline treatment normalized the reduced functional connectivity between the dorsal anterior cingulate cortex and limbic regions, thus restoring the balance of the fronto-limbic circuitry in MDD (Anand et al. Reference Anand, Li, Wang, Wu, Gao, Bukhari, Mathews, Kalnin and Lowe2005). However, it is important to note that functional connectivity measures the temporal synchronization between two spatially independent regions and provides little information about changes within the local regions. It is therefore of interest to examine the local brain changes in MDD patients following antidepressant treatment to identify target sites of antidepressant drugs.
In the current study we used a combination of R-fMRI and a recently validated approach called regional homogeneity (ReHo; Zang et al. Reference Zang, Jiang, Lu, He and Tian2004) to assess cross-sectional brain function in first-episode drug-naive patients with MDD, along with longitudinal changes following 8 weeks of treatment with escitalopram, the most selective SSRI. The ReHo method measures the synchronization between time series of a given voxel and its nearest neighbors, thus providing an effective tool to characterize brain function at the local level (Zang et al. Reference Zang, Jiang, Lu, He and Tian2004). Based on the present literature, we hypothesized that 8 weeks of escitalopram treatment would modulate the brain activity of MDD patients in certain regions within the fronto-limbic circuit, such as the dorsal prefrontal cortex, the thalamus and the insula.
Method
Subjects
Patients with first-episode drug-naive MDD were recruited from the Peking University Institute of Mental Health and the Beijing Anding Hospital of Capital Medical University between May 2010 and July 2011. A diagnosis of MDD was made by two psychiatrists based on the Mini-International Neuropsychiatric Interview (MINI; Sheehan et al. Reference Sheehan, Lecrubier, Sheehan, Amorim, Janavs, Weiller, Hergueta, Baker and Dunbar1998), a short structured diagnostic interview for DSM-IV (APA, 2000) diagnoses. Inclusion criteria for MDD subjects were: a current acute episode of depression; a total score on the 17-item Hamilton Depression Rating Scale (HAMD; Hamilton, Reference Hamilton1967) of at least 24; a duration of depression ⩾1 month but ⩽24 months; and no cognitive behavioral therapy (CBT) or other forms of psychotherapy performed during the study period. Patients diagnosed with a concurrent co-morbid Axis I psychiatric disorder or Axis II personality disorders and mental retardation within the past year were excluded.
According to these criteria, 18 currently depressed patients were recruited into this study and completed baseline fMRI scans. Of the 18 patients, three changed to other drugs because of non-response to escitalopram treatment during 5–7 weeks, and were therefore automatically withdrawn from the study. One patient refused to participate in the longitudinal study so we could not perform the clinical assessment of this patient at 8 weeks. The remaining 14 patients completed the second scans. All 14 patients showed a clinical response to escitalopram treatment, defined as at least a 50% decrease from the baseline HAMD score. Nine of these 14 patients achieved clinical remission, defined as a HAMD score ⩽7. The HAMD scores for each patient before and after treatment are listed in online Supplementary Table S1. Final doses of escitalopram were 20 mg/day (n = 10) or 15 mg/day (n = 4). No concomitant medications or treatments, including benzodiazepine and psychotherapies, were used during the study period. All patients had detectable plasma escitalopram levels, with a mean concentration of 31.63 μg/l (standard error 3.29 μg/l).
Control subjects matched for age (±5 years), gender and years of education (±5 years) with the patients were recruited from the local community. The controls with HAMD scores > 7 and any current or lifetime psychiatric or neurological disorders were excluded. We also excluded, as far as possible, first-degree relatives of the control subjects with a history of major psychiatric or neurological illness by asking the subjects for the relevant information.
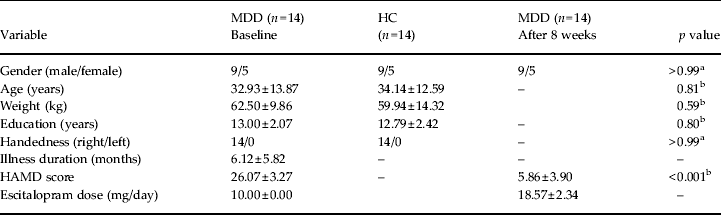
Exclusion criteria for all the subjects were: age < 18 years or > 60 years; serious medical or neurological illness; a history of significant head trauma; substance dependence or abuse within the past year; current or previous use of antidepressant or antipsychotic drugs; a history of electroconvulsive therapy; acutely suicidal or homicidal; current pregnancy or breastfeeding; and any contraindications to an MRI scan. All the participants were right-handed as determined by the Edinburgh Handedness Scale (Oldfield, Reference Oldfield1971). All subjects took part after signing a written informed consent form approved by the local Institutional Review Boards. Each individual was compensated for their participation in the study. The demographic and clinical characteristics of the subjects are presented in Table 1.
Table 1. Demographic and clinical characteristics of the subjects

MDD, Major depressive disorder; HC, healthy controls; HAMD, Hamilton Depression Rating Scale.
Values given as number or mean ± standard deviation.
a p values for the χ 2 test;
b p values for the two-sample t test.
Longitudinal study and antidepressant treatment
In this study, patients received treatment with escitalopram according to the clinical judgment of the treating psychiatrists and the patient's consent. Treatment of patients commenced with escitalopram 10 mg/day, initiated immediately following the baseline fMRI scan. Subsequent dose adjustment was determined by each patient's response and tolerance. Adherence to treatment was confirmed by measuring the plasma concentration of escitalopram on the day of the second scan.
MRI data acquisition
Baseline clinical assessments and fMRI scans were completed prior to patients receiving medication for the first time. After 8 weeks of treatment, patients were rescanned with the same process. Controls were scanned on only one occasion for comparison purposes. Image data were acquired with a 3.0-T Siemens MRI system (Siemens Medical Solutions, Germany) at the 306th Hospital of the People's Liberation Army. First, a structural scan was acquired for the localization of functional scans. Then, the resting-state functional images were recorded axially over 7 min and 6 s with the following parameters: repetition time (TR)/echo time (TE) = 2000 ms/30 ms, flip angle = 90°, 30 slices, thickness/gap = 4.0 mm/0.8 mm, voxel size = 3.3 × 3.3 × 4.0 mm3, resolution = 64 × 64 matrix, field of view (FOV) = 210 × 210 mm2, and bandwidth = 2232 Hz/pixel. The subjects were instructed to keep their eyes closed, remain still, and not think of anything in particular. After the functional scans, three-dimensional T1-weighted magnetization prepared rapidly acquired gradient echo (MPRAGE) images were acquired sagittally using the following parameters: TR/TE = 2300 ms/3.01 ms, flip angle = 9°, 176 slices, slice thickness = 1 mm, voxel size = 1 × 1 × 1 mm3; FOV = 256 × 240 mm2.
The subjects' compliance was determined when the scan was completed. None of the subjects reported discomfort during or after the procedure. No subjects fell asleep during the scan. No obvious structural damage was found in any subject based on conventional MRI scans, which were examined by two experienced radiologists.
Data analysis
Functional image preprocessing
Preprocessing was performed using the Data Processing Assistant for Resting-State fMRI (DPARSF; Chao-Gan & Yu-Feng, Reference Chao-Gan and Yu-Feng2010). The first 10 volumes were discarded to allow for scanner calibration and adaptation of the participants to the scanning environment. The remaining 200 volumes were analyzed. The steps included slice timing, head-motion correction, spatial normalization in Montreal Neurological Institute (MNI) space and resampling with 3 × 3 × 3 mm3 resolution. The head motion of all subjects was less than 1.5 mm maximum displacement in any direction of x, y and z and 1.5° in any angular dimension. No significant differences were found in the maximum movement values in each plane of translation (x, y or z) or each plane of rotation (roll, pitch or yaw) between any two groups (patients at baseline, patients after treatment, and healthy controls) (all p's > 0.05). Several sources of spurious variance were removed, including the six motion parameters, linear drift, signals from the ventricular system and white matter. The residuals of these regressions were bandpass filtered (0.01–0.08 Hz) to reduce low-frequency drift and high-frequency noise.
ReHo computation
ReHo analysis was performed using DPARSF software. The detailed procedure for ReHo computation has been described previously (Zang et al. Reference Zang, Jiang, Lu, He and Tian2004). In brief, based on the assumption that the hemodynamic characteristics of every voxel are similar within a functional cluster, individual ReHo maps were generated by assigning to each voxel a value for Kendall's coefficient of concordance (KCC; Kendall & Gibbons, Reference Kendall and Gibbons1990), which measures the similarity between the time series of a given voxel and those of its nearest neighbors. (We adopted the recommended neighborhood size of 26; Li et al. Reference Li, Kadivar, Pluta, Dunlop and Wang2012.) In this way, ReHo can map the level of regional activity across the whole brain of an individual (Kiviniemi, Reference Kiviniemi2008). This method has been successfully applied in studies of several psychiatric illnesses, such as schizophrenia (Yu et al. Reference Yu, Hsieh, Wang, Liu, Liu, Hwang, Chien, Hwu and Tseng2013), depression (Yao et al. Reference Yao, Wang, Lu, Liu and Teng2009), attention deficit hyperactivity disorder (Cheng et al. Reference Cheng, Ji, Zhang and Feng2012) and Alzheimer's disease (AD; Reference He, Wang, Zang, Tian, Zhang, Li and JiangHe et al. 2007). The findings of these ReHo studies were essentially compatible with other R-fMRI studies (Liu et al. Reference Liu, Liang, Zhou, He, Hao, Song, Yu, Liu, Liu and Jiang2008; Tomasi & Volkow, Reference Tomasi and Volkow2012; Zhao et al. Reference Zhao, Liu, Wang, Liu, Xi, Guo, Jiang, Jiang and Wang2012; Tao et al. Reference Tao, Guo, Ge, Kendrick, Xue, Liu and Feng2013) of these illnesses, most of which used the functional connectivity method and examined the brain changes at the network level. These studies suggest that the ReHo method could efficiently locate regional brain activity changes and contribute to understanding the network-level disturbances related to the illness. For example, in AD patients, ReHo alterations were detected in the medial prefrontal cortex, posterior cingulate gyrus/precuneus and inferior parietal lobule (He et al. Reference He, Wang, Zang, Tian, Zhang, Li and Jiang2007). Abnormalities in network connectivity of these regions have been frequently reported in other studies of AD (Sato et al. Reference Sato, Hoexter, Fujita and Rohde2012; Zhao et al. Reference Zhao, Liu, Wang, Liu, Xi, Guo, Jiang, Jiang and Wang2012).
In the current study, ReHo computation was performed within a gray-matter (GM) mask that was generated by setting a threshold of 0.15 on the mean GM map of the patients before and after treatment, and also in healthy controls. Each individual ReHo map was divided by its own mean KCC values within this mask. The standardized ReHo images were then smoothed with a Gaussian kernel of 4 mm at full-width at half-maximum (FWHM).
Statistical analysis
Demographic and clinical data were analyzed using SPSS, version 15.0 (SPSS Inc., USA). Normally distributed data were analyzed using two-tailed t tests and categorical variables were analyzed using χ 2 tests.
Using the Resting-State fMRI Data Analysis Toolkit (Song et al. Reference Song, Dong, Long, Li, Zuo, Zhu, He, Yan and Zang2011), a statistical analysis of the imaging data was implemented: we conducted a comparison of the ReHo maps of patients before and after treatment using a two-tailed paired t test (to examine the differences between baseline and after treatment) and a comparison of the ReHo maps of patients at pre- and post-treatment with the ReHo maps from healthy controls using two-tailed t tests (to examine the differences between patients and controls) controlling for age and gender. The results were thresholded using a combination of p < 0.05 for each voxel and a minimum volume of 1566 mm3, which resulted in a corrected threshold of p < 0.05 as determined by the Monte Carlo simulation (see AlphaSim in AFNI http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf; parameters were: single voxel p = 0.05, 4-mm FWHM, within the GM mask).
Because all patients included in the analysis responded to escitalopram treatment, we failed to demonstrate any difference in ReHo between responders and non-responders. However, we compared the baseline ReHo images between those who had and had not remitted to identify any brain regions associated with the differences in clinical efficacy. The results were restricted within a mask covering the clusters displaying significant ReHo differences between the patients at baseline and controls, and also between the patients before and after treatment.
Finally, partial correlation analysis was performed to examine the association between changes in ReHo following treatment and reduction in the HAMD score, controlling for age and gender. The levels of significance were set at p < 0.05 without correction for multiple tests.
Results
ReHo comparisons (Fig. 1 and Table 2)
Patients before treatment versus controls (Fig. 1a)
Compared to the controls, the MDD patients before treatment exhibited decreased ReHo in the frontal lobe (a cluster in the right superior frontal gyrus), the temporal lobe (left middle and right inferior temporal gyri), the parietal lobe (right precuneus) and the occipital lobe (left superior occipital gyrus and right cuneus). Increased ReHo in the MDD patients was observed in the left dorsal medial prefrontal gyrus and the left anterior lobe of the cerebellum. All p values of the peak voxels (voxels showing the most significant group difference) within these regions were < 0.001, except for the right cuneus, which had a p value of 0.0015.

Fig. 1. Statistical maps of voxel t values of regional homogeneity (ReHo) comparisons of patients versus controls and pre- versus post-treatment measurements in patients with major depressive disorder (MDD). (a) Patients at baseline compared with controls. (b) Patients before treatment versus after treatment. (c) Patients after treatment compared with controls. In all case warm colors indicate increased ReHo and cold colors indicate the opposite. The numbers at the bottom left of each image refer to the z coordinates in Montreal Neurological Institute (MNI) space. The threshold was set at a corrected p < 0.05. t-score bars are shown at the right for each map. The left side of the images corresponds to the right side of the brain, and vice versa.
Table 2. Regions that showed significant changes in ReHo between patients and controls and between pre- and post-treatment measurements in MDD patients

ReHo, Regional homogeneity; BA, Brodmann area; t, statistical value of the peak voxel; MNI, Montreal Neurological Institute; R, right; L, left.
Patients before treatment versus after treatment (Fig. 1b)
Following treatment, the MDD patients exhibited significantly decreased ReHo in the left dorsal medial prefrontal gyrus, the right insula and the bilateral thalamus, whereas increased ReHo was observed in the right superior frontal gyrus. All p values of the peak voxels within these regions were < 0.001. No significant correlations were found between changes in ReHo and reduction in the HAMD score.
Patients after treatment versus controls (Fig. 1c)
Compared to the controls, the MDD patients after treatment still exhibited some brain abnormalities, including decreased ReHo in the right precuneus and increased ReHo in the left anterior lobe of the cerebellum. All p values of the peak voxels within these regions were < 0.001. No significant difference was present in the baseline ReHo images between patients who had remitted and those who had not.
Discussion
To our knowledge, this is the first study to investigate the short-term effects of escitalopram treatment on regional brain function of MDD patients as indexed by ReHo. We observed widespread alterations in ReHo in the frontal, temporal, parietal and occipital cortices, and in the cerebellum, in MDD patients. Abnormalities in these regions have been reported in earlier PET studies (Kennedy et al. Reference Kennedy, Evans, Kruger, Mayberg, Meyer, McCann, Arifuzzman, Houle and Vaccarino2001; Kimbrell et al. Reference Kimbrell, Ketter, George, Little, Benson, Willis, Herscovitch and Post2002) and in more recent R-fMRI studies using the ReHo method (Yao et al. Reference Yao, Wang, Lu, Liu and Teng2009; Liu et al. Reference Liu, Xu, Xu, Wang, Zhao, Lv, Cao, Zhang and Du2010; Guo et al. Reference Guo, Liu, Xue, Yu, Ma, Tan, Sun, Chen, Liu, Xiao, Chen and Zhao2011a ,Reference Guo, Sun, Liu, Xu, Wu, Liu, Tan, Chen and Zhao b ; Wu et al. Reference Wu, Li, Kuang, Zhang, Lui, Huang, Chan, Kemp and Gong2011; Liu et al. Reference Liu, Hu, Wang, Guo, Zhao, Li, Xun, Long, Zhang, Wang, Zeng, Gao, Wooderson, Chen and Chen2012; Peng et al. Reference Peng, Shen, Zhang, Huang, Liu, Liu, Jiang, Xu and Fang2012). We have summarized the reported results of ReHo studies of MDD to demonstrate the comparability and reproducibility of these studies (see online Supplementary Table S2). Importantly, we found that escitalopram treatment was associated with increased ReHo in the right superior frontal gyrus, coupled with decreased ReHo in the left dorsal medial prefrontal gyrus, the right insula and the bilateral thalamus.
MDD is characterized by negative emotional bias, which has been associated with reduced activity in the prefrontal cortical brain regions, such as the superior frontal gyrus, and increased activity in limbic regions, such as the thalamus and insula (Mayberg, Reference Mayberg1997, Reference Mayberg2003; Mayberg et al. Reference Mayberg, Liotti, Brannan, McGinnis, Mahurin, Jerabek, Silva, Tekell, Martin, Lancaster and Fox1999). These findings have been incorporated into the fronto-limbic dysregulation model of MDD (Seminowicz et al. Reference Seminowicz, Mayberg, McIntosh, Goldapple, Kennedy, Segal and Rafi-Tari2004). In MDD patients responding to SSRIs such as paroxetine and fluoxetine, studies (Mayberg et al. Reference Mayberg, Liotti, Brannan, McGinnis, Mahurin, Jerabek, Silva, Tekell, Martin, Lancaster and Fox1999; Kennedy et al. Reference Kennedy, Evans, Kruger, Mayberg, Meyer, McCann, Arifuzzman, Houle and Vaccarino2001) have reported a reversal of decreased metabolism in the prefrontal regions but also, conversely, an inhibition of metabolism in limbic regions from previously normal levels. In addition, increased metabolism in limbic regions was reported in the fluoxetine non-responders (Mayberg et al. Reference Mayberg, Liotti, Brannan, McGinnis, Mahurin, Jerabek, Silva, Tekell, Martin, Lancaster and Fox1999). The findings of our study are compatible with the reported pattern of brain alteration in treatment responders. Together, it could be speculated that inhibition of limbic regions is necessary for the normalization of neocortical regions and, ultimately, for clinical efficacy, which may be a mechanism by which SSRI drugs modulate the fronto-limbic circuit.
Neural models of MDD have proposed that another brain region, the medial prefrontal cortex, which integrates both cognitive and emotional information, functions as a mediator of fronto-limbic circuit dysregulation (Mayberg et al. Reference Mayberg, Liotti, Brannan, McGinnis, Mahurin, Jerabek, Silva, Tekell, Martin, Lancaster and Fox1999; Seminowicz et al. Reference Seminowicz, Mayberg, McIntosh, Goldapple, Kennedy, Segal and Rafi-Tari2004). It has been demonstrated that the dorsal medial prefrontal cortex (DMPFC) in MDD patients has increased resting-state functional connectivity (RSFC) with the precuneus, the dorsolateral prefrontal cortex and the subgenual anterior cingulate cortex (Sheline et al. Reference Sheline, Price, Yan and Mintun2010). A subsequent study (McCabe et al. Reference McCabe, Mishor, Filippini, Cowen, Taylor and Harmer2011) in healthy volunteers found that citalopram, another SSRI, could downregulate the RSFC between the left DMPFC and the left hippocampus. The authors proposed that inhibiting the activity of the DMPFC may be a therapeutic mechanism for MDD. In support of that proposed mechanism, we found that escitalopram treatment could reverse the abnormally increased ReHo in the left DMPFC of MDD patients. Thus, the DMPFC may be another target mediating the effects of escitalopram treatment on the fronto-limbic circuit.
It is of interest to speculate on the potential mechanisms of alterations in ReHo. Because escitalopram increases the availability of 5-hydroxytryptamine (5-HT), the simplest explanation would be an increase in levels of this monoamine. Several other processes have been implicated in the antidepressant effects, such as neuroplasticity (Lee & Kim, Reference Lee and Kim2010), neurogenesis (Manev et al. Reference Manev, Uz and Manev2003) and anti-inflammatory actions (Hashioka, Reference Hashioka2011). Further studies of receptor mapping and electrophysiological recordings in animal models would be helpful in identifying the biochemical mechanisms underlying the treatment-related imaging effects.
After treatment, most of the alterations in the brain activity in MDD patients were no longer apparent. However, abnormalities did remain in the right precuneus and the left cerebellum. This is not surprising, as clinically, the maximal effects of antidepressants may take up to 8–12 weeks to occur. Thus, the residual abnormalities may be eliminated with longer treatment, and this should be further examined. Another possibility is that the alterations in ReHo may be trait markers of MDD. In support of this, studies have demonstrated decreased ReHo in the right precuneus of MDD patients (Liu et al. Reference Liu, Hu, Wang, Guo, Zhao, Li, Xun, Long, Zhang, Wang, Zeng, Gao, Wooderson, Chen and Chen2012), in addition to decreased ReHo in the left cerebellum in MDD patients (Guo et al. Reference Guo, Liu, Xue, Yu, Ma, Tan, Sun, Chen, Liu, Xiao, Chen and Zhao2011a ) and in subjects at high risk for MDD (Liu et al. Reference Liu, Xu, Xu, Wang, Zhao, Lv, Cao, Zhang and Du2010).
No significant difference was found in the baseline ReHo between MDD patients who had remitted and those who had not. Intuitively, this suggests that ReHo cannot be used as a predictor of clinical remission, although it can be helpful to locate brain regions with abnormal activity. However, this may be due to several factors. One may be an insufficient sample size after dividing patients into remission and non-remission subgroups. Another possible reason is that the full effects of antidepressants may take 8–12 weeks to occur. It is possible that the patients would show remission following continued treatment. A further follow-up study will help to verify this.
There were several limitations in our study. First, the sample size was relatively small, which may limit the ability of these results to explain the medication effects and the observation of significant clinical correlations with the alterations in ReHo. Second, given that MDD affects women in greater numbers than men, the sex distribution of the patient sample is not representative of most MDD groups, potentially limiting the generalizability of our findings. Third, no neuropsychological data were collected, so we cannot exclude the possibility that, even within patients who responded to treatment, there could be differential cognitive biases that reflect the differences in brain activity. Fourth, we cannot fully assign the alterations in ReHo to a pharmacological effect, state changes following symptomatic improvement, a placebo effect, or some combination of these possibilities. Further studies are needed to clarify the roles of these confounds. Fifth, the control subjects were scanned only once. Ideally, they should be rescanned after 8 weeks. Multiple studies have addressed this issue, as a high level of consistency in R-fMRI measurements over time has been reported in healthy groups (Shehzad et al. Reference Shehzad, Kelly, Reiss, Gee, Gotimer, Uddin, Lee, Margulies, Roy, Biswal, Petkova, Castellanos and Milham2009; Li et al. Reference Li, Kadivar, Pluta, Dunlop and Wang2012).
Despite these limitations, this study has demonstrated that successful treatment with escitalopram was associated with a modulation of resting-state brain activities in regions within the fronto-limbic mood circuit of MDD patients. This finding provides important insights into the effects of antidepressant treatment on nervous system function in MDD patients.
Supplementary material
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0033291713002031.
Acknowledgments
This study was supported by research grants from the National Key Basic Research Program of China (973 Program: 2013CB531305 to T. Si, X. Yu and G. Wang; 2012CB720704 to Z. Jin, K. Li and Y. Zeng) and the Capital Foundation of Medicine Research and Development (2009–2026, T. Si).
Declaration of Interest
None.