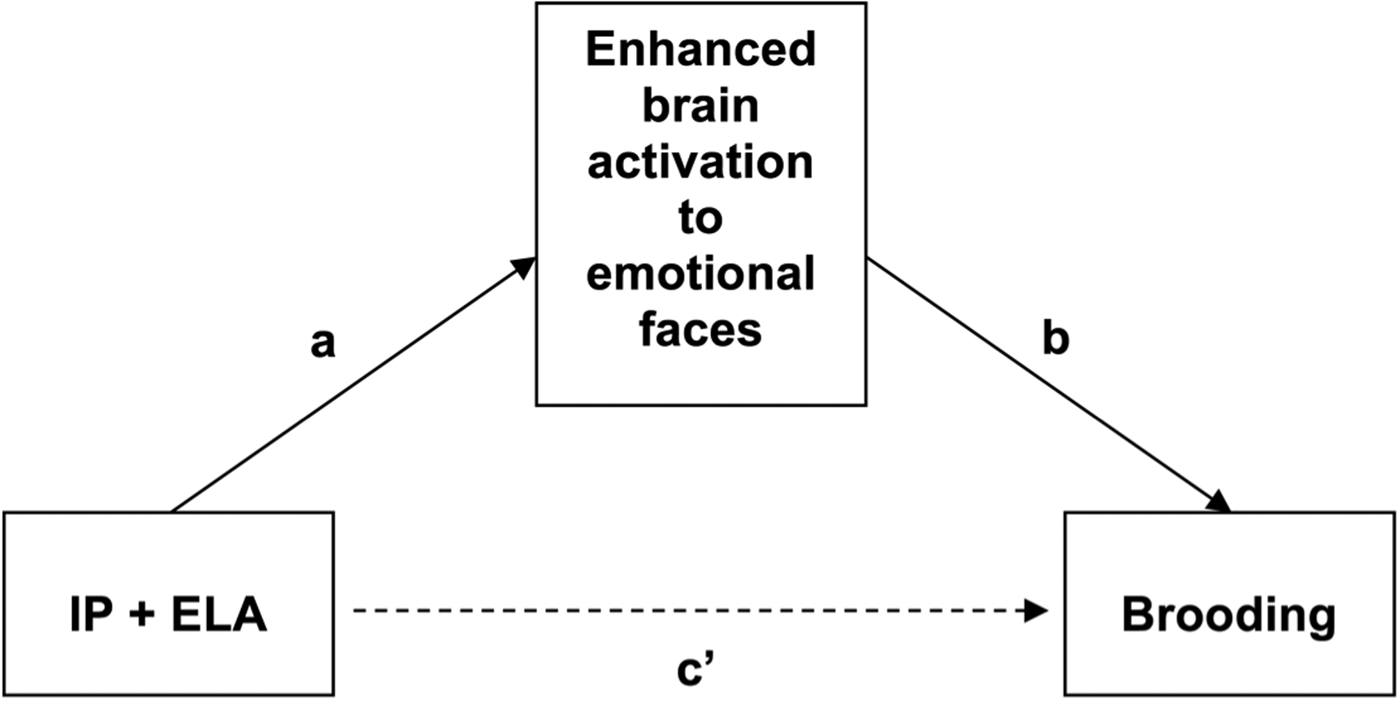
Introduction
Childhood maltreatment is a damaging form of early-life adversity (ELA) and stress broadly defined as the intentional or unintentional commission of acts (e.g. verbal, physical, or sexual abuse) or withholding of resources (emotional or physical neglect) by caregivers that adversely influence the emotional and physical health, growth, or adaptation of a child (Egeland, Reference Egeland2009). ELA is a particularly potent risk factor for many internalizing psychopathologies (IPs) in adulthood, such as depression, post-traumatic stress disorder (PTSD), and other anxiety disorders (Widom et al., Reference Widom, DuMont and Czaja2007; Gilbert et al., Reference Gilbert, Widom, Browne, Fergusson, Webb and Janson2009). Moreover, IP patients with a history of ELA demonstrate a particularly chronic course of illness and treatment resistance (Arnow, Reference Arnow2004; Wegman and Stetler, Reference Wegman and Stetler2009). Developing a better understanding of the mechanisms by which ELA may foster a predisposition to poor long-term mental outcomes is paramount for improving targeted prevention and intervention for this vulnerable population.
From a theoretical standpoint, ELA may increase vulnerability to IPs via altered brain function underlying processing and modulation of emotion (Teicher et al., Reference Teicher, Samson, Anderson and Ohashi2016; McCrory et al., Reference McCrory, Gerin and Viding2017). Specifically, this latent vulnerability theory postulates that ELA is thought to enhance neurobiological sensitivity to sources of potential threat or negative valence (McCrory et al., Reference McCrory, Gerin and Viding2017; Heany et al., Reference Heany, Groenewold, Uhlmann, Dalvie, Stein and Brooks2018). In a childhood environment saturated with constant threats to wellbeing, heightened responsiveness to social signals of possible adverse experiences may evolve as an adaptive coping mechanism, initially facilitating early identification and avoidance of potentially harmful interactions with a caregiver (McCrory and Viding, Reference McCrory and Viding2015). However, if increased stress susceptibility is instantiated during a malleable period of brain development and endures over time, the success of this compensatory mechanism may break down or become maladaptive with repeated overuse (McCrory and Viding, Reference McCrory and Viding2015). It is this chronic ‘wear and tear’ to regulatory and compensatory emotional processes that is suspected to increase vulnerability to emotional dysregulation and mental health problems later in life (Dannlowski et al., Reference Dannlowski, Stuhrmann, Beutelmann, Zwanzger, Lenzen, Grotegerd, Domschke, Hohoff, Ohrmann, Bauer, Lindner, Postert, Konrad, Arolt, Heindel, Suslow and Kugel2012; Danese and Baldwin, Reference Danese and Baldwin2017).
In initial support of this hypothesis, there is an extant literature documenting structural alterations in frontal and limbic brain regions involved in the experience and regulation of emotion, such as the anterior cingulate cortex, medial and dorsolateral prefrontal cortex (Lim et al., Reference Lim, Radua and Rubia2014; Paquola et al., Reference Paquola, Bennett and Lagopoulos2016), hippocampus (Riem et al., Reference Riem, Alink, Out, Van Ijzendoorn and Bakermans-Kranenburg2015), and amygdala (Ahmed-Leitao et al., Reference Ahmed-Leitao, Spies, van den Heuvel and Seedat2016). Building on these structural findings, task-based functional magnetic resonance imaging (fMRI) has allowed for more direct inferences regarding the neural correlates of emotion processing via the use of socio-affective cues deliberately designed to signal threat, stimulate mentalizing, or elicit negative/positive affective states. One recent meta-analysis summarizing these studies revealed that, in whole-brain analyses, adults exposed to childhood maltreatment consistently demonstrated altered activation in the superior frontal gyrus, middle temporal gyrus, hippocampus, and superior parietal lobule across a variety of socio-affective stimuli (Heany et al., Reference Heany, Groenewold, Uhlmann, Dalvie, Stein and Brooks2018). Additionally, in region of interest analyses, amygdala and anterior cingulate hyper-responsivity to socio-affective cues were replicated, but marked by more heterogeneity across studies and sampling design (Heany et al., Reference Heany, Groenewold, Uhlmann, Dalvie, Stein and Brooks2018). These results converge with another meta-analysis of neural response during emotional faces tasks, where across all emotions, the right but not left parahippocampal gyrus and amygdala activation was a correlate of maltreatment in whole-brain studies (Hein and Monk, Reference Hein and Monk2017).
Understanding the clinical significance of these functional brain changes in ELA is limited by the fact that many existing studies are undertaken in healthy individuals or small disease-based case–control samples designed around one primary diagnosis of interest (Hein and Monk, Reference Hein and Monk2017; Heany et al., Reference Heany, Groenewold, Uhlmann, Dalvie, Stein and Brooks2018). This approach lays the foundation for possible candidate mechanisms, but reduces explanatory power and fragments conclusions that may cut across psychological disorders, which commonly co-occur (Cuthbert, Reference Cuthbert2014). An additional caveat to the interpretation is that adults with IPs (Buff et al., Reference Buff, Brinkmann, Neumeister, Feldker, Heitmann, Gathmann, Andor and Straube2016; Feldker et al., Reference Feldker, Heitmann, Neumeister, Tupak, Schrammen, Moeck, Zwitserlood, Bruchmann and Straube2017), as well as those exposed to ELA (Hein and Monk, Reference Hein and Monk2017; Heany et al., Reference Heany, Groenewold, Uhlmann, Dalvie, Stein and Brooks2018), both display alterations of distributed affective corticolimbic networks in response to stimuli conveying threat or negative emotionality. Particularly, there is an extensive literature assessing the neural correlates of facial emotion processing in IPs (Etkin and Wager, Reference Etkin and Wager2007; Hamilton et al., Reference Hamilton, Etkin, Furman, Lemus, Johnson and Gotlib2012; Gentili et al., Reference Gentili, Cristea, Angstadt, Klumpp, Tozzi, Phan and Pietrini2016). Illustratively, our group has previously demonstrated that both depression and anxiety symptoms are linked with activation in paralimbic, cingulate, and lateral prefrontal regions in response to negative facial expressions (MacNamara et al., Reference MacNamara, Klumpp, Kennedy, Langenecker and Phan2017). Given this overlap, it is prudent to identify whether internalizing symptoms or ELA are the primary driver of neural alterations associated with emotion processing. If a neural signature specific to individuals with IPs and ELA is identified, this level of precision could promote the early identification of individuals with ELA at highest risk for psychopathology or offer novel intervention targets for this subgroup of patients.
Seeing as ELA constitutes risk for a number of IPs (Widom et al., Reference Widom, DuMont and Czaja2007; Gilbert et al., Reference Gilbert, Widom, Browne, Fergusson, Webb and Janson2009) and in alignment with the Research Domain Criteria initiative to understand how biologically based constructs explain the core, shared features of diagnostic categories (Cuthbert, Reference Cuthbert2015), linking neural phenotypes of ELA to patterns of thought or behavior common to IPs is also of substantial clinical utility (Insel, Reference Insel2014; Sharp et al., Reference Sharp, Fowler, Salas, Nielsen, Allen, Oldham, Kosten, Mathew, Madan, Frueh and Fonagy2016). One shared feature of IPs is rumination (Nolen-Hoeksema and Watkins, Reference Nolen-Hoeksema and Watkins2011), a maladaptive cognitive style, defined as the tendency to constantly focus on a negative thought, problem, or mood state and on the possible causes and implications of negative feelings (Smith and Alloy, Reference Smith and Alloy2009). As childhood adversity is not often easily discussed and is suppressed (Levy and Anderson, Reference Levy and Anderson2008), internal extortion and re-production of ELA events may further increase rumination amongst individuals with IPs (Grierson et al., Reference Grierson, Hickie, Naismith and Scott2016). In fact, in a non-clinical sample, rumination was associated with depression and anxiety symptoms in individuals with ELA (Kim et al., Reference Kim, Jin, Jung, Hahn and Lee2017), implicating a possible role of rumination in the maintenance of IPs. More specifically, brooding is the component of rumination most strongly associated with IP (Nolen-Hoeksema et al., Reference Nolen-Hoeksema, Wisco and Lyubomirsky2008; Aldao et al., Reference Aldao, Nolen-Hoeksema and Schweizer2010; McLaughlin and Nolen-Hoeksema, Reference McLaughlin and Nolen-Hoeksema2011) and also involved in the maintenance of symptoms in individuals exposed to ELA and other types of adversity, such as interpersonal trauma (Raes and Hermans, Reference Raes and Hermans2008; Allbaugh et al., Reference Allbaugh, Wright and Folger2016). At the neural level, it is noteworthy that there is substantial overlap between the functional correlates of ruminative thought patterns and emotion processing in ELA, including in the amygdala, anterior and posterior cingulate, medial and dorsolateral prefrontal cortex, (para-) hippocampus, medial and inferior temporal gyri, and inferior parietal lobule (Cooney et al., Reference Cooney, Joormann, Eugene, Dennis and Gotlib2010; Burkhouse et al., Reference Burkhouse, Jacobs, Peters, Ajilore, Watkins and Langenecker2017). Additionally, rumination has been linked to activation in visual and somatosensory brain regions, such as the insula, precuneus, and cuneus, which are involved in visual cortical facial emotion processing (Burkhouse et al., Reference Burkhouse, Jacobs, Peters, Ajilore, Watkins and Langenecker2017). Put together, ELA may heighten corticolimbic sensitivity to negative emotional cues, in turn, promoting increased attention to symptoms and possible causes of one's own distress. However, the integration of ELA, neural emotion processing, and rumination has yet to be examined in a clinical sample.
To address these gaps in the literature, we sought to examine the neural correlates of facial emotion processing in a large, heterogeneous, and clinically diverse population of patients with multiple and comorbid IPs, with and without exposure to ELA (IP + ELA), and healthy controls (HCs). As ELA shares similar proclivity for depression, mixed anxiety disorders, and PTSD, this approach was intentionally designed to maximize generalizability. We hypothesized that IP + ELA would demonstrate the most pronounced activation in the superior frontal gyrus, lateral temporal gyri, medial temporal lobe, anterior/posterior cingulate cortex, and inferior parietal lobule. We also expected that IP + ELA patients would report a higher degree of ruminative brooding and that enhanced brain activation in these regions would account for (mediate) brooding in IP + ELA.
Method
Participants and procedures
The current study was designed in-line with, and funded by, the NIMH RDoC initiative (RFA-MH-13-080). The aims of the larger study sought the enrollment of a clinically representative patient population, with a full range of IP and symptoms, which consented to begin treatment with pharmacotherapy [selective serotonin reuptake inhibitors (SSRIs)] or cognitive behavioral therapy. All data used in the current study were collected prior to treatment. Eligibility criteria included: (1) age 18–65; (2) current full threshold DSM-5 depression or anxiety disorder; and (3) total score of ⩾23 on the Depression, Anxiety, and Stress Scale [DASS-21; (Lovibond and Lovibond, Reference Lovibond and Lovibond1995)], and ⩽60 on the Global Assessment of Functioning [GAF; (Jones et al., Reference Jones, Thornicroft, Coffey and Dunn1995)]. Exclusion criteria included: (1) inability to provide consent and read and write in English; (2) active medical or neurological problem; (3) history of mania or psychosis; current obsessive–compulsive disorder; (4) current substance dependence; (5) intellectual disability; (6) contraindication to SSRIs; (7) ongoing treatment with psychiatric medications or psychotherapy; (8) medication use (psychiatric and other), besides oral contraceptive, within the past 4 months; (9) history of traumatic brain injury with loss of consciousness; and (10) being pregnant. The University of Illinois at Chicago Institutional Review Board approved this study and informed consent was obtained from all participants.
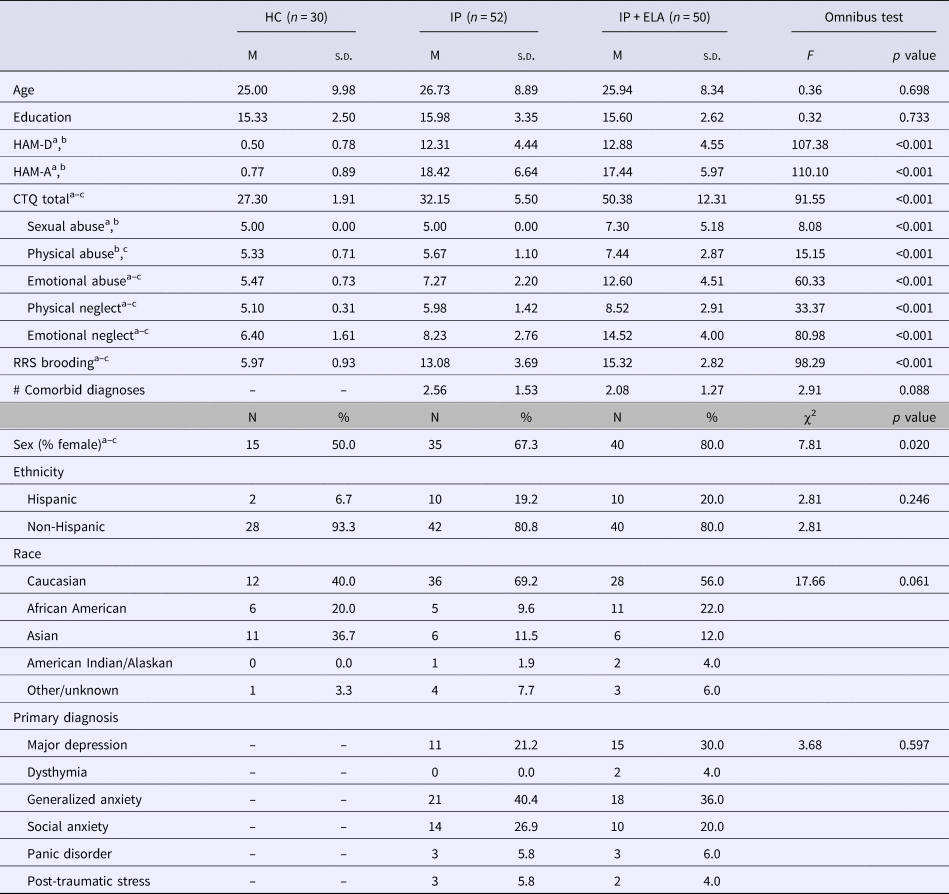
Participant clinical demographic characteristics are reported in Table 1. Diagnoses were made according to the Structured Clinical Interview for DSM-5 Disorders [SCID-5; (First et al., Reference First, Williams, Karg and Spitzer2015)] by trained research staff. In-line with the strategy encouraged by RDoC (Kozak and Cuthbert, Reference Kozak and Cuthbert2016), individuals were not excluded for comorbid disorders, but instead classified by their clinician-determined principal diagnosis, as determined by the most severe and impairing symptoms (Table 1). Current symptom severity of depression and anxiety based on clinician-administered interviews was evaluated by trained raters using the Hamilton Depression Rating Scale [HAM-D; (Hamilton, Reference Hamilton1960)] and Hamilton Anxiety Rating Scale [HAM-A; (Hamilton, Reference Hamilton1959)].
Table 1. Sample characteristics
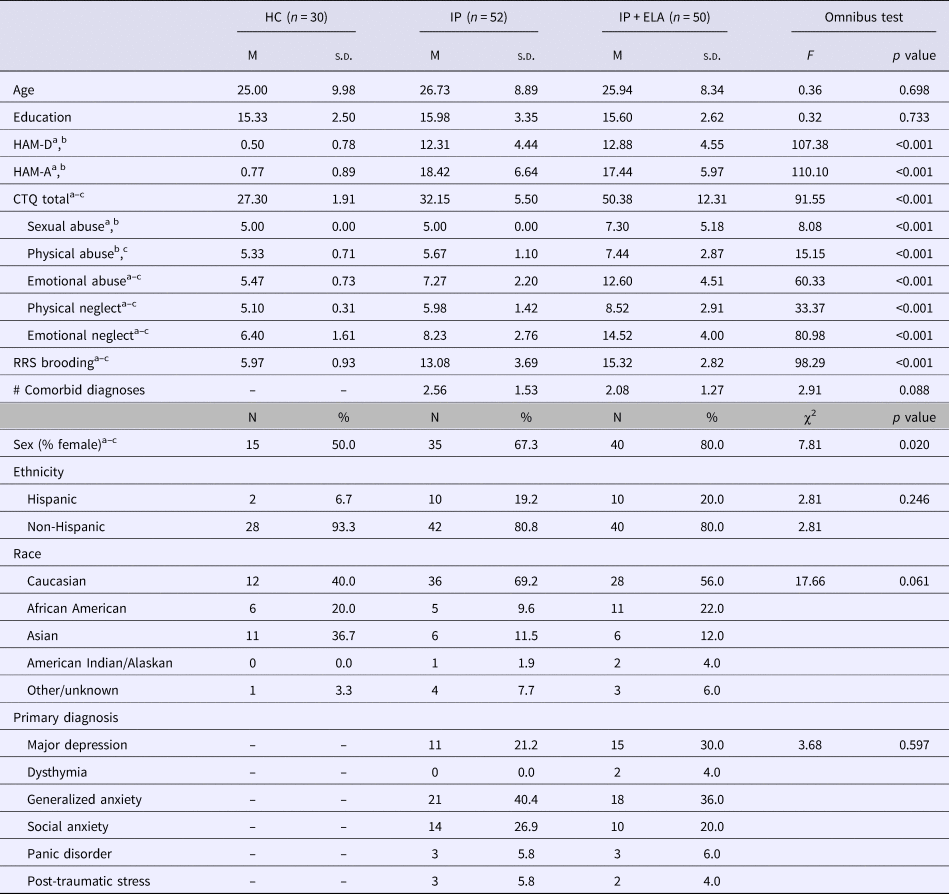
HC, healthy control; IP, internalizing psychopathology patients; IP + ELA, internalizing psychopathology patients with early-life adversity; HAM-D, Hamilton Depression Rating Scale; HAM-A, Hamilton Anxiety Rating Scale; CTQ, Childhood Trauma Questionnaire; RRS, Rumination Response Scale.
a Significant (p < 0.05) post-hoc pairwise comparison, IP v. HC.
b Significant (p < 0.05) post-hoc pairwise comparison, IP + ELA v. HC.
c Significant (p < 0.05) post-hoc pairwise comparison, IP v. IP + ELA.
Assessment of ELA
The Childhood Trauma Questionnaire (CTQ) is a 28-item self-report measure that provides brief, reliable, and valid screening for histories of abuse and neglect (Bernstein et al., Reference Bernstein, Ahluvalia, Pogge and Handelsman1997). It inquires about five subscales of maltreatment – emotional, physical, and sexual abuse, and emotional and physical neglect. The total score of each sub-scale ranges from 5 to 25, thus the total score of CTQ fluctuates from 25 to 125. Although the total score of the CTQ is intended to represent the cumulative severity of childhood adversity exposure, the distribution of the measure is often skewed by the base rate of childhood adversity (proportion of respondents reporting little to no childhood adversity experiences). In the current sample, the skewness value was 1.52. To adjust for the degree of skewness, existence of childhood adversity exposure can be determined by a cut-off score of each CTQ subscale. Participants who score higher than the threshold of any one subscale are treated as a positive case of ELA. Consistent with previous studies (Bernstein et al., Reference Bernstein, Ahluvalia, Pogge and Handelsman1997; Walker et al., Reference Walker, Unutzer, Rutter, Gelfand, Saunders, VonKorff, Koss and Katon1999; Bernstein et al., Reference Bernstein, Stein, Newcomb, Walker, Pogge, Ahluvalia, Stokes, Handelsman, Medrano, Desmond and Zule2003; Gibb et al., Reference Gibb, Schofield and Coles2009; Johnson et al., Reference Johnson, Benas and Gibb2011; Bevilacqua et al., Reference Bevilacqua, Carli, Sarchiapone, George, Goldman, Roy and Enoch2012; Lu et al., Reference Lu, Peng, Wang, Vasish, Zhang, Gao, Wu, Liao, Wang, Tang, Li, Li, Li, Zhou, Zhang and Li2013; Kudinova et al., Reference Kudinova, Gibb, McGeary and Knopik2015), the cut-offs of each subscale for moderate exposure which best differentiate clinically significant adversity exposure were used: (1) emotional abuse ⩾13, (2) emotional neglect ⩾15, (3) sexual abuse ⩾8, (4) physical abuse ⩾10, and (5) physical neglect ⩾10. In this sample, emotional abuse α = 0.83, physical abuse α = 0.67, sexual abuse α = 0.95, emotional neglect α = 0.91, and physical neglect α = 0.63. Relatively low Cronbach's α for physical abuse and neglect is likely influenced by particularly high skewness values for these subscales (Sheng and Sheng, Reference Sheng and Sheng2012).
Within the patient sample, 50 participants (49.0%) had a positive history of ELA based on the cut-off scores, forming a sub-group of IPs with significant ELA (IP + ELA). Importantly, we also verified none of the HC participants reported significant ELA based on these criteria. The convergent validity of these cut-off scores was also evaluated using an exploratory cluster analysis of the study sample. This was performed with CTQ raw subscale scores as input using Wards method of minimum variance with a squared Euclidean distance measure. Ward's method is distinct from other methods because it uses an analysis of variance (ANOVA) approach to evaluate the distances between clusters. The cluster solution was determined from inspection of the dendrogram. The cases in the resulting cluster solution identified the same three groups, with the subset of n = 50 IP + ELA patients as most similar to each other.
Assessment of rumination
Self-reported rumination was evaluated with the Ruminative Response Scale [RRS; (Treynor et al., Reference Treynor, Gonzalez and Nolen-Hoeksema2003)], which contains subscales representing brooding and reflective pondering components of rumination. As noted earlier, brooding is the component of rumination most strongly associated with IP (Nolen-Hoeksema et al., Reference Nolen-Hoeksema, Wisco and Lyubomirsky2008; Aldao et al., Reference Aldao, Nolen-Hoeksema and Schweizer2010; McLaughlin and Nolen-Hoeksema, Reference McLaughlin and Nolen-Hoeksema2011) and particularly relevant to the development of IP symptoms in trauma-exposed individuals (Raes and Hermans, Reference Raes and Hermans2008). The brooding subscale consists of five Likert-type items ranging from 1 (almost never) to 4 (almost always). The measure has demonstrated good internal consistency and 1-year retest reliability (Treynor et al., Reference Treynor, Gonzalez and Nolen-Hoeksema2003). In this study, brooding α = 0.87.
Task
Participants completed a version of the Emotional Face-Matching Task (Hariri et al., Reference Hariri, Tessitore, Mattay, Fera and Weinberger2002) previously validated for use with fMRI of blood-oxygen-level-dependent (BOLD) signal (Hariri et al., Reference Hariri, Tessitore, Mattay, Fera and Weinberger2002; Phan et al., Reference Phan, Coccaro, Angstadt, Kreger, Mayberg, Liberzon and Stein2013; Gorka et al., Reference Gorka, Fitzgerald, Labuschagne, Hosanagar, Wood, Nathan and Phan2015; MacNamara et al., Reference MacNamara, Klumpp, Kennedy, Langenecker and Phan2017). Angry, fearful, happy, and sad faces were selected from the Gur emotional faces set (Gur et al., Reference Gur, Schroeder, Turner, McGrath, Chan, Turetsky, Alsop, Maldjian and Gur2002). There were three angry, three fearful, three happy, and three sad blocks of trials, interspersed with shape-matching blocks. Each block lasted 20 s and consisted of four back-to-back 5 s trials. Shapes were used as control stimuli instead of neutral faces because they may provide a more truly neutral baseline for comparison, particularly when patients are involved (Filkowski and Haas, Reference Filkowski and Haas2017).
fMRI data acquisition and processing
fMRI based on BOLD contrast was performed on a three 3 T GE (General Electric Healthcare, Waukesha, WI) MR750 scanner at the University of Illinois at Chicago (UIC). Scanning was performed using an eight-channel phased-array radio frequency head coil, using either a gradient-echo echo planar imaging sequence, with the following parameters: repetition time 2s, echo time 25 ms, flip angle 90°, field of view 22 cm, acquisition matrix 64 × 64, 3 mm slices with no gap, 44 axial slices per volume.
All data met our criteria for image quality with minimal motion correction (movements ⩽3 mm in any direction across the run). The first four volumes were discarded to allow for the magnetization to reach equilibrium. Statistical Parametric Mapping (SPM8) software (Wellcome Trust Centre for Neuroimaging, London, www.fil.ion.ucl.ac.uk/spm) was used to perform conventional image pre-processing steps. In brief, slice-time correction was performed to account for temporal differences between slice collection order and images were spatially realigned to the first image of the run, normalized to a Montreal Neurological Institute (MNI) template using the EPI template, resampled to 2 mm3 voxels, and smoothed with an 8 mm isotropic Gaussian kernel.
The time-series data were subjected to a general linear model, convolved with the canonical hemodynamic response function, and filtered with a 128 s high-pass filter. Because of the heterogeneous IP sample and that we did not have any particular predictions about specific emotional expressions, angry, fearful, happy, sad, and shapes conditions were modeled separately, with effects estimated for each voxel for each participant. Individual motion parameters were entered in the model as covariates of no interest. Angry > shapes, fearful > shapes, sad > shapes, and happy > shapes, contrasts, created separately for each participant, were included as random effects for the second-level analysis. Each emotion contrast was modeled as a separate dependent variable due to violations of generalized linear model assumptions that occur in repeated-measures fMRI designs, particularly when covariates of non-interest are included. Therefore, this approach allowed us to maximize explanatory power by covarying for brain activation related to symptoms of depression and anxiety.
Data analytic approach
Clinical analyses
Clinical and demographic measures comparing IP + ELA, IP, and HC participants were inspected for normal distribution and analyzed using ANOVA and χ2 tests, as appropriate.
fMRI analyses
Whole-brain group (IP + ELA, IP, and HC) differences were modeled using a one-way ANOVA in SPM8, with follow-up t-contrasts for all possible pairwise differences. Symptoms of depression (HAM-D scores) and anxiety (HAM-A scores) were included in the model as covariates of no interest. To threshold results, we used a whole-brain mask encompassing all gray matter regions excluding cerebellum owing to limited coverage [created with MARINA; (Walter et al., Reference Walter, Blecker, Kirsch, Sammer, Schienle, Stark and Vaitl2003)]. Clusters of activation were identified using an uncorrected voxel threshold of p < 0.001 and then subjected to correction for multiple comparisons via simulation using 3dClustSim utility (16 December 2015, updated release; 10 000 iterations; http://afni.nimh.nih.gov/pub/dist/doc/program_help/3dClustSim.html). Given the smoothness estimates of the data, family-wise error correction at α<0.05 was achieved using a voxel threshold of p < 0.001, with minimum cluster sizes of 93 (angry > shapes), 91 (fearful > shapes), 76 (sad > shapes), and 92 (happy > shapes) voxels. To clarify the direction of significant between-group differences, we extracted parameter estimates of BOLD signal responses (β-weights, in arbitrary units of activation) averaged across voxels within a 5-mm radius sphere surrounding each peak maxima.
Mediation analyses
After identifying foci of between-group differences in brain activation, a series of mediation analyses tested whether brain activation to emotions v. shapes (extracted using the 5-mm radius sphere surrounding each peak maxima from group contrasts) was, in turn, associated with ruminative brooding in IP + ELA (relative to HC and IP). A separate mediation model was run for each cluster of between-group differences in brain activation. Mediation analyses were conducted using the SPSS macro PROCESS (Hayes, Reference Hayes2012), including covariates of no interest for depression (HAM-D) and anxiety (HAM-A) symptoms, age, and sex. Multiple parallel tests were controlled using a false-discovery rate-adjusted α threshold of p < 0.036 (Benjamini and Hochberg, Reference Benjamini and Hochberg1995). Tests of mediation employed a bootstrapping approach with N = 5000 bootstrap resamples and a 95% confidence interval to assess indirect effects using PROCESS (Preacher and Hayes, Reference Preacher and Hayes2004). Bootstrapping is a non-parametric resampling procedure that generates an approximation of the sampling distribution of a statistic from the available data. Sampling distributions of indirect effects are generated by taking a sample (with replacement) of size N from the full data set and calculating the indirect effects (i.e. conducting mediation analyses) in each of the resamples. Thus, the 95% CI represents that of each of those 5000 resample analyses, 95% of the generated indirect effects fall between the given two estimates.
Results
Demographic and clinical characteristics
IP + ELA (n = 50), IP (n = 52), and HCs (n = 30) were equivalent in terms of age, education, and distribution of race/ethnicity (Table 1). IP + ELA, IP, and HC groups did differ in sex distribution, with a greater proportion of females in the IP + ELA group relative to both IP and HC (Table 1). HAM-A scores were higher in IP + ELA (t = −19.38, p < 0.001) and IP (t = −18.88, p < 0.001) relative to HC, but IP + ELA and IP did not differ (t = 0.79, p = 0.433). HAM-D scores were higher in IP + ELA (t = −18.78, p < 0.001) and IP (t = −18.67, p < 0.001) relative to HC, but IP + ELA and IP did not differ (t = −0.64, p = 0.522). IP + ELA reported higher levels of ruminative brooding than IP (t = −3.46, p = 0.001) and HC participants (t = −21.56, p < 0.001). Ruminative brooding was also higher in IP relative to HC (t = −13.20, p < 0.001).
ELA and brain activation to emotional faces
Overall activation for each emotion condition is presented in online Supplementary Fig. S1. There were no differences in brain activation between IP and HC groups for any of the emotion contrasts. All findings below pertain to increased activation in IP + ELA relative to IP and HC; there were no significant foci of decreased activation in IP + ELA.
Angry > shapes
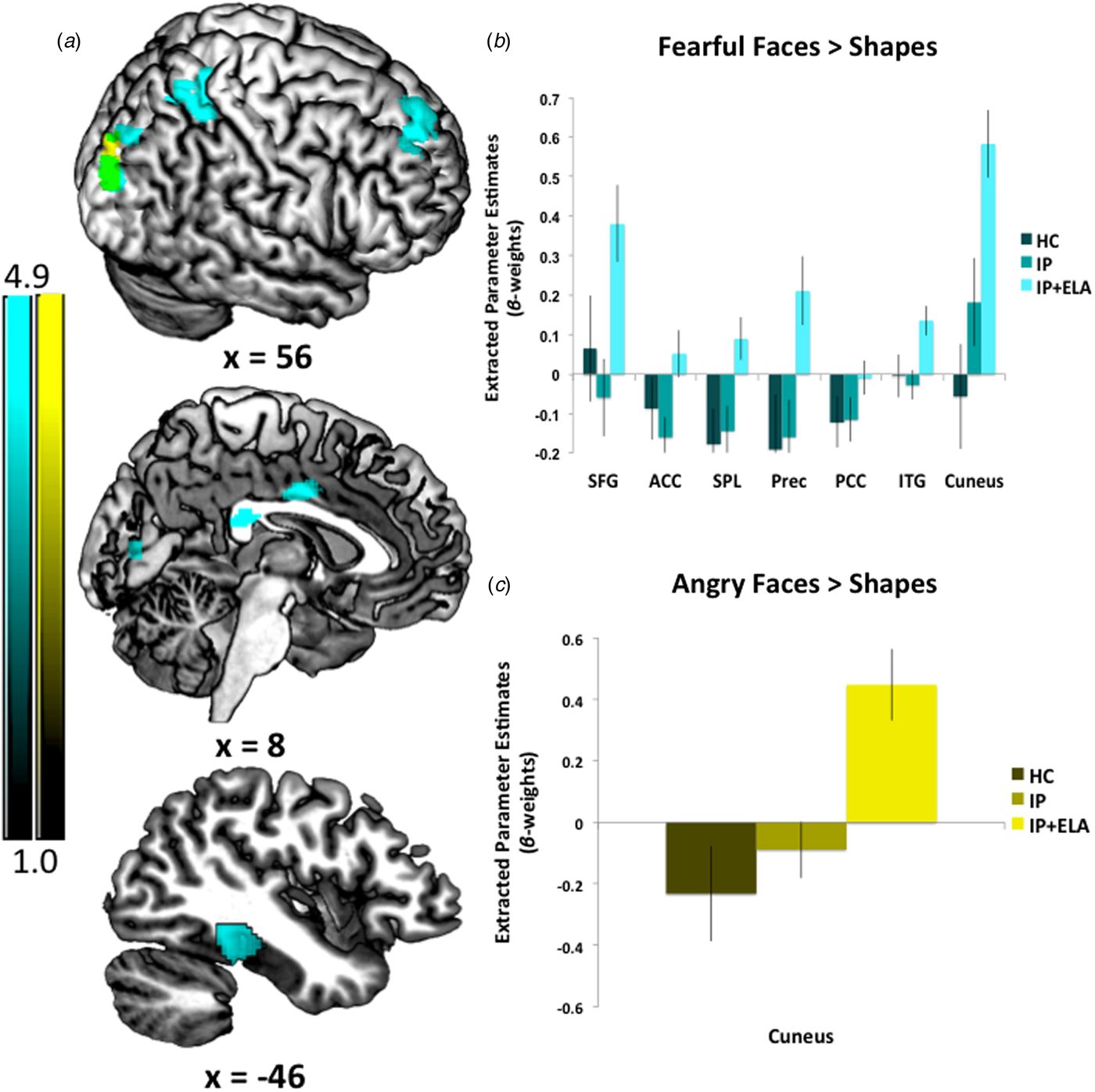
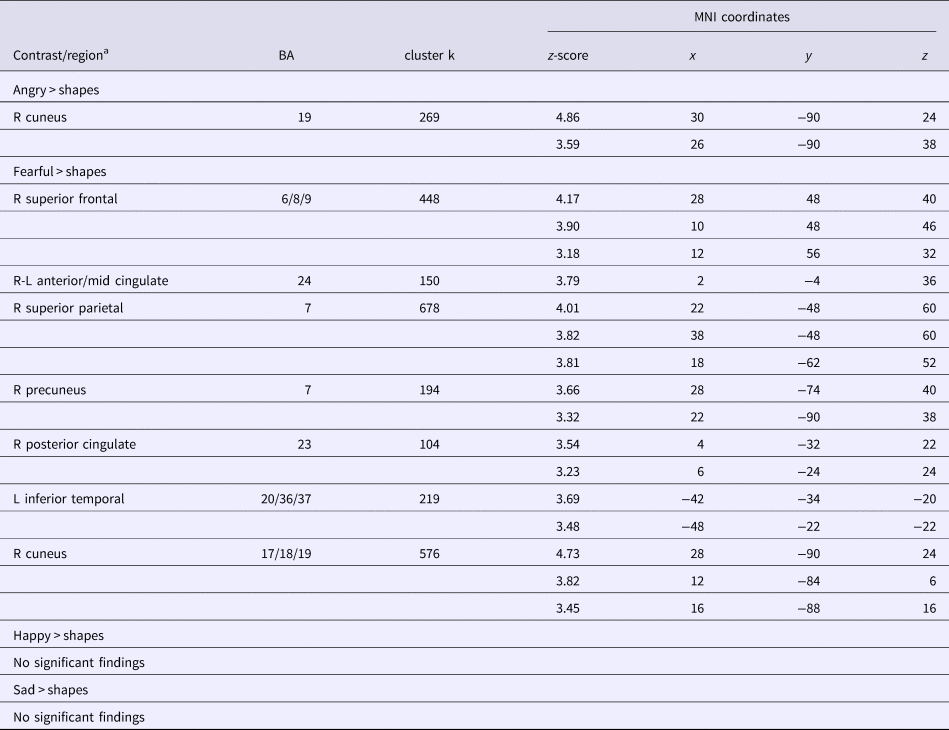
IP + ELA demonstrated greater activation (Fig. 1c, Table 2) in the right cuneus [BA 19].
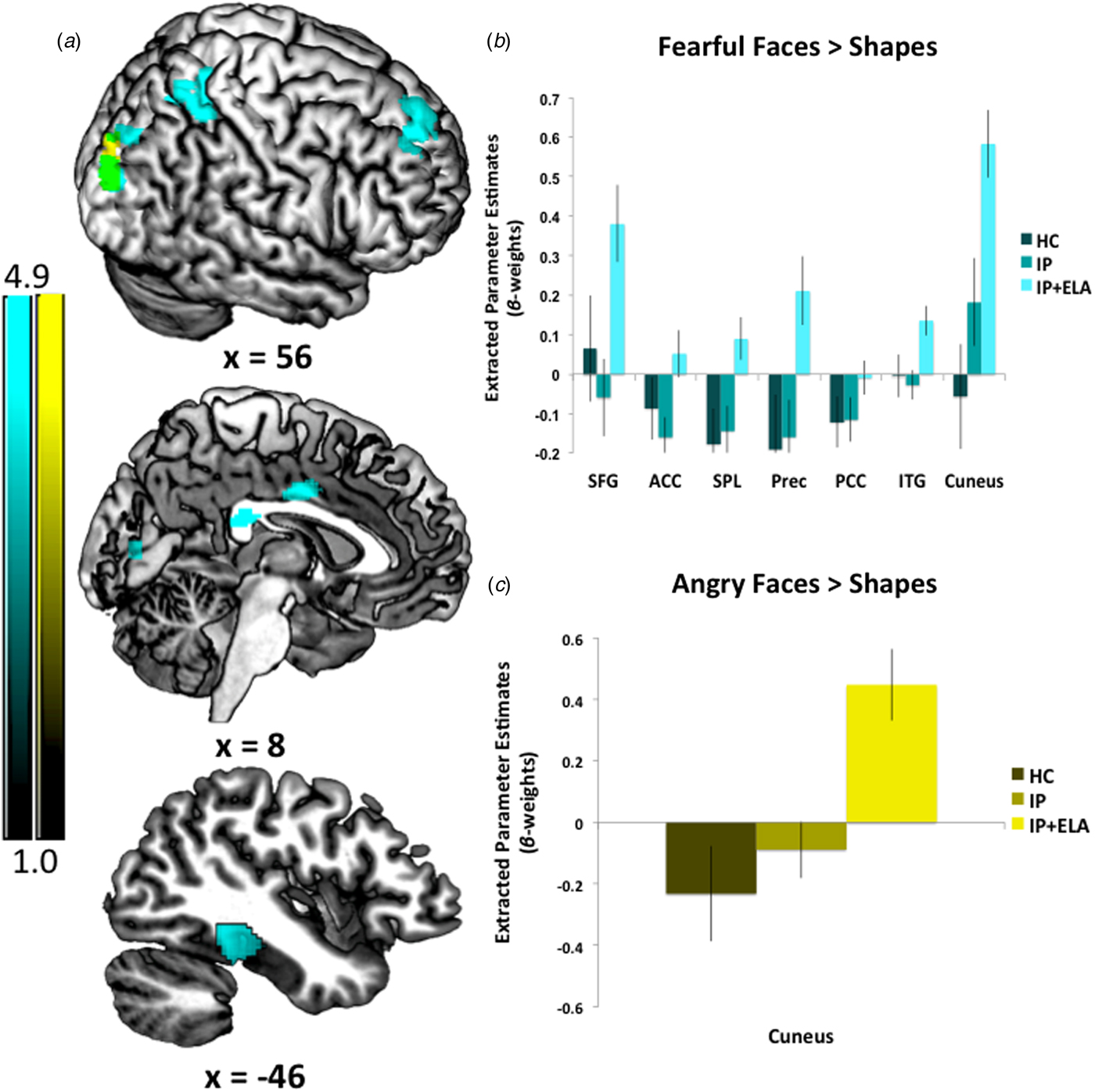
Fig. 1. (a) Foci of increased neural response for fearful faces > shapes, angry faces > shapes, and their overlap. IP + ELA, IP, and HC extracted parameter estimates of activation (β-weights) for (b) fearful > shapes and (c) angry > shapes. SFG, superior frontal gyrus; ACC, anterior cingulate cortex; PCC, posterior cingulate cortex; Prec, precuneus; SPL, superior parietal lobule; ITG, inferior temporal gyrus.
Table 2. Whole-brain activation during facial emotion processing in IP + ELA relative to IP and HC
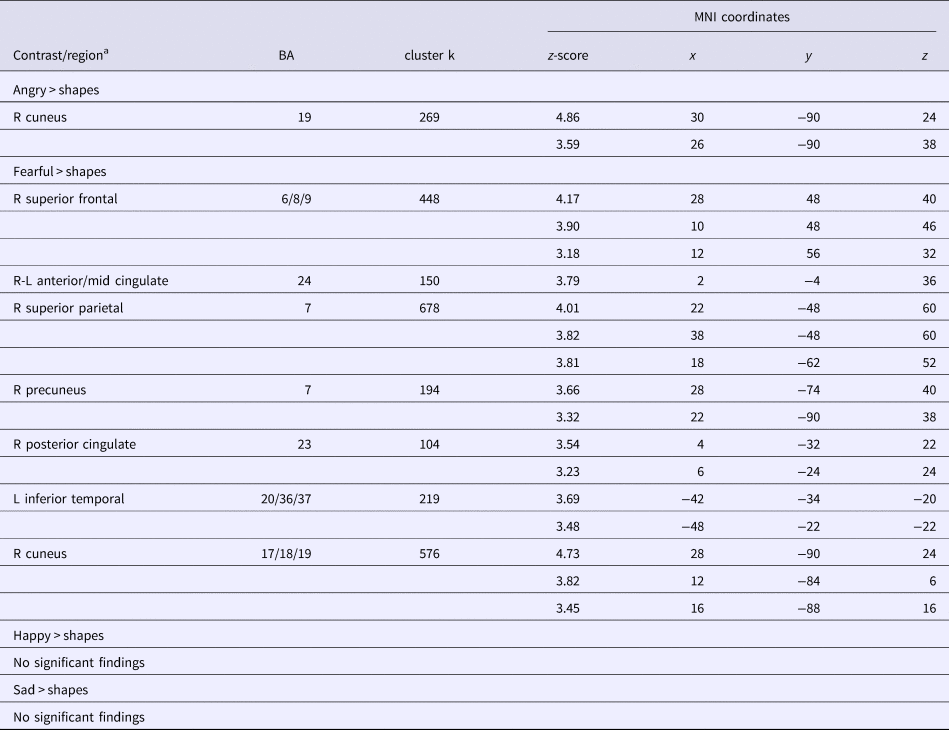
MNI, Montreal Neurological Institute; BA, Brodmann's area; k, number of contiguous voxels; L, left; R, right.
a Results are unchanged when re-performed without HAM-D and HAM-A as covariates. Results are also maintained when including sex as a covariate of non-interest in the SPM8 analysis.
Fearful > shapes
IP + ELA demonstrated greater activation (Fig. 1a, Table 2) in the right superior frontal gyrus (encompassing aspects of the dorsolateral and dorsomedial prefrontal cortex; [BA 6, 8, 9]), bilateral anterior/mid cingulate [BA 24], and right posterior cingulate [BA 23]. Greater activation was also observed in the left temporal lobe [BA 20, 36, 37], including the inferior temporal, fusiform, and parahippocampal gyri, right precuneus [BA 7], right superior parietal lobule [BA 7], and right cuneus/lingual gyrus [BA 17, 18, 19].
Sad > shapes
No significant findings.
Happy > shapes
No significant findings.
Post-hoc analyses
A post-hoc multivariate general linear model of extracted peak BOLD signal indicated there were no significant main effects of sex [F (11, 132) 0.14, p = 0.999] or sex by group interactions [F (22, 132) = 0.978, p = 0.494] in relation to BOLD activation of any of these clusters. Findings were also maintained when including sex as a covariate of non-interest in the SPM8 analysis.
Brain activation to emotional faces as a mediator of rumination in IP + ELA
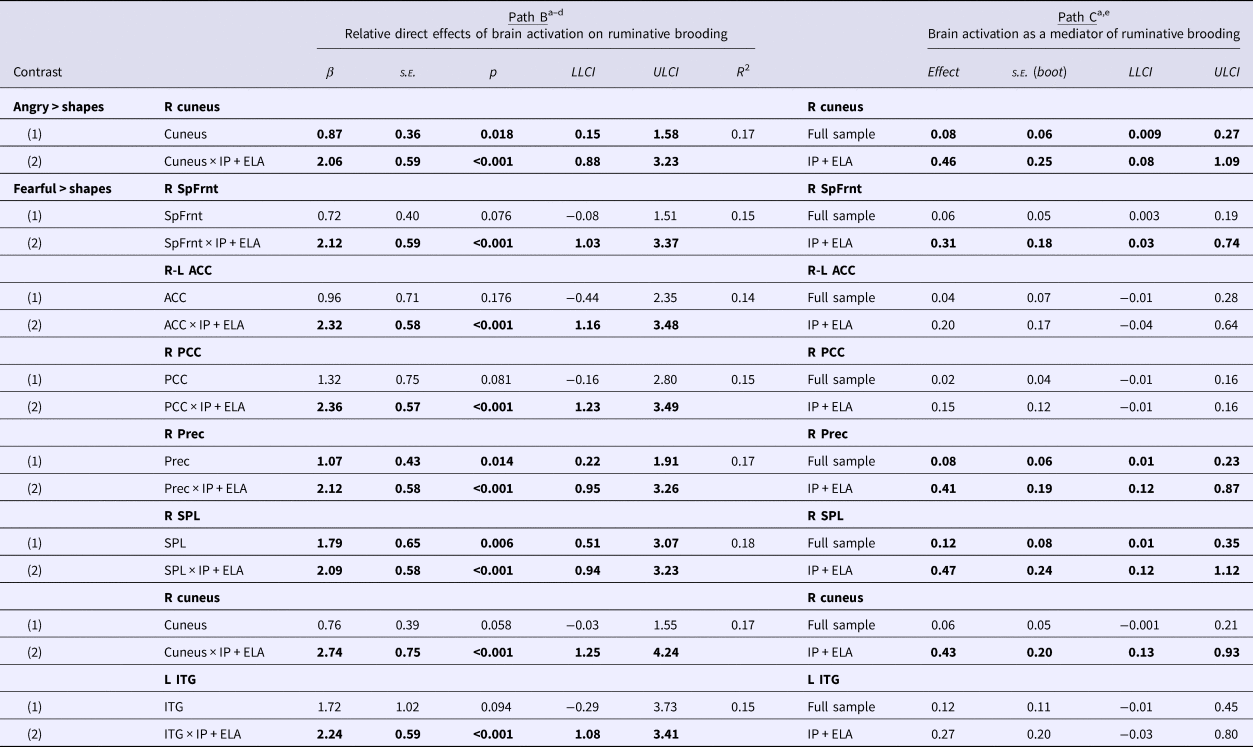
Table 3 reports BOLD response to emotional faces in relation to ruminative brooding (Path B: Illustratively, Fig. 2) and tests of brain activation to emotional faces as a mediator of ruminative brooding in IP + ELA participants (Path C: For a conceptual model, Fig. 3). Eight separate mediation models were conducted (one for angry > shapes, even for fearful > shapes).
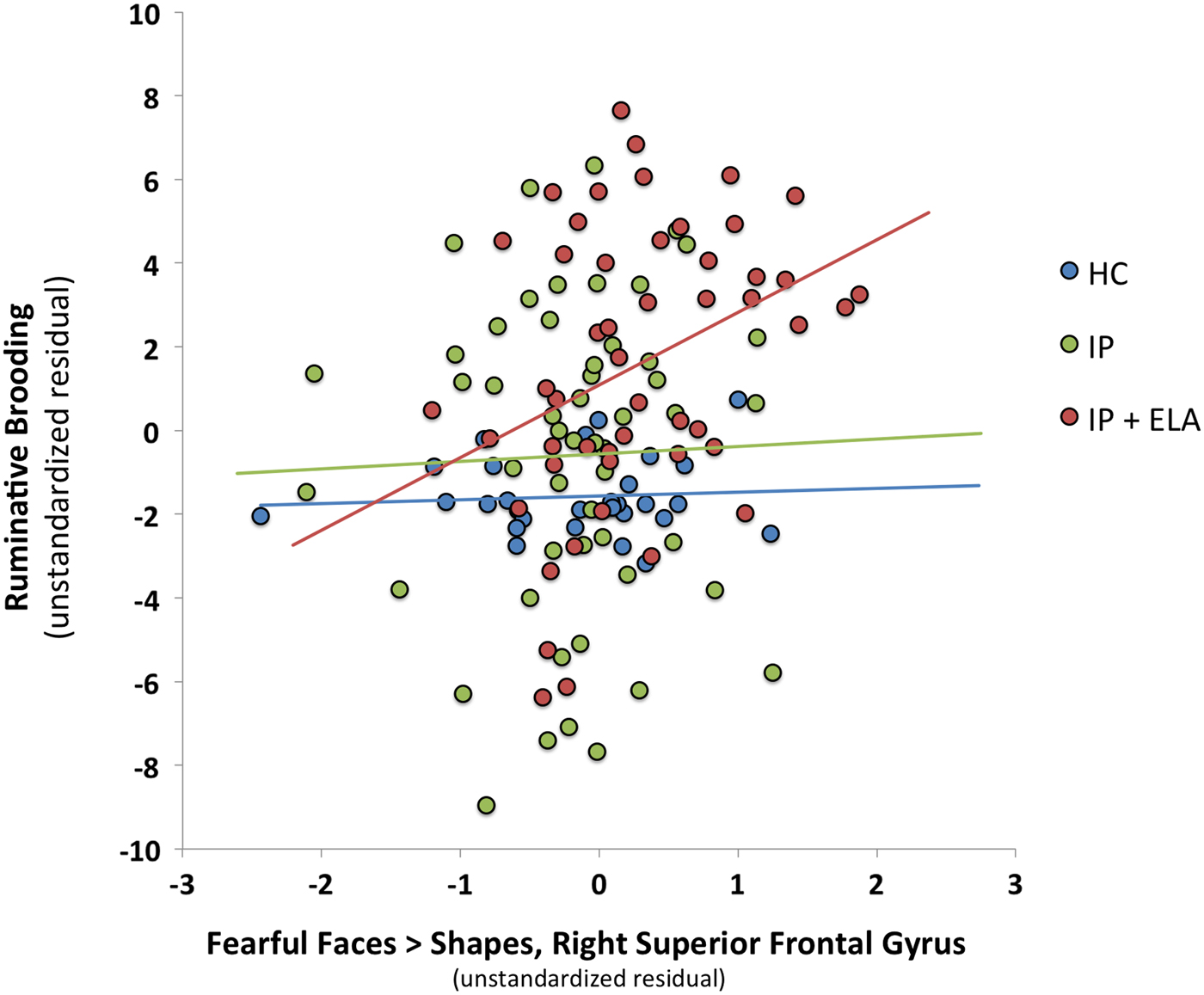
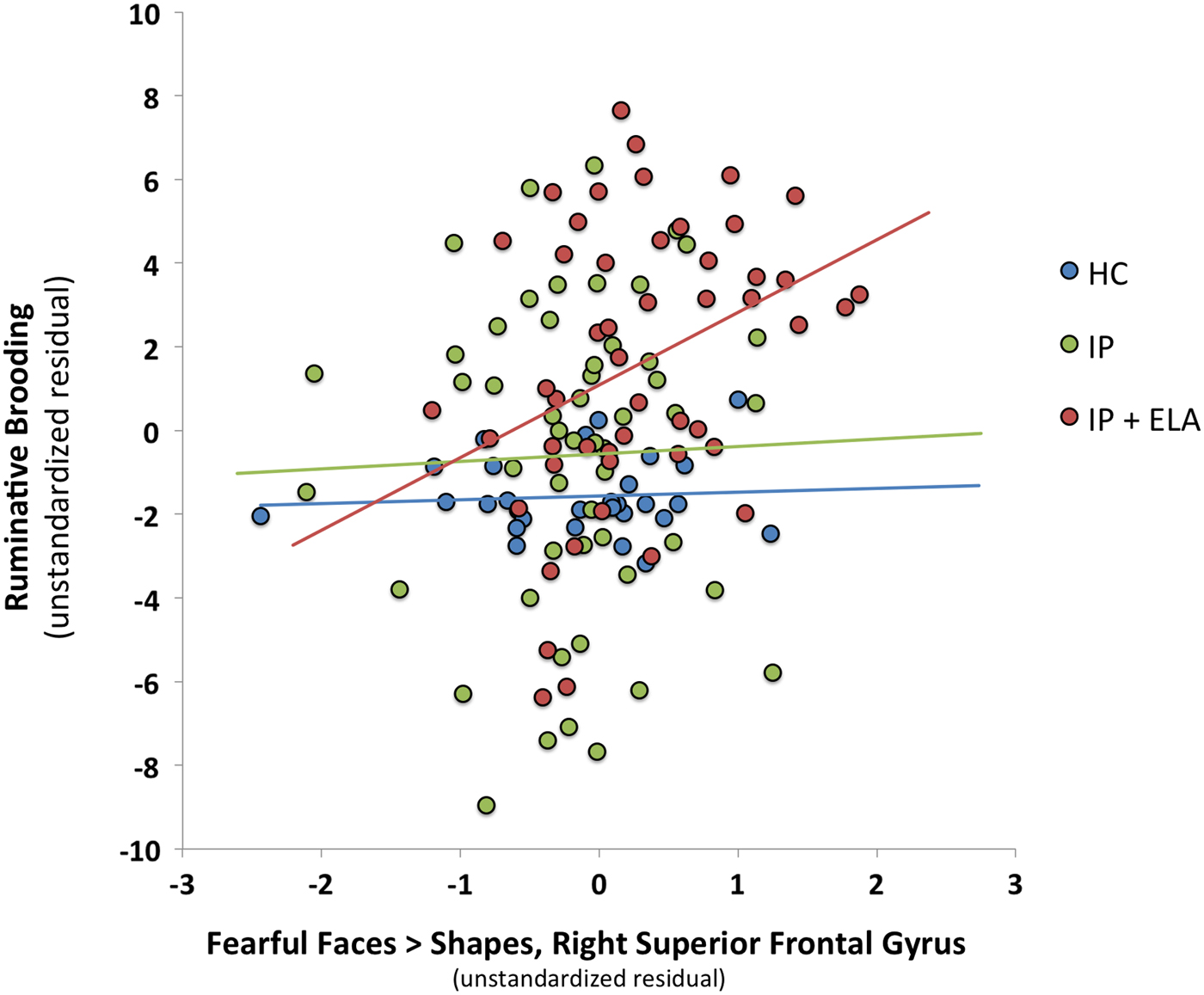
Fig. 2. Scatterplot of association between fear-related brain activation in the right superior frontal gyrus and ruminative brooding (as unstandardized residuals after controlling for covariates). HC, healthy control; IP, internalizing psychopathology patients; IP + ELA, internalizing psychopathology patients with early-life adversity history.
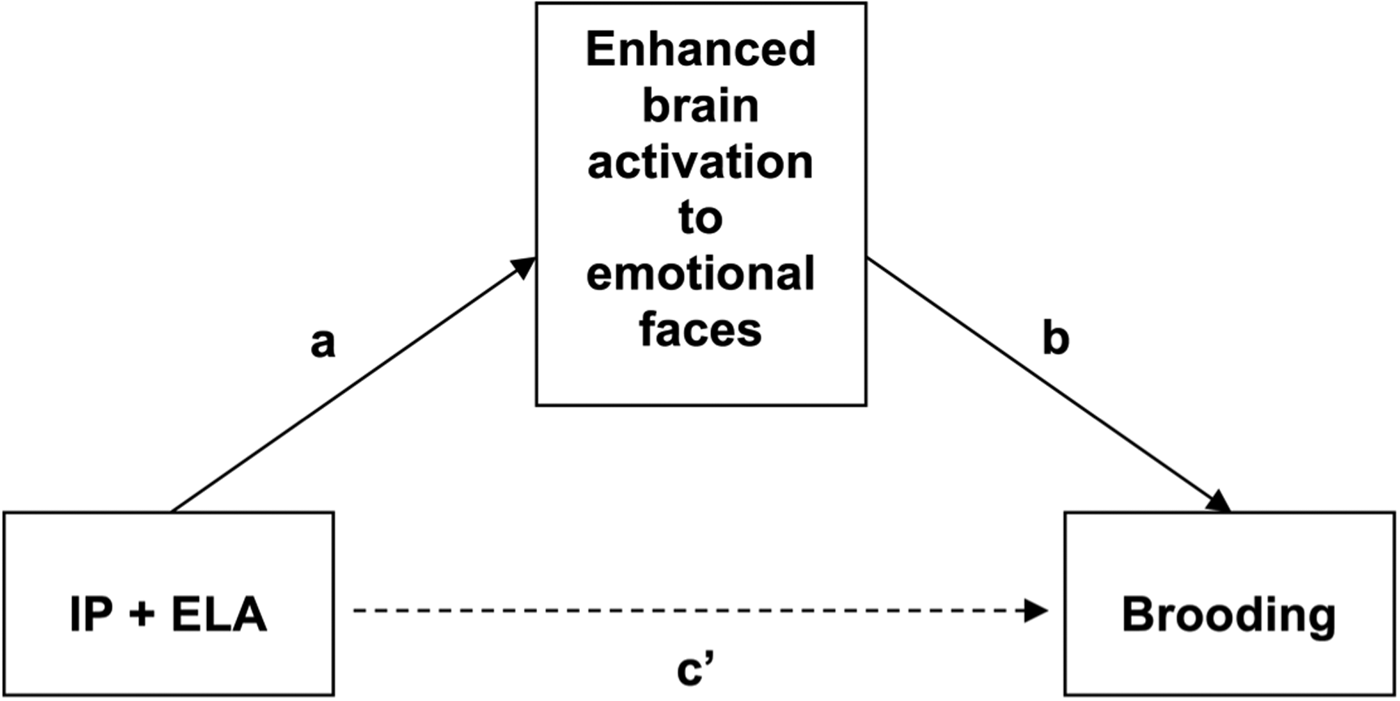
Fig. 3. Conceptual mediation model evaluating enhanced brain activation to emotional faces as a mediator of increased ruminative brooding in IP + ELA. IP + ELA, internalizing psychopathology patients with early-life adversity history.
Table 3. Mediation analyses evaluating brain activation to fearful and angry faces as a mechanism of increased rumination in IP + ELA
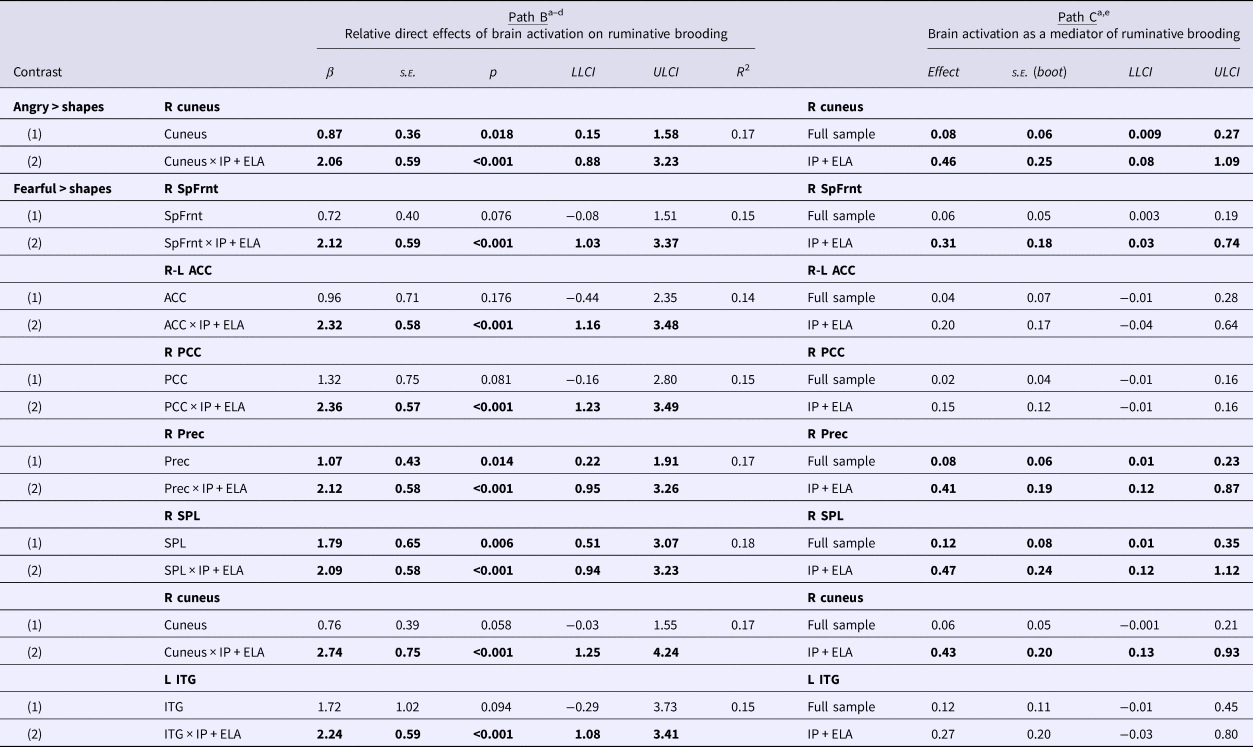
s.e., standard error; LLCI, lower limit confidence interval; ULCI, upper limit confidence interval; R, right; L, left; SpFrnt, superior frontal gyrus; ACC, anterior cingulate cortex; PCC, posterior cingulate cortex; Prec, precuneus; SPL, superior parietal lobule; ITG, inferior temporal gyrus.
Bolded text denotes significant results using a false-discovery rate-adjusted α threshold of p < 0.036.
a Covariates: age, sex, HAM-D, HAM-A.
b Estimates (1) association between extracted parameter estimates of brain activation and brooding across the entire sample and (2) association between extracted brain activation and brooding in IP + ELA, with HC + IP as a combined reference group. There are no significant associations between brain activation and brooding in IP alone with HC as the reference group.
c All correlations between extracted parameter estimates of brain activation and brooding are non-significant in HC.
d Exploratory, post-hoc analyses also evaluated whether brain activation to fearful and angry faces was significantly related to the other component of rumination, self-reflection; there were no significant associations.
e Estimates (1) mediation in the full sample and (2) mediation in IP + ELA relative to HC + IP. There are no significant tests of mediation in IP alone with HC as the reference group.
Angry > shapes
The indirect pathway (test of mediation) was significant in IP + ELA for the right cuneus; the total direct effect also remained significant indicating partial mediation.
Fearful > shapes
Indirect pathways were significant in IP + ELA for the right superior frontal gyrus, right precuneus, right superior parietal lobule, and right cuneus. Tests of mediation for the bilateral anterior cingulate, right posterior cingulate, and left inferior temporal gyrus did not yield significant indirect pathways in IP + ELA; the total direct effects also remained significant indicating partial mediation.
Discussion
Recent initiatives underscore the importance of examining neurobiological signatures that span diagnostic boundaries (Cuthbert, Reference Cuthbert2014, Reference Cuthbert2015), particularly those that might be amenable to early detection and prevention (Garvey et al., Reference Garvey, Avenevoli and Anderson2016), yet few studies have comprehensively accomplished this task. In this study, we utilized functional neural activation patterns to facial emotions across a large, heterogeneous, and clinically diverse sample of IP diagnoses with and without exposure to ELA and HCs, in effort to understand whether ELA is a correlate of aberrant neural activity relevant to behavioral constructs involved in IP. Specifically, we tested the hypothesis that enhanced neural activity in response to negative facial emotions would characterize IP + ELA and that these patterns of brain activity would, in turn, relate to brooding in IP + ELA. The present findings, which were most pronounced for fearful faces, implicate hyperactivation of superior frontal gyrus, cingulate cortex, inferior temporal gyrus, and the inferior and superior aspects of the parietal and occipital lobes, respectively, as a correlate of ELA in IPs. We speculate that ELA is candidate etiological mechanism of these neural alterations such that the observed brain changes may be due to the effects of ELA exposure on the brain. Moreover, no group differences were observed between HC and IP only, further implicating ELA exposure as a possible driver of corticolimbic hyperactivity to negative emotions; however, future studies are needed to demonstrate this amongst an ELA-exposed control group. Interestingly, the aforementioned brain regions were also consistently correlated with brooding rumination, and many of these neural regions mediated the link between IP + ELA and brooding. Taken together, these findings provide preliminary evidence that greater corticolimbic reactivity to negative affective stimuli may explain the relationship between ELA and rumination in IPs.
On the whole, we observed corticolimbic responsivity to especially fearful facial expressions, with some key regions that warrant attention. Namely, fearful faces elicited activation of the right middle anterior cingulate and superior frontal gyrus amongst IP + ELA. These findings are broadly in accordance with existing emotional regulatory theories implicating the anterior cingulate in the recognition, experience, and appraisal of emotional and the dorsolateral and medial prefrontal cortex in regulating emotional reactivity (Etkin et al., Reference Etkin, Egner and Kalisch2011). However, as there are some opposing findings regarding activation directionality in anxiety v. depression (MacNamara et al., Reference MacNamara, Klumpp, Kennedy, Langenecker and Phan2017), our design offers some confidence that enhanced anterior cingulate and dorsolateral prefrontal engagement may transcend internalizing diagnoses and symptoms when ELA is present. Additionally, and consistent with recent meta-analytic work (Heany et al., Reference Heany, Groenewold, Uhlmann, Dalvie, Stein and Brooks2018), we observed increased activation in the left inferior temporal gyrus, including the fusiform gyrus and extending to the parahippocampal gyrus in IP + ELA. This cluster of regions is typically activated by a broad array of social cognitive tasks requiring abstract reasoning and perspective taking (Schurz et al., Reference Schurz, Radua, Aichhorn, Richlan and Perner2014), implicating their involvement with an excessive focus on negative social judgment. Collectively, these results underscore convergent neurobiological alterations amongst IP + ELA related to emotion perception and social reasoning, particularly to fearful stimuli.
Processing of fearful faces was also associated with activation in the posterior cingulate, aspects of the medial prefrontal cortex, and superior parietal lobule amongst IP + ELA. Although these regions have not been extensively reported in relation to ELA amongst healthy samples (Dannlowski et al., Reference Dannlowski, Stuhrmann, Beutelmann, Zwanzger, Lenzen, Grotegerd, Domschke, Hohoff, Ohrmann, Bauer, Lindner, Postert, Konrad, Arolt, Heindel, Suslow and Kugel2012; Holz et al., Reference Holz, Buchmann, Boecker, Blomeyer, Baumeister, Wolf, Rietschel, Witt, Plichta, Meyer-Lindenberg, Banaschewski, Brandeis and Laucht2015), they are linked to rumination (Cooney et al., Reference Cooney, Joormann, Eugene, Dennis and Gotlib2010; Burkhouse et al., Reference Burkhouse, Jacobs, Peters, Ajilore, Watkins and Langenecker2017) in IPs. The observed correlations with rumination may suggest that individuals with ELA who develop IPs are particularly prone to engage in affect-congruent self-focus when faced with external cues of fear (Waters and Craske, Reference Waters and Craske2016). It is also noteworthy that ELA patients demonstrated greater activation in visual processing and somatosensory areas during fear (and to a lesser extent anger) processing, such as the cuneus and precuneus. Since IP + ELA were more likely to engage in ruminative brooding relative to IPs and HCs, rumination may also be a more elaborative process for these individuals, particularly when confronted with social signals of threat (Burkhouse et al., Reference Burkhouse, Jacobs, Peters, Ajilore, Watkins and Langenecker2017). That is, IP + ELA may be more likely to ruminate on social signals of threat more extensively and vividly, as supported by superior occipital and inferior parietal recruitment (Vuilleumier and Pourtois, Reference Vuilleumier and Pourtois2007). Indeed, these regions, in addition to the superior frontal gyrus, superior parietal lobule, partially mediated the extent of rumination present amongst IP + ELA patients. Notably, the observed patterns of brain activation accounted for 13–18% of the variance in rumination, consistent with a moderate-to-large effect size, suggesting that targeting this neurocircuitry amongst IP + ELA could have measureable effects on maladaptive coping styles known to increase proneness to or persistence of internalizing mental health problems (Kim et al., Reference Kim, Jin, Jung, Hahn and Lee2017). Of particular interest, in light of the emotional faces eliciting the neural response is to evaluate whether cognitive therapy emphasizing appraisals of other's emotional responses and interpretation bias, would have effects on these brain alterations. Additionally, another possibility would be to target regulatory neural circuits (e.g. cognitive control network) with brain stimulation (e.g. transcranial magnetic stimulation) as a top-down approach to reduce the observed corticolimbic reactivity to negative affective stimuli.
There are aspects of the present findings that were inconsistent with our hypotheses. Namely, in contrast to the expectation that IP + ELA would exhibit similar neural alterations to all negative facial emotions, the overwhelming pattern of results showed dominance for fearful faces; ELA-specific neural correlates were surprisingly sparse for angry faces. One possible reason for this discrepancy relates prior work implicating neural sensitivity to angry faces as a mediator of aggressive behavior (Shackman and Pollak, Reference Shackman and Pollak2014), of which base rates in this IP sample were very low. It may be the case that maltreated children who tend to respond to threat with reactive aggression are more likely to develop forms of externalizing psychopathology (Lee and Hoaken, Reference Lee and Hoaken2007) and hypervigilance to facial expressions of anger is therefore more relevant in that context. Equally, sensitivity to angry faces is particularly pronounced in victims of physical, as opposed to other forms of abuse (Pollak et al., Reference Pollak, Cicchetti, Hornung and Reed2000; Pollak and Tolley-Schell, Reference Pollak and Tolley-Schell2003). Although we were not powered to undertake within- and between-group comparisons of specific types of abuse and neglect, higher levels of emotional than physical maltreatment characterized the current sample and the emotion -specificity of our findings may reflect this variability. On the other hand, we must also consider that corticolimbic hyper-responsivity to angry faces has nevertheless been reported in both depression and anxiety disorders (MacNamara et al., Reference MacNamara, Klumpp, Kennedy, Langenecker and Phan2017); this may be a broader marker of psychological distress that simply is not a specific etiological pathway related to ELA.
Additionally, it was also somewhat surprising that activation in the amygdala was not enhanced in ELA. Amygdala hyperactivity has been reported in association with ELA (Teicher et al., Reference Teicher, Andersen, Polcari, Anderson and Navalta2002; McCrory et al., Reference McCrory, De Brito and Viding2012) and in the pathophysiology of IPs (Shin and Liberzon, Reference Shin and Liberzon2010; Heller, Reference Heller2016); nonetheless, many of these studies are small, single-disorder case–control designs (Hein and Monk, Reference Hein and Monk2017; Heany et al., Reference Heany, Groenewold, Uhlmann, Dalvie, Stein and Brooks2018). In fact, whole-brain evidence in support of amygdala hyperactivity is actually somewhat inconsistent, identified in certain meta-analyses (Hein and Monk, Reference Hein and Monk2017), but not others (Hein and Monk, Reference Hein and Monk2017; Heany et al., Reference Heany, Groenewold, Uhlmann, Dalvie, Stein and Brooks2018). Both recent meta-analyses identified increased amygdala activation using a region of analysis (ROI) approach, lending credence to the need for small-volume correction of this small anatomical region. However, an ROI-driven approach to threshold results can also increase the likelihood of identifying significant areas of activation; thus, amygdala activation may be preferentially present in experiments that opt to report ROI v. whole-brain analyses. Alternatively, as there is great heterogeneity in social-affective stimuli and tasks reporting amygdala hyperactivation in ELA and IPs (Etkin and Wager, Reference Etkin and Wager2007; Hamilton et al., Reference Hamilton, Etkin, Furman, Lemus, Johnson and Gotlib2012; Gentili et al., Reference Gentili, Cristea, Angstadt, Klumpp, Tozzi, Phan and Pietrini2016; Heany et al., Reference Heany, Groenewold, Uhlmann, Dalvie, Stein and Brooks2018), we also consider the possibility that faces are less evocative, depersonalized elicitors of emotion for some patients. For instance, facial expressions of others are likely to have less emotional significance compared with a personal narrative of adversity experiences (MacNamara et al., Reference MacNamara, Klumpp, Kennedy, Langenecker and Phan2017). Particularly in the case of ELA-exposed IPs, recruitment of the amygdala for emotional learning of facial expressions could be superseded by the salience of ongoing personal fear memories (Clark and Mackay, Reference Clark and Mackay2015).
We are also cautious not to over interpret null findings for the amygdala, as amygdala activation in response to the emotional stimuli in this task was present, but across all participants and not specific to the IP or IP + ELA groups (online Supplemental Fig. S1). Likewise, it is also noteworthy that IP and HC groups did not demonstrate other differences in brain activation. One explanation relates to the fact that most case–control designs have compared single disorders to controls rather than a heterogeneous group of different internalizing diagnoses. Accordingly, the lack of group differences identified could simply represent there are not shared neural alterations across different anxiety disorders and depression diagnoses relative to controls. Alternatively, as demonstrated in our prior work (MacNamara et al., Reference MacNamara, Klumpp, Kennedy, Langenecker and Phan2017) and in line with the RDoC initiative, transdiagnostic neural alterations may be more likely to covary with depression and anxiety symptoms dimensionally, rather than discrete diagnostic categories.
There were several limitations to the current study. First, ELA assessment was retrospective by means of a self-report measure. This kind of reporting could be subject to inaccuracies or mood congruent recall, which might be particularly relevant in IP + ELA (Gaddy and Ingram, Reference Gaddy and Ingram2014; Ono et al., Reference Ono, Devilly and Shum2016; Schonfeld and Ehlers, Reference Schonfeld and Ehlers2017). Second, while the present analysis benefited from an HC comparison and IP reference group without ELA equivalent in anxiety and depression symptoms, this design could be further strengthened by inclusion of an ELA-exposed HC group. That is, although ELA substantially increases the risk for IPs, not all individuals exposed to ELA develop IPs and this kind of comparison could elucidate key determinants of adaptive coping and resilience (Kim-Cohen and Turkewitz, Reference Kim-Cohen and Turkewitz2012; Bowes and Jaffee, Reference Bowes and Jaffee2013). Moreover, there may also be unique markers involved in the propensity for ELA to develop into other forms of psychopathology, such as substance use (Puetz and McCrory, Reference Puetz and McCrory2015) or externalizing disorders (Busso et al., Reference Busso, McLaughlin and Sheridan2017), that were not the emphasis of the current study. A third limitation is that our IP sample, and particularly the IP + ELA group, constituted a greater proportion of female participants relative to controls. Although this is reflective of the sex differences that characterizes sensitivity to stress and prevalence of internalizing disorders (Bekhbat and Neigh, Reference Bekhbat and Neigh2018), these group differences called for careful post-hoc analysis to ensure sex effects did not drive our significant findings (footnote, Table 2). The null results of such post-hoc analyses notwithstanding, additional confidence in these findings would be procured in a design matched on sex across groups. Additionally, the patients in this study were seeking treatment and we do not know if these findings are generalizable to what might be observed in naturalistic, community settings. Last, the present study was cross-sectional; therefore, the results are correlational in nature and we are unable to draw definitive conclusions regarding causal effects of ELA on brain function or how any such effects may influence susceptibility to rumination. Although the analytic approach of the current study was informed by theory and prior evidence (Hart and Rubia, Reference Hart and Rubia2012; Heany et al., Reference Heany, Groenewold, Uhlmann, Dalvie, Stein and Brooks2018), they are hypothesis generating and future longitudinal designs are needed to establish temporal precedence of these constructs.
To close, this study provides evidence supporting a neural mechanism (i.e. corticolimbic hyper-responsivity) linking ELA to rumination amongst adults with current IPs. Findings may suggest that for individuals experiencing childhood adversity, enhanced corticolimbic reactivity to negative socio-emotional stimuli may enhance vulnerability to psychological characteristics involved in the maintenance of internalizing symptoms, such as rumination. Therefore, targeting aberrant emotion neurocircuitry in IP + ELA, possibly throughout psychological interventions, could be a novel target for modifying ruminative habits known to increase persistence of internalizing mental health problems. These results also help to close the gap between the segregated sampling designs of earlier studies (Hart and Rubia, Reference Hart and Rubia2012; Heany et al., Reference Heany, Groenewold, Uhlmann, Dalvie, Stein and Brooks2018), affirming that ELA is associated with transdiagnostic alterations to corticolimbic emotional processing, above and beyond what can be accounted for by current internalizing symptoms. This is an important step toward identifying a common pathway to prevalent and co-occurring forms of IP in adulthood. We hope that the work herein will provide further impetus for the assessment of longitudinal, developmental trajectories of risk and resilience to adversity within systems neuroscience models; it is these models that are most likely to bring the field closer to early detection of etiological factors involved in IP across the lifespan.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291718003203
Acknowledgements
None.
Financial support
This work was supported by the National Institute of Mental Health (R01-MH101497-04) to KLP. ATP is supported by the National Institute of Mental Health T32 MH112485. KLB is supported by the National Institute of Mental Health Grant K23-MH113793-01.
Conflict of interest
None.