Introduction
Over recent years researchers have made a concerted effort to identify factors that could modify the risk of dementia in older age. Physical inactivity, diabetes, midlife obesity and hypertension, smoking and limited education have all been recognised as potential targets (Norton et al. Reference Norton, Matthews, Barnes, Yaffe and Brayne2014), although systematic attempts to address these risk factors have had only modest success (Lautenschlager et al. Reference Lautenschlager, Cox, Flicker, Foster, van Bockxmeer and Xiao2008; Ngandu et al. Reference Ngandu, Lehtisalo, Solomon, Levalahti, Ahtiluoto and Antikainen2015; Moll van Charante et al. Reference Moll van Charante, Richard, Eurelings, van Dalen, Ligthart and van Bussel2016). Nonetheless, reports of a decline in age-specific incidence and prevalence rates of dementia are some regions, concurrent with improvements in living conditions and healthcare, suggest that dementia may be preventable (Wu et al. Reference Wu, Beiser, Breteler, Fratiglioni, Helmer and Hendrie2017). More recently, clinical conditions such as depression, bipolar disorder and hearing loss have been associated with an increase in the risk of dementia (Almeida et al. Reference Almeida, McCaul, Hankey, Yeap, Golledge and Flicker2016; Livingston et al. Reference Livingston, Sommerlad, Orgeta, Costafreda, Huntley and Ames2017), but at this stage it remains unclear whether their successful management would reduce the incidence of dementia in later life (Almeida et al. Reference Almeida, Hankey, Yeap, Golledge and Flicker2017; Kessing et al. Reference Kessing, Gerds, Knudsen, Jorgensen, Kristiansen and Voutchkova2017).
Psychotic disorders, such as schizophrenia and delusional disorder, are also thought to confer greater risk of cognitive and functional impairment (Almeida et al. Reference Almeida, Howard, Levy, David, Morris and Sahakian1995; Heinrichs & Zakzanis, Reference Heinrichs and Zakzanis1998; Harvey et al. Reference Harvey, Silverman, Mohs, Parrella, White and Powchik1999). However, it is difficult to draw firm conclusions from available data, as most of the evidence has been derived from small case-series, cross-sectional studies or brief follow-up surveys that are difficult to generalise to the entire population. A decade ago, a systematic review of 53 longitudinal studies that examined the course of adults with schizophrenia showed widespread deficits in cognitive performance, but inconsistent evidence of progressive decline (Szoke et al. Reference Szoke, Trandafir, Dupont, Meary, Schurhoff and Leboyer2008). Although the cognitive deficits afflicting people with schizophrenia-like psychosis seem to meet criteria for the clinical diagnosis of dementia in later life (de Vries et al. Reference de Vries, Honer, Kemp and McKenna2001), it is not known whether they are caused by pathologies that commonly cause dementia (e.g. Alzheimer's disease, vascular disease) or whether they are caused by the disease underpinning the psychosis or its associated behaviours and treatments (Chung et al. Reference Chung, Nakajima, Plitman, Iwata, Uy and Gerretsen2016).
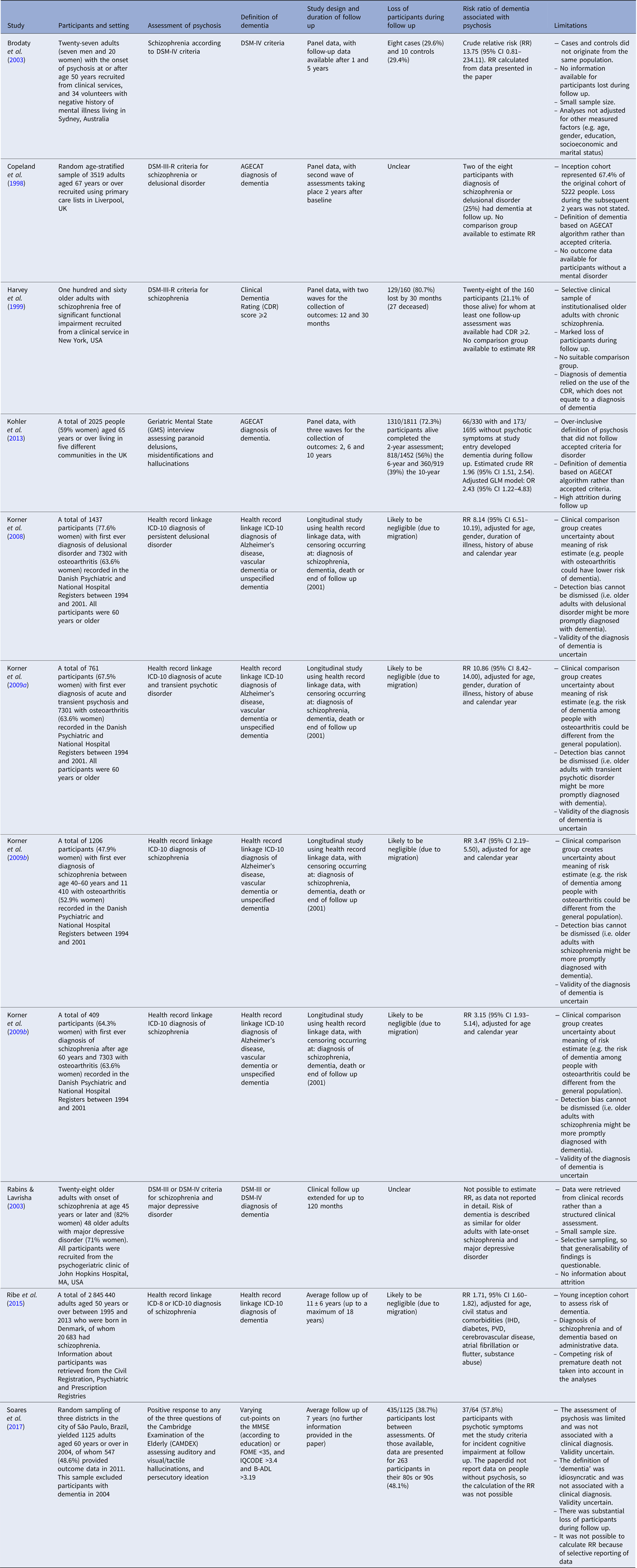
We aimed to explore the association between psychotic disorders (including schizophrenia) and risk of incident dementia by first undertaking a systematic review of longitudinal studies that have been designed to investigate the association. Details about the search strategy and inclusion of studies appear in the online supplementary material. Table 1 summarises the key features of the 11 longitudinal studies that we identified (two studies were reported in the same paper) (Copeland et al. Reference Copeland, Dewey, Scott, Gilmore, Larkin and Cleave1998; Harvey et al. Reference Harvey, Silverman, Mohs, Parrella, White and Powchik1999; Brodaty et al. Reference Brodaty, Sachdev, Koschera, Monk and Cullen2003; Rabins & Lavrisha, Reference Rabins and Lavrisha2003; Korner et al. Reference Korner, Lopez, Lauritzen, Andersen and Kessing2008; Reference Korner, Lopez, Lauritzen, Andersen and Kessing2009a, Reference Korner, Lopez, Lauritzen, Andersen and Kessingb; Kohler et al. Reference Kohler, Allardyce, Verhey, McKeith, Matthews and Brayne2013; Ribe et al. Reference Ribe, Laursen, Charles, Katon, Fenger-Gron and Davydow2015; Soares et al. Reference Soares, Dos Santos, Bottino and Elkis2017). Risk ratio information could be retrieved from seven studies, all of which indicated that dementia was more frequent among people with than without psychotic disorders (Brodaty et al. Reference Brodaty, Sachdev, Koschera, Monk and Cullen2003; Korner et al. Reference Korner, Lopez, Lauritzen, Andersen and Kessing2008; Reference Korner, Lopez, Lauritzen, Andersen and Kessing2009a, Reference Korner, Lopez, Lauritzen, Andersen and Kessingb; Kohler et al. Reference Kohler, Allardyce, Verhey, McKeith, Matthews and Brayne2013; Ribe et al. Reference Ribe, Laursen, Charles, Katon, Fenger-Gron and Davydow2015). However, there was marked heterogeneity among the studies (I 2 = 93.7%), which precluded a meaningful formal meta-analysis of the published tabulated data. Furthermore, methodological limitations outlined in Table 1 potentially compromised the validity of the findings. In particular, the two population-based studies did not adjust their results for death as a competing risk (Kohler et al. Reference Kohler, Allardyce, Verhey, McKeith, Matthews and Brayne2013; Ribe et al. Reference Ribe, Laursen, Charles, Katon, Fenger-Gron and Davydow2015).
Table 1. Summary of published longitudinal studies of older adults investigating the association between psychosis and dementia
Given the constraints of currently available evidence, we concluded that the association between psychotic disorders and dementia is yet to be established. We designed the present study to investigate if the presence of a psychotic disorder increased the risk of incident dementia in later life. We hypothesised that the risk of prevalent and incident dementia would be higher among older men with than without a psychotic disorder and that this association would not be due to differences in age, sex or concurrent medical morbidities, hearing loss, depressive and bipolar disorders, and alcohol use disorders. In addition, we predicted that this increase in risk would not be attributable to differential mortality or censoring.
Methods
Study design and setting
Longitudinal cohort study of a community representative sample of older men living in the Perth metropolitan region of Western Australia. Participants were identified and recruited between 1996 and 1998, and followed prospectively until they received the diagnosis of dementia, died or reached 31 December 2013 alive and free of dementia.
Participants
We used the Australian Electoral Roll to identify all men aged 65–85 years living in the Perth metropolitan region in 1996–1998 (enrolment for voting in mandatory for all Australian citizens aged 18 years or older). We retrieved the contact details for 49 801 men, of whom 1839 had died by the time the study started and another 9482 were excluded because they were living outside the study region. Of the remaining 38 480 men, 307 were excluded because they were younger than 65 years (these men were invited in error), leaving a total study sample of 38 173 older men.
The Ethics Committees of the University of Western Australia and of the Department of Health of Western Australia approved the study procedures. In addition, the Legal Data Custodian of Western Australia has oversight of the study to ensure that all data are de-identified at the time of analysis and used for the purposes of the approved research protocol only. The Legal Data Custodian is also responsible for granting access to the data to the named investigators alone in order to safeguard the confidentiality of participants.
Outcome measures
Dementia (from any cause) was the primary outcome of interest of the study. We used the Western Australian Data Linkage System (WADLS) to retrieve relevant clinical information about participants. Briefly, WADLS links health service data from inpatient and outpatient mental health services, hospital morbidity data, community aged care services, as well as cancer and death registries (Holman et al. Reference Holman, Bass, Rosman, Smith, Semmens and Glasson2008). WADLS uses the International Classification of Diseases (ICD) system for the coding of clinical diagnoses and procedures: ICD-8 from 1 January 1966 to 31 December 1969, ICD-9 from 1 January 1970 to 30 June 1999 and ICD-10 from the 1 July 1999. WADLS records also show the date when the occasion of service started and finished, and allows for the logging of multiple diagnoses for each contact.
We used the following codes to establish the diagnosis of dementia among participants: ICD-8 code 290; ICD-9 codes 290, 294.1, 294.2, 331.0, 331.1, 331.2, 331.82; ICD-10 codes F00-F03, G30, G31.0, G31.1, G31.83. As indicated before, men with a diagnosis of dementia prior to the date of enrolment (1996–1998) were excluded from the follow-up study.
Exposure: psychotic disorders
The diagnosis of psychotic disorder was coded according to the ICD, as outlined above, and included: schizophrenia, persistent delusional disorders, acute and transient psychotic disorders, schizoaffective disorders, other non-organic psychotic disorders, unspecified non-organic psychotic disorders. These were the ICD codes used: ICD-8 and ICD-9 codes 295 and 297, and ICD-10 codes F20, F22, F23, F25, F28 and F29.
Other study measures
We calculated the age of participants (in years) by subtracting the date of birth from the date of enrolment into the study. We also retrieved from WADLS data on cardiovascular events, cancers (except skin cancer), chronic respiratory diseases, gastrointestinal and renal diseases, hearing loss, depressive and bipolar disorders, and alcohol use disorder using the following codes:
– Cardiovascular diseases ICD-8 and 9 codes 390–398, 401, 402, 403, 404, 410-429, 430–434, 436–438, 440–448, and ICD-10 codes I00–09, I10, I11, I12, I13, I20–51, I60–69, I70–78;
– Cancers ICD-8 and ICD-9 codes 140–209, and ICD-10 codes C00–C97;
– Respiratory diseases ICD-8 and ICD-9 codes 490–496 and 507–519, and ICD-10 codes J00–09, J20–39, J40–47 and J60–99;
– Gastrointestinal diseases ICD-8 and ICD-9 codes 520–537, 540–543, 550–553, 555–589, and ICD-10 codes K00–K99;
– Renal diseases ICD-8 and ICD-9 codes 580-589, and ICD-10 codes N00-07, N17-19 and N25-27;
– Hearing loss ICD-8 and ICD-9 codes 388.12, 388.2 and 389, and ICD-10 codes H90 and H91;
– Depressive disorder ICD-8 and ICD-9 codes 296.2, 296.3, 300.4 and 311, and ICD-10 codes F32, F33, F34.1 and F38.10;
– Bipolar disorder ICD-8 and ICD-9 codes 296.0, 296.1, 296.4–7 and 296.80–81, and ICD-10 codes F30 and F31;
– Alcohol use disorder ICD-8 and ICD-9 codes 291, 303 and 305.0, and ICD-10 code F10.
Statistical analyses
We used the statistical software Stata 15.0 to manage and analyse the data. Descriptive statistics summarised categorical variables as count and proportions (%), and continuous variables as mean, range and standard deviation of the mean (s.d.). We employed t tests to compare the age of participants with and without a psychotic disorder, and reported the t-statistic, the number of degrees of freedom (df) and p value. We used logistic regression to investigate the odds of dementia in men with compared with those without psychosis, and Cox regression (Breslow method) to investigate the hazard ratio (HR) of dementia during follow up. In these models, we split and joined time-span sets according to the diagnosis of psychosis, so that men without psychosis contributed data as controls until the time of diagnosis and as cases thereafter. We used age as the time scale in the Cox regression models in order to control as accurately as possible the effect of age on psychosis and mortality (Breslow et al. Reference Breslow, Lubin, Marek and Langholz1983). In addition, we investigated and modelled the potential contribution of other measured factors on the risk estimates of dementia associated with psychosis (i.e. prevalent cardiovascular diseases, cancer, chronic respiratory diseases, digestive and renal diseases, hearing loss, depressive or bipolar disorder, alcohol use disorder). Finally, we used competing risk regression, with death entered in the models as a competing risk (the risk estimate is expressed as sub-HR), and the Mantel–Haenszel method to calculate the rate ratios (RR). Alpha was set at 5% and all risk estimates were reported alongside their respective 95% confidence interval (95% CI).
Results
Among the 38 173 older men who entered the study, 435 (1.1%) had a recorded history of non-organic psychotic disorder. Men with and without a psychotic disorder had similar mean ± s.d. age (72.5 ± 4.6 v. 72.5 ± 4.6, t = 0.51, df = 38 171, p = 0.812). Table 2 shows the clinical characteristics of participants at the time of enrolment. There was an excess of chronic respiratory and gastrointestinal diseases among men with psychosis, as well as greater prevalence of hearing loss, alcohol use disorder and depressive and bipolar disorders. The odds ratio (OR) of dementia was greater for men with than without psychotic disorders: 3.42 (95% CI 2.23–5.25) – adjusted for concurrent respiratory and gastrointestinal diseases, as well as hearing loss, depressive and bipolar disorders and alcohol use disorder. Online Supplementary Table S1 shows the associations between clinical measures and dementia at study entry.
Table 2. Clinical characteristics of the population and of men with a recorded history of psychotic disorder at study entry

95% CI, 95% confidence interval of the odds ratio.
Cardiovascular diseases: recorded medical history of myocardial infarction, angina or stroke.
a Odds ratio adjusted for statistically significant concurrent morbidities: 3.42 (2.23–5.25).
The longitudinal study included the 37 770 men who were free of dementia at the start of the follow-up period. During the subsequent 17.7 years, 8068 (21.4%) men developed dementia and 23 999 (63.5%) died. Ninety men experienced an incident psychotic disorder during follow up (in the absence of dementia). The HR of dementia among men with a prevalent psychotic disorder at the start of the follow-up period was 2.57 (95% CI 2.23–2.96) – adjusted for prevalent cardiovascular, respiratory, gastrointestinal and renal diseases, cancer, and hearing loss, depressive and bipolar disorders, and alcohol use disorder. The adjusted RR of dementia for those with compared with those without a psychotic disorder was 2.38 (95% CI 2.07–2.74).
We re-ran our analyses after taking into account incident cases of psychotic disorder recorded during follow up before the diagnosis of dementia, death or end of the study – the adjusted HR of dementia was 2.73 (95% CI 2.39–3.11) and the RR 2.61 (95% CI 2.28–2.99). In addition, we completed a competing risk regression analysis using death as the competing risk – the adjusted sub-hazard ratio (SHR) of dementia associated with psychotic disorders (including incident cases) was 2.67 (95% CI 2.30–3.09). We then completed a sensitive analysis considering the cases of incident psychosis (i.e. those with the onset of symptoms after the start of the follow up) as prevalent cases. The rationale, in this case, was that the same pathophysiological mechanisms could be contributing to the development of both psychosis and dementia. The adjusted HR of dementia associated with psychosis was 2.61 (95% CI 2.07–3.28).
We then calculated the time (in years) elapsed between the diagnosis of a psychotic disorder and dementia, death or the end of the study, and distributed men with psychosis into three groups: those who had lived with a psychotic disorder for <5 years, 5–10 years and 10 years or more. Figure 1 shows the proportion of participants who survived free of dementia during the 17.7 years of follow up according to the time lived with a psychotic disorder. Men with a recent history of psychosis had the highest adjusted HR of dementia, followed by older men who had lived with a psychotic disorder between 5 and 10 years and those with ⩾10 years since diagnosis (Table 3). The adjusted HR of dementia was higher in the group with illness duration <5 years (HR 2.07, 95% CI 1.29–3.31) and 5–10 years (HR 1.71, 95% CI 1.04–2.81) compared with those with illness duration ⩾10 years. Similarly, we calculated the age (in years) of participants at the time of the first ever recorded diagnosis of a psychotic disorder by subtracting the date of birth from the date of diagnosis and dividing the result by 365.25. The risk ratios according to age of onset (65 years cut-point) appear in Table 3. Figure 2 shows the age at the time of the diagnosis of dementia for participants with and without a recorded diagnosis of psychotic disorder, and Table 3 the HR according to the presence of a psychotic disorder, time lived with psychosis and age at the time of onset of the psychotic disorder.

Fig. 1. The figure depicts the proportion of men who remained alive and free of dementia over a follow-up period of up to 17.7 years. Compared with men without a recorded diagnosis of psychotic disorder, the hazard ratio (HR) of dementia varied according to the duration of the disorder: <5 years (HR 4.78, 95% CI 3.05, 7.50), 5–10 years (HR 3.96, 95% CI 2.46–6.38) and ⩾10 years (HR 2.31, 95% CI 2.01–2.67). The regression model was adjusted for prevalent cardiovascular, respiratory, renal and gastrointestinal diseases, as well as hearing loss, depressive and bipolar disorders and alcohol use disorder. The risk table includes prevalent and incident cases of psychosis (see ‘Statistical analyses’ section).

Fig. 2. The figure depicts the age of participants at the time of the diagnosis of dementia in older men with (right panel) and without (left panel) a recorded history of a psychotic disorder. The vertical axis shows the cumulative proportion of those who developed dementia during follow up (combined for men with and without a psychotic disorder).
Table 3. Risk ratio of dementia associated with psychosis, duration of the psychotic disorder and age at onset

HR, hazard ratio; SHR, sub-hazard ratio (death used as a competing risk); 95% CI, 95% confidence interval of the ratio. The reference group consisted of men with no recorded history of psychotic disorder
Adjusted for age (in years) and prevalent cardiovascular diseases, cancer, chronic respiratory diseases, gastrointestinal and renal diseases, hearing loss, alcohol use disorders, depressive and bipolar disorder
Finally, we limited our analyses of the exposure to men with a diagnosis of schizophrenia (rather than the broader syndromic diagnosis of psychotic disorder). The crude HR of dementia associated with the diagnosis of schizophrenia (n = 180) was 3.26 (95% CI 2.58–4.13) and the adjusted HR 2.61 (95% CI 2.05–3.31) (adjusted for age, cardiovascular diseases, cancer, chronic respiratory diseases, gastrointestinal and renal diseases, hearing loss, alcohol use disorders, depressive and bipolar disorder).
Discussion
The results of this 18-year longitudinal study of 37 770 dementia-free older men showed that the RR of incident dementia was more than twice as large among those who had received a diagnosis of psychotic disorder at baseline or were diagnosed with a psychotic disorder during follow-up. The association between psychosis and incident dementia could not be attributed to concurrent medical morbidities (including other mental health and alcohol use disorders), hearing loss or premature death. In addition, the number of years lived with a psychotic disorder seemed to have an inverse relationship with the risk of dementia, with the risk being greatest among those with illness onset <5 years, intermediate for 5–10 years, and slightly smaller for those who had lived with psychosis for 10 years or more.
Before discussing the implications of these results, we will consider the strengths and limitations of our study. We recruited a large community-representative sample of older Western Australians, which comprised a population at risk of dementia because of their age. The follow-up period was long and this facilitated the accrual of a sufficiently large number of incident cases of dementia, which in turn allowed us to calculate precise risk estimates associated with a psychotic disorder. Moreover, the use of WADLS enabled us to adjust our risk estimates for other measures commonly associated with increased risk of dementia, such as age, diabetes, cardiovascular disease, hearing loss and other mental disorders. These adjustments showed that the association between the diagnosis of a psychotic disorder and incident dementia was robust. Another common problem with longitudinal studies is the non-random loss of participants during follow up, although the use of WADLS allowed us to minimise the risk for this type of bias, as we were able to keep track of all deaths and because the migratory movement of older adults away from the state of Western Australia is negligible. Finally, and in contrast with previous reports (Table 1), the approach we used to analyse our data took into account incident cases of psychosis that occurred during follow up and considered death as a competing risk – this offered a more complete and accurate appraisal of the role that psychotic disorders might have in increasing the risk of dementia in older age.
A related issue to consider is the age of our cohort at the start of the follow-up period. As schizophrenia is associated with increased mortality, a considerable number of people with schizophrenia may have died before reaching the older age (i.e. they would not have been available for this study). Consequently, survivors with a diagnosis of psychotic disorder may not necessarily represent well the population of adults living with schizophrenia in the community, so that it would not be appropriate to conclude from our findings alone that schizophrenia itself increases the risk of dementia.
This study involved only older men – while this allowed us to circumvent the issue of confounding due to gender, it limits our ability to generalise our results to women. We also acknowledge that our study measures were derived from electronic health records and not a structured clinical assessment. There is empirical evidence that WADLS identifies psychotic disorders, such as schizophrenia, accurately (Jablensky et al. Reference Jablensky, Morgan, Zubrick, Bower and Yellachich2005), but we accept that administrative health measures cannot be seen as ‘gold standard’. Consequently, the residual error could partly account for our results, although this effect would have to be rather large in order to explain away our findings. Of note, we chose to group participants under the general umbrella of ‘psychotic disorders’ because of the instability of the diagnosis of schizophrenia and schizoaffective disorders over time. For example, Chen et al. (Reference Chen, Swann and Burt1996) reported the results of a 7-year follow-up study showing that 22% of the 256 participants with a diagnosis of schizophrenia received a different diagnosis during a subsequent contact with mental health services. In addition, 33% of the 680 adults with a mental health diagnosis other than schizophrenia at the start of follow up later received a diagnosis of schizophrenia. Moreover, limiting our analyses to schizophrenia did not change the risk estimates of dementia substantially.
Confounding due to unmeasured factors, such as apolipoprotein E (Apo E) ε4 allele genotype or limited education (Jones et al. Reference Jones, Rodgers, Murray and Marmot1994), is likely to have influenced some of the effects observed but their impact would have had to be unusually strong to dismiss our observed association between psychotic disorders and incident dementia. Finally, while our study design enabled us to minimise the risk of bias due to differential loss to follow up, it could have introduced some detection bias. People with psychotic disorders utilise health services more frequently than the general population (Carr et al. Reference Carr, Johnston, Lewin, Rajkumar, Carter and Issakidis2003), thereby increasing the opportunity for a diagnosis of dementia to be established. In that case, an artificial association between psychotic disorders and dementia could arise. We are unable to dismiss this possibility outright but believe our data suggest that this is unlikely to be a satisfactory explanation for our findings. Detection bias would be expected to bring forward the diagnosis of dementia among people with psychotic disorders, which would lower the age at the time of diagnosis and shift the curve of onset of dementia to the left (i.e. to an earlier age of onset). We found no evidence of this (Fig. 2). In fact, our data suggest that premature mortality may be responsible for any age-difference that may exist at the time of the diagnosis of dementia between people with and without psychosis (few people with psychotic disorders reach the late 1980s or 1990s, so all but no people with psychosis will develop dementia at an advanced age).
After considering the various methodological aspects of the study, and acknowledging the potential for systematic error due to confounding and reverse causality, we would suggest that the magnitude and consistency of the association that we have observed between psychotic disorders and incident dementia is unlikely to be fully explained by systematic error. If the findings are valid, how might one explain the link between psychotic disorders and dementia? The answer to this question is not clear at this stage, but several possibilities should be considered. Schizophrenia and other psychotic disorders have been associated with decreased cognitive reserve (Barnett et al. Reference Barnett, Salmond, Jones and Sahakian2006), and this may render people with these conditions more vulnerable to the effects of increasing age on brain structure and function, which in turn might facilitate the onset of dementia. In this case, we would expect people with a psychotic disorder to develop dementia earlier than the remainder of the population, a hypothesis that is not supported by our data (Fig. 2). Alternatively, the increased risk of dementia among older adults with a psychotic disorder could be due to their high exposure to adverse risk factors that contribute to cognitive decline, such as low educational attainment, physical inactivity, drug use, smoking, obesity, diabetes and cardiovascular diseases (Morgan et al. Reference Morgan, McGrath, Jablensky, Badcock, Waterreus and Bush2014). In this instance, the association between psychotic disorders and dementia would be due to factors other than the psychosis itself. We discussed above the issue of confounding associated with unmeasured factors, and although we accept that they may explain some of the association observed, it is difficult to see how they could account for all of it. In addition, the risk factors listed above are commonly associated with Alzheimer's disease and strokes (Livingston et al. Reference Livingston, Sommerlad, Orgeta, Costafreda, Huntley and Ames2017). However, the dementia of older people with a history of psychosis does not seem to be associated with Alzheimer's disease or cerebrovascular pathology (Purohit et al. Reference Purohit, Perl, Haroutunian, Powchik, Davidson and Davis1998; Jellinger & Gabriel, Reference Jellinger and Gabriel1999), which suggests that the dementia syndrome of these cases may be due to some other mechanism. One such mechanism could be neurotoxicity associated with antipsychotic treatment. The Iowa Longitudinal Study undertook repeated brain scanning of 211 adults with schizophrenia over a period of up to 14 years (Ho et al. Reference Ho, Andreasen, Ziebell, Pierson and Magnotta2011). The results showed that generalised and focal brain tissue loss was associated with the intensity of the exposure to antipsychotic treatment (greater volume loss associated with greater exposure) and that these changes in brain volume could not be adequately explained by the severity of the illness (Ho et al. Reference Ho, Andreasen, Ziebell, Pierson and Magnotta2011). It is unclear whether these changes would be sufficient to explain the increased risk of dementia associated with psychotic disorders, but they do highlight the importance of careful risk–benefit analysis when using these medications (Wisniewski et al. Reference Wisniewski, Constantinidis, Wegiel, Bobinski and Tarnawski1994). There are two further possibilities that should be considered: (1) dementia (i.e. the acquired decline of higher cortical functions and of daily activities) may be part of the natural course of psychotic disorders, at least for some people (Kahn & Keefe, Reference Kahn and Keefe2013; Rajji et al. Reference Rajji, Miranda and Mulsant2014); (2) psychotic symptoms with onset in later life represent an early clinical expression of an underlying neurodegenerative disorder that ultimately leads to the development of dementia (Almeida et al. Reference Almeida, Howard, Levy, David, Morris and Sahakian1995; Fischer & Aguera-Ortiz, Reference Fischer and Aguera-Ortiz2017). In the first case, one would expect that individuals with very long history of psychosis (i.e. prolonged exposure) would be at the greatest risk of dementia – this is not consistent with our results. In contrast, the second alternative would suggest that older people with a recent history of psychosis would be the most vulnerable to developing dementia. Our findings support this interpretation (i.e. reverse causality), although they also show that recency of psychosis is but a partial explanation for the link between psychotic disorders and dementia.
Our results have potential implications for practice and research. Psychotic disorders increase the risk of dementia in older men. When symptoms arise in later life, their presence may herald the early stages of a dementia syndrome, at least in some cases. Consequently, a detailed dementia work-up should be considered at the time of the initial assessment. There is also troubling, albeit preliminary, evidence that the prolonged use of antipsychotics could lead to brain changes that ultimately facilitate the development of dementia (it is unclear if this association is limited to people with psychotic disorders). If future studies confirm this link, users will need to be advised about the risk of dementia and safer new treatments will need to be developed. Furthermore, it is important not to lose sight of the factors that have been associated with increased dementia risk, as risk reduction strategies should be made available for people with and without psychotic disorders. Finally, it is possible that other as yet poorly understood mechanisms explain the association between psychotic disorders and dementia – if that is the case, clarifying these pathways may not only improve the clinical outcomes of people with psychotic disorders but also offer new insights into how to prevent cognitive decline in older age.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S003329171800065X
Acknowledgements
The authors thank study participants and research staff for their contribution.
This project was supported by the National Health and Medical Research Council of Australia (NHMRC), project grant numbers 0634492 and 1045710.
Conflict of interest
The authors declare they have no conflict of interest.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.







