Schizophrenia (SZ) is a severe neuropsychiatric disorder that has been characterized by disrupted connectivity within distributed large-scale neural networks (Stephan et al. Reference Stephan, Baldeweg and Friston2006, Reference Stephan, Friston and Frith2009). Recently, a number of studies using functional connectivity-based neuroimaging methods reported abnormalities within the thalamo-cortical-cerebellar network (e.g. Woodward et al. Reference Woodward, Karbasforoushan and Heckers2012; Anticevic et al. Reference Anticevic, Cole, Repovs, Murray, Brumbaugh and Winkler2014a; Damaraju et al. Reference Damaraju, Allen, Belger, Ford, McEwen and Mathalon2014; Klingner et al. Reference Klingner, Langbein, Dietzek, Smesny, Witte and Sauer2014; Cheng et al. Reference Cheng, Palaniyappan, Li, Kendrick, Zhang and Luo2015; Li et al. Reference Li, Wang, Zhang, Rolls, Yang and Palaniyappan2016). In the majority of cases, studies have corroborated initial reports of hypoconnectivity between the thalamus and both prefrontal and cerebellar regions, as well as hyperconnectivity between the thalamus and sensory regions. As these findings continue to be reported across studies that differ in sample size, patient characteristics, and analysis techniques, thalamo-cortical-cerebellar dysconnectivity is emerging as a reliable neurobiological marker for SZ.
Despite replications across laboratories, however, some inconsistencies remain that warrant further investigation. For example, initial reports of hyperconnectivity between the thalamus and cortical regions in SZ patients were limited to somatosensory cortices (Woodward et al. Reference Woodward, Karbasforoushan and Heckers2012). More recently, however, studies reported hyperconnectivity between thalamus and occipital and/or temporal cortices (e.g. Çetin et al. Reference Çetin, Christensen, Abbott, Stephen, Mayer and Cañive2014; Klingner et al. Reference Klingner, Langbein, Dietzek, Smesny, Witte and Sauer2014; Cheng et al. Reference Cheng, Palaniyappan, Li, Kendrick, Zhang and Luo2015; Li et al. Reference Li, Wang, Zhang, Rolls, Yang and Palaniyappan2016), which may suggest that SZ is characterized by hyperconnectivity between the thalamus and sensory regions more generally. Inconsistencies in patterns of connectivity create a challenge for using thalamic dysconnectivity as a reliable biomarker for SZ. Additional methodologically rigorous and well-powered studies are required to elucidate the most robust dysconnectivity patterns characterizing SZ.
More critically, fundamental questions remain regarding the clinical significance of these connectivity abnormalities, as correlations with clinical symptoms have largely been absent in prior reports (e.g. Woodward et al. Reference Woodward, Karbasforoushan and Heckers2012; Wang et al. Reference Wang, Rau, Li, Chen and Yu2015; Woodward & Heckers, Reference Woodward and Heckers2015). It has been suggested that difficulties in identifying relationships between thalamic dysconnectivity and SZ symptoms may be due to a number of factors, including small sample sizes and medication confounds (Mathalon & Ford, Reference Mathalon and Ford2012) as well as the functional heterogeneity of brain regions abnormally connected to the thalamus (Giraldo-Chica & Woodward, Reference Giraldo-Chica and Woodward2016).
In line with this notion, a small number of studies using large sample sizes identified relationships between overall dysconnectivity and symptom severity. Anticevic et al. (Reference Anticevic, Cole, Repovs, Murray, Brumbaugh and Winkler2014a) reported a positive correlation between SZ total symptom severity, and a single region of interest (ROI) comprising all regions displaying hyperconnectivity with the thalamus in schizophrenia patients (n = 90). Anticevic et al. (Reference Anticevic, Haut, Murray, Yang, Diehl and McEwen2015) also reported that thalamic hyperconnectivity was directly related to positive symptom severity prior to psychosis onset in the psychosis-risk syndrome. However, this correlation was only found when collapsing across healthy controls and participants at clinical high risk for psychosis (N = 397) under the rationale that mild positive symptoms may exist in the general population, raising questions about whether this effect was simply driven by the difference between healthy and clinical high-risk individuals. In a large multi-site study, Cheng et al. (Reference Cheng, Palaniyappan, Li, Kendrick, Zhang and Luo2015) reported that thalamo-sensory hyperconnectivty in SZ patients (n = 415) was associated with negative symptom severity, while thalamo-frontal hypoconnectivty was associated with positive symptom severity. Finally, in a meta-analytic review, Li et al. (Reference Li, Wang, Zhang, Rolls, Yang and Palaniyappan2016) found that thalamo-frontal hypoconnectivity and thalamo-sensory/motor hyperconnectivity in chronic SZ was related to the severity of positive symptoms, including delusions, hallucinations, and suspiciousness. Together, these findings suggest that overall disruptions in information flow might be related to symptom severity, however, they also highlight a need for additional well-powered SZ studies to examine the relationships between specific symptom domains (e.g., hallucinations) and discrete brain regions exhibiting dysconnectivity with the thalamus.
In this study, we aimed to examine explore relationships between symptoms of schizophrenia and hyperconnectivity between thalamus and sensory regions and hypoconnectivity of thalamus with cerebellar and frontal regions in SZ by analyzing resting-state functional magnetic resonance imaging (fMRI) data from a large sample of SZ patients (n = 183) and HCs (n = 178) collected as part of the multi-site Functional Bio-Informatics Research Network (FBIRN).
Methods
Participants
Participants included 183 SZ patients (mean age ± s.d. = 38.9 ± 11.6, 145 males) and 178 HCs (mean age ± s.d. = 37.5 ± 11.2, 126 males) recruited from seven sites as part of the multi-site FBIRN study (Table 1). All SZ patients met diagnostic criteria for schizophrenia based on the Structured Clinical Interview for DSM-IV-TR Axis I Disorders (SCID-I/P) (First et al. Reference First, Spitzer, Gibbon and Williams2002b). All potential participants were excluded for a history of major medical illness, contraindications for MRI, insufficient eyesight to see with normal acuity with MRI compatible corrective lenses, history of drug dependence in the last 5 years or a current substance abuse disorder, or an IQ less than 75 (Uttl, Reference Uttl2002). Patients with movement disorder symptoms (i.e., significant extra-pyramidal symptoms or tardive dyskinesia), and HCs with a current or past history of major neurological or psychiatric illness (SCID-I/NP) (First et al. Reference First, Spitzer, Gibbon and Williams2002a) or with a first-degree relative with an Axis-I psychotic disorder diagnosis, were also excluded.
Table 1. Demographic information for healthy controls and schizophrenia patients. SAPS and SANS global totals are the sum of all global scales

Trained clinical assessors conducted semi-structured interviews with SZ patients to rate symptom severity using the Scale for the Assessment of Positive Symptoms (SAPS; Andreasen, Reference Andreasen1984) and the Scale for the Assessment of Negative Symptoms (SANS; Andreasen, Reference Andreasen1983). Total scores for positive and negative symptoms were created by summing across global domains within each scale. All participants were assessed with the Hollingshead Socioeconomic Status Scale (HSSS; Hollingstead, Reference Hollingstead1975), the Edinburgh Handedness Questionnaire (EDQ; Oldfield, Reference Oldfield1971), the Fagerstrom-Lifetime Smoking Form (Fagerstrom, Reference Fagerstrom1978), a basic demographics form, and the North American Adult Reading Test (NAART; Uttl, Reference Uttl2002). Clinical assessors at each FBIRN site participated in training sessions to calibrate clinical ratings.
The FBIRN study protocol was approved by the Institutional Review Boards of the University of California Irvine, the University of California Los Angeles, the University of California San Francisco, Duke University, the University of North Carolina, the University of New Mexico, the University of Iowa, and the University of Minnesota. Written informed consent, including permission to share de-identified data between the FBIRN sites and with the wider research community, was obtained from all study participants. This protocol complies with the ethical standards of national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Neuroimaging acquisition methods
Resting fMRI data were acquired at seven sites: six 3T Siemens TIM Trio scanners and one 3T GE MR750 scanner. Data were acquired using the following T2*-weighted AC-PC aligned echo planar imaging sequence: TR = 2 s, TE = 30 ms, flip angle = 77°, 32 slices (3.4 × 3.4 × 4 mm3, 1 mm gap) acquired sequentially from superior to inferior, 162 frames, 5 : 38 m acquisition time.
Image processing
Preprocessing was achieved with Statistical Parametric Mapping 8 (SPM8; http://www.fil.ion.ucl.ac.uk/spm/software/spm8/). Motion correction via affine registration realigned all images to the first image using INRIAlign (http://www.fil.ion.ucl.ac.uk/spm/ext/#INRIAlign). Images were slice-time corrected to adjust for timing differences of individual slice acquisitions within each TR. The artifact detection tools toolbox (ART; http://www.nitrc.org/projects/artifact_detect/) was then used to identify outlying volumes in the time-series based on global image intensity values (>Z = 3) and head motion (>2 mm translational movement in x, y, or z plane or >0.02°rotation in yaw, pitch, or roll). To further denoise the data, we implemented aCompCor (Behzadi et al. Reference Behzadi, Restom, Liau and Liu2007), a principal component analysis (PCA) based approach to noise reduction of fMRI time-series data. ACompCor derives principal components from the time series of voxels within noise ROIs defined on the eroded white matter and cerebrospinal fluid (CSF) parcels from participants’ segmented high-resolution T1-weighted anatomical images coregistered to their functional data. White matter noise regions were derived from FreeSurfer (http://surfer.nmr.mgh.harvard.edu/) segmentation, while CSF noise regions were derived from segmentation in SPM8. A binary union mask of noise ROIs was generated and co-registered to the mean functional scan. Time series data for the voxels in the noise ROI mask were then subjected to a PCA, and a number of noise components comprising weighted averages of white matter and CSF voxel time series were identified using a bootstrap procedure.
The first level GLM included the mean seed time series, which was calculated using reverse-normalized anatomical mask images of the thalamus. Seven motion parameters, calculated via the ART toolbox, consisting of the temporal derivatives of the six motion parameters as well as a composite measure of total motion across translation and rotation, were included as regressors to remove fluctuations in BOLD signal attributable to participant head movement. To further ensure that the data were optimally cleaned of noise, regressors were also included for (i) data points identified by the ART toolbox as outliers and (ii) statistically significant (p < 0.05) principal noise components from the ACompCor denoising routine retained from each individual fMRI run based on a Monte Carlo simulation procedure (Behzadi et al. Reference Behzadi, Restom, Liau and Liu2007).The mean functional image from the motion correction pre-processing step was normalized to standard neuroanatomical space (Montreal Neurological Institute's MNI-EPI template; http://www.bic.mni.mcgill.ca), resulting in 3 mm3 isotropic voxel dimensions, and the normalization parameters were applied to first-level Fisher r-to-z transformed correlation images. Data were then spatially smoothed with a 6 mm full-width half-maximum Gaussian kernel.
The group-level analyses compensated for subjects missing voxels due to signal dropout and only analyzed data at a given voxel at the group level if >50% of subjects in each group had usable data at that voxel. That is, if a voxel was missing data from <50% of subjects per group, the GLM was estimated using only subjects with data (Huang et al. Reference Huang, Nichols, Kim and Foster2007).
Data analysis
A bilateral thalamus seed encompassing the entire thalamus was defined anatomically using the TD atlas (Lancaster et al. Reference Lancaster, Woldorff, Parsons, Liotti, Freitas and Rainey2000). A seed-based connectivity analysis of the thalamus was conducted using the CONN toolbox (Whitfield-Gabrieli & Nieto-Castanon, Reference Whitfield-Gabrieli and Nieto-Castanon2012) implemented in Matlab (http://www.nitrc.org/projects/conn). A mean bilateral thalamic seed time series vector was generated by averaging the time series data across all voxels within the thalamus ROI from the normalized functional images prior to smoothing. Voxel-wise correlation and anti-correlation maps were generated for each subject, representing the Pearson's r correlation value between the time series of the thalamus seed and every voxel in the brain. These r values were transformed to z-scores via Fisher's transformation, resulting in voxel-wise thalamic functional connectivity z-score maps for each participant. Connectivity values were tested for significance within- and between-groups in SPM8 using a family-wise error corrected voxel-wise height threshold of p = 0.001 based on Gaussian Random Field theory.
Mean thalamic connectivity values within significant regional clusters identified in the group difference maps (SZ > HC and HC > SZ) were extracted for each participant and imported into SPSS for analysis with symptom data. Correlations were performed between the 15 regions showing differential thalamic connectivity between groups and both SAPS Total Positive and SANS Total Negative symptom scores within the schizophrenia group. Results were FDR-corrected within each symptom domain for all 15 regions. Follow-up analyses were done on any correlation between connectivity and symptoms that remained after correcting for multiple comparisons. Dummy coded site variables were included in all regression models in order to account for variance attributable to the data collection sites.
Results
Within-group thalamic connectivity
Figure 1 displays within group connectivity results for (A) HCs and (B) SZ patients. The direction of connectivity is largely positive in both groups, although HCs demonstrated negative connectivity between thalamus and occipital cortex.
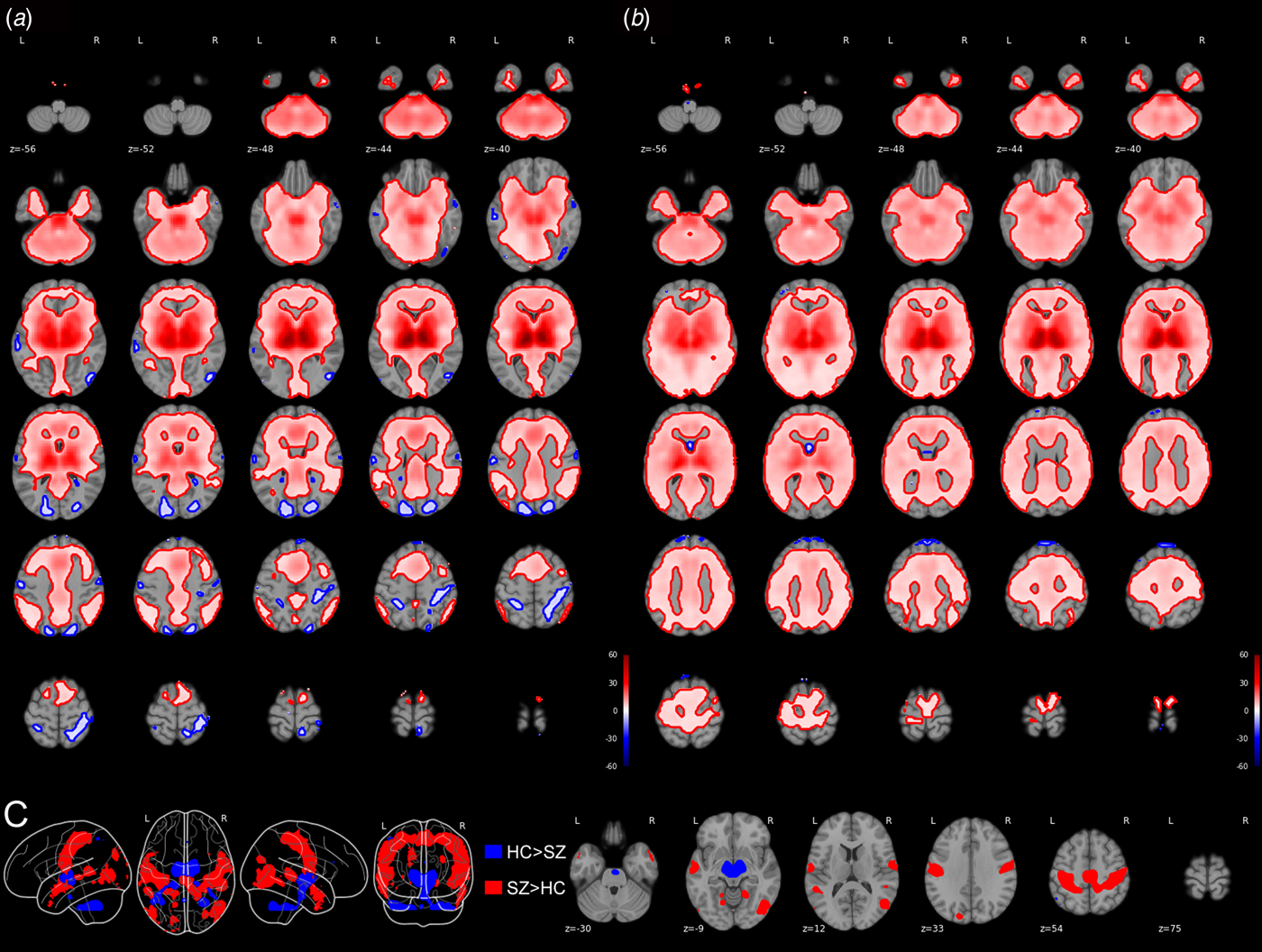
Fig. 1. Within-group connectivity maps for healthy controls (a) and individuals with schizophrenia (b). Between-group connectivity maps (c) depict regions showing greater connectivity with the thalamus in individuals with schizophrenia compared with healthy controls in red, and regions showing greater connectivity with the thalamus in healthy controls compared with individuals with schizophrenia in blue. A t-statistic scale is presented to the right of the figure. All results are presented at a voxel-wise FWE-corrected height threshold of p = 0.001.
Group differences in thalamic connectivity
Thalamic hypoconnectivity in schizophrenia
Compared with HCs, SZ patients had reduced thalamic connectivity with left and right cerebellum, as well as with bilateral thalamus itself. To determine if we also replicated reports of reduced thalamic connectivity between the anterior cingulate cortex (ACC) and prefrontal cortex in SZ patients, we lowered the voxel-wise height threshold to the family-wise error corrected p = 0.01. At this threshold, we observed reduced connectivity between thalamus and ACC in SZ patients.
Thalamic hyperconnectivity in schizophrenia
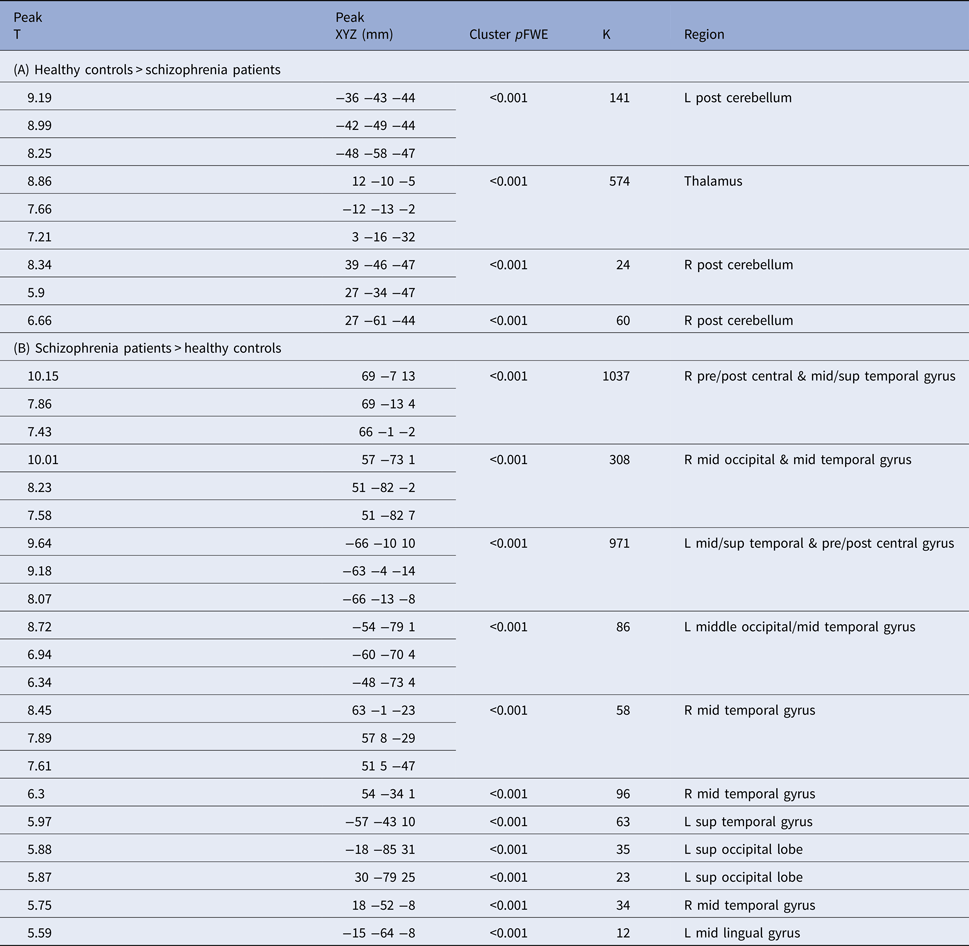
Compared with HCs, SZ patients had greater thalamic connectivity with large regions covering the right and left pre- and post-central gyrus that extended into the middle and superior temporal gyrus. Additional clusters were observed covering bilateral middle occipital gyrus extending into the middle temporal gyrus. Several discrete clusters were observed in the right middle temporal gyrus, left superior temporal gyrus, left superior occipital gyrus, and left middle lingual gyrus. Group differences in connectivity can be seen in Fig. 1c and Table 2.
Table 2. Regions of the brain showing differential connectivity with the thalamus between (A) healthy controls and (B) schizophrenia patients; FWE-corrected height threshold, p = 0.001

Associations between thalamic connectivity and symptoms
We tested the clinical significance of thalamic dysconnectivity in schizophrenia by first examining the relationship between the total scores for SAPS and SANS with each of the 15 regions showing differential connectivity patterns between the HC and SZ groups, listed in Table 2. After FDR correction, we observed a significant negative relationship between SAPS Total Positive symptom scores and thalamic connectivity with right posterior cerebellum (r = −0.234, p = 0.005) as well as a significant positive relationship between SAPS Total Positive symptom scores and thalamic connectivity with right middle temporal gyrus (r = 0.247, p = 0.002). No relationships between thalamic connectivity and negative symptoms remained significant after FDR correction for multiple comparisons.
To further explore the nature of significant relationships between thalamic connectivity and positive symptoms, we tested for correlations between each discrete brain region significantly correlated with SAPS Total Positive symptoms and the global rating for each of the positive symptom domains from the SAPS. Connectivity between thalamus and right posterior cerebellum was negatively correlated with both Delusions (r = −0.276, p = 0.001) and Bizarre Behavior (r = −0.196, p = 0.019). Connectivity between thalamus and right middle temporal gyrus was positively correlated with Hallucinations (r = 0.225, p = 0.005) and Delusions (r = 0.158, p = 0.049). Scatterplots depicting relationships between thalamic connectivity and overall positive symptoms as well as specific symptom domains are shown in Fig. 2. All reported correlations remained significant after controlling for individual differences in antipsychotic medication dose.
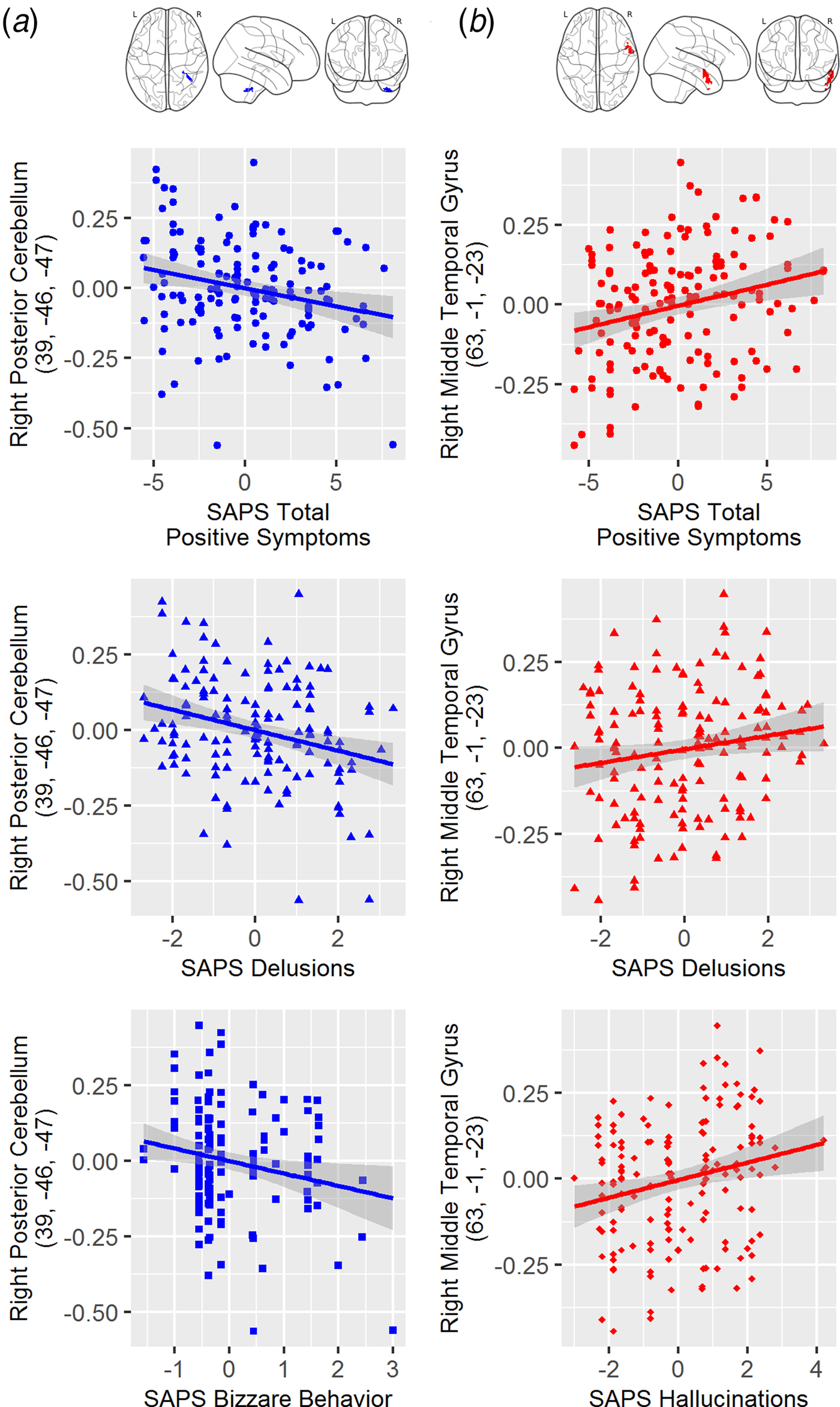
Fig. 2. (a) Scatterplots depicting the relationship between thalamic-right cerebellum connectivity and SAPS total (top), global SAPS bizarre behavior (middle) and global SAPS delusions (bottom); (b) Scatterplots depicting the relationship between thalamic-right middle temporal gyrus connectivity and SAPS total (top), global SAPS hallucinations (middle) and global SAPS delusions (bottom). Note: The SAPS scores represented residualized scores after controlling for the fixed effect of scanner site (i.e., removing the mean for each site).
Discussion
It is increasingly recognized that the thalamus plays a prominent role in cortico-cortical communication (Sherman, Reference Sherman2007, Reference Sherman2016) and higher-order processes such as cognition, emotion, and the coordination of sensory information (Kastner et al. Reference Kastner, Schneider and Wunderlich2006; Nishio et al. Reference Nishio, Hashimoto, Ishii and Mori2011; Niendam et al. Reference Niendam, Laird, Ray, Dean, Glahn and Carter2012; Watanabe & Funahashi, Reference Watanabe and Funahashi2012). As schizophrenia is characterized by a diversity of deficits in these areas, the thalamus and its widespread connections with the cortex and cerebellum have become a target of investigations aimed at elucidating the pathophysiology of SZ. This study is among the first to report correlations between specific symptom domains and discrete brain regions that show abnormal connectivity with the thalamus. In addition, we replicated previous reports of thalamic hyperconnectivity with sensory regions, and hypoconnectivity with cerebellar regions in SZ patients compared with HCs.
Replicating scientific findings is an essential part of establishing knowledge, particularly if the replication study is well-powered and methodologically sound (Brandt et al. Reference Brandt, Ijzerman, Dijksterhuis, Farach, Geller and Giner-Sorolla2014). In recent years, improving power in neuroscience studies has emerged as a priority for producing findings that are reliable, replicable, and of use to the greater scientific community (Button et al. Reference Button, Ioannidis, Mokrysz, Nosek, Flint and Robinson2013). The current study replicates previous findings of abnormal hyperconnectivity with motor and somatosensory regions and hypoconnectivity between the cerebellum and the thalamus (and ACC at lower thresholds) in a large sample (n = 183) of SZ patients. Furthermore, this study replicates the more limited number of studies that have suggested that SZ patients also display hyperconnectivity between the thalamus and the middle and superior temporal cortices (Klingner et al. Reference Klingner, Langbein, Dietzek, Smesny, Witte and Sauer2014; Cheng et al. Reference Cheng, Palaniyappan, Li, Kendrick, Zhang and Luo2015; Li et al. Reference Li, Wang, Zhang, Rolls, Yang and Palaniyappan2016), contributing to the idea that schizophrenia is characterized by thalamic hyperconnectivity with sensory regions in general. Given the role of the thalamus in the regulation and coordination of sensory information (Schmid et al. Reference Schmid, Singer and Fries2012), this enhanced connectivity potentially reflects an overabundance of sensory information or a lack of top-down control and coordination of sensory information that may contribute to the diverse symptoms of SZ. These robust and consistent replications of effects across studies suggest that abnormal connectivity may represent a core neurobiological feature and thus a potential biomarker for SZ.
While numerous studies (e.g. Collin et al. Reference Collin, Pol, Haijma, Cahn, Kahn and van den Heuvel2011; Anticevic et al. Reference Anticevic, Cole, Repovs, Murray, Brumbaugh and Winkler2014a; Wang et al. Reference Wang, Rau, Li, Chen and Yu2015), in addition to our own, have reported hypoconnectivity between the thalamus and the cerebellum, the implications of these findings remain poorly understood. The cerebellum has classically been viewed as playing a role in the coordination of motor function, but an increasing body of evidence suggests it may also play a role in higher-level cognitive processes and the coordination of cortical activity, through connections with the frontal and parietal cortex and through its role as a key node in the cortico-cerebellar-thalamic-cortical circuit or CCTCC (Andreasen, Reference Andreasen1983, Reference Andreasen1984; Andreasen et al. Reference Andreasen, Paradiso and O'leary1998; Andreasen & Pierson, Reference Andreasen and Pierson2008). Ramnani (Reference Ramnani2006) noted the cerebellum is key to ‘control theory,’ which posits the sensory consequences of our motor actions are evaluated quickly and efficiently by forward models instantiated in the cerebellum. A forward model allows the system to predict or anticipate imminent sensations resulting from actions, thereby minimizing the allocation of unnecessary processing resources. This is in contrast to slower and less efficient cortex-based proprioception. These ‘side loop’ forward models are particularly useful with over-learned responses when flexibility is not required (Ramnani, Reference Ramnani2006). Hypoconnectivity between thalamus and cerebellum could then underlie dysfunction of the forward model system that has been theorized to contribute to the pathophysiology of SZ and many of its positive symptoms (Feinberg, Reference Feinberg1978; Feinberg & Guazzelli, Reference Feinberg and Guazzelli1999; Frith et al. Reference Frith, Blakemore and Wolpert2000; Mathalon & Ford, Reference Mathalon and Ford2008).
The relevance of thalamus-cerebellar connectivity is underscored by a negative correlation between total positive symptoms and thalamic connectivity with the right posterior cerebellum. Analysis of specific positive symptom domains showed this region was negatively correlated with severity of delusions and bizarre behavior. There is a strong theoretical framework supporting the idea that the diversity of symptoms in SZ may relate to thalamus-mediated dysconnectivity between the cerebellum and cortical regions (Andreasen & Pierson, Reference Andreasen and Pierson2008). Like the thalamus itself, the cerebellum may play an important role in the coordination or modulation of cortical activity across a range of higher-order tasks through the CCTCC network, and, thus cerebellar dysfunction may potentially explain a wide range of abnormalities in the realms of emotion, perception, and cognition observed in SZ. While it is unlikely that this region, or any other single brain region, solely accounts for symptoms, ‘cognitive dysmetria’ theory identifies the cerebellum as a critically important node within a larger network that has the potential to give rise to the diverse symptoms of SZ (Andreasen & Pierson, Reference Andreasen and Pierson2008).
This study also adds to the more limited body of research suggesting that thalamic dysconnectivities with sensory regions, including the middle and superior temporal gyrus, are present in SZ. Abnormalities in temporal lobe function and structure (Cobia et al. Reference Cobia, Smith, Wang and Csernansky2012; Tang et al. Reference Tang, Liao, Zhou, Tan, Liu and Wang2012; Brent et al. Reference Brent, Thermenos, Keshavan and Seidman2013) in SZ have been reported in a variety of studies. Importantly, abnormal activation, structure, and connectivity with regions of the brain associated with language and memory, including middle and superior temporal gyrus, have been proposed to underlie some positive symptoms of SZ, including hallucinations (Allen et al. Reference Allen, Larøi, McGuire and Aleman2008; Jardri et al. Reference Jardri, Pouchet, Pins and Thomas2011). In this study, we also observed that the magnitude of connectivity between right middle temporal gyrus and the thalamus was positively correlated with total positive symptoms, and specifically with the positive symptom domains of hallucinations and delusions.
One limitation of this study is the use of the whole thalamus as a seed region. Previous examinations of thalamic connectivity have typically either investigated connectivity of large regions of the cortex to the thalamus (Woodward et al. Reference Woodward, Karbasforoushan and Heckers2012) or the whole thalamus with the rest of the brain (Anticevic et al. Reference Anticevic, Cole, Repovs, Murray, Brumbaugh and Winkler2014a). We chose the latter method to increase the likelihood of detecting connectivity relationships with the cortex; however, we recognize that the thalamus is a heterogeneous region encompassing many sub-nuclei with distinct connections to specific cortical regions that subserve specific functions. When examining sub-nuclei specifically, however, similar connectivity patterns have emerged when using either the lateral geniculate nucleus (LGN) or the medial dorsal nucleus (MDN) as seed regions (Anticevic et al. Reference Anticevic, Yang, Savic, Murray, Cole and Repovs2014b). Other limitations include potential confounds resulting from antipsychotic medication use, the chronicity of illness, history of substance use and current nicotine use.
This study adds to the growing body of research that collectively implicates thalamo-cortical-cerebellar disturbances in connectivity as a core feature of the pathophysiology underlying SZ. In this investigation, we replicate and extend this research by finding that thalamic connectivity, particularly with the cerebellum and middle temporal gyrus, is related to the severity of positive symptoms of SZ. These correlations suggest that too much connectivity to sensory association areas and too little connectivity to the cerebellum may set the stage for some of the cardinal symptoms of SZ–specifically, hallucinations and delusions. Future investigations are recommended to improve our understanding of when these effects emerge, how they relate to the progression of the illness, how they reflect its diverse symptoms, and ultimately, how they may be used to track effects of current treatments and they may provide pathophysiological targets for the development of novel treatments.
Acknowledgements
This work was supported by the National Center for Research Resources at the National Institutes of Health (grant number: NIH 1 U24 RR021992 (Function Biomedical Informatics Research Network). All authors report no conflicts interest with the current research. Dr. Van Erp consulted for Roche Pharmaceuticals and has a contract with Otsuka Pharmaceutical Co., Ltd. (OPCJ). Dr. Bustillo consulted with Novartis and Otsuka Pharmaceuticals. Dr. Mathalon serves as a consultant for Boehringer Ingelheim, Alkermes, Takeda, and Upsher-Smith Laboratories. Dr. Preda consulted for Boehringer-Ingelheim. Dr. Potkin has financial interests in Bristol-Myers Squibb, Eisai, Inc., Eli Lilly, Forest Laboratories, Genentech, Janssen Pharmaceutical, Lundbeck, Merck, Novartis, Organon, Pfizer, Roche, Sunovion, Takeda Pharmaceutical, Vanda Pharmaceutical, Novartis, Lundbeck, Merck, Sunovion and has received grant funding from Amgen, Baxter, Bristol-Myers Squibb, Cephalon, Inc., Eli Lilly, Forest Laboratories, Genentech, Janssen Pharmaceutical, Merck, Otsuka, Pfizer, Roche, Sunovion, Takeda Pharmaceutical, Vanda Pharmaceutical, NIAAA, NIBIB, NIH/NCRR, University of Southern California, UCSF, UCSD, Baylor College of Medicine.