Introduction
In 2009, an estimated 9.3% of all children living in the USA experienced maltreatment (U.S. Department of Health and Human Services, 2009). Emotional maltreatment involves any act or series of acts of commission (i.e. verbal abuse) or omission (i.e. emotional neglect) by a parent or other caregiver that results in harm, potential for harm, or threat of harm to a child's emotional development (Leeb et al. Reference Leeb, Paulozzi, Melanson, Simon and Arias2008; Egeland, Reference Egeland2009). The experience of emotional neglect and emotional abuse has a substantial impact on an individual's life. This impact is enhanced when the maltreatment is experienced in childhood, partly due to the dependence of children on the perpetrator for various necessities of life, such as food, shelter and protection from harm. Consequences of childhood emotional maltreatment (CEM) include effects on mental well-being (Gibb, Reference Gibb2002; Teicher et al. Reference Teicher, Samson, Polcari and McGreenery2006; Leeb et al. Reference Leeb, Paulozzi, Melanson, Simon and Arias2008; Egeland, Reference Egeland2009; Wright et al. Reference Wright, Crawford and Del Castillo2009), internalizing attribution styles (Taussig & Culhane, Reference Taussig and Culhane2010), emotion regulation (Rellini et al. Reference Rellini, Vujanovic, Gilbert and Zvolensky2012) and behavior (Gilbert et al. Reference Gilbert, Widom, Browne, Fergusson, Webb and Janson2009). In addition, the experience of CEM increases the chance of developing various psychopathologies (Egeland, Reference Egeland2009), including anxiety and depression (Gibb et al. Reference Gibb, Chelminski and Zimmerman2007; Spinhoven et al. Reference Spinhoven, Elzinga, Hovens, Roelofs, Zitman, van Oppen and Penninx2010). These consequences have been found to continue or become evident long after the maltreatment ended, even after the child reaches adulthood. Although CEM has not received as much attention as physical abuse and sexual abuse, it has become increasingly clear that CEM occurs more frequently and has its own specific disruptive effects on the development, functioning and attachment styles of an individual (Finzi et al. Reference Finzi, Cohen, Sapir and Weizman2000; McLewin & Muller, Reference McLewin and Muller2006; Egeland, Reference Egeland2009; van Harmelen et al. Reference van Harmelen, de Jong, Glashouwer, Spinhoven, Penninx and Elzinga2010a).
From animal studies it is known that paradigms resembling emotional maltreatment in humans, such as maternal separation, have a profound effect on brain morphology and behavior of animals (McEwen, Reference McEwen2001; Fabricius et al. Reference Fabricius, Wortwein and Pakkenberg2008). Regions of the brain predominantly being affected by maternal separation include the hippocampus (McEwen, Reference McEwen2001; Fabricius et al. Reference Fabricius, Wortwein and Pakkenberg2008; Joels et al. Reference Joels, Krugers and Karst2008), the amygdala (Joels et al. Reference Joels, Krugers and Karst2008) and the medial prefrontal cortex (mPFC) (Muhammad et al. Reference Muhammad, Carroll and Kolb2012). In line with these animal studies, previous work by our group found CEM to be associated with abnormalities of regional brain morphology in humans (van Harmelen et al. Reference van Harmelen, van Tol, van der Wee, Veltman, Aleman, Spinhoven, van Buchem, Zitman, Penninx and Elzinga2010b). We demonstrated reduced gray matter volumes in the left dorsal mPFC (dmPFC) in subjects who reported having experienced CEM.
The amygdala, hippocampus and mPFC are important constituents of a limbic network known to be involved in stress responses and emotion regulation (Vermetten & Bremner, Reference Vermetten and Bremner2002; Bremner, Reference Bremner2007a; Shin & Liberzon, Reference Shin and Liberzon2010). The hippocampus is involved in declarative memory and is connected reciprocally to the amygdala, which plays a crucial role in the acquisition of fear responses and in memory consolidation of emotional experiences and stimuli (Bremner, Reference Bremner2007b). The mPFC has a more controlling function in the neural circuitry of stress and emotion, as it inhibits fear responses and emotional responsiveness mediated by the amygdala, and is important for self-referential processes (Bremner, Reference Bremner2007b; Roy et al. Reference Roy, Shehzad, Margulies, Kelly, Uddin, Gotimer, Biswal, Castellanos and Milham2009). Amygdala activation has been found to increase during and after stressful situations (van Marle et al. Reference van Marle, Hermans, Qin and Fernandez2009; van Wingen et al. Reference van Wingen, Geuze, Vermetten and Fernandez2011; Oei et al. Reference Oei, Veer, Wolf, Spinhoven, Rombouts and Elzinga2012). Moreover, we found increased amygdala activation in individuals reporting CEM during the processing of faces (van Harmelen et al. Reference van Harmelen, van Tol, Demenescu, van der Wee, Veltman, Aleman, van Buchem, Spinhoven, Penninx and Elzinga2012). An increase in functional connectivity between the amygdala and cortical midline structures was found during a recovery period after the induction of social stress (Veer et al. Reference Veer, Oei, Spinhoven, van Buchem, Elzinga and Rombouts2011), highlighting the importance of functional connectivity for understanding responsiveness to (chronic) stress.
Functional magnetic resonance imaging (fMRI) is widely used to study functional connectivity within the context of task paradigms, but it is also being used increasingly to study activation and connectivity during the resting state, that is in the absence of an externally controlled task or stimulus (Biswal et al. Reference Biswal, Yetkin, Haughton and Hyde1995; Raichle et al. Reference Raichle, MacLeod, Snyder, Powers, Gusnard and Shulman2001). During the resting state, several networks of functionally connected brain areas have been identified consistently (Damoiseaux et al. Reference Damoiseaux, Rombouts, Barkhof, Scheltens, Stam, Smith and Beckmann2006). Given the influence of a history of CEM on brain structure and on emotional processing and regulation, episodic memory and self-referential processing, it can be hypothesized that resting-state networks of brain areas involved in these processes show abnormalities in individuals reporting CEM (Danese & McEwen, Reference Danese and McEwen2012). This is especially the case for the default-mode network (DMN), the salience network and limbic network. The DMN is a network containing the precuneus cortex, posterior cingulate cortex (PCC), mPFC, lateral and inferior parietal cortex and ventral anterior cingulate cortex (vACC) (Raichle et al. Reference Raichle, MacLeod, Snyder, Powers, Gusnard and Shulman2001; Greicius et al. Reference Greicius, Krasnow, Reiss and Menon2003). The DMN is thought to be involved in the retrieval and manipulation of episodic memories and semantic knowledge, self-referential processing and prospective memory (Raichle et al. Reference Raichle, MacLeod, Snyder, Powers, Gusnard and Shulman2001; Buckner et al. Reference Buckner, Andrews-Hanna and Schacter2008; Kim, Reference Kim2012). The function of the salience network is the identification of the most important internal and extrapersonal stimuli with respect to reaching or protecting a state of homeostatic equilibrium (Seeley et al. Reference Seeley, Menon, Schatzberg, Keller, Glover, Kenna, Reiss and Greicius2007). The salience network contains the dorsal anterior cingulate cortex (dACC) and orbital frontoinsular cortex, along with several subcortical and limbic structures (Seeley et al. Reference Seeley, Menon, Schatzberg, Keller, Glover, Kenna, Reiss and Greicius2007). The limbic network is involved in emotional processing and regulation and contains structures such as the amygdala and hippocampus and medial prefrontal structures such as the ACC.
Abnormalities in resting-state functional connectivity (RSFC) have been found in a variety of (neuro)psychiatric disorders known to involve aberrant stress system reactivity and disturbed emotion regulation and self-processing, such as depression and anxiety (Greicius, Reference Greicius2008; Broyd et al. Reference Broyd, Demanuele, Debener, Helps, James and Sonuga-Barke2009; Liao et al. Reference Liao, Chen, Feng, Mantini, Gentili, Pan, Ding, Duan, Qiu, Lui, Gong and Zhang2010; Veer et al. Reference Veer, Beckmann, van Tol, Ferrarini, Milles, Veltman, Aleman, van Buchem, van der Wee and Rombouts2010). Moreover, CEM has been identified as an important risk factor for these disorders (Egeland, Reference Egeland2009; Spinhoven et al. Reference Spinhoven, Elzinga, Hovens, Roelofs, Zitman, van Oppen and Penninx2010). However, at present it is unknown whether exposure to CEM is associated with altered RSFC in adulthood.
Therefore, in the current study we aimed to evaluate whether there are differences in RSFC between individuals who reported having experienced CEM compared to individuals who reported not having experienced CEM. Taking into consideration the role of the limbic network in the neural circuitry of stress and emotion, we hypothesized that individuals with a history of CEM would show aberrant connectivity in the limbic network during the resting state. In addition, we hypothesized that individuals with a history of CEM would typically also display altered RSFC within the salience network and the DMN, given the roles of these networks in emotional processing, episodic memory and self-processing. Finally, given our previous finding of morphological abnormalities in the left dmPFC in individuals reporting CEM (van Harmelen et al. Reference van Harmelen, van Tol, van der Wee, Veltman, Aleman, Spinhoven, van Buchem, Zitman, Penninx and Elzinga2010b), we also expected to find differences in RSFC of this area.
Method
Assessment of CEM
Childhood maltreatment was assessed through the use of The Netherlands Mental Health Survey and Incidence Study (NEMESIS) trauma interview (Robins et al. Reference Robins, Wing, Wittchen, Helzer, Babor, Burke, Farmer, Jablenski, Pickens and Regier1988; De Graaf et al. Reference De Graaf, Bijl, Smit, Vollebergh and Spijker2002). In this interview, respondents were asked whether they had experienced emotional neglect, emotional abuse, physical abuse and/or sexual abuse before the age of 16 years, how often the childhood maltreatment had occurred (responses were recorded as: ‘never’, ‘once’, ‘sometimes’, ‘regularly’, ‘often’ or ‘very often’) and what their relationship to the perpetrator was. Emotional neglect was described as: ‘people at home didn't listen to you, your problems were ignored, you felt unable to find any attention or support from the people in your house’. Emotional abuse was described as: ‘you were cursed at, unjustly punished, your brothers and sisters were favored – but no bodily harm was done’. Our definition of CEM (i.e. emotional neglect and/or emotional abuse before the age of 16 years) is based on the definition from the American Professional Society on the Abuse of Children (APSAC; Binggeli et al. Reference Binggeli, Hart and Brassard2001; Egeland, Reference Egeland2009). This definition states that emotional child maltreatment consists of acts of commission (emotional abuse such as degrading, terrorizing, belittling, blaming, exploiting) and/or omission (emotional neglect, for example isolation, rejection, denying emotional responsiveness) that convey to the child that they are worthless, unloved and unwanted, and are harmful to the child's emotional developmental needs.
Sample
Participants were drawn from the large-scale longitudinal Netherlands Study of Depression and Anxiety (NESDA; Penninx et al. Reference Penninx, Beekman, Smit, Zitman, Nolen, Spinhoven, Cuijpers, De Jong, Van Marwijk, Assendelft, Van Der Meer, Verhaak, Wensing, De Graaf, Hoogendijk, Ormel and Van Dyck2008). From the 301 subjects who underwent the MRI scanning protocol, 97 reported having experienced CEM (emotional neglect and/or emotional abuse) more than once before the age of 16 years. We discarded data from 15 subjects due to excessive head motion (>3 mm in any direction) during resting-state data acquisition. Next, 38 subjects who reported having experienced either sexual or physical abuse or both once or more before the age of 16 were removed from the data to obtain a CEM group without sexual and physical abuse. This resulted in a CEM group of 44 subjects. In the CEM group, 97.7% (n=43) reported having been emotionally neglected and 29.5% (n=13) reported having experienced emotional abuse. The control group, NoCEM (n=44), consisted of subjects who reported having experienced no childhood maltreatment of any kind before the age of 16 and was group-wised matched to the CEM group for age, gender, handedness and presence of psychopathology. The demographics of each group together with the distribution of psychiatric diagnoses are reported in Table 1.
Table 1. Demographic and clinical characteristics of individuals reporting CEM versus individuals reporting no CEM
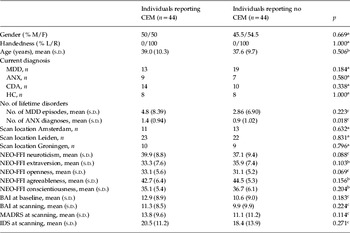
CEM, Childhood emotional maltreatment; M, male; F, female; L, left; R, right; MDD, major depressive disorder; ANX, anxiety disorder; CDA, co-morbid MDD and ANX; HC, healthy control subjects; NEO-FFI, Neuroticism–Extroversion–Openness Five-Factor Inventory; BAI, Beck Anxiety Inventory; MADRS, Montgomery–Åsberg Depression Rating Scale; IDS, Inventory of Depressive Symptomatology; s.d., standard deviation.
a χ2 test.
b Independent-sample t test.
c Mann–Whitney U test.
Data acquisition
Imaging data were acquired at one of the three participating scanning locations, situated in the University Medical Centers in Leiden, Amsterdam and Groningen, using Philips 3-T MR systems (Philips Healthcare, The Netherlands). These systems were equipped with a SENSE-8 (Leiden and Groningen) and a SENSE-6 (Amsterdam) channel head coil respectively. A recent study demonstrated that multi-center datasets can be aggregated and shared, even when different scan sequences were used (Biswal et al. Reference Biswal, Mennes, Zuo, Gohel, Kelly, Smith, Beckmann, Adelstein, Buckner, Colcombe, Dogonowski, Ernst, Fair, Hampson, Hoptman, Hyde, Kiviniemi, Kotter, Li, Lin, Lowe, Mackay, Madden, Madsen, Margulies, Mayberg, McMahon, Monk, Mostofsky, Nagel, Pekar, Peltier, Petersen, Riedl, Rombouts, Rypma, Schlaggar, Schmidt, Seidler, Siegle, Sorg, Teng, Veijola, Villringer, Walter, Wang, Weng, Whitfield-Gabrieli, Williamson, Windischberger, Zang, Zhang, Castellanos and Milham2010). As part of a fixed imaging protocol, resting-state fMRI (RS-fMRI) data were acquired for each subject. Subjects were instructed to lie as still as possible and not to fall asleep. After completion of the scan, all subjects confirmed not having fallen asleep. To obtain RS-fMRI data, T2*-weighted gradient-echo echo-planar imaging (EPI) was used with the following scan parameters in Amsterdam and Leiden: 200 whole-brain volumes, repetition time (TR) 2300 ms, echo time (TE) 30 ms, flip angle 80°, 35 transverse slices, no slice gap, matrix 220 × 220 mm, voxel size 2.3 × 2.3 mm, slice thickness 3 mm. The scan parameters in Groningen were similar except for: TE 28 ms, 39 axial slices, voxel size 3.45 × 3.45 mm. For registration purposes and for gray matter density analysis, anatomical images were acquired using a sagittal three-dimensional (3D) gradient-echo T1-weighted sequence with the following scan parameters: TR 9 ms, TE 3.5 ms, flip angle 80°, 170 sagittal slices, no slice gap, matrix 256 × 256 mm, voxel size 1 mm isotropic. All anatomical images were examined by a neuroradiologist. No abnormalities were found.
Data preprocessing
The structural and RS-fMRI images were preprocessed using FEAT (FMRIB's Expert Analysis Tool) version 5.90, part of FSL (FMRIB's Software Library, www.fmrib.ox.ac.uk/fsl) (Smith et al. Reference Smith, Jenkinson, Woolrich, Beckmann, Behrens, Johansen-Berg, Bannister, De Luca, Drobnjak, Flitney, Niazy, Saunders, Vickers, Zhang, De Stefano, Brady and Matthews2004). Non-brain tissue removal was applied to the structural images. Motion correction was applied to the RS-fMRI data along with non-brain tissue removal, spatial smoothing using a 6-mm full-width at half-maximum (FWHM) Gaussian kernel, grand-mean intensity normalization of the entire 4D dataset by a single multiplicative factor and high-pass temporal filtering (Gaussian-weighted least-squares straight line fitting, 0.01 Hz cut-off). RS-fMRI data were registered to the high-resolution structural image (T1) and subsequently the T1 image was registered to the 2-mm isotropic MNI-152 (T1 standard brain average over 152 subjects; Montreal Neurological Institute, Canada) images. The resulting transformation matrices derived from these registration steps were then combined to obtain a native to MNI space transformation matrix and its inverse (MNI to native space).
Statistical analysis
After preprocessing, the data were analyzed using seed-based correlations assessing three networks of interest: the limbic network, the DMN and the salience network. The following seed regions of interest (ROIs) were selected: the bilateral amygdala (limbic network), the bilateral dACC (salience network) (Margulies et al. Reference Margulies, Kelly, Uddin, Biswal, Castellanos and Milham2007) and the PCC (DMN) (Fox et al. Reference Fox, Snyder, Vincent, Corbetta, Van Essen and Raichle2005). The bilateral seeds for the amygdala were created in standard space using the Harvard–Oxford Subcortical Structural Probability Atlas. In addition, a mask was created for the area showing decreased gray matter density earlier identified in individuals reporting CEM, in the left dmPFC (van Harmelen et al. Reference van Harmelen, van Tol, van der Wee, Veltman, Aleman, Spinhoven, van Buchem, Zitman, Penninx and Elzinga2010b), along with a white matter mask and a cerebrospinal fluid (CSF) mask. MNI coordinates for each of the masks are reported in Table 2.
Table 2. MNI coordinates of the seed regions

MNI, Montreal Neurological Institute; dACC, dorsal anterior cingulate cortex; PCC, posterior cingulated cortex; dmPFC, dorsomedial prefrontal cortex; CSF, cerebrospinal fluid.
A sphere with 4-mm radius was created around the single voxel seed. These spheres were then transformed to the native space using the inverse transformation matrices obtained during registration in the preprocessing phase. Spatially averaged time series were extracted for each seed and each subject. A time series was also extracted for the global mean signal. For each subject and for each network separately, a multiple regression analysis was performed using the general linear model implemented in FSL (Smith et al. Reference Smith, Jenkinson, Woolrich, Beckmann, Behrens, Johansen-Berg, Bannister, De Luca, Drobnjak, Flitney, Niazy, Saunders, Vickers, Zhang, De Stefano, Brady and Matthews2004). The time courses that were extracted from the voxels in all of our seed regions were entered as a regressor in a general linear model for each network. Nine nuisance regressors were included in the model: the signal from the white matter, the CSF signal and the global signal, and six motion parameters (three translations and three rotations). The global signal was included to reduce artifacts associated with physiological signal sources (i.e. cardiac and respiratory) (Birn et al. Reference Birn, Diamond, Smith and Bandettini2006; Fox & Raichle, Reference Fox and Raichle2007). After reslicing the resulting parameter estimate maps and their corresponding within-subject variance maps into 2-mm isotropic MNI space, they were entered into a higher-level within- and between-groups mixed effects analysis (one- and two-sample t tests). For each subject, gray matter density maps were derived from the anatomical scans using FSL. Subjects in this study were drawn from the same sample (the NESDA) as the subjects used to investigate the structural abnormalities of CEM (van Harmelen et al. Reference van Harmelen, van Tol, van der Wee, Veltman, Aleman, Spinhoven, van Buchem, Zitman, Penninx and Elzinga2010b). Therefore, to control for structural differences possibly confounding differences in functional connectivity and to correct for the effects of possible misregistration (Oakes et al. Reference Oakes, Fox, Johnstone, Chung, Kalin and Davidson2007), information about gray matter density of each subject was included as a voxelwise confound regressor. Groups were compared using the general linear model including age and scan location as additional confound regressors in each comparison. Cluster correction was applied in all group analyses with an initial cluster-forming threshold of z > 2.3 and a corrected p < 0.05.
Results
Psychometric data
There was no significant difference between the CEM group and the control group in anxiety rates based on Beck Anxiety Inventory (BAI) scores (Beck et al. Reference Beck, Epstein, Brown and Steer1988) both at baseline and immediately before scanning or in depressive symptoms measured by the Montgomery–Åsberg Depression Rating Scale (MADRS; Montgomery & Asberg, Reference Montgomery and Asberg1979) and the Inventory of Depressive Symptomatology (IDS; Rush et al. Reference Rush, Gullion, Basco, Jarrett and Trivedi1996). No differences between the groups were found in neuroticism, extraversion, agreeableness, openness or conscientiousness as measured by the subscales of the Neuroticism–Extroversion–Openness Five-Factor Inventory (NEO-FFI; Costa & McCrea,Reference Costa and McCrea1992). In terms of past psychopathology, no between-group differences were found in the number of episodes of major depressive disorder (MDD). However, our CEM group did report experiencing significantly more episodes of anxiety disorders in the past (Table 1).
RSFC
Analysis of the main effects of both the CEM group and the NoCEM group showed connectivity between the seed chosen for the specific networks and other structures known to be implicated in these networks in both groups, indicating correct positioning of our seeds. Analysis of the amygdala seeds showed a decrease in negative connectivity between the right amygdala and the superior division of the bilateral occipital cortex and the bilateral precuneus cortex in the CEM group (Fig. 1). Furthermore, a decrease in positive connectivity was found in the CEM group between the right amygdala and a large cluster stretching from the orbitofrontal cortex and the insular to subcortical structures including the hippocampus and the putamen of the left hemisphere of the brain (Fig. 1). The left amygdala seed yielded no differences between the two groups but, when taken together, the bilateral amygdala seeds showed a decrease in negative connectivity with the cuneus, the superior division of the lateral occipital cortex and the precuneus in the left hemisphere of the brain in the CEM group.

Fig. 1. Right amygdala connectivity. (a) The main effect of childhood emotional maltreatment (CEM) for negative connectivity with the right amygdala, (b) the main effect of NoCEM for negative connectivity with the right amygdala, (c) the between-group effect of negative connectivity with the right amygdala, (d) the main effect of CEM for positive connectivity with the right amygdala, (e) the main effect of NoCEM for positive connectivity with the right amygdala and (f) the between-group effect of positive connectivity with the right amygdala. Images are z statistics, overlaid on the MNI-152 1 mm standard brain. The left hemisphere of the brain corresponds to the right side of the image.
Analysis of the RSFC of the bilateral dACC seeds probing the salience network showed decreased negative connectivity between the left dACC seed and the angular cortex and the precuneus of the right hemisphere in CEM (Fig. 2). Furthermore, decreased positive connectivity was found in the CEM group between the left dACC seed and a bilateral frontal cluster containing the mPFC, the paracingulate gyrus and the frontal pole (Fig. 2). Contrasts for the right dACC seed and the left and the right dACC seeds together yielded no differences between the CEM group and the NoCEM group.

Fig. 2. Left dorsal anterior cingulate cortex (dACC) connectivity. (a) The main effect of childhood emotional maltreatment (CEM) for negative connectivity with the left dACC, (b) the main effect of NoCEM for negative connectivity with the left dACC, (c) the between-group effect of negative connectivity with the left dACC, (d) the main effect of CEM for positive connectivity with the left dACC, (e) the main effect of NoCEM for positive connectivity with left dACC and (f) the between-group effect of positive connectivity with the left dACC. Images are z statistics, overlaid on the MNI-152 1 mm standard brain. The left hemisphere of the brain corresponds to the right side of the image.
Analysis of the seed in the left dmPFC, the area implicated in the structural effects of CEM, and also the PCC seed probing the DMN yielded no differences between the CEM and the control group. Information about all significant between-group effects is listed in the online Supplementary Tables S1 and S2.
Discussion
The aim of this study was to investigate differences in RSFC between adult individuals reporting CEM and a control group without maltreatment and matched for the presence of psychopathology, using a seed-based correlation approach. We hypothesized aberrant connectivity of seed regions in the limbic network (amygdala), salience network and DMN seeds and of a dmPFC region previously found to exhibit significant gray matter loss in this group of individuals (van Harmelen et al. Reference van Harmelen, van Tol, van der Wee, Veltman, Aleman, Spinhoven, van Buchem, Zitman, Penninx and Elzinga2010b). We found aberrant connectivity of the amygdala and salience network seeds but, contrary to our hypotheses, no aberrant connectivity was found for the seed in the DMN and the previously identified brain region within the dmPFC that showed structural abnormalities in the CEM group.
Of note, we found decreased negative RSFC in individuals reporting CEM between the right amygdala and a brain region containing the precuneus and parts of the superior division of the lateral occipital cortex. Task-related neuroimaging studies have shown the precuneus to be involved in visuospatial imagery (Frings et al. Reference Frings, Wagner, Quiske, Schwarzwald, Spreer, Halsband and Schulze-Bonhage2006), episodic memory encoding and retrieval (Fletcher et al. Reference Fletcher, Frith, Baker, Shallice, Frackowiak and Dolan1995; Cavanna & Trimble, Reference Cavanna and Trimble2006) and self-referential processing (Kircher et al. Reference Kircher, Senior, Phillips, Benson, Bullmore, Brammer, Simmons, Williams, Bartels and David2000; Kjaer et al. Reference Kjaer, Nowak and Lou2002; Lou et al. Reference Lou, Luber, Crupain, Keenan, Nowak, Kjaer, Sackeim and Lisanby2004). Studies have shown that a history of CEM is associated with specific disturbances in emotional and cognitive processing, including negative explicit and automatic self-associations and increased amygdala reactivity (van Harmelen et al. Reference van Harmelen, van Tol, Demenescu, van der Wee, Veltman, Aleman, van Buchem, Spinhoven, Penninx and Elzinga2012). Taking into account the role of the amygdala in the acquisition of fear responses and in the memory consolidation of emotional experiences, the decrease in connectivity between the right amygdala and the precuneus in individuals reporting CEM could reflect or underlie specific disturbances in emotional and cognitive (self) processing in individuals with a history of CEM.
Another finding in our study was decreased positive connectivity in the CEM group between the right amygdala and a large area in the left hemisphere stretching from the orbitofrontal cortex and the insula to subcortical structures including the hippocampus and the putamen. The hippocampus and the insula are regions known to be involved in emotion processing and affect regulation (Pessoa, Reference Pessoa2008; Veer et al. Reference Veer, Oei, Spinhoven, van Buchem, Elzinga and Rombouts2011). Of note, reduced connectivity in a resting-state network containing the insular cortex and the amygdala has also been found in patients with MDD (Veer et al. Reference Veer, Beckmann, van Tol, Ferrarini, Milles, Veltman, Aleman, van Buchem, van der Wee and Rombouts2010). Because of the matching for presence of psychopathology, our results cannot be attributed to a higher prevalence of depression in our CEM group, suggesting a possible shared RSFC abnormality between CEM and MDD that could be associated with, or underlie, the elevated risk for developing recurrent and persistent depressive episodes (Nanni et al. Reference Nanni, Uher and Danese2012).
With regard to the altered RSFC of the right amygdala with the putamen and the orbitofrontal cortex, it should be noted that both are part of an intricate functional network also containing the dorsolateral PFC, the ventral medial pallidum and thalamic regions (Bennett, Reference Bennett2011). This prefrontal-limbic network is thought to be involved in goal-directed activity and also insight into an individual's well-being (Bennett, Reference Bennett2011). The latter function includes the ability to suppress negative feelings, an ability that is usually found to be reduced in individuals who have experienced CEM (Taussig & Culhane, Reference Taussig and Culhane2010).
Analysis of the left and right amygdala seeds together demonstrated a decrease in negative connectivity with a brain region including the cuneus, the superior division of the lateral occipital cortex and the precuneus cortex in the left hemisphere of the brain in the CEM group. As this region was also found in the analysis for the right amygdala seed, we conclude that this result is mostly driven by the differences in connectivity with the right amygdala.
Functional connectivity analysis of the bilateral dACC seeds, probing the salience network, showed altered RSFC in individuals reporting CEM. Decreased negative RSFC was found between the left dACC and the right angular cortex and the right precuneus. As self-referential processing is an important function ascribed to the precuneus, a decrease in connectivity with the precuneus within the salience network might be related to the disturbances in relating internal and external stimuli to oneself in individuals reporting CEM (Gibb, Reference Gibb2002; Wright et al. Reference Wright, Crawford and Del Castillo2009; van Harmelen et al. Reference van Harmelen, de Jong, Glashouwer, Spinhoven, Penninx and Elzinga2010a). We also found a decrease in positive connectivity between the left dACC seed and a bilateral frontal region containing both the mPFC and the frontal pole in individuals reporting CEM. Previous studies implicate the ACC, the mPFC and the frontal pole in reward-guided learning, decision making and adjusting problem-solving strategies (Kahnt et al. Reference Kahnt, Grueschow, Speck and Haynes2011; Koechlin, Reference Koechlin2011; Tsujimoto et al. Reference Tsujimoto, Genovesio and Wise2011). The altered connectivity of the left dACC with these regions might be interpreted as underlying certain disturbances of reward-guided learning and decision-making strategies such as those reported by Guyer et al. (Reference Guyer, Kaufman, Hodgdon, Masten, Jazbec, Pine and Ernst2006), who showed that maltreated children made more risky decisions and responded less quickly as the chance of winning increased.
As the precuneus cortex is an important part of the DMN, it could be argued that differences in RSFC with the precuneus cortex are caused by group differences in DMN activity, rather than in connectivity with the precuneus cortex. However, both groups showed similar patterns of DMN connectivity and no between-group differences were found. Similarly, the seed derived from our previous study showing structural effects of CEM did not yield group differences.
To the best of our knowledge, this is the first study examining RSFC in individuals reporting CEM. Our sample size (n=88) was fairly large with respect to MRI studies in the field of psychiatry. We matched the groups for presence of psychopathology, improving homogeneity of our two groups, which did not differ in neurotic personality characteristics, anxiety symptoms, depressive symptoms and history of experienced depressive episodes. Finally, this study facilitates replication as a seed-based ROI approach was used to analyze the data. There are also some limitations to consider. The cross-sectional design of this study precludes any claim of causality or developmental trajectory, as we cannot establish whether the differences found were already present before the experience of CEM or were a consequence of the experience of CEM or its developmental and social sequelae. The presence of CEM was assessed retrospectively based on self-report and not corroborated with other sources. A bias in recall, either over- or under-reporting the experiences, cannot therefore be excluded. Clearly, the interpretation of abnormalities in RSFC in our cross-sectional observational design is more speculative, as the relationship between abnormalities in RSFC and abnormalities in task-related functional connectivity in CEM has not yet been studied directly. Our seed-based analysis is also a possible limitation as it focuses on certain networks, ignoring possibly valuable information about other networks in the brain. Another possible limitation is the influence of between-group differences in heart rate variability and respiratory rate on the results. As this physiological activity was not monitored in the current study, it remains unclear whether any differences between the two groups have influenced the results. However, regressing out global signal changes has been shown to at least partly filter out the effects of cardiac and respiratory fluctuations (Birn et al. Reference Birn, Diamond, Smith and Bandettini2006; Fox & Raichle, Reference Fox and Raichle2007). Finally, our RS-fMRI data were acquired at the end of a fixed imaging protocol: after completion of three task-related fMRI runs and the acquisition of an anatomical scan (scan sequence: Tower of London, word encoding, T1-weighted scan, word recognition, perception of facial expression, resting-state scan; van Tol et al. Reference van Tol, van der Wee, Demenescu, Nielen, Aleman, Renken, van Buchem, Zitman and Veltman2011). It is therefore possible that the facial expression task influenced the RSFC (i.e. carryover effect), with subjects from our CEM group showing aberrant connectivity in areas involved in the processing of emotional faces while the facial stimulus was no longer present.
In summary, this study is the first study to demonstrate patterns of aberrant RSFC in adult individuals reporting CEM, between areas in the brain known to be involved in (emotional) stimulus processing, emotion regulation, decision making and self-referential processing. The aberrant connectivity of the precuneus with both the limbic network and the salience network in CEM is a novel finding and its possible relationship with disturbances of self-referential processing, typically found in CEM, should be investigated in future studies.
Supplementary material
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0033291712002942.
Acknowledgments
The infrastructure for the NESDA is funded through the Geestkracht program of the Netherlands Organization for Health Research and Development (ZonMw, grant no. 10-000-1002) and is supported by participating universities and mental health care organizations (VU University Medical Center, GGZ inGeest, Arkin, Leiden University Medical Center, GGZ Rivierduinen, University Medical Center Groningen, Lentis, GGZ Friesland, GGZ Drenthe, IQ Healthcare, Netherlands Institute for Health Services Research (NIVEL) and Netherlands Institute of Mental Health and Addiction (Trimbos Institute). Part of this research was supported through the Netherlands Organization for Scientific Research – National Initiative Brain and Cognition project (NWO-NIHC, project no. 056-25-010) and VIDI grants from the Netherlands Organization for Scientific Research (NWO) to the authors S. A. R. B. Rombouts (Grant no. 917. 863.68) and B. M. Elzinga (Grant no. 016.085.353).
Declaration of Interest
None.






