Introduction
The subcallosal region (SCR) mainly includes the subcallosal area (BA25 and prelimbic BA32) and the perigenual ventromedial prefrontal cortex (vmPFC) (BA10m) (Hamani et al., Reference Hamani, Mayberg, Snyder, Giacobbe, Kennedy and Lozano2009, Reference Hamani, Mayberg, Stone, Laxton, Haber and Lozano2011) and is an extensively connected component of the limbic system, including the subcortical cortex, amygdala and hippocampus, and is involved in autonomic regulation (Gianaros et al., Reference Gianaros, Van Der Veen and Jennings2004), emotion regulation (Wager et al., Reference Wager, Davidson, Hughes, Lindquist and Ochsner2008), autobiographical memory (Addis et al., Reference Addis, Pan, Vu, Laiser and Schacter2009; van der Meer et al., Reference van der Meer, Costafreda, Aleman and David2010), and reward-based learning (Valentin et al., Reference Valentin, Dickinson and O'doherty2007). Growing evidence from structural imaging studies has suggested that there are volume reductions in the SCR in major depressive disorder (MDD) (Kempton et al., Reference Kempton, Salvador, Munafo, Geddes, Simmons, Frangou and Williams2011; Du et al., Reference Du, Wu, Yue, Li, Liao, Kuang, Huang, Chan, Mechelli and Gong2012; Bijanki et al., Reference Bijanki, Hodis, Brumm, Harlynn and Mccormick2014; Rodriguez-Cano et al., Reference Rodriguez-Cano, Sarro, Monte, Maristany, Salvador, Mckenna and Pomarol-Clotet2014; Jaworska et al., Reference Jaworska, Yucel, Courtright, Macmaster, Sembo and Macqueen2016). A recent, large meta-analysis of cortical abnormalities from the ENIGMA MDD working group confirmed thinning of the bilateral medial PFC of depressed adult patients and a reduction in the left medial PFC surface area (SA) in depressed adolescent patients (Schmaal et al., Reference Schmaal, Hibar, Samann, Hall, Baune, Jahanshad, Cheung, Van Erp, Bos, Ikram, Vernooij, Niessen, Tiemeier, Hofman, Wittfeld, Grabe, Janowitz, Bulow, Selonke, Volzke, Grotegerd, Dannlowski, Arolt, Opel, Heindel, Kugel, Hoehn, Czisch, Couvy-Duchesne, Renteria, Strike, Wright, Mills, De Zubicaray, Mcmahon, Medland, Martin, Gillespie, Goya-Maldonado, Gruber, Kramer, Hatton, Lagopoulos, Hickie, Frodl, Carballedo, Frey, Van Velzen, Penninx, Van Tol, Van Der Wee, Davey, Harrison, Mwangi, Cao, Soares, Veer, Walter, Schoepf, Zurowski, Konrad, Schramm, Normann, Schnell, Sacchet, Gotlib, Macqueen, Godlewska, Nickson, Mcintosh, Papmeyer, Whalley, Hall, Sussmann, Li, Walter, Aftanas, Brack, Bokhan, Thompson and Veltman2017). In addition, the SCR is an important target for deep brain stimulation (DBS) treatment for treatment-resistant depression (Lozano et al., Reference Lozano, Mayberg, Giacobbe, Hamani, Craddock and Kennedy2008; Hamani et al., Reference Hamani, Mayberg, Stone, Laxton, Haber and Lozano2011, Holtzheimer et al., Reference Holtzheimer, Kelley, Gross, Filkowski, Garlow, Barrocas, Wint, Craighead, Kozarsky, Chismar, Moreines, Mewes, Posse, Gutman and Mayberg2012, Riva-Posse et al., Reference Riva-Posse, Choi, Holtzheimer, Crowell, Garlow, Rajendra, Mcintyre, Gross and Mayberg2018). Taken together, these results strongly suggested that the SCR plays a key role in the pathophysiology of depression and in treatment effects (Riva-Posse et al., Reference Riva-Posse, Choi, Holtzheimer, Crowell, Garlow, Rajendra, Mcintyre, Gross and Mayberg2018).
Previous structural imaging studies examining the effects of depression on the SCR have largely been based on measures of cortical volume (CV). For example, left subgenual PFC volume reductions were found in young women with adolescent-onset MDD (Botteron et al., Reference Botteron, Raichle, Drevets, Heath and Todd2002) and in patients with a family history of depression (Bijanki et al., Reference Bijanki, Hodis, Brumm, Harlynn and Mccormick2014). Depressed participants with comorbid anxiety also had smaller subgenual PFC volumes than those without anxiety (Jaworska et al., Reference Jaworska, Yucel, Courtright, Macmaster, Sembo and Macqueen2016). Thus, decreased subgenual PFC volumes may suggest delayed or altered neurodevelopment in a key emotion regulation region (Jaworska et al., Reference Jaworska, Yucel, Courtright, Macmaster, Sembo and Macqueen2016). In addition, CV is a product of cortical thickness (CT) and SA (Panizzon et al., Reference Panizzon, Fennema-Notestine, Eyler, Jernigan, Prom-Wormley, Neale, Jacobson, Lyons, Grant, Franz, Xian, Tsuang, Fischl, Seidman, Dale and Kremen2009), and some neuroimaging studies have found that CT and SA differentially contributed to volume loss in neuropsychiatric disorders (Dickerson et al., Reference Dickerson, Feczko, Augustinack, Pacheco, Morris, Fischl and Buckner2009; Rimol et al., Reference Rimol, Nesvag, Hagler, Bergmann, Fennema-Notestine, Hartberg, Haukvik, Lange, Pung, Server, Melle, Andreassen, Agartz and Dale2012; Ecker et al., Reference Ecker, Ginestet, Feng, Johnston, Lombardo, Lai, Suckling, Palaniyappan, Daly, Murphy, Williams, Bullmore, Baron-Cohen, Brammer, Murphy and Consortium2013). For example, widespread reduction in CV in frontal, temporal, occipital, and parietal regions in schizophrenia was mainly driven by cortical thinning (Rimol et al., Reference Rimol, Nesvag, Hagler, Bergmann, Fennema-Notestine, Hartberg, Haukvik, Lange, Pung, Server, Melle, Andreassen, Agartz and Dale2012). In contrast, the abnormal volumes in autism spectrum disorder were driven by SA rather than CT (Ecker et al., Reference Ecker, Ginestet, Feng, Johnston, Lombardo, Lai, Suckling, Palaniyappan, Daly, Murphy, Williams, Bullmore, Baron-Cohen, Brammer, Murphy and Consortium2013). Thinning of the medial temporal lobe (MTL) contributed to small volumes of the MTL in Alzheimer's disease (Dickerson et al., Reference Dickerson, Feczko, Augustinack, Pacheco, Morris, Fischl and Buckner2009). In contrast, the small volume of the MTL was associated with diminished MTL SA in normal ageing (Dickerson et al., Reference Dickerson, Feczko, Augustinack, Pacheco, Morris, Fischl and Buckner2009). It is possible that SA and CT have distinct developmental pathways that are modulated by different neurobiological mechanisms (Ecker et al., Reference Ecker, Ginestet, Feng, Johnston, Lombardo, Lai, Suckling, Palaniyappan, Daly, Murphy, Williams, Bullmore, Baron-Cohen, Brammer, Murphy and Consortium2013). Thus, it is not clear whether volumetric reductions in the SCR in MDD patients are due to thinning, loss of SA, or both.
CT and SA represent distinct genetic aetiologies (Panizzon et al., Reference Panizzon, Fennema-Notestine, Eyler, Jernigan, Prom-Wormley, Neale, Jacobson, Lyons, Grant, Franz, Xian, Tsuang, Fischl, Seidman, Dale and Kremen2009) and cellular processes (Chenn and Walsh, Reference Chenn and Walsh2002) and show unique developmental trajectories in children and adolescents (Wierenga et al., Reference Wierenga, Langen, Oranje and Durston2014) across the adult life span (Hogstrom et al., Reference Hogstrom, Westlye, Walhovd and Fjell2013; Storsve et al., Reference Storsve, Fjell, Tamnes, Westlye, Overbye, Aasland and Walhovd2014). For instance, the CT was reduced and negatively correlated with increasing age during late childhood and across the adult life span (Storsve et al., Reference Storsve, Fjell, Tamnes, Westlye, Overbye, Aasland and Walhovd2014). The SA was positively correlated with age until late childhood, and then smaller, steady decreases were observed with increasing age (Amlien et al., Reference Amlien, Fjell, Tamnes, Grydeland, Krogsrud, Chaplin, Rosa and Walhovd2016). The dominant contributor to CV reductions during adolescence (Tamnes et al., Reference Tamnes, Herting, Goddings, Meuwese, Blakemore, Dahl, Guroglu, Raznahan, Sowell, Crone and Mills2017) and with ageing (Storsve et al., Reference Storsve, Fjell, Tamnes, Westlye, Overbye, Aasland and Walhovd2014) was cortical thinning. In addition, individuals with depression had significant differences in CV that may be differentially affected by depression at various stages of life (Schmaal et al., Reference Schmaal, Hibar, Samann, Hall, Baune, Jahanshad, Cheung, Van Erp, Bos, Ikram, Vernooij, Niessen, Tiemeier, Hofman, Wittfeld, Grabe, Janowitz, Bulow, Selonke, Volzke, Grotegerd, Dannlowski, Arolt, Opel, Heindel, Kugel, Hoehn, Czisch, Couvy-Duchesne, Renteria, Strike, Wright, Mills, De Zubicaray, Mcmahon, Medland, Martin, Gillespie, Goya-Maldonado, Gruber, Kramer, Hatton, Lagopoulos, Hickie, Frodl, Carballedo, Frey, Van Velzen, Penninx, Van Tol, Van Der Wee, Davey, Harrison, Mwangi, Cao, Soares, Veer, Walter, Schoepf, Zurowski, Konrad, Schramm, Normann, Schnell, Sacchet, Gotlib, Macqueen, Godlewska, Nickson, Mcintosh, Papmeyer, Whalley, Hall, Sussmann, Li, Walter, Aftanas, Brack, Bokhan, Thompson and Veltman2017). For example, a reduction in the left medial PFC SA was found in depressed adolescent (⩽21 years) patients compared with controls (Schmaal et al., Reference Schmaal, Hibar, Samann, Hall, Baune, Jahanshad, Cheung, Van Erp, Bos, Ikram, Vernooij, Niessen, Tiemeier, Hofman, Wittfeld, Grabe, Janowitz, Bulow, Selonke, Volzke, Grotegerd, Dannlowski, Arolt, Opel, Heindel, Kugel, Hoehn, Czisch, Couvy-Duchesne, Renteria, Strike, Wright, Mills, De Zubicaray, Mcmahon, Medland, Martin, Gillespie, Goya-Maldonado, Gruber, Kramer, Hatton, Lagopoulos, Hickie, Frodl, Carballedo, Frey, Van Velzen, Penninx, Van Tol, Van Der Wee, Davey, Harrison, Mwangi, Cao, Soares, Veer, Walter, Schoepf, Zurowski, Konrad, Schramm, Normann, Schnell, Sacchet, Gotlib, Macqueen, Godlewska, Nickson, Mcintosh, Papmeyer, Whalley, Hall, Sussmann, Li, Walter, Aftanas, Brack, Bokhan, Thompson and Veltman2017). A meta-analysis showed grey matter volume reductions in the bilateral medial PFC in late-life depression (Du et al., Reference Du, Liu, Chen, Huang, Li, Kuang, Yang, Zhang, Zhou, Bi, Kendrick and Gong2014). Thus, whether the alterations in SA or CT of the SCR in MDD were associated with age through the adult life span remain unclear.
The aim of the current study was to identify SCR differences in the CT, SA, and CV across the adult lifespan in a large sample of patients with depressive disorders (age range: 18–74 years) and healthy control (HC) subjects (age range: 19–72 years). We used an automated method of regional parcellation (FreeSurfer, Destrieux atlas) to measure the CT, SA, and CV of the bilateral SCR region; specifically, the SCR was region 32 in the Destrieux atlas (Destrieux et al., Reference Destrieux, Fischl, Dale and Halgren2010). Based on neurobiological knowledge as well as previous work, CT and SA are known to represent distinct morphometric features of the cortex that may be differentially affected by depression at various stages of life. We hypothesised that reductions in SCR volume and SA would be observed in patients with depressive disorders compared with HC subjects, while SCR thinning would be associated with ageing.
Materials and methods
Samples
Initially, 383 consecutively recruited MDD outpatients and 260 HC participants were included and underwent a resting-state functional and structural magnetic resonance imaging (MRI) scan as part of a project investigating human neuroimaging markers of MDD (Cheng et al., Reference Cheng, Rolls, Qiu, Liu, Tang, Huang, Wang, Zhang, Lin, Zheng, Pu, Tsai, Yang, Lin, Wang, Xie and Feng2016). They underwent a diagnostic interview by experienced doctors using the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, 4th edition, for Axis I Disorders. For this study, the final sample included 114 individuals with MDD (41 males, 73 female) and 112 matched control individuals (40 males, 72 females). The main exclusion criteria were as follows: (1) participants were excluded if they had not completed the personality test (Eysenck Personality Questionnaire, EPQ), resulting in 214 MDDs and 150 HCs remaining; (2) 16 patients with bipolar disorders were excluded, resulting in 198 MDDs and 150 HCs remaining; (3) 32 participants with hypertension, diabetes and cardiovascular disorders were excluded, resulting in 185 MDDs and 131 HCs remaining; (4) participants aged younger than 18 years and older than 75 years were excluded, resulting in 165 MDDs and 126 HCs remaining; (5) 63 participants who had not completed the Short Ruminative Responses Scale test were excluded, resulting in 116 MDDs and 112 HCs remaining; and (6) participants with bad imaging data and bad segments (by visual inspection) were excluded, resulting in the final sample of 114 MDDs and 112 HCs (see Fig. 1). Neuroticism and rumination have important implications for understanding the development and maintenance of depressive episodes. A previous study indicated that ruminative self-focus was associated with enhanced activity of the subgenual anterior cingulate cortex in depression (Mandell et al., Reference Mandell, Siegle, Shutt, Feldmiller and Thase2014). We also wanted to test whether alterations in the structure of the SCR were associated with neuroticism or rumination in depressive disorders. In the end, 112 matched control individuals and 114 individuals with MDD were retained. Of the 114 MDD patients included, 95 were first episode and 19 were recurrence; 29 of the patients had depression with anxiety, and 50 of the patients were medicated for MDD (see Table 1). In the current study, the mean age of the patients with depression was 37.12 years (s.d. = 13.27, range = 18–74), and the mean age of the HC subjects was 39.07 years (s.d. = 12.79, range = 19–72). Depression severity was rated using the 17-item Hamilton Depression Rating Scale (HDRS-17) by interview, as well as the self-report scales in the Beck Depression Inventory-II (BDI-II). Participants provided written informed consent to participate. The study was approved by the Institutional Review Board of Chongqing Medical University for the protection of human subjects and was performed in accordance with the Declaration of Helsinki.

Fig. 1. The pipeline of selecting the sample.
Table 1. Demographic and clinical characteristics of major depression disorders and healthy comparison subjects

MRI data acquisition
High-resolution T1-weighted structural images were acquired on a 3.0-T Siemens Trio MRI scanner using a 12-channel whole-brain coil (Siemens Medical, Erlangen, Germany) using magnetisation-prepared rapid acquisition gradient-echo sequence (MPRAGE) (echo time = 2.52 ms; repetition time = 1900 ms; inversion time = 900 ms; flip angle = 9°; slices = 176; field of view = 256 × 256; voxel size = 1 × 1 × 1 mm3).
Cortical surface reconstruction and measures
All cortical parcellations and surface-based cortical reconstruction were performed by using FreeSurfer software (Version 5.3, https://surfer.nmr.mgh.harvard.edu). In brief, T1-weighted images first underwent a series of preprocessing steps that involved intensity non-uniformities, skull stripping, tissue classification, and surface extraction. In each hemisphere, the white matter was segmented, and the surface was generated by tessellation. After correcting for topological defects, the pial surface was produced by nudging the white surface outwards. During the reconstruction, several check points (skull strip, white matter segments and pial surface) were visually inspected, and segmentation errors were corrected. CT was measured by calculating the shortest distance from the grey/white boundary to the grey/cerebrospinal fluid (CSF) boundary at each vertex. Next, the surface was divided into separate cortical regions using an automated labelling approach. Finally, the mean CV (in mm3), SA (in mm2), and CT (in mm) were extracted for each of the 148 regions (74 per hemisphere) in the parcellation scheme (i.e. Destrieux atlas) (Destrieux et al., Reference Destrieux, Fischl, Dale and Halgren2010).
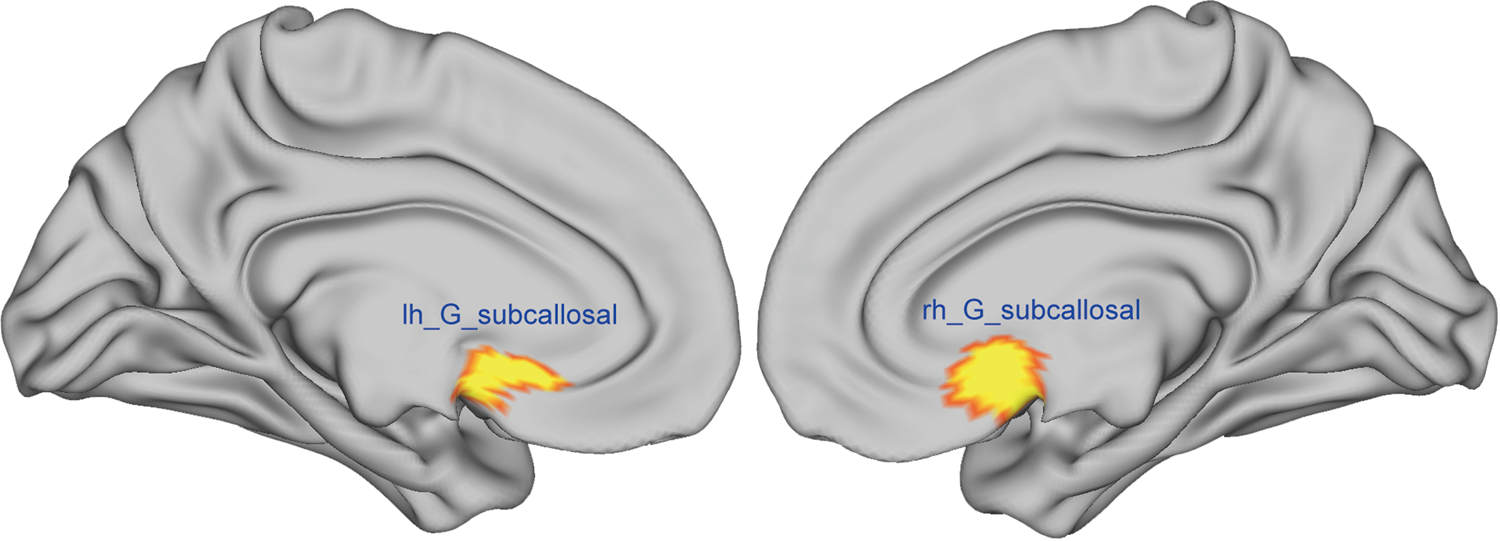
In the current study, we computed the CV, SA, and CT of the SCR using the cortical parcellation based on the Destrieux atlas. Specifically, the SCR/gyrus was region 32 in the Destrieux atlas (see Fig. 2) (Destrieux et al., Reference Destrieux, Fischl, Dale and Halgren2010). In addition, the intracranial volume (ICV) was also obtained for each participant.
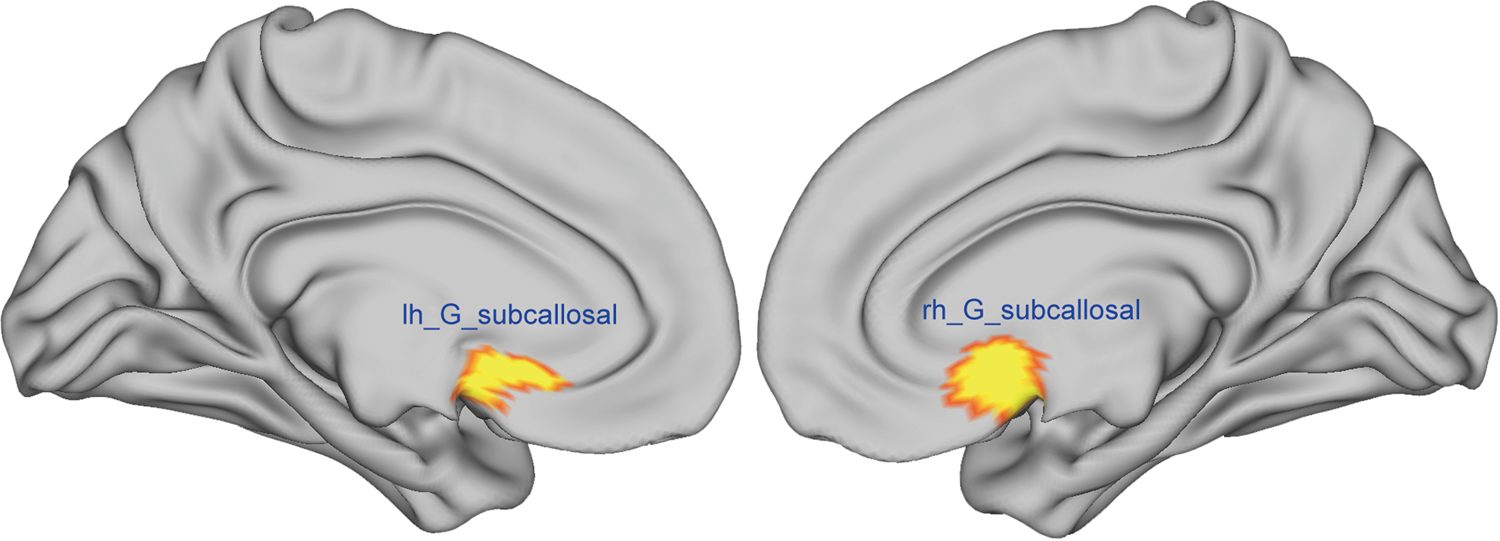
Fig. 2. The left and right SCR/gyrus was region 32 in the Destrieux atlas. The mean CV, SA and CT of this region were computed using cortical parcellation based on FreeSurfer software.
Statistics
We examined group differences in CV, CT and SA between the MDDs and HCs. To control the type-1 error rate, first, two separate multivariate analyses of covariance (MANCOVA) models were tested on the left and right SCR (CT, SA and CV), with group (MDD = 1, HC = 0) as the between-subject factor while controlling for age, sex, and total ICV. Subsequent univariate analyses were performed for each variable (CT, SA and CV). p values were adjusted for the number of variables measured (i.e. corrected for three measures in total). Furthermore, we examined associations between symptom severity and different measurement indices of the SCR (CV, CT and SA) within the MDD patient group.
To investigate the effects of age on the SCR in the MDDs and HCs, we used multiple linear regression models to test age-by-group interaction effects while controlling for sex and total ICV. In addition, a generalised additive model (GAM) was also employed to assess the association between left SCR (CT, SA and CV) and age in the MDDs and HCs. GAM was proposed by Hastie and Tibshirani (Reference Hastie and Tibshirani1995) as an effective method to tackle the problem of rapidly increasing variance in estimates when there is a large number of variables to model. Given that brain development is known to be a non-linear process, we modelled both linear and non-linear age effects using a GAM (Wood, Reference Wood2004, Reference Wood2006). The GAM was implemented to assess a penalty on non-linearity using restricted maximum likelihood to avoid over-fitting and thus captures both linear and non-linear effects in a data-driven fashion. In addition, GAM provides accurate delineations of developmental trajectories, as it avoids some of the inherent weaknesses of global polynomial models, e.g. quadratic and cubic models, where the timing of peaks and the end points of the trajectories may be substantially affected by irrelevant factors, such as the age range of the samples (Fjell et al., Reference Fjell, Walhovd, Westlye, Østby, Tamnes, Jernigan, Gamst and Dale2010). Furthermore, to detect potentially different effects of major depression with age, the participants were separated into three groups: early adulthood (18–30 years old; MDD = 35, HC = 50), middle adulthood (31–49 years old; MDD = 55, HC = 38) and later adulthood (50–75 years old; MDD = 24, HC = 24). We set the age cut-off for early adulthood MDD at ⩽30 based on (1) the first phase of early adulthood comes to a close at approximately 28–33 years or at the age 30 transition (Levinson, Reference Levinson1986) and (2) some neuroimaging studies have indicated that in several brain regions, structural growth curves and maturation had not plateaued even by the age of 30 (Amlien et al., Reference Amlien, Fjell, Tamnes, Grydeland, Krogsrud, Chaplin, Rosa and Walhovd2016; Somerville, Reference Somerville2016). Two-factor covariance analysis was performed, using the CT, SA and CV of the SCR as the dependent variables and group (MDD, HC) and age group as the independent variables, while controlling for age, sex, and total ICV; post hoc multiple comparison tests were performed to determine which means differed among these groups. p values were adjusted for the number of variables measured (i.e. corrected for three measures in total).
Statistical analyses were conducted using statistical software (R, Statistical Package version 3.3.2; R Foundation for Statistical Computing; www.R-project.org). The mgcv packages were used to apply the GAM function.
Results
Demographic and clinical measures
A total of 228 participants entered the study. The demographic, clinical, symptom severity and personality data of the MDDs and HCs are presented in Table 1. No significant differences between groups were observed in the demographic characteristics. There were no significant differences in the scores from the HDRS-17 and BDI-II between unmedicated and medicated depression patients. Compared with the recurrent patients, the first-episode patients showed no significant difference in either the HDRS-17 and BDI-II scores.
Multivariate analysis of covariance (MANCOVA)
A significant main effect of group (MDD v. HC) was found with the left SCR [F (3, 223) = 2.85, p = 0.03]. Subsequent univariate analyses showed that patients with depression had a smaller CV [F (1, 225) = 6.24, p = 0.013; Fig. 3] and SA [F (1, 225) = 8.09, p = 0.005; Fig. 3] of the left SCR than the HC subjects. There was no main effect of group on CT, SA or CV in the right SCR [F (3, 223) = 1.01, p = 0.39].

Fig. 3. Left column: The reductions in left SCR volume (top), SA (middle), and thickness (bottom) in the MDD group compared with the HC group after controlling for age, sex and total ICV. Right column: The age-related changes in left SCR volume (top), SA (middle), and thickness (bottom) both in the MDD and HC groups across the adult life span after controlling for sex and total ICV.
We also tested the effects of age on the left SCR in MDDs and HCs. There was no significant interaction effect of age-by-diagnosis on the left/right subcallosal SA, CV and CT. In addition, using the GAMs, we found a significant main effect of age on the left subcallosal CT (see Table 2, Fig. 3) in each group (MDDs and HCs) after controlling for sex and ICV.
Table 2. Results of nonparametric regression models

a Data were calculated using the GAMs.
b Degrees of freedom refers to the curvature of the fitted GAM line relative to a simple straight line. Some variables were automatically forced to a linear relationship (df = 1).
To detect potentially different effects of major depression with age, the participants were separated into three groups: early adulthood (18–30 years old; MDD = 35, HC = 50), middle adulthood (31–49 years old; MDD = 55, HC = 38) and later adulthood (50–75 years old; MDD = 24, HC = 24). Two-factor covariance analysis was performed, and there was a significant interaction effect between age group (early, middle, and later adulthood) and group (MDD and HC) for CV (F = 2.51, p = 0.03) and SA (F = 3.08, p = 0.01) of the left SCR. Then, post hoc multiple comparisons showed that the reductions in the CV (t = −2.9, p = 0.004) and SA (t = −3.2, p = 0.002) of the SCR were observed only in the patients with an adult age of illness onset compared with the early adulthood controls (see Fig. 4). We did not detect significant differences in CV (t = 0.22, p = 0.82) and SA (t = −0.15, p = 0.88) of SCR in middle adulthood patients compared with age-matched controls or in later adulthood patients compared with their age-matched controls (CV: t = −1.27, p = 0.22; SA: t = −1.39, p = 0.17).

Fig. 4. Post hoc multiple comparisons showed that the reduction in SCR volume (t = −2.9, p = 0.004) was observed only in patients in the early adult age range (18 years ⩽ age ⩽ 30 years, N = 35) compared with early adulthood controls (18 years ⩽ age ⩽ 30 years, N = 50).
Recurrence, medication, duration of illness, neuroticism, rumination and symptom severity
The CV, SA and CT of the SCR were not correlated with symptom severity using the HDRS-17 and BDI-II questionnaires. There are no significant differences on the cortical measurement of left SCG between unmedicated depression patients and medicated depression patients (F = 0.21, p = 0.97), and between first and recurrent episode patients (F = 1.36, p = 0.26). Two MANCOVA models were performed on the left and right SCRs (the dependent variables were CT, SA and CV), with diagnostic group (depression patients and depression patients with anxiety) as the between-subject factor while controlling for age, sex, and total ICV. There were no significant differences between depressed patients and depressed patients with anxiety [right: F (1, 109) = 0.21, p > 0.05; left: F (1, 109) = 0.22, p > 0.05]. In addition, our correlation analysis revealed no relationship between neuroticism, rumination and the structural brain measures of SCR within the MDD group.
Discussion
This study examined differences in the CV, CT and SA in the SCR across the adult life span in patients with depressive disorder relative to HC subjects. We also tested the effects of age on the CV, CT and SA of the SCR across the adult life span. We found that patients with depressive disorder had significant differences in the CV and SA of the SCR (Fig. 1) after controlling for the effects of brain size, age and sex. Furthermore, the reductions in the CV and SA of the left SCR were observed only in early adulthood patients (18–30 years) with depressive disorders compared with early adulthood HC groups. However, we found a significant age-related CT reduction in the SCR in both the group with depressive disorders and the HC group (see Fig. 2).
Consistent with our prediction and with prior voxel-based morphometry research (, Botteron et al., Reference Botteron, Raichle, Drevets, Heath and Todd2002, Du et al., Reference Du, Wu, Yue, Li, Liao, Kuang, Huang, Chan, Mechelli and Gong2012, Bijanki et al., Reference Bijanki, Hodis, Brumm, Harlynn and Mccormick2014; Jaworska et al., Reference Jaworska, Yucel, Courtright, Macmaster, Sembo and Macqueen2016), we observed left SCR CV reductions in patients with depressive disorders relative to HC subjects. The SCR has extensive connections with the PFC, nucleus accumbens, hypothalamus and brainstem and has been implicated in the pathophysiology of MDD (Lozano et al., Reference Lozano, Mayberg, Giacobbe, Hamani, Craddock and Kennedy2008, Hamani et al., Reference Hamani, Mayberg, Stone, Laxton, Haber and Lozano2011). Interestingly, the SCR CV reduction was mainly driven by its SA rather than CT in the current study. In terms of phylogeny, the size of the SA is related to the number of ontogenetic columns, whereas CT is contributed by the number of cells within a column (Rakic, Reference Rakic1988). Previous studies indicated that cortical area expansion might be more efficient to facilitate brain connectivity and functional development (Ruppin et al., Reference Ruppin, Schwartz and Yeshurun1993; Murre and Sturdy, Reference Murre and Sturdy1995; Hogstrom et al., Reference Hogstrom, Westlye, Walhovd and Fjell2013). The current results suggest that the reductions in the SA of the SCR may influence abnormal structural and functional connectivity between the SCR and cortical/subcortical areas in MDD. In addition, the SA and CT have distinct developmental pathways that are modulated by different neurobiological mechanisms and at different stages of life (Ecker et al., Reference Ecker, Ginestet, Feng, Johnston, Lombardo, Lai, Suckling, Palaniyappan, Daly, Murphy, Williams, Bullmore, Baron-Cohen, Brammer, Murphy and Consortium2013; Wierenga et al., Reference Wierenga, Langen, Oranje and Durston2014). For example, the CT decreased non-linearly across ages 4 to 87 (Storsve et al., Reference Storsve, Fjell, Tamnes, Westlye, Overbye, Aasland and Walhovd2014; Tamnes et al., Reference Tamnes, Herting, Goddings, Meuwese, Blakemore, Dahl, Guroglu, Raznahan, Sowell, Crone and Mills2017). In contrast, the SA expanded until approximately 12 years of age, followed by a relatively stable size and then a steady decrease with increasing age (Amlien et al., Reference Amlien, Fjell, Tamnes, Grydeland, Krogsrud, Chaplin, Rosa and Walhovd2016). Recently, the ENIGMA MDD group found cortical thinning of the bilateral medial PFC of depressed adult patients (>21 years) and a reduction in the left medial PFC SA in depressed adolescent (⩽21 years) patients (Schmaal et al., Reference Schmaal, Hibar, Samann, Hall, Baune, Jahanshad, Cheung, Van Erp, Bos, Ikram, Vernooij, Niessen, Tiemeier, Hofman, Wittfeld, Grabe, Janowitz, Bulow, Selonke, Volzke, Grotegerd, Dannlowski, Arolt, Opel, Heindel, Kugel, Hoehn, Czisch, Couvy-Duchesne, Renteria, Strike, Wright, Mills, De Zubicaray, Mcmahon, Medland, Martin, Gillespie, Goya-Maldonado, Gruber, Kramer, Hatton, Lagopoulos, Hickie, Frodl, Carballedo, Frey, Van Velzen, Penninx, Van Tol, Van Der Wee, Davey, Harrison, Mwangi, Cao, Soares, Veer, Walter, Schoepf, Zurowski, Konrad, Schramm, Normann, Schnell, Sacchet, Gotlib, Macqueen, Godlewska, Nickson, Mcintosh, Papmeyer, Whalley, Hall, Sussmann, Li, Walter, Aftanas, Brack, Bokhan, Thompson and Veltman2017). Their results indicated that cortical development may be dynamically impacted by depression at different stages of life. In addition, the reduced cortical area may be associated with a disturbance of neurodevelopment in schizophrenia (Rimol et al., Reference Rimol, Nesvag, Hagler, Bergmann, Fennema-Notestine, Hartberg, Haukvik, Lange, Pung, Server, Melle, Andreassen, Agartz and Dale2012) and a delay in cortical maturation in adolescent MDD (Schmaal et al., Reference Schmaal, Hibar, Samann, Hall, Baune, Jahanshad, Cheung, Van Erp, Bos, Ikram, Vernooij, Niessen, Tiemeier, Hofman, Wittfeld, Grabe, Janowitz, Bulow, Selonke, Volzke, Grotegerd, Dannlowski, Arolt, Opel, Heindel, Kugel, Hoehn, Czisch, Couvy-Duchesne, Renteria, Strike, Wright, Mills, De Zubicaray, Mcmahon, Medland, Martin, Gillespie, Goya-Maldonado, Gruber, Kramer, Hatton, Lagopoulos, Hickie, Frodl, Carballedo, Frey, Van Velzen, Penninx, Van Tol, Van Der Wee, Davey, Harrison, Mwangi, Cao, Soares, Veer, Walter, Schoepf, Zurowski, Konrad, Schramm, Normann, Schnell, Sacchet, Gotlib, Macqueen, Godlewska, Nickson, Mcintosh, Papmeyer, Whalley, Hall, Sussmann, Li, Walter, Aftanas, Brack, Bokhan, Thompson and Veltman2017). In our study, we found the reductions in the CV and SA of the left SCR only in early adulthood patients with depressive disorders (18–30 years) compared with the early adulthood HC group (see Figs 4 and 5). The SA of the SCR did not show a significant age-related decline across the adult life span (see Fig. 3). Thus, the alteration of functional/structural connections between the SCR and the PFC, nucleus accumbens, hypothalamus and brainstem may be influenced by delayed maturation through decreases in growth and branching of dendritic trees and the number of synapses associated with grey matter volume (Anderson, Reference Anderson2011), which may persist in adult MDD patients with an early age of onset of depression.

Fig. 5. Post hoc multiple comparisons showed that the reduction in subcallosal SA (t = −3.2, p = 0.002) was observed only in patients in the early adult age range (18 years ⩽ age ⩽ 30 years, N = 35) compared with early adulthood controls (18 years ⩽ age ⩽ 30 years, N = 50).
It has been suggested that SCR dysfunction may disturb stress-autonomic and neuroendocrine responses and reward-related mesolimbic dopamine function (Drevets et al., Reference Drevets, Ongur and Price1998; Myers-Schulz and Koenigs, Reference Myers-Schulz and Koenigs2012). Anxious/depressed scores were negatively associated with vmPFC CT at healthy younger ages (<9 years) (Ducharme et al., Reference Ducharme, Albaugh, Hudziak, Botteron, Nguyen, Truong, Evans and Karama2014). Childhood experiences of maltreatment were associated with lower subgenual anterior cingulate cortex-hippocampus connectivity in adolescence (Herringa et al., Reference Herringa, Birn, Ruttle, Burghy, Stodola, Davidson and Essex2013). Moreover, early life stress and symptoms of anxiety/depression in childhood and adolescence were involved in the pathogenesis of early-onset MDD (Herringa et al., Reference Herringa, Birn, Ruttle, Burghy, Stodola, Davidson and Essex2013; Ducharme et al., Reference Ducharme, Albaugh, Hudziak, Botteron, Nguyen, Truong, Evans and Karama2014). In the current study, laterality of the SCR reduction was found, which is consistent with left-lateralised changes reported in the PFC in MDD. For example, a recent meta-analysis showed a reduction in the left medial PFC SA in depressed adolescent (⩽21 years) patients (Schmaal et al., Reference Schmaal, Hibar, Samann, Hall, Baune, Jahanshad, Cheung, Van Erp, Bos, Ikram, Vernooij, Niessen, Tiemeier, Hofman, Wittfeld, Grabe, Janowitz, Bulow, Selonke, Volzke, Grotegerd, Dannlowski, Arolt, Opel, Heindel, Kugel, Hoehn, Czisch, Couvy-Duchesne, Renteria, Strike, Wright, Mills, De Zubicaray, Mcmahon, Medland, Martin, Gillespie, Goya-Maldonado, Gruber, Kramer, Hatton, Lagopoulos, Hickie, Frodl, Carballedo, Frey, Van Velzen, Penninx, Van Tol, Van Der Wee, Davey, Harrison, Mwangi, Cao, Soares, Veer, Walter, Schoepf, Zurowski, Konrad, Schramm, Normann, Schnell, Sacchet, Gotlib, Macqueen, Godlewska, Nickson, Mcintosh, Papmeyer, Whalley, Hall, Sussmann, Li, Walter, Aftanas, Brack, Bokhan, Thompson and Veltman2017), decreased CT in the left medial PFC associated with an increased number of episodes in MDD (Treadway et al., Reference Treadway, Waskom, Dillon, Holmes, Park, Chakravarty, Dutra, Polli, Iosifescu, Fava, Gabrieli and Pizzagalli2015), and reduced volume in the left subgenual PFC in young patients with early-onset MDD in comparison to control subjects (Botteron et al., Reference Botteron, Raichle, Drevets, Heath and Todd2002). Furthermore, we did not find that the CV, CT, and SA of the SCR were associated with depressive symptoms within the MDD groups, possibly due to depression being a heterogeneous clinical syndrome, and the alterations in SCR structure may be associated with specific depressive symptoms. The SCR is an important hub in a network that includes cortical structures, the limbic system, the thalamus, the hypothalamus, and brainstem nuclei, and dysfunction in the SCR-subcortical pathways may influence the observed depressive symptoms. Recently, a study indicated that the functional connectivity of SCR was significantly correlated with patient's percent change in the HDRS by treatment (Dunlop et al., Reference Dunlop, Rajendra, Craighead, Kelley, Mcgrath, Choi, Kinkead, Nemeroff and Mayberg2017). Further studies are required to clarify the relationship between the structural/functional connectivity of SCR and clinical symptoms of depression.
Interestingly, the CT of the SCR showed significant non-linear atrophy with ageing both in the MDD and HC groups. Previous studies indicated that CT changes showed a marked age-related reduction in extensive regions of brain compared with the SA changes across the adult life span (Lemaitre et al., Reference Lemaitre, Goldman, Sambataro, Verchinski, Meyer-Lindenberg, Weinberger and Mattay2012), and SA showed a relatively subtle decrease with age from late childhood to early adulthood (Tamnes et al., Reference Tamnes, Herting, Goddings, Meuwese, Blakemore, Dahl, Guroglu, Raznahan, Sowell, Crone and Mills2017). CT might be a more sensitive indicator of morphological ageing than SA in the subgenual PFC. The different trajectories between the CT and SA of the SCR with age may provide valuable information to distinguish pathological processes and normal ageing in MDD.
The current study has some limitations. The first limitation of our study is inherent in its cross-sectional design. In such studies, age-related changes in brain structure may be affected by potential cohort effects. The cross-sectional design of the study limits our ability to test the direction of causality, whether differences in the SCR SA or CV precede and confer vulnerability to or are the consequence of MDD. Second, alterations in the SCR structure are not specific to MDD, as these changes have been observed in other psychiatric disorders, such as post-traumatic stress disorder (Keding and Herringa, Reference Keding and Herringa2015), first-episode psychosis (Hirayasu et al., Reference Hirayasu, Shenton, Salisbury, Kwon, Wible, Fischer, Yurgelun-Todd, Zarate, Kikinis and Jolesz1999) and first-episode schizophrenia (Koo et al., Reference Koo, Levitt, Salisbury, Nakamura, Shenton and Mccarley2008). Thus, a future study design comparing patients with these disorders is clearly needed to resolve this issue. Third, in the current study, the assessment of the effects of recurrent episodes was based on patients’ subjective reports (yes/no). We have not collected data on the number of episodes, the duration of episodes, and the total time spent depressed. This study did not allow an investigation of antidepressant medication effects and the number of episodes on brain structure across the life span because of a lack of detailed information on the number of episodes, duration of episodes and type of antidepressant treatment. Fourth, our MDD samples were adults, and we do not know for certain whether and to what extent there was cortical maturation delay in MDD from early adolescence to adulthood. Future research is needed to investigate the CT and SA changes in depressive disorders in early adolescence, and longitudinal studies are required to understand the relationship between the depressive illness and neural development. In addition, the general intelligence and socioeconomic status of the participants were not collected in our study, and future research is needed to take into account these factors. Finally, we specifically focused on the SCR, which is an important target for DBS treatment for treatment-resistant depression and has extensive connections with the subcortical structures implicated in the pathophysiology of MDD. Therefore, the study did not evaluate the abnormal SA and CT of other brain structures.
Taken together, the left SCR CV reduction was mainly driven by SA rather than CT in early adulthood MDD. The SCR CT might be a more sensitive indicator of morphological ageing than SA both in MDD patients and HCs. The different trajectories between the CT and SA of the SCR with age may provide valuable information to distinguish pathological processes and normal ageing in MDD. Future studies are needed to examine the functional and structural connectivity of the SCR with other brain regions and to relate such connectivity to different dimensions of depressive symptoms and treatment response in MDD.
Author ORCIDs
Dongtao Wei, 0000-0003-2544-8015
Conflict of interest
All authors declare no competing interests.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (31571137; 31500885; 31600878; 31771231), Project of the National Defense Science and Technology Innovation Special Zone, Chang Jiang Scholars Program, National Outstanding Young People Plan, the Program for the Top Young Talents by Chongqing, the Fundamental Research Funds for the Central Universities (SWU1609177).