Introduction
Major depressive disorder (MDD) is often a chronic and/or recurrent disorder that can have major consequences over the entire lifespan. For clinical practice, it is important to be able to predict the risk of recurrence. In patients with a high risk of recurrence, it would seem reasonable to treat the depression as a chronic disorder (Andrews, Reference Andrews2001), for example with antidepressants as maintenance treatment (Geddes et al. Reference Geddes, Carney, Davies, Furukawa, Kupfer, Frank and Goodwin2003; Kaymaz et al. Reference Kaymaz, van Os, Loonen and Nolen2008), whereas treatment of those with a low risk of recurrence may be limited to the index episode. If the most important risk factors for recurrence are known, subgroups could be selected that need more intensive or long-term treatment.
Over the past 20–30 years, several studies have been able to assess putative risk factors for the recurrence of MDD. The factors that have been considered include sociodemographic data (e.g. age, gender and level of education), psychosocial characteristics (e.g. personality and social support) and clinical characteristics of the depressive disorder (e.g. the number, duration and severity of previous episodes). Clinical factors, such as subclinical residual symptoms and the number of previous episodes, have been found to be the most important predictors whereas sociodemographic factors do not seem to predict the recurrence of MDD (Hardeveld et al. Reference Hardeveld, Spijker, De Graaf, Nolen and Beekman2010). However, the prediction of recurrence is complex; it is likely that multiple predictors may be identified and that predictors interact with one another producing an aversive cascade of synergetic events resulting in the recurrence of a depressive state. Consequently, it may be possible to identify a combination of risk factors that have a high overall predictive value.
A major limitation of the current knowledge is that the majority of previous studies on risk factors for recurrence were performed in specialized mental health care (with more severe, complex, recurrent and long-lasting disorders) whereas the natural course of depressive disorder and the risk factors for recurrence are best studied using a general population sample without any selection bias (Eaton et al. Reference Eaton, Shao, Nestadt, Lee, Bienvenu and Zandi2008). Moreover, as most depressed patients are treated in primary care, it could be that risk factors for recurrence in specialized mental health care are different from those in primary care.
Large-scale general population-based follow-up studies addressing the recurrence of MDD are scarce. The best-known studies are the Zurich cohort (Merikangas et al. Reference Merikangas, Zhang, Avenevoli, Acharyya, Neuenschwander and Angst2003), the Lundby Study (Mattison et al. Reference Mattison, Bogren, Horstmann, Munk-Jorgensen and Nettelbrandt2007) and the Baltimore Epidemiologic Catchment Area (ECA) follow-up (Eaton et al. Reference Eaton, Anthony, Gallo, Cai, Tien, Romanoski, Lyketsos and Chen1997, Reference Eaton, Shao, Nestadt, Lee, Bienvenu and Zandi2008). These studies focused mainly on the risk of recurrence of MDD and not on the determinants of recurrence. Therefore, knowledge about the predictors of recurrence is still incomplete.
The aim of this study was to determine the incidence and risk factors for the recurrence of MDD among people who had recovered from their last episode of MDD, using data from The Netherlands Mental Health Survey and Incidence Study (NEMESIS), a large prospective naturalistic psychiatric survey (n=7076) among the general population.
Method
Study sample
Data derived from the NEMESIS (Bijl et al. Reference Bijl, Van Zessen, Ravelli, De Rijk and Langendoen1998b), which surveyed the Dutch adult general population aged 18–64 years, were recorded at three waves: at baseline in 1996, at the 12-month follow-up in 1997 and after 3 years (1999). The methodology is described in detail elsewhere (Bijl et al. Reference Bijl, Van Zessen, Ravelli, De Rijk and Langendoen1998b). In brief, the NEMESIS is based on a multistage, stratified, random sampling procedure. The first step of this study was to draw a sample from 90 Dutch municipalities. The stratification criteria were urbanicity (five categories as classified by Statistics Netherlands) and adequate distribution over the 12 provinces. The second step was to draw a sample of private households (addresses) from post office registers. The number of households selected in each municipality was governed by the size of its population. The third step was to choose which individuals to interview. The selected households were sent a letter of introduction signed by the Minister of Public Health requesting their participation. Shortly thereafter, they were contacted by telephone by the interviewers. Households with no telephone or with ex-directory numbers (18%) were visited in person. A respondent was selected randomly in each household: the member with the most recent birthday, on the condition that they were between 18 and 64 years of age and sufficiently fluent in Dutch to be interviewed. Persons who were not immediately available (owing to circumstances such as hospitalization, travel or imprisonment) were contacted later in the year. If necessary, the interviewers made a maximum of 10 telephone calls or visits to an address at different times and on different days. Respondents received no remuneration but only a token of appreciation at the end of the interview. At baseline (T0), 7076 persons were eligible for inclusion, with a response rate of 69.7% (Bijl et al. Reference Bijl, Ravelli and Van Zessen1998a). After 12 months (T1), 1458 respondents (20.6%) were lost to attrition and, after 3 years (T2), a further 822 (14.6%) were lost. In total, 4796 respondents (67.8%) were interviewed at all three waves.
Cohort
We aimed to study the time to recurrence of a major depressive episode (MDE) and associated risk factors, in respondents who had recovered from an MDE. The design relied on both retrospective and prospective data.
The recency and severity of the last depressive episode was assessed retrospectively at baseline (T0) using the Composite International Diagnostic Interview (CIDI); respondents were asked at what age the last period of MDD ended. The time to recurrence was assessed prospectively between T0 and T2.
To prevent contaminating the risk factor data with current levels of depression, we only included respondents without a current MDD and/or dysthymia at baseline (T0). Dysthymia was defined according to DSM-III-R criteria but we could not assess whether dysthymia was also present before the onset of MDD. An advantage of including people with remitted depression at baseline was that the measurement of the predictors was not influenced by the presence of an MDE. Furthermore, respondents had to be free of these diagnoses for 6 months. As a consequence, a duration of partial or full remission of at least 6 consecutive months was chosen. This term was based on a 10-year follow-up study (Furukawa et al. Reference Furukawa, Fujita, Harai, Yoshimura, Kitamura and Takahashi2008) that concluded that the best duration to declare remission is probably 4 or 6 months. Therefore, recurrence of MDD was operationalized as a return of symptoms after (partial or complete) remission of at least 6 months and symptoms that were sufficiently severe to satisfy criteria for MDD according to DSM-III-R. It is important to note that respondents with partial remission were also included because this is a well-known risk factor for recurrence (Hardeveld et al. Reference Hardeveld, Spijker, De Graaf, Nolen and Beekman2010). Respondents with a lifetime diagnosis of bipolar disorder or schizophrenia or for whom the diagnosis had changed during the follow-up period from unipolar to bipolar disorder or schizophrenia were excluded, as they are likely to have a different recurrence risk. To contribute to the prospective data, respondents had to be reinterviewed at least once during follow-up.
Consequently, the study sample was assembled by first identifying all respondents with a lifetime diagnosis of MDD (first or recurrent cases) at T0 (n=1153). Three cases had missing data on the variable ‘age of the last depressive episode’. Of the 1150 cases identified, at T0 (6-month prevalence), 289 respondents had an ongoing episode of MDD and 17 had dysthymia and were excluded from further evaluation. For eight respondents, the diagnosis had changed during the follow-up period from unipolar to bipolar disorder or schizophrenia, and they were also excluded. Thus, 836 respondents were at risk for a recurrent episode of MDD. Of the 836 eligible subjects, 687 (82.2%) were reinterviewed at T1 and 590 (70.1%) at T2. Hence, 687 (82.2%) respondents were reinterviewed at least once and constitute the study sample (Table 1). Drop-out (30.0%) of the eligible subjects was lower than that in the total sample (35.2%) and was not associated with demographic factors (age, gender), severity of the last episode of MDD (χ2 = 2.53, df = 2, p = 0.28) or the number of previous episodes (χ2 = 0.63, df = 1, p = 0.43).
Table 1. Characteristics of 687 included subjects
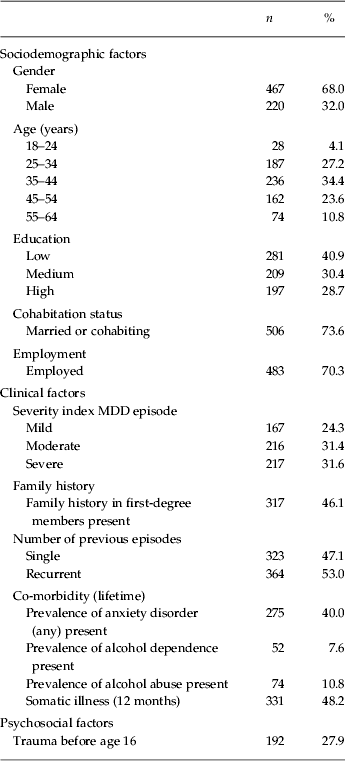
MDD, Major depressive disorder.
The sociodemographic and clinical characteristics of the study sample (n = 687) were as follows (Table 1): 68.0% (n = 467) were female; median age was 40.7 years; 73.6% (n = 506) were married or cohabiting; 70.3% (n = 483) were employed; mean age of onset was 29.9 years (s.d. = 10.5); 47.0% (n = 323) had experienced a single MDE and 53.0% (n = 364) had two or more episodes; in 31.6% (n = 217) the last MDE was severe, in 31.4% (n = 216) moderate and in 24.3% (n = 167) mild; 40.0% (n = 275) had a lifetime anxiety disorder at T0; 7.6% (n = 52) had a lifetime alcohol dependence; and 2.0% (n = 14) had a lifetime drug dependence.
Measurements
Diagnostic instrument
Diagnoses of psychiatric disorders according to DSM-III-R (APA, 1987) were based on the CIDI version 1.1 (computerized version) (Smeets & Dingemans, Reference Smeets and Dingemans1993). The CIDI is a structured interview developed by the World Health Organization (WHO, 1990) and has been found to have high inter-rater reliability and high test–retest reliability for most diagnoses (Wittchen, Reference Wittchen1994). The following DSM-III-R diagnoses were recorded in NEMESIS: mood disorders (bipolar disorder, major depression, dysthymia), anxiety disorders (panic disorder, agoraphobia, simple phobia, social phobia, generalized anxiety disorder, obsessive–compulsive disorder), psycho-active substance use disorders (alcohol or drug abuse and dependence, including sedatives, hypnotics and anxiolytics), eating disorders, schizophrenia and other non-affective psychotic disorders. Prevalence rates were calculated using the hierarchical rules of DSM, thus excluding MDEs occurring in schizophrenic and other psychotic disorders or in bipolar disorders.
Time to recurrence
A mentioned previously, the time to recurrence was assessed retrospectively at baseline and prospectively between T0 and T2. Using the prevalence rates (1-month, 6-month, 1-year and 2-year) at T1 or T2, a period could be defined in which the recurrence of an MDE occurred and the average time of this period was calculated. For example, if a respondent had a 6-month prevalence of MDD at T1 but no 1-month prevalence at T1 and reported the last MDE 36 months before T0, it was estimated that the time to recurrence was 45 months (36 months before T0 plus 9 months between T0 and T1).
Potential predictors for time to recurrence
Sociodemographic, clinical and psychosocial factors were assessed, along with personality characteristics.
Sociodemographic factors
These included gender, age, cohabitation status (categorized into living alone or not), educational attainment (categorized into low, medium and high) and employment status (categorized into paid employment or not).
Clinical factors
With the CIDI, information was obtained on age of onset, severity of the last MDE (categorized into mild, moderate and severe, with or without psychotic features), number of MDEs (categorized into single or recurrent), history of MDD in first-degree family members (categorized into yes or no) and lifetime co-morbidity with other mental disorders. The co-morbid disorders we deemed relevant were anxiety disorders, alcohol dependence and abuse. Co-morbidity with somatic illnesses was assessed with a questionnaire listing 31 mostly chronic somatic conditions.
Psychosocial factors
Several psychosocial factors were measured at T1. Life events and ongoing difficulties were recorded with a semi-structured interview-based questionnaire (De Graaf et al. Reference De Graaf, Bijl, Ravelli, Smit and Vollebergh2002; Spijker et al. Reference Spijker, De Graaf, Bijl, Beekman, Ormel and Nolen2004) based on information about life events and difficulties in the manual of the Life Events and Difficulties Schedule (LEDS; Brown & Harris, Reference Brown and Harris1987). The occurrence of the following nine negative life events in the 12 months preceding T1 were recorded: adverse change in health status, adverse change in health status of a significant other, adverse change in important domains (such as loss of employment, divorce), adverse change in important domains of a significant other, adverse change in living conditions, expected adverse changes in the future, failure to attain an important goal, another important self-reported distressing event (such as physical threat or assault, sexual violence, discrimination), or another important distressing event of a significant other. The presence of three distressing ongoing conflicts or difficulties in the 12 months preceding T1 were recorded: relationship problems, conflicts at work or school, and private or occupational problems (such as noise exposure, financial difficulties). These are persisting situations that usually develop gradually and form a continuous source of daily problems and concerns. Because the impact of specific events can vary, depending on their context or their meaning for the individual, we questioned respondents on their subjective perception of the effects that each event had had on their own mental health.
Furthermore, the recency of life events and ongoing difficulties were assessed. Only ongoing difficulties or life events experienced as ‘a significant influence’ preceding recurrence were included. The outcome was categorized into ‘yes’ or ‘no’. The subjective experience of social support between T0 and T1 was measured by the Social Support Questionnaire for Satisfaction with the supportive transactions (SSQS; Doeglas et al. Reference Doeglas, Suurmeijer, Briancon, Moum, Krol, Bjelle, Sanderman and van den Heuvel1996; Suurmeijer et al. Reference Suurmeijer, Doeglas, Briancon, Krijnen, Krol, Sanderman, Mourn, Bjelle and Van den Heuvel1996) with 23 items. The internal reliability of the SSQS in the research cohort was high (Cronbach's α = 0.88). Childhood experiences of emotional neglect, emotional or physical abuse or sexual abuse before age 16 were recorded. The answers for neglect and for emotional and physical abuse were categorized into ‘once to sometimes’ versus ‘regular to frequent’ and the answers for sexual abuse were categorized into ‘never’ versus ‘once or more’. Negative youth experiences were present if the respondent confirmed emotional or physical abuse on a regular to frequent basis or if the respondent had experienced sexual abuse once or more.
Personality characteristics
Neuroticism was assessed with the Groningen Neuroticism Questionnaire containing 14 items (Ormel, Reference Ormel1980). The internal reliability of the questionnaire in our research cohort (Cronbach's α = 0.75) was satisfactory. Locus of control was assessed with the five-item Mastery Scale (Pearlin & Schooler, Reference Pearlin and Schooler1978), with high scores on mastery corresponding to an internal locus of control. The internal reliability of this questionnaire in our research cohort (Cronbach's α = 0.78) was satisfactory.
Statistical methods
We used Kaplan–Meier survival curves to estimate the time to recurrence of MDD during the follow-up period. The primary outcome variable was the recurrence of MDD (yes/no) during the 3-year follow-up period. Hazard ratios (HRs) and their 95% confidence intervals (CIs) were calculated using Cox regression analyses in SPSS version 17.0 (SPSS Inc., USA). Predictors that had a p value ⩽0.1 in the bivariate analyses were included in multivariate analyses. The stepwise backward method (p in ⩽0.05 and p out ⩾0.10) was used. In these analyses, censored data included subjects who had not met the criteria for the endpoint event of analysis, either by the end of the follow-up period or by the time they left the study.
Results
Time to recurrence
Because we could assess the timing of the last MDE retrospectively, it was possible to assess the occurrence of recurrence over a long period of time. A total of 135 respondents had an MDE during follow-up (Fig. 1). The median time to recurrence was 6 years (s.d. = 5.5). The estimated cumulative recurrence percentage of MDD, after a duration of remission of at least 6 months, was 2.5% after 1 year, 4.5% after 2 years, 13.2% at 5 years, 23.2% at 10 years and 42.0% at 20 years.

Fig. 1. Survival curve of time to recurrence of a major depressive episode (MDE) in a cohort from The Netherlands Mental Health Survey and Incidence Study (NEMESIS). +, Censored.
Predictors
Table 2 shows the potential risk factors, corrected for age and gender, of time to recurrence of MDD using bivariate and multivariate Cox proportional regression analyses respectively. In bivariate analyses of the sociodemographic factors, age was found to be associated with recurrence of MDD, with each additional year of age lowering the risk for recurrence by 0.04 (95% CI 0.94–0.98, p < 0.001). The younger age group (18–30 years) had an HR of 3.8 in comparison with the older age group (50–65 years). In addition, a younger age of onset was also related to a faster recurrence. Because there was a correlation between age of onset and age (Pearson's correlation 0.68, p < 0.001), this variable was not analyzed in the multivariate Cox proportional hazards model. Among the clinical factors, a history of recurrent episodes and severity of the last depressive episode were significant predictors for a shorter time to recurrence. However, the percentage of missing data was high in the variable ‘severity of the last depressive episode’ (12.8%, n = 88). We analyzed the missing data and found that this was more common in women (χ2 = 5.16, df = 1, p = 0.02) and respondents who had (only) experienced a single episode of MDD (χ2 = 5.01, df = 1, p = 0.03). High neuroticism was also associated with a shorter time to recurrence of MDD. Of the psychosocial factors, negative youth experiences and ongoing difficulties were significant predictors.
Table 2. Bivariable and multivariable analyses of potential predictors of time to recurrence of MDE in a cohort from the NEMESIS

MDE, Major depressive episode; NEMESIS, The Netherlands Mental Health Survey and Incidence Study; MDD, major depressive disorder; HR, hazard ratio; CI, confidence interval.
a Corrected for age and gender.
Bold type indicates significance, p < 0.05.
In multivariate Cox proportional hazards analyses (adjusted for gender), the missing data of the variable ‘severity of the last MDE’ were excluded. Younger age, a high number of previous episodes, a severe last depressive episode, negative youth experiences and the presence of ongoing difficulties before recurrence remained significant predictors of time to recurrence.
Furthermore, we assessed the cumulative recurrence percentage of MDD (posteriori hypotheses) of the respondents who had zero, one, two, three, or four or more of the predictors found in multivariate Cox proportional hazards analyses (Fig. 2). The number of respondents with five predictors was too small to analyze (n = 5). The predictor ‘age’ was dichotomized into younger or older than 30 years. We found that the risk of recurrence depended on the number of predictors the respondents had; after 10 years the cumulative recurrence percentage of MDD was 3.4% for respondents with no predictors (n = 78), 19.0% for respondents with one predictor (n = 175), 26.6% for those with two predictors (n = 197), 56.5% for those with three predictors (n = 97) and 65% for those with four or more predictors (n = 49) (log rank, χ2 = 63.77, df = 4, p < 0.001).

Fig. 2. Survival curve of time to recurrence of a major depressive episode (MDE) in a cohort from The Netherlands Mental Health Survey and Incidence Study (NEMESIS) divided into respondents with zero (+, n = 78), one (◂, n = 175), two (o, n = 197), three (▾, n = 97) or four or more (▴, n = 49) predictors respectively [age <30 years, ongoing difficulties present, traumatic youth experiences present, severe last depressive episode, recurrent major depressive disorder (MDD)].
Discussion
This is one of the few studies, using a large general population cohort, on the incidence and predictors for recurrence of MDD among people who had recovered from their last episode of MDD. Several conclusions can be drawn. First, the cumulative incidence of recurrence after 20 years was found to be 42.0%. This percentage is higher than that found in previous research (Mattison et al. Reference Mattison, Bogren, Horstmann, Munk-Jorgensen and Nettelbrandt2007; Eaton et al. Reference Eaton, Shao, Nestadt, Lee, Bienvenu and Zandi2008) but it is difficult to compare these studies because of differences in duration of remission, follow-up, diagnosis and population. The study by Eaton et al. (Reference Eaton, Shao, Nestadt, Lee, Bienvenu and Zandi2008) is the most similar to ours, with a percentage of recurrence of 35% over 23 years. However, one of the major differences between their study and ours is that their cohort contained exclusively first incidence cases; the inclusion criteria of remission were also different, with at least 1 year in their study compared with 6 months in ours. As a consequence, our cohort contained more respondents, with a shorter time to recurrence, and also respondents with multiple recurrences. Mattison et al. (Reference Mattison, Bogren, Horstmann, Munk-Jorgensen and Nettelbrandt2007) observed a lifetime recurrence percentage of 40% but used a broader definition of depression, also including dysthymia. Our percentage for recurrence of MDD is, however, still lower than that found in a specialized mental health care setting (85% in 15 years) (Mueller et al. Reference Mueller, Leon, Keller, Solomon, Endicott, Coryell, Warshaw and Maser1999). Evidently, the discrepancy in recurrence risk of MDD between settings (general population versus specialized mental health care) is considerable. Second, the following variables predicted shorter time to recurrence in the bivariate models: younger age, younger age of onset, a large number of previous episodes, a more severe last depressive episode, negative youth experiences, the presence of ongoing difficulties before recurrence, and high neuroticism. Multivariably, younger age, a greater number of previous episodes, a severe last depressive episode, negative youth experiences and ongoing difficulties remained significant. Because previous large-scale general population-based follow-up studies have mainly focused on the risk of recurrence of MDD and not on the determinants of recurrence, it was not possible to fully compare our results with previous research. However, Eaton et al. (Reference Eaton, Shao, Nestadt, Lee, Bienvenu and Zandi2008) also studied the main sociodemographic factors and found similar results: the 18–29-year-old group had a higher risk for recurrence, a younger age of onset was a significant predictor of time to recurrence and no other sociodemographic variables were related to a shorter time to recurrence. Third, our data suggests that the risk for recurrence of MDD depends on the number of predictors that are present. However, because this is a data-derived model, in other words we have included those risk factors that are modeled to be most strongly associated with recurrence, no firm conclusions can be drawn and these observations should be tested in future studies.
Previous research in the NEMESIS (De Graaf et al. Reference De Graaf, Bijl, Ravelli, Smit and Vollebergh2002) found that female gender, negative life events, ongoing difficulties and a high level of neuroticism in multivariate analysis were associated with first incidence of MDD. By contrast, our study did not find that female gender was related to recurrence. However, both studies found that stress-related factors seem to play a role, and additional clinical factors related to past MDD episodes (number of previous episodes, severity of the last episode) emerged.
Our study has several strengths. It was performed in a large cohort from the general population, thereby avoiding selection bias. In addition, as all respondents were in remission from an MDE at baseline, the measurement of the predictors was not influenced by the presence of an MDE, apart from the psychosocial predictors (life events, social support, ongoing difficulties), which were measured at follow-up (T1).
In interpreting the results of this study, its limitations should be noted. First, we assessed cases with a lifetime diagnosis of MDD, and assessed the last depressive episode in retrospect using the CIDI. As a consequence, the assessment of the age of onset and recency of the last depressive episode may not have been measured exactly, and therefore the risk of recurrence may have been selectively underestimated in subjects who experienced an MDD at an earlier point in time. This would lead to an overestimation of the time to recurrence and an underestimation of the number of recurrences a respondent had. In addition, the severity of the MDD may not be measured accurately, especially among respondents who had an MDE at an earlier point in time. Furthermore, the difference in time between the end of the last MDE and the measurement of predictors may have led to an overestimation of the effect of the predictor on recurrence. Second, only respondents were included who had a duration of remission of at least 6 months, implying that respondents with a shorter time to recurrence were not included. As the literature on this subject is still scarce, we also analyzed the recurrence rate of MDD in respondents with a duration of remission of at least 1 month. A total of 764 respondents were included in this analysis, of whom 155 had an MDE during follow-up. The number of respondents included was higher in this analysis because fewer respondents were excluded at baseline. As expected, the incidence of recurrence was slightly higher: 3.9% after 1 year, 5.9% after 2 years, 15.1% after 5 years, 25% after 10 years, and 43.3% after 20 years. Third, despite the inclusion of a large number of predictors, subclinical residual symptoms, which is an important predictor of recurrence of MDD (Hardeveld et al. Reference Hardeveld, Spijker, De Graaf, Nolen and Beekman2010), could not be assessed. It was also not possible to study dysthymia (as an equivalent) because the number of respondents with a 6-month prevalence of dysthymia at T0 was small (n = 17) and we could not assess whether the dysthymia had occurred before or after the MDD. Fourth, the resulting HRs were an average for the whole period but it is likely that the hazard changes over time, being greater in the beginning. Previous literature (Mueller et al. Reference Mueller, Leon, Keller, Solomon, Endicott, Coryell, Warshaw and Maser1999) has also suggested that the risk of recurrence is greater earlier on. If the risk of recurrence declines over time, the effect of the factors determining this risk may also not be constant. Moreover, the Cox regression model is built on the assumption that predictors remain constant during follow-up, which may not be true for some predictors. Furthermore, although the study is in part a prospective cohort, it is less capable of confirming causality between a predictor and recurrence of MDD. Therefore, the possibility remains that the previous depressive episode has affected the presence and the effect of potential predictors, or even that some of these risk factors are a consequence of previous episodes. The question remains, for example, as to whether ongoing difficulties contribute to the recurrence of MDD, or multiple recurrences generate ongoing difficulties. Finally, our cohort contained respondents with varying numbers of previous episodes. It could be that the predictors of recurrence change over subsequent episodes. For example, previous research (Kendler et al. Reference Kendler, Thornton and Gardner2000) reported that life events were a significant risk factor during the first episodes, but when the numbers of episodes increased, life events were no longer a relevant predictor.
What directions does this study provide for future research? Various possible genetic and other biological predictors were not studied, but it seems likely that both genetic and environmental factors are involved and interact with one another (Caspi et al. Reference Caspi, Sugden, Moffitt, Taylor, Craig, Harrington, McClay, Mill, Martin, Braithwaite and Poulton2003), and future research should focus on this interaction. Furthermore, ongoing difficulties preceding the recurrence of an MDE seems to be an important predictor. The link between ongoing difficulties, which could cause (chronic) stress, and negative youth experiences, which were also a predictor of recurrence, is of particular interest. Previous research (Heim et al. Reference Heim, Newport, Wagner, Wilcox, Miller and Nemeroff2002) concluded that the hypothalamic–pituitary–adrenal (HPA) axis could be dysregulated following traumatic childhood exposure. Subjects who have a dysregulated HPA axis and experience ongoing difficulties might be at a higher risk for recurrence. Our data support this association, but long-term prospective studies should further elucidate this finding.
What are the implications of our findings? Long-term naturalistic studies performed in mental health care have underscored the importance of understanding depression as a lifelong and recurrent illness, with several possible courses. It also seems that, in the general population, subjects who have experienced an MDE have a long-term vulnerability for recurrence that could be triggered under certain circumstances. Subjects were found to have a higher risk for an earlier recurrence of MDE if they were younger, had negative youth experiences, had multiple previous episodes, experienced ongoing difficulties, and had a more severe last episode. Clinicians should be aware of these risk factors and consider long-term treatment with antidepressants (Geddes et al. Reference Geddes, Carney, Davies, Furukawa, Kupfer, Frank and Goodwin2003; Kaymaz et al. Reference Kaymaz, van Os, Loonen and Nolen2008) or additional psychological treatment, for example preventive cognitive therapy (Bockting et al. Reference Bockting, Spinhoven, Wouters, Koeter and Schene2009). Preventive programs for recurrence of MDD are also reported to be cost-effective (Scott et al. Reference Scott, Palmer, Paykel, Teasdale and Hayhurst2003). Moreover, is it important to know not only who will have a fast recurrence but also which group of patients will experience multiple recurrences in their lives. With this knowledge we might be better able to classify and stage the different course patterns of this disabling disorder and choose the appropriate treatment.
Acknowledgments
The NEMESIS is supported by The Netherlands Ministry of Health, Welfare and Sport (VWS), the Medical Sciences Department of The Netherlands Organization for Scientific Research (NWO), and the National Institute for Public Health and Environment (RIVM).
Declaration of Interest
Dr Nolen has received speaking fees from AstraZeneca, Eli Lilly, Pfizer, Servier and Wyeth; unrestricted grants from The Netherlands Organization for Health Research and Development, the European Union, the Stanley Medical Research Institute, AstraZeneca, Eli Lilly, GlaxoSmithKline and Wyeth; and served on advisory boards for AstraZeneca, Cyberonics, Pfizer and Servier. Dr Spijker has received speaking fees from AstraZeneca, Wyeth, Servier, Eli Lilly and GlaxoSmithKline.






