Introduction
Repetitive transcranial magnetic stimulation (rTMS) is increasingly applied as an alternative method in the treatment of non-psychotic major depressive disorder (MDD). Following countries including Canada and Israel, NeuroStar TMS Therapy® was recently approved by the US Food and Drug Administration for the treatment of MDD. In addition, an increasing number of private clinics around the world already offer rTMS as a regular form of treatment and hospitals are setting up ambulant TMS treatment units.
The main principle of TMS relies on Faraday's law of electromagnetic induction. Brief but strong magnetic pulses that result from rapid electric discharges in an induction coil can penetrate the cortical layers several centimetres and cause secondary electric currents in the vicinity of neurons and glial cells (Hallett, Reference Hallett2001). The secondary currents constitute the physical basis for local (direct) and remote (indirect) modulation of brain physiology. The first evidence for antidepressant effects of magnetic brain stimulation involved fast-frequency rTMS (⩾10 Hz) to the left prefrontal cortex (George et al. Reference George, Wassermann, Williams, Callahan, Ketter, Basser, Hallett and Post1995). A recent meta-analysis on the efficacy of fast-frequency rTMS to the left prefrontal cortex in MDD demonstrated significantly larger effects of real as compared with sham rTMS (Schutter, Reference Schutter2009). Even though the cumulative effect size of the thirty double-blind sham-controlled studies is moderate in magnitude, the result can be considered as evidence that fast-frequency rTMS is effective in improving depression severity. This notion is further strengthened by the fact that the observed effect size is comparable with several commercially available antidepressants (Moncrieff et al. Reference Moncrieff, Wessely and Hardy2004).
In addition to fast-frequency rTMS, the progressive emergence of random trials suggested that slow-frequency rTMS (⩽1 Hz) might have antidepressant properties as well (Höflich et al. Reference Höflich, Kasper, Hufnagel, Ruhrmann and Möller1993; Grisaru et al. Reference Grisaru, Yeroslavsky, Abrabanel, Lamberg and Belmaker1994). However, as regards the efficacy of slow-frequency rTMS, no systematic research of the available literature is yet available. The aim of the present meta-analysis was therefore to examine the antidepressant efficacy of slow-frequency rTMS by analysing the effects of sham and real treatment in MDD patients. In addition, a comparison was made with the effect size of high-frequency rTMS reported in a previous meta-analysis (Schutter, Reference Schutter2009).
Method
Study selection
Articles for inclusion were identified starting with conducting a literature search in the databases PubMed and Web of Science in the period between January 1994 and July 2009. The search criteria were ‘depression’ and ‘transcranial magnetic stimulation’.
Studies had to satisfy the following quality criteria based on the Cochrane Reviewers' Handbook 4.1.4. and the Users' Guide to the Medical Literature for inclusion (e.g. Couturier, Reference Couturier2005; Schutter, Reference Schutter2009):
(1) Study validity: random allocation, patients and clinical raters were blind to treatment (double-blind), sham-controlled, parallel-design, intent-to-treat analysis.
(2) Adults with major depressive episode according to the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV).
(3) Slow-frequency (⩽1 Hz) rTMS, intensity ⩾80% motor threshold (MT), at least five treatment sessions, sham condition: 45° and 90° from scalp or sham coil.
(4) Primary outcome measure: percentage change from baseline or end-point scores on the Hamilton Depression Rating Scale or Montgomery–Åsberg Depression Rating Scale when depression scores between treatment conditions were not different at baseline (i.e. p⩾0.3).
(5) Participants' treatment completion within 6 weeks after first session.
(6) Article published in a peer-reviewed English-language journal.
(7) Study approved by a medical ethical committee or review board.
Of the initially selected studies, nine fulfilled the criteria for inclusion in the meta-analysis. Characteristics of the studies can be found in Table 1.
Table 1. Study characteristics
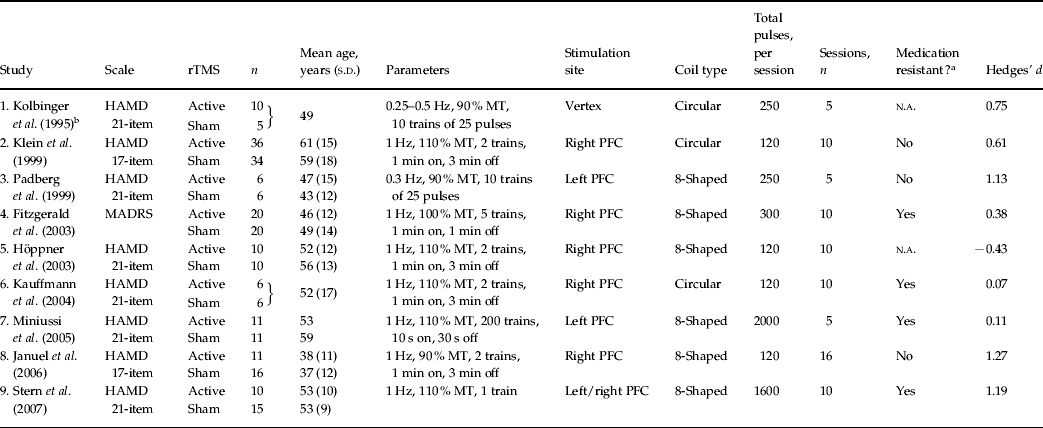
rTMS, Repetitive transcranial magnetic stimulation; s.d., standard deviation; HAMD, Hamilton Depression Rating Scale; MT, motor threshold; N.A., not available; PFC, prefrontal cortex; MADRS, Montgomery–Åsberg Depression Rating Scale.
a Medication resistance is defined as the failure to respond to two or more trials of antidepressants or history of failed responses to electroconvulsive therapy.
b Stimulation of the vertex with a circular coil will affect both the left and right prefrontal cortex.
Data synthesis and analysis
End-point scores were used for studies 1, 3, 4, 5, 7, 8 and 9, and the percentage baseline-corrected difference score was used in studies 2 and 6 to compute the effect size for each study. In the Stern et al. (Reference Stern, Tormos, Press, Pearlman and Pascual-Leone2007) study (i.e. study 8) two patient groups were treated with either slow-frequency rTMS to the left (n=10) or slow-frequency rTMS to the right frontal cortex (n=10) and compared with a patient group treated with sham rTMS (n=15). Effect sizes were calculated for each treatment condition as compared with sham rTMS. The cumulative effect size was calculated based on the ten data entry points and a random-effects model analysis was performed to estimate the ‘true’ antidepressant effect of slow-frequency rTMS and the 95% confidence interval (95% CI) (Hedges & Olkin, Reference Hedges and Olkin1985). Total heterogeneity of the effect sizes, QT, was determined and tested against the χ2 distribution with 9 (n−1) degrees of freedom (Hedges & Olkin, Reference Hedges and Olkin1985). Due to the relatively small sample sizes in many of the studies, non-parametric variances were chosen for the meta-analysis. The fail-safe number of studies (NR) was calculated according to Rosenthal's method to estimate the number of additional non-significant or missing studies needed to render the cumulative effect size non-significant (α⩾0.05).
Finally, to compare the present cumulative effect size of slow-frequency rTMS with the cumulative effect size of fast-frequency rTMS reported in Schutter (Reference Schutter2009), the effect size and the non-parametric variance of each study (n=39) were entered in a categorical random-effects model analysis. All analyses were performed with metawin version 2 (Arizona State University, USA; Rosenberg et al. Reference Rosenberg, Adams and Gurevitch2000).
Results
A total of 252 patients with major depression [mean age 50 (s.d.=7) years] were enrolled in the meta-analysis of which 134 patients [mean age 50 (s.d.=6.3) years] were treated with real rTMS and 118 patients [mean age 51 (s.d.=7.3) years] received sham rTMS treatment. The cumulative effect size (E++) for treatment was 0.63 (95% CI 0.03–1.24). The test for heterogeneity was not significant (QT=9.63, p=0.38), implying that the variance among the effect sizes was not greater than expected by sampling error. The fail-safe number of studies was 119.3, indicating that at least 119 unpublished null-findings are needed to render the effect of real treatment statistically non-significant. Additional analyses demonstrated that no reliable cumulative effect size estimates could be determined for left (n=3, E++=0.26, 95% CI −1.30 to 1.82, QT=2.16, p=0.33) and right frontal slow-frequency rTMS (n=6, E++=0.76, 95% CI −0.22 to 1.75, QT=6.17, p=0.29). Last, results of the categorical random-effects model analysis yielded no significant difference between slow and fast rTMS treatment (QBetween=1.50, p=0.22). This finding suggests that slow and fast rTMS treatments are equally effective in ameliorating MDD severity (Table 2).
Table 2. Main outcomes
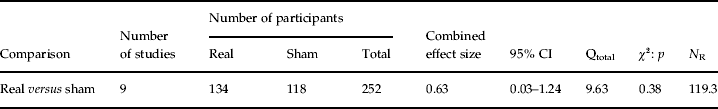
CI, Confidence interval; Qtotal, total heterogeneity; N R, fail-safe number of studies.
Discussion
Non-invasive magnetic stimulation of the brain has been proposed as a novel way of treating MDD, and may be an alternative for patients who do not tolerate the side-effects of antidepressant medication or simply do not respond to drug treatment. Recent meta-analyses have shown that fast-frequency rTMS applied to the left frontal cortex produces antidepressant effects comparable with several commercially available drug agents (Moncrieff et al. Reference Moncrieff, Wessely and Hardy2004; Schutter, Reference Schutter2009). Because fast-frequency rTMS can be rather uncomfortable during high-intensity stimulation and is associated with an increased risk of adverse events, slow-frequency rTMS has been put forward as an alternative stimulation option. The aim of the present meta-analysis was to examine the antidepressant properties of slow-frequency rTMS.
Nine double-blind sham-controlled studies were entered in a random-effects model and results showed that real rTMS was more effective than sham rTMS. Even though the cumulative effect size (0.63) of slow-frequency rTMS was larger than the cumulative effect size (0.39) of fast-frequency rTMS (Schutter, Reference Schutter2009), an additional contrast demonstrated that the difference was not statistically reliable. This finding indicates that both treatments are equally effective in ameliorating MDD severity. In spite of similar therapeutic efficacy, strong inferences on slow-frequency rTMS as being equally effective as fast rTMS cannot be made at this point. In addition, at this stage the positive results on the antidepressant effects of slow-frequency rTMS should be viewed as preliminary rather than definitive. Several limitations should be taken into account when interpreting the present findings.
The first limitation concerns the small number of double-blind sham-controlled studies that have investigated the effects of slow-frequency rTMS in MDD. Even though the observed variance among the effect sizes was not greater than one would expect by sampling error, the 95% CI of 0.03–1.24 nonetheless suggests that the present meta-analysis is underpowered. The second limitation pertains to the large variation of target stimulation sites across the studies. Unfortunately, the available data were not suitable to reliably determine effect sizes for slow-frequency rTMS applied over the left and right frontal cortex separately. Several lines of research, however, suggest that particularly targeting the right frontal cortex would be effective in treating MDD with slow-frequency rTMS. This idea fits several proposed biological mechanisms suggested to underlie the antidepressant effects of slow and fast rTMS to the respective right and left frontal cortex. According to the ‘frontal hypoactivity’ hypothesis of MDD, fast-frequency rTMS over the left prefrontal lobe increases levels of cortical excitability (George et al. Reference George, Wassermann, Williams, Callahan, Ketter, Basser, Hallett and Post1995; Pascual-Leone et al. Reference Pascual-Leone, Rubio, Pallardo and Catala1996). Others have raised the possibility that antidepressant responses may arise from restoring a functional imbalance between the left and right frontal cortex rather than ‘boosting’ the left prefrontal cortex per se (Kimbrell et al. Reference Kimbrell, Little, Dunn, Frye, Greenberg, Wassermann, Repella, Danielson, Willis, Benson, Speer, Osuch, George and Post1999). This idea concurs with the assumed antidepressant effects associated with inhibitory slow-frequency rTMS to the right frontal cortex and recent work on sequential bilateral stimulation of the left and right frontal cortex with fast and slow rTMS, respectively (Fitzgerald et al. Reference Fitzgerald, Benitez, de Castella, Daskalakis, Brown and Kulkarni2006). Moreover, research in healthy subjects has shown that the left frontal cortex is associated with approach- and reward-related motivational tendencies, whereas the right frontal cortex is linked to avoidance- and punishment-related motivational tendencies (Harmon-Jones, Reference Harmon-Jones2003; Schutter et al. Reference Schutter, de Weijer, Meuwese, Morgan and van Honk2008). Taken together, this suggests that targeting the right frontal cortex would be more effective than targeting the left frontal cortex in treating MDD with slow-frequency rTMS. On the other hand, antidepressant effects of slow-frequency rTMS to the left frontal cortex have also been reported (Rosenberg et al. Reference Rosenberg, Mehndiratta, Mehndiratta, Wamer, Rosse and Balish2002). Despite the study having an open-label character, the results may hint towards the notion that location may not be crucial in obtaining antidepressant effects. For example, rTMS to the left dorsolateral prefrontal cortex has been found to increase dopamine release in deep interconnected brain regions (Strafella et al. Reference Strafella, Paus, Barrett and Dagher2001), and augmenting dopamine turnover in the striatum and ventral tegmental area has been proposed to promote hedonia and reduce depression severity (Dunlop & Nemeroff, Reference Dunlop and Nemeroff2007). According to this idea, the frontal lobe constitutes a gateway for accessing the core motivational circuits located in deep brain regions. A possible alternative way to target distal regions may involve the H-coil that, unlike the circular and eight-shaped coils, can reach deep brain structures directly (Levkovitz et al. Reference Levkovitz, Roth, Harel, Braw, Sheer and Zangen2007; for a discussion, see Fadini et al. Reference Fadini, Matthäus, Rothkegel, Sommer, Tergau, Schweikard, Paulus and Nitsche2009).
Further ways to improve rTMS efficacy have been proposed along the lines of prolonging treatment duration and using higher stimulation intensities (Loo & Mitchell, Reference Loo and Mitchell2005; Fitzgerald et al. Reference Fitzgerald, Hoy, McQueen, Herring, Segrave, Been, Kulkarni and Daskalakis2008). The mean number of treatment sessions in the current studies was 9 (s.d. 3.6), which may have been too low to elicit clinically relevant effects. In fact, several fast-frequency rTMS studies that used longer stimulation periods (up to 6 weeks) found incremental effects over time (e.g. Avery et al. Reference Avery, Holtzheimer, Fawaz, Russo, Neumaier, Dunner, Haynor, Claypoole, Wajdik and Roy-Byrne2006; Fitzgerald et al. Reference Fitzgerald, Benitez, de Castella, Daskalakis, Brown and Kulkarni2006; O'Reardon et al. Reference O'Reardon, Solvason, Janicak, Sampson, Isenberg, Nahas, McDonald, Avery, Fitzgerald, Loo, Demitrack, George and Sackeim2007). Thus, increasing the number of sessions may also produce beneficial effects in slow-frequency rTMS treatment. Moreover, the mean intensity of stimulation was 102% MT (s.d.=9.7) and there is some evidence suggesting a positive relationship between intensity and antidepressant efficacy (Avery et al. Reference Avery, Holtzheimer, Fawaz, Russo, Neumaier, Dunner, Haynor, Claypoole, Wajdik and Roy-Byrne2006). Even though stimulation at higher intensities may have a positive influence on outcome, patient discomfort associated with stimulation including site pain and muscle contractions may be a drawback.
Additional means to improve efficacy may include the use of preconditioning paradigms prior to ‘regular’ slow-frequency rTMS (Iyer et al. Reference Iyer, Schleper and Wassermann2003; Siebner et al. Reference Siebner, Lang, Rizzo, Nitsche, Paulus, Lemon and Rothwell2004; Huang et al. Reference Huang, Edwards, Bhatia and Rothwell2005). For example, theta-bursting in which series of 50 Hz pulses of stimulation are applied to the cortex in a repeated 5 Hz fashion has proven highly effective in establishing acute changes of neural excitability levels (Huang et al. Reference Huang, Edwards, Bhatia and Rothwell2005). Thus, preconditioning paradigms may lay a physiological foundation for more effective modulation of the frontal cortex with slow-frequency rTMS, which in turn may yield higher antidepressant effects.
In addition, examining and using individual differences in the patients' brain physiology may ultimately serve as a proxy for selecting the best rTMS treatment. For example, recent findings suggest that the efficacy of slow- or fast-frequency rTMS may depend on baseline perfusion levels of the brain as measured with positron emission tomography imaging (Speer et al. Reference Speer, Benson, Kimbrell, Wassermann, Willis, Herscovitch and Post2009). Others have proposed that individual differences in steroid hormone levels may influence the impact of rTMS on cortical tissue and its subsequent antidepressant effects (e.g. Huang et al. Reference Huang, Wei, Chou and Su2008; Schutter & van Honk, Reference Schutter and van Honk2010). Finally, as prior research found evidence that monophasic slow-frequency rTMS produces greater effects on cortical excitability than biphasic slow-frequency rTMS (Sommer et al. Reference Sommer, Lang, Tergau and Paulus2002), single current stimulation and its higher stimulation intensity may play a significant role in the efficacy when treating with slow-frequency rTMS.
In conclusion, both slow- and fast-frequency rTMS treatments have very few negative side-effects and the likelihood of serious adverse events is low (Rossini & Rossi, Reference Rossini and Rossi2007). On the basis of previous and present findings, rTMS to the frontal cortex may be an alternative treatment option for patients who do not tolerate the negative side-effects of antidepressant medication or are unresponsive to drug treatment and/or cognitive behavioural therapy. The fact that slow-frequency rTMS is usually better tolerated than fast-frequency rTMS and permits longer safe stimulation periods within one session can be considered arguments in favour of applying slow-frequency rTMS. In sum, preliminary findings suggest that slow-frequency rTMS can improve MDD and additional clinical trials aimed at optimizing treatment are worthwhile.
Acknowledgements
The author thanks Carlo Miniussi, Dennis Hofman, Jiska S. Peper, and two anonymous reviewers. This work was supported by an Innovational Research Grant (VIDI 452-07-012) from the Netherlands Organization for Scientific Research (NWO).
Declaration of Interest
None.




