Introduction
Cognitive dysfunction is considered a core feature of psychosis that significantly impacts social, functional and clinical treatment outcomes (Green, Reference Green1996; Green et al., Reference Green, Kern, Braff and Mintz2000; Bora et al., Reference Bora, Yucel and Pantelis2010; Keefe et al., Reference Keefe, Fox, Harvey, Cucchiaro, Siu and Loebel2011) A series of cross-sectional studies revealed intermediate levels of cognitive impairment in individuals at clinical-high-risk (CHR) for psychosis compared to individuals with schizophrenia (SZ) and healthy controls (HCs) (Bora et al., Reference Bora, Yucel and Pantelis2010; Giuliano et al., Reference Giuliano, Li, Mesholam-Gately, Sorenson, Woodberry and Seidman2012; Fusar-Poli et al., Reference Fusar-Poli, Deste, Smieskova, Barlati, Yung, Howes, Stieglitz, Vita, Mcguire and Borgwardt2012b); some investigators hypothesize that this reflects a progressive cognitive decline occurring prior to psychosis onset in CHR individuals (Kraemer et al., Reference Kraemer, Yesavage, Taylor and Kupfer2000). However, Bora and Murray (Bora and Murray, Reference Bora and Murray2014) examined 25 longitudinal studies and reported improving cognitive performance in CHR individuals and in the first-episode psychosis over time, with no evidence of cognitive decline in either group. Together, these findings suggest that impaired cognition is present in CHR youth prior to psychosis onset with no subsequent decline. This is consistent with a neurodevelopmental hypothesis of psychosis based on evidence from epidemiology to neuropathology studies (Murray and Lewis, Reference Murray and Lewis1987; Weinberger, Reference Weinberger1987). Cognitive impairments in CHR most likely represent a potential marker of risk that could be used to enhance early identification and intervention efforts (Fusar-Poli et al., Reference Fusar-Poli, Bonoldi, Yung, Borgwardt, Kempton, Valmaggia, Barale, Caverzasi and McGuire2012a; Velthorst et al., Reference Velthorst, Zinberg, Addington, Cadenhead, Cannon, Carrión, Auther, Cornblatt, Mcglashan, Mathalon, Perkins, Seidman, Tsuang, Walker, Woods, Reichenberg and Bearden2018).
Carrión et al. (Reference Carrión, Cornblatt, Burton, Tso, Auther, Adelsheim, Calkins, Carter, Niendam, Taylor and Mcfarlane2016) validated the North American Prodrome Longitudinal Study (NAPLS) psychosis risk calculator using a sub-dataset of CHR individuals recruited as a part of the Early Detection, Intervention, and Prevention of Psychosis Program (EDIPPP) (McFarlane et al., Reference Mcfarlane, Levin, Travis, Lucas, Lynch, Verdi, Williams, Adelsheim, Calkins, Carter, Cornblatt, Taylor, Auther, Mcfarland, Melton, Migliorati, Niendam, Ragland, Sale, Salvador and Spring2015). Based on the risk calculator, Carrión and colleagues predicted conversion outcome with 71% of accuracy using six risk factors: age, unusual thoughts and suspiciousness ideas from the Structured Interview for Prodromal Syndromes (SIPS) (McGlashan et al., Reference Mcglashan, Miller, Woods, Rosen, Hoffman and Davidson2001), family history of psychosis, decline in social functioning over the year prior to baseline on the Global Functioning: Social scale (Cornblatt et al., Reference Cornblatt, Auther, Niendam, Smith, Zinberg, Bearden and Cannon2007), processing speed on the Brief Assessment of Cognition in Schizophrenia (BACS) (Keefe et al., Reference Keefe, Goldberg, Harvey, Gold, Poe and Coughenour2004), and verbal learning on the on the Hopkins Verbal Learning Test (HVLT) (Keefe et al., Reference Keefe, Fox, Harvey, Cucchiaro, Siu and Loebel2011). Such findings represent an important step in understanding the clinical and cognitive factors that contribute to risk for psychosis conversion in CHR individuals as well as enhancing the precision of risk prediction for clinical purposes. They also highlight the importance of cognitive measurement as a key aspect of the precision psychiatry of early psychosis care.
Much of the prior literature on cognition in CHR and SZ has used multiple elements of clinical neuropsychological test batteries to identify specific deficits; however, these measures often share variance and have limited construct validity as measures of specific cognitive mechanisms related to discrete neural systems (Carter and Barch, Reference Carter and Barch2007; Guo et al., Reference Guo, Ragland and Carter2019). In response to these limitations, efforts such as the NIMH supported CNTRICS (Carter and Barch, Reference Carter and Barch2007) initiative and subsequent work by the CNTRACS consortium (Gold et al., Reference Gold, Barch, Carter, Dakin, Luck, Macdonald, Ragland, Ranganath, Kovacs, Silverstein and Strauss2012) led to the development and validation of a number of tasks from basic cognitive neuroscience that have established links to know cognitive and neural systems and can be readily administered in a clinical setting. Among these tasks is the AX-CPT, which has been widely shown to reveal performance deficits in schizophrenia and other psychotic disorders and is linked to the function of a frontal parietal cognitive control network as well as to clinical symptoms of disorganization and poor functioning. Cognitive neuroscience studies in healthy individuals show that a broad range of cognitive functions that cut across traditional cognitive and neuropsychological domains are regulated by the frontal-parietal cognitive control network (Frith and Dolan, Reference Frith and Dolan1996; Niendam et al., Reference Niendam, Laird, Ray, Dean, Glahn and Carter2012). Dysfunction in this network leads to a range of cognitive deficits in neuroimaging studies of schizophrenia (Minzenberg et al., Reference Minzenberg, Laird, Thelen, Carter and Glahn2009; Lesh et al., Reference Lesh, Niendam, Minzenberg and Carter2011; Birur et al., Reference Birur, Kraguljac, Shelton and Lahti2017) and the psychosis risk syndrome (Allen et al., Reference Allen, Luigjes, Howes, Egerton, Hirao, Valli, Kambeitz, Fusar-Poli, Broome and Mcguire2012; Niendam et al., Reference Niendam, Lesh, Yoon, Westphal, Hutchison, Ragland, Solomon, Minzenberg and Carter2014), although alterative accounts of the neural underpinnings of AX-CPT performance have been proposed (Dias et al., Reference Dias, Butler, Hoptman and Javitt2011). Since the CNTRACS version of the AX-CPT is brief (15 min or less) and readily administered on a computer without specialized training, for pragmatic and theoretical reasons, we sought to incorporate this measure into the CHR risk calculator.
It has become increasingly clear that a binary prediction of conversion status does not accurately capture the full range of potentially negative clinical and functional outcomes that are observed in CHR populations (Lin et al., Reference Lin, Nelson and Yung2012). Indeed, a recent large (NCHR = 173 and NHCs = 384) 24-month longitudinal study examined baseline cognitive performance and its longitudinal trajectory according to four CHR outcomes: converters v. non-converters and remitters v. non-remitters, which includes CHR individuals with persistent attenuated symptoms and converters (Lam et al., Reference Lam, Lee, Rapisarda, See, Yang, Lee, AbdulRashid, Kraus, Subramaniam, Chong and Keefe2018). They reported significant impaired baseline cognition in converters, non-converters and non-remitters compared to controls, whereas preserved baseline cognition was shown in remitters, which suggested CHR with persistence of attenuated symptoms showed similar levels of impairment on various cognitive measures (e.g. social cognition, attention, and general cognitive function at baseline and during follow-up) in comparison with converters.
Building upon innovative prior work (Cannon et al., Reference Cannon, Cadenhead, Cornblatt, Woods, Addington, Walker, Seidman, Perkins, Tsuang, Mcglashan and Heinssen2008; Cornblatt et al., Reference Cornblatt, Carrión, Addington, Seidman, Walker, Cannon, Cadenhead, Mcglashan, Perkins, Tsuang, Woods, Heinssen and Lencz2012; Addington et al., Reference Addington, Liu, Buchy, Cadenhead, Cannon, Cornblatt, Perkins, Seidman, Tsuang, Walker, Woods, Bearden, Mathalon and Mcglashan2015; Seidman et al., Reference Seidman, Shapiro, Stone, Woodberry, Ronzio, Cornblatt, Addington, Bearden, Cadenhead, Cannon, Mathalon, Mcglashan, Perkins, Tsuang, Walker and Woods2016), the current study explores cognitive impairments in CHR using a single, well-established computer-administered measure of cognitive control, the AX-CPT, which is sensitive to impaired cognition in schizophrenia and has been shown to have robust psychometric properties (Henderson et al., Reference Henderson, Poppe, Barch, Carter, Gold, Ragland, Silverstein, Strauss and Macdonald2012). We hypothesize that baseline AX-CPT performance will perform as well as the multi-factorial NAPLS psychosis risk calculator in predicting 12-month conversion outcomes in a CHR sample. Furthermore, we hypothesize that prediction accuracy will show further improvement when three-subgroup clinical outcomes (remission, persistence, conversion) are examined.
Methods
Participants
The present study combined CHR individuals from two population-based cohort studies: the Early Detection and Intervention for the Prevention of Psychosis (EDIPPP) study and the Understanding Early Psychosis (EP) study. EDIPPP enrolled CHR participants between 2007 and 2010 in a large clinical trial at six centers. Participants completed clinical and cognitive assessments at baseline, 6-, 12-, and 24-month follow-ups over 2 years. Detailed sample characteristics and the trajectories of clinical outcomes have been reported elsewhere (McFarlane et al., Reference Mcfarlane, Levin, Travis, Lucas, Lynch, Verdi, Williams, Adelsheim, Calkins, Carter, Cornblatt, Taylor, Auther, Mcfarland, Melton, Migliorati, Niendam, Ragland, Sale, Salvador and Spring2015). CHR participants in the EP study were recruited between 2005 and 2010 from the University of California, Davis EDAPT Clinic. They completed clinical, cognitive, and imaging assessments at baseline and 12-month intervals for 2 years. CHR individuals from both cohorts were included if they had no history of psychosis and met criteria for one of the three syndromes according to the SIPS (McGlashan et al., Reference Mcglashan, Miller, Woods, Rosen, Hoffman and Davidson2001) (1) attenuated psychotic symptoms (APS); (2) brief and self-limited psychotic symptoms (BIPS); (3) substantial drop in global functioning over past year with schizotypal personality disorder or first-degree relative with psychotic disorder (GRD). Of the 508 and 122 CHR individuals from EDIPPP and EP respectively, 133 EDIPPP CHR and 53 EP CHR participants performed AX-CPT task and met SIPS criteria. 170 HCs were randomly selected from the EP cohort to demographically match the CHR group. Informed consent or assent for the study was provided by all participants or by the guardians for minors with compensation for participating. Study protocols and informed consent procedures were approved by the University of California at Davis IRB and the six participating EDIPPP sites.
In addition to EDIPPP and EP-specific exclusion criteria (Niendam et al., Reference Niendam, Lesh, Yoon, Westphal, Hutchison, Ragland, Solomon, Minzenberg and Carter2014), the current study excluded 54 CHR participants for: age younger than 12 years and older than 25 years, unclear CHR diagnosis at baseline or follow-ups as verified by an experienced clinical psychologist (T.A.N.), IQ below 70, and poor performance on the AX-CPT (>55% AX errors, 100% AY or BX errors, and >50% BY errors) (Henderson et al., Reference Henderson, Poppe, Barch, Carter, Gold, Ragland, Silverstein, Strauss and Macdonald2012). Overall, the exclusion rate was 31.4% (Fig. 1). Of the participants included at baseline, 35 (29.7%) of the CHRs and 52 HCs (30.6%) completed the AX-CPT task at follow-up. Notably, there is no selection bias at baseline, although attrition bias was observed with younger CHR at baseline showing better follow-up rates (online Supplementary Table S1).

Fig. 1. Flow chart illustrated participants' recruitment procedure at baseline and follow-up.
AX-CPT data collection protocol
In the AX-CPT, participants are instructed to respond to the probe letter ‘X’ only when it is preceded by the ‘A’ cue (Rosvold et al., Reference Rosvold, Mirsky, Sarason, Bransome and Beck1956); all other stimuli require a non-target response, including trials in which (1) probe X is preceded by any letter other than A (Cue B trials) or (2) trials that use any other letter than X as a probe (e.g. AY or BY trials). Sustained cognitive control is required to support correct responses to high-frequency AX target trials and inhibition in the face of non-target trials (AY and BX) (Braver et al., Reference Braver, Barch, Keys, Carter, Cohen, Kaye, Janowsky, Taylor, Yesavage, Mumenthaler, Jagust and Reed2001). D prime context (d’ context) indexes (MacDonald et al., Reference MacDonald, Carter, Kerns, Ursu, Barch, Holmes, Stenger and Cohen2005) represents the ability to use contextual information from the cue (A or B) to respond appropriately to the probe (X or Y). Multiple practice trials were completed with criteria of 80% accuracy and no less than one 1 BX trial correct to perform the actual task.
The version of the AX-CPT used by EDIPPP study contained four blocks of 63 trials [252 trials total – 200 AX (79.37%), 20 AY (7.94%), 20 BX (7.94%), 12 BY trials (4.76%)], a fixed inter-stimulus-interval (ISI) of 5489 ms, and a cue or probe presentation time of 500 ms. This task takes 15 min of testing time, including instructions and practice (online Supplementary Fig. S1, left panel). EP study utilized a shorter version of the AX-CPT task that contained 4 blocks of 38 trials [152 trials total – 120 AX (78.95%), 12 AY (7.89%), 12 BX (7.89%), and 8 BY trials (5.26%)], a shorter fixed ISI (5000 ms), and the same cue or probe presentation time (500 ms) (online Supplementary Fig. S2, right panel). This minor difference between two AX-CPT versions failed to show effects on task performance (online Supplementary Table S2). Thus, any significant results revealed in the current study were not attributable to the utilization of different AX-CPT versions. 32.2% of CHR participants completed the AX-CPT task at follow-up.
Clinical and social functioning data collection protocol
The severity of symptoms was calculated by summing the individual SIPS Positive, Negative, Disorganized, and General item scores to create total scores within each domain at baseline and 12-month follow-up. The Global Functioning: Social (GF:S) and Role (GF:R) scales (Cornblatt et al., Reference Cornblatt, Auther, Niendam, Smith, Zinberg, Bearden and Cannon2007) were completed at baseline and 12-month follow-up. In addition, GF:S and GF:R decline was computed by subtracting current score at baseline from the highest score in the year prior to baseline assessments (Carrión et al., Reference Carrión, Cornblatt, Burton, Tso, Auther, Adelsheim, Calkins, Carter, Niendam, Taylor and Mcfarlane2016).
Statistical analysis
Prediction models
We evaluated the predictive utility of the AX-CPT D prime context (d’ context) for psychosis conversion using binary logistic regression, together with other four predictors employed in the NAPLS psychosis risk calculator (Addington et al., Reference Addington, Liu, Buchy, Cadenhead, Cannon, Cornblatt, Perkins, Seidman, Tsuang, Walker, Woods, Bearden, Mathalon and Mcglashan2015). Specifically, five predictors included age at consent, d’ context, modified sum P1 and P2 scores from the SIPS, and GF:S decline. This model is referred to as the GF:S d’ context validation model. We also examined the predictive utility of GF:R decline in the d’ context validation model because of previously reported relationships between role functioning and negative symptoms in psychosis converters with longer duration prior to conversion (Cornblatt et al., Reference Cornblatt, Carrión, Addington, Seidman, Walker, Cannon, Cadenhead, Mcglashan, Perkins, Tsuang, Woods, Heinssen and Lencz2012). This model is referred to as the GF:R d’ context validation model. Missing values in GF:S decline (N = 18) and GF:R decline (N = 17) were imputed by calculating k-nearest neighbors of GF:S and GF:R baseline scores respectively using kNN: VIM package in R (Kowarik and Templ, Reference Kowarik and Templ2016). Finally, we examined whether GF:S and GF:R d’ context models could be improved by examining three subgroups of CHR outcome at 12 months using multinomial logistic regression: CHR remission (CHR-R) with all SIPS positive symptoms scored below 3, CHR persistence (CHR-P) with at least 1 SIPS positive symptoms scored in the attenuated 3–5 range, and conversion to psychosis (CHR-C). Prediction accuracy was evaluated with a micro-average measure, which takes into account the proportional contributions of all classes to compute the average metric given the imbalanced sample size in the current study. Above-mentioned prediction models were performed in R version 3.5.1 (https://cran.r-project.org/mirrors.html). In a series of exploratory analyses, we tested several additional predictive models utilizing other common clinical and social functioning measures in order to establish the specificity of the above prediction models (online Supplementary Figs S1 and S2, and Table S3).
Group differences in AX-CPT performance at baseline
D’ context was calculated as the Z-score of AX hits (% correct) – the Z-score of BX false alarms (% errors) (Servan-Schreiber et al., Reference Servan-Schreiber, Cohen and Steingard1996). D’ context differences between converters and non-converters were explored using a 2-tailed non-parametric Mann-Whitney U test with alpha set at p ⩽ 0.05. Then, d’ context differences among three CHR subgroups were examined via a non-parametric Kruskal-Wallis test. Post hoc analyses were performed with three pairwise comparisons, adjusting p-values manually for multiple comparisons using False Discovery Rate correction (FDR).
Group differences in Clinical and social functioning at baseline and over the follow-up
Baseline clinical scores in four domains, baseline modified P1 + P2 scores and baseline functioning scores (GF:S, GF:S decline, GF:R, and GF:R decline) were compared among CHR-R, CHR-P and CHR-C using ANOVA. Post hoc tests were conducted with Bonferroni correction adjusting for multiple comparisons. To evaluate improvement or decline in symptoms or functioning over follow up, change scores for SIPS in four domains, GF:S, and GF:R were computed by subtracting scores at baseline from scores at 12-month follow-up. ANOVAs were performed to examine the difference of those change scores among CHR-R, CHR-P, and CHR-C and post hoc tests were conducted with Bonferroni correction adjusting for multiple comparisons. Above-mentioned group comparisons were performed in SPSS version 25 (SPSS, 2017).
Results
Demographics
Demographic data for CHR participants including age, gender, education history, and race are presented in Table 1. No differences were found among CHR three subgroups. Demographic data for HCs were presented in online Supplementary Table S4.
Table 1. Demographic data: two and three groups respectively
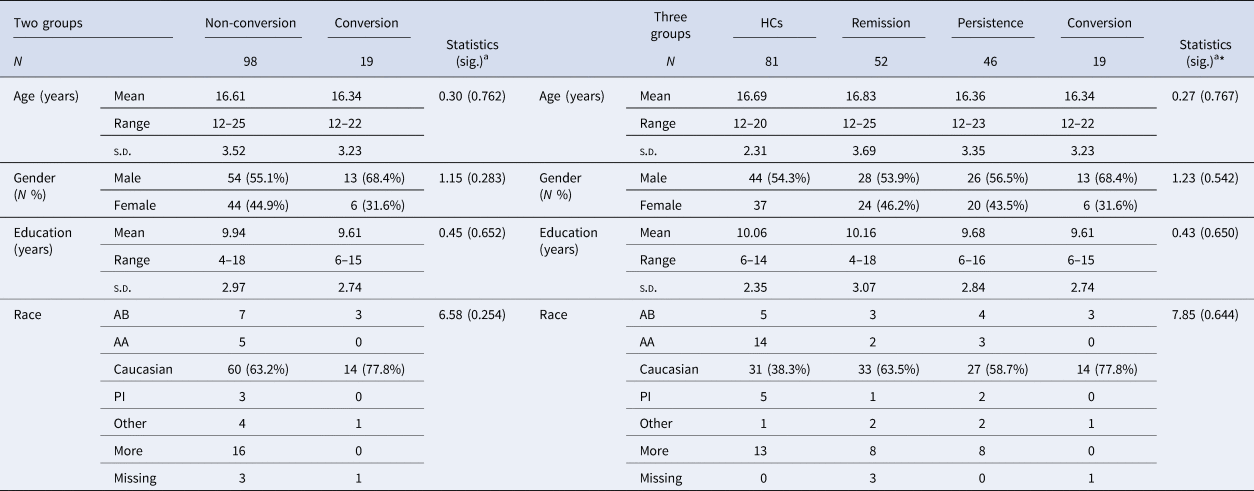
AB, African American/Black; AA, Asian American; AN, American Indian/Alaskan Native; PI, Native Hawaiian or Other Pacific Islander; More, More than one race
a Kruskal-Wallis H test or χ2 test for nonparametric continuous and categorical data analyses
Binary logistic regression models
The GF:S d’ context validation model showed significant discrimination between CHR-C and CHR non-converters with AUC = 0.723 (p = 0.001, 95% CI 0.60–0.84) (Fig. 2, left panel, blue line), while the GF:R d’ context validation model yielded good prediction performance (AUC = 0.716, p = 0.002, 95% CI 0.58–0.85) (Fig. 2, left panel, yellow line). Importantly, two out of five selected risk factors significantly and independently predict psychosis conversion, including modified P1 and P2 baseline scores (AUC = 0.711, p = 0.002, 95% CI 0.59–0.83) and d’ context at baseline (AUC = 0.639, p = 0.028, 95% CI 0.52–0.76).

Fig. 2. d’ context validation models (Left panel) showed fair prediction accuracy for psychosis conversion with slightly better discrimination of GF:S (left panel, blue line) than GF:R d’ context validation model (left panel, yellow line). While discriminating CHR three subgroups, both GF:S (middle panel) and GF:R (right panel) d’ context validation models illustrated improved prediction accuracy.
Multinomial logistic regression models
Going beyond the prediction of conversion alone, we examined the prediction performance for the 3 subgroups (CHR-R, CHR-P, and CHR-C). Both the GF:S d’ context and the GF:R d’ context validation models showed improved discrimination accuracy. Specifically, micro average prediction performance was AUC = 0.757 with 95% confidence interval ranging from 0.70 to 0.83 for the GF:S d’ context validation model (Fig. 2, middle panel). The GF:R d’ context validation model revealed the best prediction performance with micro average of AUC = 0.771 (95% CI 0.71–0.84) (Fig. 2, right panel). Intriguingly, d’ context significantly predicts CHR-C from CHR-R (AUC = 0.648, p = 0.021, 95% CI 0.53–0.74); however, it failed to distinguish CHR-C from CHR-P (AUC = 0.573, p = 0.387, 95% CI 0.47–0.70).
d’ context in CHR clinical outcomes and healthy controls at baseline
D’ context was lower in CHR converters (median = 0.63, Q1: 0.24 – Q3: 0.74) than CHR non-converters (median = 0.73, Q1: 0.47 – Q3: 0.85) at baseline, however, the difference was not significant (U = 672, p = 0.056) (Fig. 3, left panel). When comparisons were conducted among the three CHR subgroups and HCs, significant d’ context differences were revealed (χ2 (3) = 43.62, p = 0.000) with a mean rank d’ context of 129.20 for HCs, 93.60 for CHR-R, 70.98 for CHR-P and 58.11 for CHR-C. Post-hoc tests showed higher d'context in HCs (median = 0.90, Q1: 0.75 – Q3: 0.96) compared to CHR-R (median = 0.77, Q1: 0.56 – Q3: 0.91, p = 0.000, r = 35.60), CHR-P (median = 0.59, Q1: 0.44 – Q3: 0.82, p = 0.000, r = 58.22), and CHR-C (median = 0.63, Q1: 0.24 – Q3: 0.74, p = 0.000, r = 71.09). Importantly, CHR-R showed significantly higher d’ context at baseline compared to CHR-C (p = 0.021, r = 35.49), and to CHR-P (p = 0.051, r = 22.62). D’ context did not differ between CHR-P and CHR-C (p = 0.410, r = 12.87) (Fig. 3, right panel).
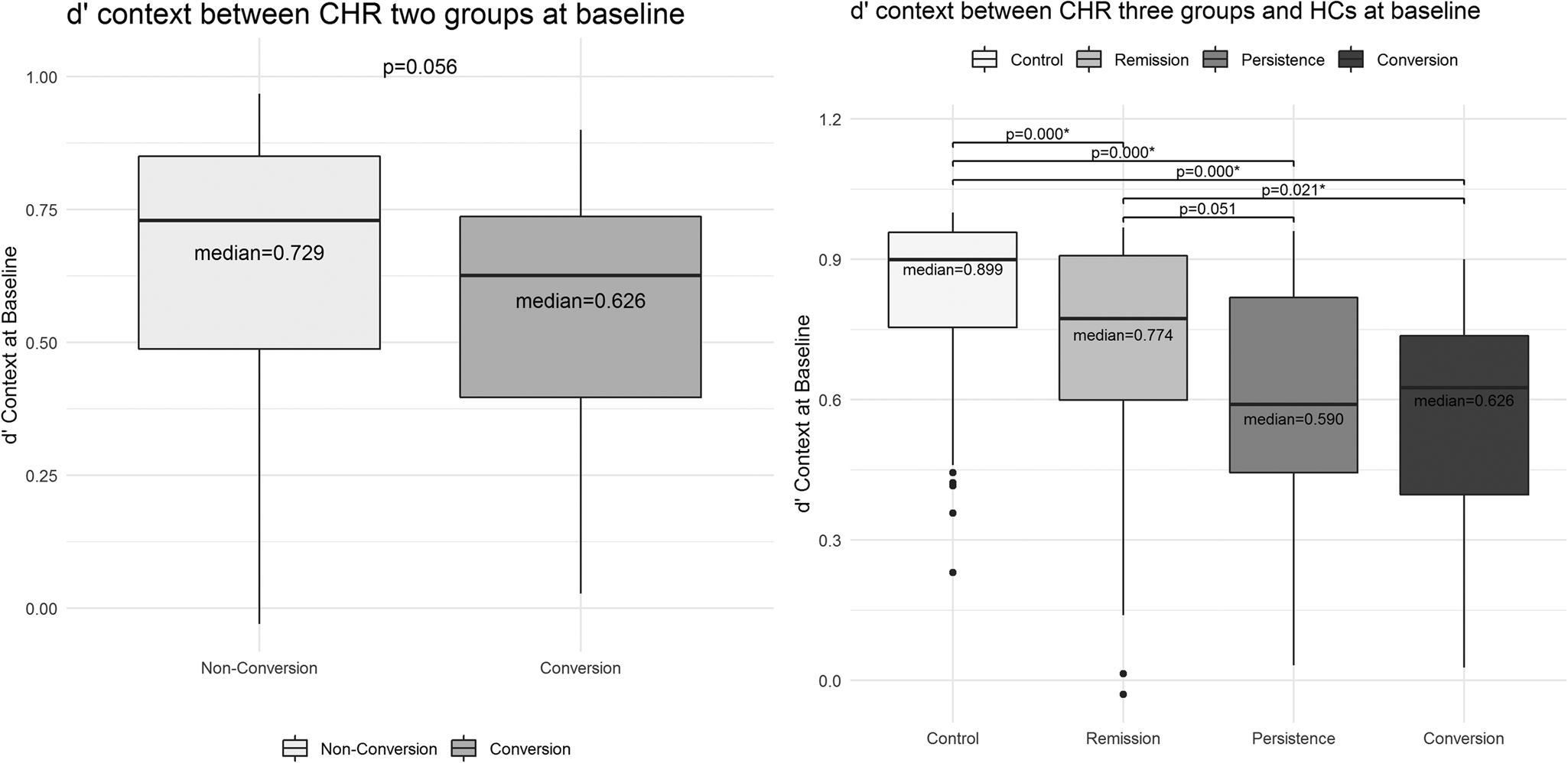
Fig. 3. There was no significant d’ context difference between CHR conversion and non-conversion groups (Left panel). Significant baseline d’ context difference was revealed among CHR-R, CHR-P, CHR-C and HCs (Right panel).
Clinical and functioning scores change from baseline to 12-month follow-up
CHR-R showed less severe positive symptoms at baseline compared to CHR-P and CHR-C (P total score at baseline: F (2,114) = 7.48, p = 0.001; modified P1 + P2 sum at baseline: F (2,114) = 4.41, p = 0.0014) (Fig. 4, Left panel). Other baseline clinical symptoms and social functioning did not differ among the three subgroups. Longitudinal analyses revealed a significant reduction in SIPS positive symptoms in CHR-R compared to the other two CHR sub-groups. Decreased SIPS general symptoms and improved GF:S was significantly different between CHR-R and CHR-P; whereas CHR-R showed significantly improved GF:R compared only to CHR-C (Fig. 4, Right panel). Detailed statistical results are presented in the supplementary materials (online Supplementary Table S5)

Fig. 4. SIPS positive symptoms at baseline differed significantly between CHR-R and CHR-P or between CHR-R and CHR-C. The majority of clinical and social functioning score changes differed between CHR-R and other two subgroups.
Discussion
In the current study, we found good prediction accuracy for psychosis conversion (AUC = 0.723 for GF:S model and AUC = 0.716 for GF:R model) utilizing prediction models with d’ context and four other risk factors from the NAPLS psychosis risk calculator (Cannon et al., Reference Cannon, Cadenhead, Cornblatt, Woods, Addington, Walker, Seidman, Perkins, Tsuang, Mcglashan and Heinssen2008). Importantly, AUC was improved to 0.757 with the GF:S model and to 0.771 with the GF:R model when CHR clinical outcomes were separated into three subgroups. In addition, we revealed significantly reduced severity of clinical symptoms and improved psychosocial functioning during 12-month follow-up in CHR-R compared to the other two subgroups.
The current study showed better proactive control at baseline in CHR-R than in CHR-P and CHR-C, while proactive control at baseline was not different between CHR-P and CHR-C. The twelve-month longitudinal analysis did not reveal any significant main effects or interactions involving diagnostic group or time, suggesting that cognitive control impairments in CHR remained stable over time. These results are in line with the consensus of longitudinal studies that cognitive impairments are already evident in the high-risk phase of psychosis, with limited evidence of any deterioration over time (Bora and Murray, Reference Bora and Murray2014). Such pre-morbid cognitive impairment in CHR suggests that cognitive abnormalities in psychosis are not solely consequences of psychotic symptoms, but may reflect specific neurodevelopmental processes leading to the development of psychosis (Kahn and Keefe, Reference Kahn and Keefe2013). Alternatively, premorbid cognitive deficits might reflect non-specific neurodevelopmental abnormalities that increase the risk of developing psychosis as well as other serious forms of psychopathology (Owen et al., Reference Owen, O'donovan, Thapar and Craddock2011). Indeed, the current study revealed heterogeneous levels of cognitive deficits in CHR, in which the sub-group of CHR individuals with worse premorbid proactive control deficits more often converted to psychosis over a 12-month follow-up. Accumulating evidence suggests an ongoing, but reduced rate of psychosis conversion for up to 10 years for CHR individuals (Nelson et al., Reference Nelson, Yuen, Wood, Lin, Spiliotacopoulos, Bruxner, Broussard, Simmons, Foley, Brewer, Francey, Amminger, Thompson, McGorry and Yung2013). Consistent with this, the current study found significantly worse proactive control in CHR-P than CHR-R individuals at baseline. This suggests that CHR-P individuals may be at ongoing risk of developing psychosis and longer-term monitoring and clinical treatment may be warranted.
The novelty of the proposed work includes the use of an experimental cognitive task, the AX-CPT, with established links to a domain general cognitive control deficit with known neural mechanisms, that is present in individuals with psychosis, and the advantage of brief, computerized administration that makes it pragmatically well suited to the clinical or community setting. Inclusion of a single measure of cognitive control (d’ context), instead of the traditional neuropsychological tests of cognition utilized in the NAPLS risk calculator, provided discrimination of psychosis conversion with GF:S (AUC of 0.72) and validated the prediction probability of the original NAPLS-2 psychosis risk calculator (AUC of 0.71) (Cannon et al., Reference Cannon, Yu, Addington, Bearden, Cadenhead, Cornblatt, Heinssen, Jeffries, Mathalon, Mcglashan, Perkins, Seidman, Tsuang, Walker, Woods and Kattan2016). Besides SIPS unusual thoughts and suspiciousness baseline scores, d’ context is the only other single risk factor that significantly predicted psychosis conversion, specifically distinguishing converters from remitters. Together with the reliability of AX-CPT examination (Strauss et al., Reference Strauss, Mclouth, Barch, Carter, Gold, Luck, Macdonald, Ragland, Ranganath, Keane and Silverstein2014), these results suggest that d’ context is sensitive to capturing cognitive impairments in CHR individuals, and provides a time-efficient alternative to traditional neuropsychological measures for use in CHR research, and potentially in clinical practice.
Specifically, the traditional neuropsychological battery requires trained clinicians, typically clinical psychologists, and necessitates extensive time for testing (e.g. up to 2 h) depending on the breadth of assessments. In contrast, the AX-CPT task can be administered by a variety of staff after brief training and takes less than 15 min to complete. Therefore, the AX-CPT provides a feasible option for psychosis risk screening that requires fewer clinical resources for administration and minimizes the burden on participants in both research and clinical settings. Further, it is important to note that screening is not the same as a comprehensive assessment of cognition. The cognitive screen and a full neuropsychological assessment have complementary, yet distinct roles, and the Working Group on Screening and Assessment (WGSA) provides information in distinguishing screening from comprehensive assessment for mental and behavioral health problems (American Psychological Association Practice Organization, 2014). Based on this statement, Roebuck-Spencer et al. (Reference Roebuck-Spencer, Glen, Puente, Denney, Ruff, Hostetter and Bianchini2017) extensively reviewed application protocols (e.g. early detection of individuals at high-risk for a specific disorder) and limitations (e.g. false-positive test results) for cognitive screening tests, and suggested a combination of cognitive screen and other clinical risk factors to determine if further neuropsychological assessments or corresponding treatments are needed. Consistent with this approach, the psychosis risk calculator uses a combination of cognitive and clinical variables to identify individuals who may be at increased risk, which is then followed by a comprehensive clinical evaluation. For treatment planning and outcome measurement, the use of comprehensive cognitive assessments in individuals who are identified at increased risk may yield important clinical value. Ongoing research is needed to confirm the combination of clinical and cognitive variables that are most accurate in predicting psychosis outcomes.
Of note, two independent samples validated the NAPLS-2 psychosis risk calculator with slightly different prediction accuracy. While Carrión and colleagues (Carrión et al., Reference Carrión, Cornblatt, Burton, Tso, Auther, Adelsheim, Calkins, Carter, Niendam, Taylor and Mcfarlane2016) reported AUC of 0.79 using part of the EDIPPP dataset, Zhang et al. (Zhang et al. Reference Zhang, Li, Tang, Niznikiewicz, Shenton, Keshavan, Stone, Mccarley, Seidman and Wang2018) revealed AUC of 0.63 using the Shanghai At Risk for Psychosis (SHARP) dataset. Although such discrepancy was interpreted as statistical model overfitting in the EDIPPP dataset (Zhang et al., Reference Zhang, Li, Tang, Niznikiewicz, Shenton, Keshavan, Stone, Mccarley, Seidman and Wang2018), these authors utilized GAF decline instead of GF:S decline, which was used in the previous NAPLS-2 psychosis risk calculator validation studies and did not describe the potential implications. In the current study, the GF:S d’ context validation model (AUC of 0.722) showed a higher prediction accuracy for psychosis conversion compared to the model utilizing GAF decline (AUC of 0.709). The GF:S scale was specifically designed to examine changes in social functioning associated with psychosis (Cornblatt et al., Reference Cornblatt, Auther, Niendam, Smith, Zinberg, Bearden and Cannon2007), so the use of a broader functioning scale like the GAF by Zhang and colleagues may have negatively impacted their prediction models. Furthermore, prediction accuracy generally improved when discriminating the 3 CHR subgroups compared to predicting psychosis conversion alone, with the GF:S d’ context validation model delivering the highest AUC of 0.773. That is, the 3 CHR subgroups may more reliably reflect the magnitude of the neurocognitive deficits in CHR than the conventional two-class psychosis conversion outcomes, suggesting that three-group outcomes should be considered for future neurocognitive prediction models.
Could cognitive deficits serve as a target for future interventions studies aimed at the prevention of emerging psychosis? Accumulating evidence emphasizes that cognition-enhancing interventions in the clinically high-risk phase have the potential to prevent psychosis conversion in CHR subjects. A study demonstrated reduced conversion rates for CHR individuals treated with cognitive behavioral therapy (CBT) in 58 CHR individuals compared to a monitoring (placebo) group (Morrison et al., Reference Morrison, French, Walford, Lewis, Kilcommons, Green, Parker and Bentall2004). An 18 month longitudinal multi-center study (N = 196 CHR) demonstrated significantly lower psychosis conversion rate (10.5%) in the CBT treated group than a comparison group receiving routine treatment (23.8%) (Ising et al., Reference Ising, Smit, Veling, Rietdijk, Dragt, Klaassen, Savelsberg, Boonstra, Nieman, Linszen, Wunderink and Van Der Gaag2015). Cognitive remediation represents another promising approach (Harvey and Bowie, Reference Harvey and Bowie2012). A recent review paper reported that cognitive remediation is effective in improving attention, processing speed, and verbal memory, social functioning in CHR individuals (Glenthøj et al., Reference Glenthøj, Hjorthøj, Kristensen, Davidson and Nordentoft2017). In one paper examining the effects of cognitive remediation on psychosis conversion (Bechdolf et al., Reference Bechdolf, Wagner, Ruhrmann, Harrigan, Putzfeld, Pukrop, Brockhaus-Dumke, Berning, Janssen, Decker, Bottlender, Maurer, Möller, Gaebel, Häfner, Maier and Klosterkötter2012), results showed reduced transition to psychosis in CHR participants who received cognitive remediation in combination with additional psychological interventions (including CBT, group skills training, psychoeducational multifamily group, compared to participants with supportive counselling). Therefore, future studies are needed to evaluate the effects of cognition-enhancing treatments on CHR conversion for psychosis, as well as on CHR-R and CHR-P. With such refined clinical outcomes, we may advance our knowledge of the association between cognitive enhancement and CHR transition to psychosis.
In addition, CHR-R showed significant improvement in social and role functioning in comparison to CHR-C and CHR-P. Our results suggested impairments in social functioning may be less responsive to treatment in individuals developing psychosis (CHR-C) or showing a persistent state of attenuated psychosis symptoms (CHR-P) over follow-up. In agreement with the original NAPLS study, we observed better prediction of psychosis conversion for GF:S decline (AUC of 0.723) than GF:R decline (AUC of 0.716) (Cannon et al., Reference Cannon, Cadenhead, Cornblatt, Woods, Addington, Walker, Seidman, Perkins, Tsuang, Mcglashan and Heinssen2008). However, GF:R decline (AUC of 0.757) showed better prediction accuracy to discriminate the three subgroups than GF:S decline (AUC of 0.771). These results agree with prior studies showing impaired social functioning as a long-standing trait for psychosis with limited or no impact from demographic factors such as social class and IQ (Cornblatt et al., Reference Cornblatt, Auther, Niendam, Smith, Zinberg, Bearden and Cannon2007). Additional research is needed to determine which global functional measures lead to more reliable discrimination of psychosis conversion and the CHR three subgroups.
Conclusion
The results of the present study used a measure of cognitive control linked to the integrity of a frontal-parietal network in the brain, d’ context from the AX-CPT task, to predict clinical outcomes in CHR individuals. Use of this measure alone showed comparable performance to studies using additional clinical measures from the NAPLS risk algorithm and lengthier clinical neuropsychological measurements of cognition. Prediction of outcomes was enhanced when clinical outcomes distinguished between CHR remitters and non-remitters as well as conversion to psychosis. CHR-C individuals also have reductions in social functioning and role scales compared to improvements in CHR-P and CHR non-converters. This brief computer-administered measure also performed well when used as an alternative to traditional neuropsychological tests of cognition in the NAPLS risk calculator. These data suggest that the AX-CPT task may be included as a scientifically informative and cost-effective routine cognitive task in future studies of cognition in CHR.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291719002332








