Introduction
The fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) defines a traumatic event as direct or indirect exposure to threatened death, serious injury, or sexual violence and includes a new category for trauma- and stressor-related disorders (i.e. disorders in which exposure to a traumatic or stressful event is listed explicitly as a diagnostic criterion) (American Psychiatric Association, 2013). Posttraumatic stress disorder (PTSD) is the most recognized among these diagnoses. According to the DSM-5, PTSD diagnosis includes multiple criteria (Table 1): stressor, intrusion symptoms, avoidance, negative alterations in cognitions and mood, alterations in arousal and reactivity, duration, functional significance, and exclusion. In addition to the diagnostic criteria, there are two additional specifications PTSD-affected patients are expected to experience: dissociative specification (depersonalization: being an outside observer of or detached from oneself; derealization: experience of unreality, distance, or distortion) and delayed specification (full diagnostic criteria are not met until at least 6 months after the trauma(s), although the onset of symptoms may occur immediately). Due to the presence of multiple diagnostic symptoms, PTSD is among the most heterogeneous psychiatric diagnoses. There are 636 120 possible PTSD diagnostic combinations (i.e. any set of symptoms for a disorder such that an individual meets criteria for that disorder if he or she exhibits that set of symptoms) and 52% of them (N = 336 000) are disjoint combinations (i.e. diagnostic combinations occurring among sets of symptoms that have no overlap) (Olbert, Gala, & Tupler, Reference Olbert, Gala and Tupler2014). Since its introduction in the DSM classification, several critiques were made with respect to PTSD diagnosis (Ball & Stein, Reference Ball, Stein, Beck and Sloan2012). These include: symptom overlap, high rates of comorbidity with other psychiatric disorders, the inability of PTSD diagnostic criteria to reflect the complexity of trauma response, and the variability of PTSD construct across contexts and cultures (Frueh, Elhai, & Acierno, Reference Frueh, Elhai and Acierno2010; Papa, Neria, & Litz, Reference Papa, Neria and Litz2008). Although these criticisms represent valid viewpoints, DSM diagnostic criteria are used in most of the human studies of PTSD. Molecular studies of PTSD can help disentangle the complexities of PTSD diagnosis through the understanding of the biological basis linking exposure to traumatic events to psychiatric disorders and physical health outcomes. Here, we review the progress made by genomic research of PTSD from twin studies to large-scale genome-wide association studies (GWAS; Figure 1). We also describe investigations focused on other omics domains and brain imaging and their contributions to understanding the molecular changes associated with PTSD in brain and non-brain tissues. Finally, we conclude by discussing the clinical and therapeutic implications of PTSD and trauma genomic research.
Table 1. Diagnostic and statistical manual of mental disorders (DSM-5) diagnostic criteria for posttraumatic stress disorder


Fig. 1. Summary of multifaceted investigations into the etiology of posttraumatic stress disorder ranging from environmental effects, genetics, multi-omics, and neuroimaging efforts.
Epidemiology
General population studies have shown that a large proportion of people in developed countries have been exposed to at least one traumatic event in their lifetime (estimates from 28 to 90%) with 82.7% prevalence of exposure to any traumatic event in the USA (Benjet et al., Reference Benjet, Bromet, Karam, Kessler, McLaughlin, Ruscio and Koenen2016). There are known differences among trauma types with respect to the consequent PTSD risk. In surveys from the World Health Organization (WHO), the investigators obtained representative data on trauma-specific PTSD from 24 countries (68 894 subjects) and assessed 29 lifetime traumas (Kessler et al., Reference Kessler, Aguilar-Gaxiola, Alonso, Benjet, Bromet, Cardoso and Koenen2017). Trauma involving interpersonal violence had the highest risk. PTSD burden, determined by multiplying trauma prevalence by trauma-specific PTSD risk and persistence, was 77.7 person-years/100 respondents. The trauma types with the highest proportions of this burden were rape (13.1%), other sexual assault (15.1%), being stalked (9.8%), and unexpected death of a loved one (11.6%). The broad category of intimate partner sexual violence accounted for nearly 42.7% of all person-years with PTSD. Due to trauma-specific PTSD risk, the disease prevalence varies depending on population and trauma type. In the North American general adult population, lifetime PTSD prevalence ranges from 6% to 9% while the 1-year prevalence is between 3.5% and 5% (Sareen, Reference Sareen2020). However, ~2% prevalence was reported by WHO for upper-middle income and lower-middle income countries included in their survey (Koenen et al., Reference Koenen, Ratanatharathorn, Ng, McLaughlin, Bromet, Stein and Kessler2017a). In contrast, a recent systematic review of PTSD prevalence studies in Africa found an overall current pooled prevalence of PTSD of 25% (Ng et al., Reference Ng, Stevenson, Kalapurakkel, Hanlon, Seedat, Harerimana and Koenen2020).
In addition to trauma-specific PTSD risk, several pre-trauma risk factors can influence PTSD development: gender, age at trauma, education, socioeconomic status, psychiatric comorbidities, being in a confiding relationship as an adult, history of previous traumatic experience, childhood adversity and abuse, social support, and initial reaction severity to the traumatic event (Wild et al., Reference Wild, Smith, Thompson, Bear, Lommen and Ehlers2016). Accordingly, the interplay between traumatic events and pre-trauma risk factors can consistently affect the frequency with which PTSD occurs. For instance, PTSD risk shows evident differences between sexes: women are four times more likely to develop PTSD when compared with men when accounting for the exposure to traumatic events. Considering specific traumas, PTSD rates between women and men are similar for accidents, natural disasters, and the sudden death of a loved one (Sareen, Reference Sareen2020). Differently, although women are >10-times more likely as men to be raped, PTSD incidence after rape is higher in men than that observed in women. An opposite scenario for sex-specific PTSD incidence is present for molestation and physical assault (Chivers-Wilson, Reference Chivers-Wilson2006). PTSD is associated with several psychiatric comorbidities including depression (Dunn, Nishimi, Powers, & Bradley, Reference Dunn, Nishimi, Powers and Bradley2017), substance abuse and dependence (Roberts, Roberts, Jones, & Bisson, Reference Roberts, Roberts, Jones and Bisson2015), and suicidal behaviors (Victor & Klonsky, Reference Victor and Klonsky2014). Additionally, PTSD has also been implicated in the etiology of various physical disorders (Boscarino, Reference Boscarino2004; Gupta, Reference Gupta2013; Lohr et al., Reference Lohr, Palmer, Eidt, Aailaboyina, Mausbach, Wolkowitz and Jeste2015) such as cancer (Roberts et al., Reference Roberts, Huang, Koenen, Kim, Kubzansky and Tworoger2019; Shand, Cowlishaw, Brooker, Burney, & Ricciardelli, Reference Shand, Cowlishaw, Brooker, Burney and Ricciardelli2015), gastrointestinal disorders (Savas et al., Reference Savas, White, Wieman, Daci, Fitzgerald, Laday Smith and El-Serag2009), and cardiovascular disease (Koenen et al., Reference Koenen, Sumner, Gilsanz, Glymour, Ratanatharathorn, Rimm and Kubzansky2017b). However, the studies regarding the physical health sequelae of PTSD are in some cases conflicting and there is still an open debate about possible explanations.
Pedigree analyses
The study of PTSD familiarity permitted researchers to understand the genetic and environmental factors involved in the propensity to traumatic events and the vulnerability to PTSD. In particular, studies comparing monozygotic (MZ) and dizygotic (DZ) twins partitioned genetic factors into additive and non-additive effects to understand shared environmental and non-shared environmental effects (Afifi, Asmundson, Taylor, & Jang, Reference Afifi, Asmundson, Taylor and Jang2010). In line with the sex difference observed in PTSD epidemiology (higher prevalence in women) (Rivollier et al., Reference Rivollier, Peyre, Hoertel, Blanco, Limosin and Delorme2015), twin studies observed that, while sex-combined cohorts presented a 40%–60% heritability, all-female cohorts showed higher PTSD heritability estimates than all-male cohorts (~70% v. ~30%, respectively) (Duncan, Cooper, & Shen, Reference Duncan, Cooper and Shen2018). As mentioned, PTSD risk is influenced by the type of trauma and several pre-trauma risk factors. Accordingly, the variation of heritability estimates observed across different cohorts is likely to be partially affected by the characteristics of the samples investigated. Additionally, exposure to certain traumatic experiences appears to present a consistent familiarity (Stein, Jang, Taylor, Vernon, & Livesley, Reference Stein, Jang, Taylor, Vernon and Livesley2002). A study of 222 MZ and 184 DZ twin pairs demonstrated that the variance of assaultive traumatic events (e.g. robbery, being held captive, being beaten up, and sexual assault) is accounted by 20% additive genetic factors, 21% shared environmental factors, and 58% non-shared environmental factors (Stein et al., Reference Stein, Jang, Taylor, Vernon and Livesley2002). Conversely, non-assaultive traumatic events (e.g. sudden death of a family member, motor vehicle accident, fire, tornado, flood, and earthquake) did not have a detectable genetic component and were accounted by shared and non-shared environmental effects (39% and 61%, respectively) (Stein et al., Reference Stein, Jang, Taylor, Vernon and Livesley2002). The environmental components of assaultive and non-assaultive traumatic events appear to be mostly independent of each other: the shared environmental correlation was 0.31 and the non-shared environmental correlation was estimated at −0.20 (Stein et al., Reference Stein, Jang, Taylor, Vernon and Livesley2002). On the other hand, the genetic components of PTSD and the exposure to certain traumatic events are highly overlapping (Smoller, Reference Smoller2016). They also overlap with the genetic component of resilience, i.e. the ability to maintain or regain normal psychological and physical functioning in the face of adversity (Wu et al., Reference Wu, Feder, Cohen, Kim, Calderon, Charney and Mathe2013). In 3318 male twin pairs from the Vietnam Era Twin Registry assessed with the PTSD Checklist and the Connor-Davidson Resilience Scale-10, PTSD and resilience shared a single genetic factor accounting for 59% of their correlation (Wolf et al., Reference Wolf, Miller, Sullivan, Amstadter, Mitchell, Goldberg and Magruder2018c). These shared genetic factors are not unique to trauma exposure, PTSD, and resilience, but they also overlap with other psychiatric disorders. A study conducted in 2591 participants (996 female and 536 male twins; 625 female and 434 male nontwin siblings) reported a high genetic overlap of high-risk trauma exposure with both PTSD and major depressive disorder (MDD) (Sartor et al., Reference Sartor, Grant, Lynskey, McCutcheon, Waldron, Statham and Nelson2012). Recent twin studies focused their attention on the genetic overlap of PTSD with insomnia and sleep duration (Cox, Taylor, Strachan, & Olatunji, Reference Cox, Taylor, Strachan and Olatunji2020; McCall et al., Reference McCall, Turkheimer, Tsang, Avery, Duncan and Watson2019). A consistent phenotypic covariance of PTSD symptoms and insomnia was explained by genetic factors (36–44%) with a significant genetic correlation of insomnia with PTSD re-experiencing and avoidance symptoms (Cox et al., Reference Cox, Taylor, Strachan and Olatunji2020). In a cohort including 1865 MZ and 758 DZ twin pairs from the community-based Washington State Twin Registry, the variance in sleep duration attributable to the shared environment was moderated by PTSD severity, while the variance in PTSD symptoms attributable to additive genetics was moderated by sleep duration (McCall et al., Reference McCall, Turkheimer, Tsang, Avery, Duncan and Watson2019).
In addition to twin-based studies, family-based investigations contribute to characterizing the genetic vulnerability to PTSD (Skelton, Ressler, Norrholm, Jovanovic, & Bradley-Davino, Reference Skelton, Ressler, Norrholm, Jovanovic and Bradley-Davino2012). For example, adult children of PTSD cases exposed to extremely severe traumatic events (e.g. Holocaust survivors and Cambodian refugees) received more frequently a PTSD diagnosis than adult children of individuals without PTSD that were exposed to the same traumatic experience (Sack, Clarke, & Seeley, Reference Sack, Clarke and Seeley1995; Yehuda, Halligan, & Bierer, Reference Yehuda, Halligan and Bierer2001). An important limitation of pedigree analyses is that PTSD can be assessed only in individuals that experience a traumatic event and we cannot determine the PTSD status of trauma-unexposed subjects.
From candidate genes to genome-wide investigations
Genetic liability to PTSD is characterized by the effect of thousands of loci across the genome. These variants present individual small effects on the overall disease risk. To identify these effects, genetic association studies test the allele frequency of genetic variants with respect to binary and quantitative traits (e.g. PTSD diagnosis and PTSD severity, respectively). Over the years, the designs of association studies were developed based on the genotyping technologies available. Early genetic association studies were based on the ability to genotype a limited number of variants in small cohorts. The genetic variants of interest were selected considering genes included in biological pathways known from the scientific literature to be related to the pathogenesis of PTSD and related psychiatric disorders. This particular approach is known as ‘candidate gene’. The first candidate gene study of PTSD observed a positive association of DRD2*A1 allele in two samples including a total of 37 PTSD cases and 19 controls (Comings, Muhleman, & Gysin, Reference Comings, Muhleman and Gysin1996). Because of the genotyping technology progress, larger studies reported associations of variants across multiple genes expected to play a key role in PTSD pathogenesis: serotonin transporter gene (SLC6A4) (Kilpatrick et al., Reference Kilpatrick, Koenen, Ruggiero, Acierno, Galea, Resnick and Gelernter2007), dopamine transporter gene (SLC6A3) (Segman et al., Reference Segman, Cooper-Kazaz, Macciardi, Goltser, Halfon, Dobroborski and Shalev2002), catechol-O-methyltransferase gene (COMT) (Kolassa, Kolassa, Ertl, Papassotiropoulos, & De Quervain, Reference Kolassa, Kolassa, Ertl, Papassotiropoulos and De Quervain2010), steroid receptor chaperone FK506 binding protein 5 (FKBP5) (Zhang et al., Reference Zhang, Hu, Yu, Chen, Dohl, Li and Ursano2020), adenylate cyclase activating polypeptide 1 (ADCYAP1) gene (Ressler et al., Reference Ressler, Mercer, Bradley, Jovanovic, Mahan, Kerley and May2011), and brain-derived neurotrophic factor (BDNF) gene (Zhang et al., Reference Zhang, Ozbay, Lappalainen, Kranzler, van Dyck, Charney and Gelernter2006). Similar to other complex traits, candidate gene studies of PTSD are often inconsistent across the different samples investigated (Sheerin et al., Reference Sheerin, Lind, Bountress, Marraccini, Amstadter, Bacanu and Nugent2020).
With the advent of genome-wide arrays and genotype imputation based on large reference panels, genome-wide analyses permitted psychiatric geneticists to move from hypothesis-driven studies (candidate gene design) to hypothesis-generating studies (GWAS design). Genetic studies based on such wide screening can uncover loci in molecular pathways that were not previously expected to be associated with PTSD, generating novel hypotheses about disease pathogenesis. Between 2013 and 2017, several PTSD GWAS with sample size ranging from 147 to 13 690 participants identified risk alleles in several genes, including RORA (RAR Related Orphan Receptor A) (Logue et al., Reference Logue, Baldwin, Guffanti, Melista, Wolf, Reardon and Miller2013), TLL1 (Tolloid Like 1) (Xie et al., Reference Xie, Kranzler, Yang, Zhao, Farrer and Gelernter2013), lincRNA AC068718.1 (long intergenic non-protein coding RNA AC068718.1) (Guffanti et al., Reference Guffanti, Galea, Yan, Roberts, Solovieff, Aiello and Koenen2013), PRTFDC1 (Phosphoribosyl Transferase Domain Containing 1) (Nievergelt et al., Reference Nievergelt, Maihofer, Mustapic, Yurgil, Schork, Miller and Baker2015), ANKRD55 (Ankyrin Repeat Domain 55) (Stein et al., Reference Stein, Chen, Ursano, Cai, Gelernter, Heeringa and Resilience in Servicemembers2016), and ZNF626 (zinc finger protein 626) (Stein et al., Reference Stein, Chen, Ursano, Cai, Gelernter, Heeringa and Resilience in Servicemembers2016).
Although GWAS are powerful tools, their gene discovery can be affected by confounders (systematic biases affecting the analyses) (Sul, Martin, & Eskin, Reference Sul, Martin and Eskin2018), winner's curse (overestimation of genetic effects) (Palmer & Pe'er, Reference Palmer and Pe'er2017), and polygenicity (thousands of variants with small effects) (Holland et al., Reference Holland, Frei, Desikan, Fan, Shadrin, Smeland and Dale2020). A better understanding of the genetics of complex traits permitted investigators to establish the unreliability of results generated by candidate gene studies and relatively-small GWAS of PTSD. Indeed, similar to other complex traits, findings of underpowered genetic association studies of PTSD independently from their design are likely to be false positive results. To conduct statistically powerful GWAS, investigators analyzed the massive cohorts via collaborative initiatives and large biobanks. The Psychiatric Genomics Consortium (PGC) is the largest collaborative experiment in the history of psychiatry, including >800 investigators from >150 institutions in >40 countries (Sullivan et al., Reference Sullivan, Agrawal, Bulik, Andreassen, Borglum, Breen and Psychiatric Genomics2018). Among PGC workgroups, PGC-PTSD investigators focus on the harmonization of genome-wide data from multiple studies to conduct powerful PTSD GWAS meta-analyses. In 2017, the first PGC-PTSD GWAS was finalized including 20 730 individuals from 11 cohorts (Duncan et al., Reference Duncan, Ratanatharathorn, Aiello, Almli, Amstadter, Ashley-Koc-h and Koenen2018). Although this was the first large PTSD GWAS meta-analysis, the sample size was too limited to identify associations surviving genome-wide multiple testing correction. However, these data were powerful enough to conduct the first analyses of PTSD polygenic inheritance. PGC-PTSD investigators reported higher PTSD SNP-heritability (i.e. the proportion of phenotypic variance attributable to the additive effects of common genetic variants) in women and significant genetic correlation (r g; i.e. the proportion of phenotypic variance that two traits share due to common genetic causes) of PTSD with schizophrenia and MDD (Duncan et al., Reference Duncan, Ratanatharathorn, Aiello, Almli, Amstadter, Ashley-Koc-h and Koenen2018). After this first GWAS meta-analysis, access to large biobanks rapidly increased the number of PTSD-informative individuals with genome-wide information. In 2019, a GWAS of PTSD reexperiencing symptoms was conducted in >165 000 participants of the US Million Veteran Program (MVP) (Gelernter et al., Reference Gelernter, Sun, Polimanti, Pietrzak, Levey, Bryois and Million Veteran2019). MVP investigators identified eight distinct risk alleles and 30 gene-based associations; reported 400 significant genetic correlations with psychiatric disorders, behavioral traits, and other complex phenotypes; and observed functional enrichments for cortex, hypothalamus, amygdala, hippocampus, basal ganglia medium, and spiny neurons in the striatum (Gelernter et al., Reference Gelernter, Sun, Polimanti, Pietrzak, Levey, Bryois and Million Veteran2019). Leveraging reexperiencing-symptom data from 117 900 UK Biobank participants of European descent, the MVP findings were replicated at a single-variant level and at a polygenic level (r g = 0.88, s.e. = 0.07). The same year a second PTSD GWAS meta-analysis was finalized by PGC-PTSD investigators (Nievergelt et al., Reference Nievergelt, Maihofer, Klengel, Atkinson, Chen, Choi and Koenen2019). This novel study included over 30 000 PTSD cases and 170 000 controls (combining UK Biobank with 60 other datasets), identifying ancestry- and sex-specific risk loci (African ancestry, European ancestry, and male sample) and confirming that PTSD SNP-based heritability varies by sex with estimates ranging around 5%–20% (Nievergelt et al., Reference Nievergelt, Maihofer, Klengel, Atkinson, Chen, Choi and Koenen2019). To investigate the genetics of PTSD in diverse populations, PGC-PTSD investigators developed a framework for improving the inclusion of admixed individuals in large-scale association studies, using a local-ancestry informed regression model to generate ancestry-specific effect size estimates (Atkinson et al., Reference Atkinson, Maihofer, Kanai, Martin, Karczewski, Santoro and Neale2020). Recently, MVP investigators completed a PTSD GWAS analyzing data from more than 250 000 MVP participants and testing a validated electronic health record-based algorithmically-defined PTSD diagnosis phenotype (48 221 cases and 217 223 controls), and PTSD quantitative symptom phenotypes (212 007 individuals) (Stein et al., Reference Stein, Levey, Cheng, Wendt, Harrington, Cho and Gelernter2019). Beyond the risk loci identified with respect to case-control and quantitative phenotypes, this novel MVP study showed that PTSD symptom sub-domains share most of their genetic liability (rg 0.93–0.98) and identified novel potential treatment from a drug repositioning analysis conducted with respect to the loci identified (CRHR1 antagonist; TCF4: darinaparsin; TCF4-PLXNA1: otenzepad; PLEKHM1: dopamine receptor antagonists, acetylcholine receptor antagonists, and angiotensin receptor antagonists) (Stein et al., Reference Stein, Levey, Cheng, Wendt, Harrington, Cho and Gelernter2019). Findings from PGC and MVP PTSD GWAS are summarized in Fig. 2. Novel methods are being developed to conduct multivariate genome-wide investigations of complex traits, increasing the statistical power and to model the pleiotropy widespread across the human genome (Grotzinger et al., Reference Grotzinger, Rhemtulla, de Vlaming, Ritchie, Mallard, Hill and Tucker-Drob2019). A multivariate GWAS conducted in a military cohort combining pre- and post-deployment biochemical and behavioral phenotypes identified novel loci associated with human stress response (Schijven et al., Reference Schijven, Geuze, Vinkers, Pulit, Schur, Malgaz and Luykx2019). With respect to rare variants, although whole-exome sequencing (WES) is very rarely used to investigate PTSD, a study identified rare variants located in TROVE2 gene as associated with emotional memory and PTSD (Heck et al., Reference Heck, Milnik, Vukojevic, Petrovska, Egli, Singer and Papassotiropoulos2017).
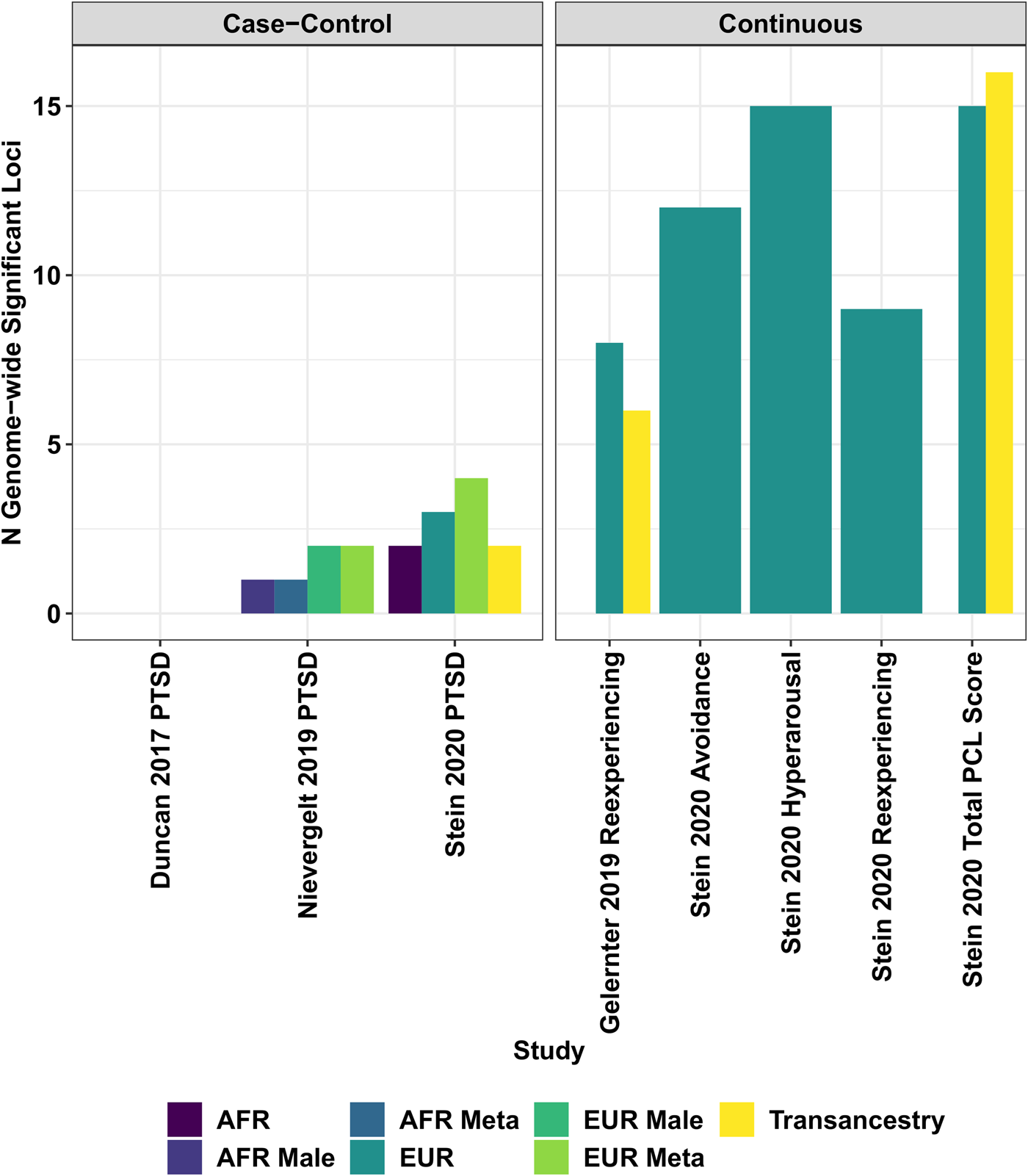
Fig. 2. Locus discovery from genome-wide association studies of biobank and consortia case-control and continuous (i.e. symptom count) measures of posttraumatic stress disorder.
In addition to the relevant biology uncovered by genome-wide analyses, the data generated are being used as a base to conduct follow-up analyses to disentangle further the pathogenesis of PTSD. In a study focused on genetically regulated gene expression comparing 29 539 PTSD cases and 166 145 controls (Huckins et al., Reference Huckins, Chatzinakos, Breen, Hartmann, Klengel, da Silva Almeida and Daskalakis2020), a substantial genetic heterogeneity based on ancestry, cohort type (military v. civilian), and sex was observed, but two significant tissue-gene associations were observed: ZNF140 (zinc finger protein 140) is predicted to be upregulated in whole blood and SNRNP35 (small nuclear ribonucleoprotein U11/U12 subunit 35) is predicted to be downregulated in the dorsolateral prefrontal cortex.
Leveraging data from large-scale GWAS, several studies have been conducted to investigate PTSD comorbidities, applying mainly two approaches: linkage score regression to calculate genetic correlation (Bulik-Sullivan et al., Reference Bulik-Sullivan, Finucane, Anttila, Gusev, Day, Loh and Neale2015) and Mendelian randomization (MR) for causal inference (Smith & Ebrahim, Reference Smith and Ebrahim2003). With respect to PTSD sex differences, the polygenic component of body shape and reproductive behaviors appear to be associated with PTSD in women with potential evidence linking body shape and sexual trauma to PTSD (Polimanti et al., Reference Polimanti, Amstadter, Stein, Almli, Baker and Bierut2017). As shown by twin studies (Cox et al., Reference Cox, Taylor, Strachan and Olatunji2020; McCall et al., Reference McCall, Turkheimer, Tsang, Avery, Duncan and Watson2019), there is a genetic overlap of PTSD with insomnia and sleep duration. GWAS data confirmed a moderate genetic correlation of PTSD with insomnia symptoms (rg range 0.36–0.49), oversleeping (rg range 0.32–0.44), undersleeping (rg range 0.48–0.49), but no causal effects were observed using the MR approach applied to the first PGC-PTSD GWAS (Lind et al., Reference Lind, Brick, Gehrman, Duncan, Gelaye and Maihofer2020). A causal inference analysis based on PGC-PTSD GWAS demonstrated that this genetic overlap between PTSD and educational attainment is due to a negative causal effect of socioeconomic status (measured as household income) on PTSD (Polimanti et al., Reference Polimanti, Ratanatharathorn, Maihofer, Choi, Stein and Morey2019). Using a similar causal-inference approach, certain blood metabolites showed putative causal effects on PTSD (Carvalho et al., Reference Carvalho, Wendt, Stein, Stein, Gelernter, Belangero and Polimanti2020). A more complex network of bidirectional associations was observed among PTSD, serum C-reactive protein, childhood support, and socioeconomic status (Muniz Carvalho et al., Reference Muniz Carvalho, Wendt, Maihofer, Stein, Stein, Sumner and Polimanti2020). Leveraging a different GWAS-based approach, investigators also reported that the comorbidity between PTSD and late-onset Alzheimer's disease may be due to common genetic mechanisms involved in immune response (Lutz, Luo, Williamson, & Chiba-Falek, Reference Lutz, Luo, Williamson and Chiba-Falek2020).
Gene × Environment interaction
The interplay of genetic susceptibility with traumatic experiences and pre-trauma risk factors is expected to play a key role in the PTSD pathogenesis. Numerous gene-by-environment (G × E) studies of PTSD have been conducted testing candidate genes (e.g. FKBP5, BDNF, and COMT) (Jin, Jeon, Hyun, & Lee, Reference Jin, Jeon, Hyun and Lee2019; van Rooij et al., Reference van Rooij, Stevens, Ely, Fani, Smith, Kerley and Jovanovic2016; Wang, Shelton, & Dwivedi, Reference Wang, Shelton and Dwivedi2018). Similarly, to candidate gene association studies, these G × E investigations present the same important limitations due to the lack of power and the potential presence of systematic bias from selection of candidate loci and environmental moderator(s) (Border et al., Reference Border, Johnson, Evans, Smolen, Berley, Sullivan and Keller2019). Some genome-wide studies explored the genetic interplay of traumatic experiences and PTSD with respect to other psychiatric traits. In a total sample of >24 000 participants, a genome-wide gene × trauma interaction analysis of alcohol misuse identified PRKG1 (protein kinase cGMP-dependent 1) as a risk locus modulating the effect of trauma exposure (Polimanti et al., Reference Polimanti, Kaufman, Zhao, Kranzler, Ursano, Kessler and Gelernter2018a). The homolog of this locus in Drosophila melanogaster (foraging gene) is well-known, because its activity controls synaptic transmission tolerance to acute stress (Burns et al., Reference Burns, Svetec, Rowe, Mery, Dolan, Boyce and Sokolowski2012). Additionally, the polygenic component of bipolar disorder and schizophrenia seemed to moderate the effect of trauma exposure on alcohol abuse with high voltage-gated calcium channel activity and Beta1/Beta2 adrenergic receptor signaling as key molecular pathways (Polimanti et al., Reference Polimanti, Kaufman, Zhao, Kranzler, Ursano, Kessler and Stein2018b). Recently, a multivariate GEWIS investigated the genetic interplay of traumatic experience and posttraumatic stress with respect to suicidality, identifying risk loci, and sex-specific cell-type transcriptome enrichments related to the potential role of extracellular matrix biology and synaptic plasticity as biological mediators (Wendt et al., Reference Wendt, Pathak, Levey, Nunez, Overstreet, Tyrrell and Polimanti2020a, Reference Wendt, Pathak, Overstreet, Tylee, Gelernter, Atkinson and Polimantib). A study showed enrichments for excitatory synaptic transmission and plasticity in the interaction between MVP re-experiencing PRS and attachment style with respect to PTSD symptoms assessed in the National Health and Resilience in Veterans Study (Tamman et al., Reference Tamman, Wendt, Pathak, Krystal, Montalvo-Ortiz, Southwick and Pietrzak2020). Investigators have also begun to characterize the genetic architecture of traumatic experiences that appears to have a strong genetic overlap with PTSD and other psychiatric disorders and may be linked to externalizing behaviors or to a greater likelihood of reporting maltreatment (Dalvie et al., Reference Dalvie, Maihofer, Coleman, Bradley, Breen, Brick and Nievergelt2020). Additionally, traumatic experiences appear to affect also the genetic liability to other psychiatric disorders. A study conducted in the UK Biobank reported that MDD SNP-heritability is higher in individuals that reported trauma with a genetic overlap among trauma exposure, body composition, and MDD (Coleman et al., Reference Coleman, Peyrot, Purves, Davis, Rayner, Choi and Breen2020). Further studies will be needed to understand how to disentangle the genetic and the environmental components of traumatic experiences and their effect on PTSD risk.
Epigenetics
The development of high-throughput technologies expanded the possibilities across different genomic features (Hasin, Seldin, & Lusis, Reference Hasin, Seldin and Lusis2017). Differently from genetic variation, other omics changes can be related to causative mechanisms (i.e. the molecular change is causal with respect to the trait-of-interest) or to downstream consequences (i.e. the molecular change is induced by the trait) with a consistent overrepresentation of the latter with respect to the former and accordingly often have higher effect size. With respect to PTSD research, epigenetic variation appears to be an obvious target because of its potential ability to reflect the molecular changes induced by traumatic events. Epigenome-wide association studies (EWAS) on brain specimens are expected to be informative for understanding PTSD pathogenesis, but there is limited availability of such samples and there may be also issues regarding the transferability of potential brain biomarkers to peripheral tissues of living participants. Accordingly, most EWAS are being conducted on peripheral tissues, mainly whole blood and saliva. Several candidate-gene and small PTSD EWAS have been performed (Zannas, Provencal, & Binder, Reference Zannas, Provencal and Binder2015), but similarly to what observed when the same designs were applied to genetic data, their results are likely to be affected by low statistical power and unaccounted confounders. Due to the well-known impact of PTSD among military personnel (Zang et al., Reference Zang, Gallagher, McLean, Tannahill, Yarvis, Foa and Consortium2017), several epigenetic studies have focused their attention on understanding whether there are specific epigenetic patterns of PTSD between individuals exposed to combat traumas and non-combat civilian traits (Hammamieh et al., Reference Hammamieh, Chakraborty, Gautam, Muhie, Yang, Donohue and Jett2017; Kuan et al., Reference Kuan, Waszczuk, Kotov, Marsit, Guffanti, Gonzalez and Luft2017b; Mehta et al., Reference Mehta, Klengel, Conneely, Smith, Altmann, Pace and Binder2013; Yang et al., Reference Yang, Zhang, Ge, Weder, Douglas-Palumberi, Perepletchikova and Kaufman2013). In a cohort of military veterans (378 lifetime PTSD cases and 135 controls), an epigenome-wide significant association at cg19534438 in the gene G0S2 (G0/G1 switch 2) was observed and replicated in other military cohorts (Logue et al., Reference Logue, Miller, Wolf, Huber, Morrison and Zhou2020). A longitudinal PTSD EWAS conducted with respect to pre- and post-deployment of 532 military participants showed that combat-related PTSD is associated with distinct methylation patterns mainly related to loci involved in the immune system (Snijders et al., Reference Snijders, Maihofer, Ratanatharathorn, Baker, Boks, Geuze and Nievergelt2020). Conversely, in civilian cohorts (545 participants), whole blood-derived DNA methylation levels at CpG sites located in HGS (hepatocyte growth factor-regulated tyrosine kinase substrate) and NRG1 (neuregulin 1) genes were associated with current PTSD (Uddin et al., Reference Uddin, Ratanatharathorn, Armstrong, Kuan, Aiello, Bromet and Smith2018). Although the findings reported were replicated in some cases, they could be still due to unaccounted confounders, the limited sample size, or to differences in temporal stability of methylation signatures over time. PGC-PTSD workgroup is leading the largest collaborative effort to identify reliable epigenetic associations. Additionally, since the epigenetic variation is expected to be affected by many potential confounders, the PGC-PTSD workgroup developed a multi-site analysis pipeline to account adequately for ancestry population stratification and type I error inflation (Ratanatharathorn et al., Reference Ratanatharathorn, Boks, Maihofer, Aiello, Amstadter, Ashley-Koch and Smith2017). In the PGC-PTSD EWAS meta-analysis (796 PTSD cases and 1100 trauma-exposed controls from military and civilian cohorts) (Smith et al., Reference Smith, Ratanatharathorn, Maihofer, Naviaux, Aiello, Amstadter and Nievergelt2020), 10 epigenome-wide significant associations were observed in genes previously linked to other psychiatric disorders. Four signals mapped within AHRR (aryl-hydrocarbon receptor repressor) locus, which is well-known to present large methylation changes in response to tobacco smoking. The AHRR epigenetic associations observed in PGC-PTSD EWAS appeared to be independent of smoking status and were stronger in non-smokers than in smokers (Smith et al., Reference Smith, Ratanatharathorn, Maihofer, Naviaux, Aiello, Amstadter and Nievergelt2020). Additionally, in a subsample with metabolomics data, AHRR methylation was associated with kynurenine level (an inflammatory marker), which was lower in PTSD subjects than in controls (Smith et al., Reference Smith, Ratanatharathorn, Maihofer, Naviaux, Aiello, Amstadter and Nievergelt2020).
Epigenetic variation can also be used to assess accelerated cellular aging. Traumatic experience and posttraumatic stress are expected to have an impact on cellular regulation accelerating certain negative outcomes. In two studies conducted on US military veterans, accelerated DNA methylation aging was associated with different PTSD symptoms (avoidance, numbing, and hyperarousal) (Wolf et al., Reference Wolf, Logue, Stoop, Schichman, Stone, Sadeh and Miller2018a, Reference Wolf, Logue, Morrison, Wilcox, Stone, Schichman and Miller2019). However, the pattern observed across the two studies was not completely concordant (i.e. the symptoms reported as associated were not the same). Additionally, differences were also observed across different algorithms used to estimate the accelerated DNA methylation aging. In 2018, a large PGC-PTSD meta-analysis across nine cohorts including a total of 2186 participants from civilian and military cohorts reported that traumatic stress is associated with advanced epigenetic age and this relationship may be due to the function of immune cells (Wolf et al., Reference Wolf, Maniates, Nugent, Maihofer, Armstrong, Ratanatharathorn and Logue2018b).
Growing evidence highlights the potential role of transgenerational effects of paternal exposure to stress v. positive stimuli on the behavioral, affective, and cognitive characteristics of their progeny (Yeshurun & Hannan, Reference Yeshurun and Hannan2019). These mechanisms appear to be related to sperm-specific epigenetic mechanisms (e.g. DNA methylation changes and variation small non-coding RNAs) (Yeshurun & Hannan, Reference Yeshurun and Hannan2019). However, transgenerational epigenomics is in its infancy and further studies will be needed to understand the role of parental traumatic stress in the progeny's physical and mental health.
Transcriptomics
Transcriptomic analyses also contribute to understand the molecular changes associated with PTSD and traumatic experiences. A blood-based transcriptomic analysis comparing 229 PTSD and 311 controls showed co-expression networks related to specific functional modules depending on sex and modes of trauma: wound-healing module downregulated in men exposed to combat traumas; IL-12-mediated signaling module upregulated in men exposed to interpersonal-related traumas; modules associated with lipid metabolism and mitogen-activated protein kinase activity upregulated in women exposed to interpersonal-related traumas (Breen et al., Reference Breen, Tylee, Maihofer, Neylan, Mehta, Binder and Glatt2018). Shared PTSD functional network modules were detected with respect to cytokine, innate immune, and type I interferon pathways (Breen et al., Reference Breen, Tylee, Maihofer, Neylan, Mehta, Binder and Glatt2018). In an independent whole-blood transcriptome-wide study conducted in 324 World Trade Center responders (Kuan et al., Reference Kuan, Waszczuk, Kotov, Clouston, Yang, Singh and Luft2017a), a polygenic expression achieved sensitivity/specificity of 0.92/0.51, respectively, for identifying current PTSD with current and past PTSD groups scoring higher than trauma-exposed controls without any history of PTSD. In a subset of the same cohort (39 World Trade Center responders) (Kuan et al., Reference Kuan, Yang, Clouston, Ren, Kotov, Waszczuk and Luft2019), cell-specific and shared differentially expressed genes across four immune cell subpopulations (CD4T, CD8T, B cells, and monocytes) and enrichments for pathways related to mast cell activation and regulation in CD4T, interferon-beta production in CD8T, and neutrophil-related gene sets in monocytes were reported. In prefrontal cortex tissues from 22 donors with PTSD and 22 matched non-PTSD control donors, a study observed lower relative expression of TSPO and microglia-associated genes TNFRSF14 and TSPOAP1 in the female PTSD subgroup (Bhatt et al., Reference Bhatt, Hillmer, Girgenti, Rusowicz, Kapinos, Nabulsi and Cosgrove2020). In a recent study analyzing four prefrontal cortex subregions, a gene network of downregulated interneuron transcripts was associated with PTSD with converging evidence with MVP GWAS results related to the interneuron synaptic gene ELFN1 (Girgenti et al., Reference Girgenti, Wang, Ji, Cruz, Traumatic Stress Brain Research, Stein and Duman2021).
Neuroimaging
To investigate further the neurobiology of PTSD, genetic investigations can integrate information regarding brain structural and functional variation from imaging techniques. The study of brain imaging phenotypes in the context of PTSD genetics can lead to a more comprehensive understanding of the interplay between traumatic experiences and PTSD vulnerability. The PGC-PTSD workgroup joined forces with the ENIGMA (Enhancing NeuroImaging Genetics through Meta-Analysis) consortium to combine their different expertise to dissect the pleiotropic mechanisms linking PTSD and brain imaging phenotypes (Nievergelt et al., Reference Nievergelt, Ashley-Koch, Dalvie, Hauser, Morey, Smith and Uddin2018). In an initial study conducted in a small sample (66 PTSD cases and 91 non-PTSD controls) (Morey et al., Reference Morey, Davis, Garrett, Haswell, Mid-Atlantic, Marx and Ashley-Koch2017), pleiotropic associations were observed between caudate volume and childhood trauma and between right lateral ventricular volume and lifetime alcohol use disorder. Leveraging ENIGMA and PGC-PTSD genome-wide association statistics, novel PTSD risk loci were identified when accounting for the genetic associations of putamen volume, supporting a possible involvement for the glutamatergic system (van der Merwe et al., Reference van der Merwe, Jahanshad, Cheung, Mufford, Groenewold, Koen and Stein2019). Recently, ENIGMA-PGC-PTSD investigators investigated hippocampal markers of PTSD, depression, and the interaction of these conditions across 31 cohorts worldwide (N = 3115) (Salminen et al., Reference Salminen, Sämann, Zheng, Dennis, Clarke-Rubright, Jahanshad and Logue2019). Their findings highlighted that the comorbidity of PTSD and depression is strongly associated with hippocampal volumetry with the latter having a larger contribution than the former.
Future perspectives
There are several challenges we need to overcome before translating molecular findings into PTSD clinical practice. There is still a consistent missing heritability (i.e. the difference between twin-based and SNP-based heritability estimates) with respect to PTSD genetics. Whole-genome sequencing data may be able to address this, improving our ability to investigate uncommon genetic variants in low LD regions (Wainschtein et al., Reference Wainschtein, Jain, Yengo, Zheng, Cupples, Shadyab and Visscher2019). Additionally, the diagnostic complexity of psychiatric disorders was associated with the predicted effect size variance for trait-associated loci (Wendt et al., Reference Wendt, Pathak, Levey, Nunez, Overstreet, Tyrrell and Polimanti2020a, Reference Wendt, Pathak, Overstreet, Tylee, Gelernter, Atkinson and Polimantib). Improving the ability to investigate genetic variation while addressing diagnostic heterogeneity will surely boost PTSD gene discovery, potentially leading to genetic instruments to identify high-risk individuals and characterize molecular targets to develop effective treatments. Similarly, the ongoing technological progress helps to conduct more powerful epigenetic, transcriptomic, and brain-imaging studies that can contribute to design PTSD biomarkers. Additionally, several approaches are being proposed to integrate data generated from different high-throughput experimental data and conduct more holistic investigations of PTSD (Chakraborty, Meyerhoff, Jett, & Hammamieh, Reference Chakraborty, Meyerhoff, Jett and Hammamieh2017; Thakur et al., Reference Thakur, Daigle, Dean, Zhang, Rodriguez-Fernandez, Hammamieh and Doyle2015). In addition to these analytic challenges, like many other human diseases and traits, PTSD research has a serious diversity imbalance where underserved minorities are under-investigated (Sirugo, Williams, & Tishkoff, Reference Sirugo, Williams and Tishkoff2019). Although findings from PGC and MVP studies were generated from cohorts including individuals with diverse ancestral background, the vast majority of the participants are individuals of European descent. To avoid the widening of health disparities, molecular studies of PTSD need to increase the diversity of the cohorts analyzed to reflect adequately human variation and generate results transferrable across worldwide populations. Large-scale efforts, such as MVP and AllOfUs, are currently recruiting more diverse populations and will provide resources useful to partially address the present disparities in PTSD molecular research.
Conclusions
Our understanding of the molecular basis of PTSD is progressing rapidly, mainly because of international collaborations and large biobanks leading to an increase in statistical power. While technological and analytic progress are improving the ability to dissect PTSD pathogenesis, investigators have to continue to be particularly careful about communicating their findings to the general public to avoid that molecular insights are distorted to support a ‘blaming the victim’ rhetoric. This is particularly important with respect to certain traumatic events like sexual assaults that are more likely to be stigmatized (Kennedy & Prock, Reference Kennedy and Prock2018). Genetic studies of PTSD should be a further opportunity to address how to reduce the burden of traumatic experiences in human societies.
Acknowledgements
The authors thank Karestan Koenen and Caroline Nievergelt for their helpful comments. The authors acknowledge support from the National Institutes of Health (R21 DC018098, R21 DA047527, and F32 MH122058).
Conflict of interest
Dr Polimanti is paid for their editorial work on the journal Complex Psychiatry. Dr Wendt declares no competing interests.






