Individuals with a borderline personality disorder (BPD) suffer from a chronic and disabling combination of emotional, cognitive, and behavioral psychiatric symptoms. Diagnostic criteria for BPD (five of which must be met for diagnosis) highlight instability and flux in various domains, including unstable moods, reactive and uncontrolled anger (exacerbated by a tendency to ruminate on angry experiences (Baer and Sauer, Reference Baer and Sauer2011; Peters et al., Reference Peters, Eisenlohr-Moul, Upton, Talavera, Folsom and Baer2017), changing identities, frequent feelings of emptiness, turbulence in interpersonal relationships, fear of abandonment, a tendency to become paranoid or dissociate during times of stress, and damaging, impulsive behaviors. Recurrent, self-injurious thoughts and behaviors are also a symptom, and roughly 10% of people with BPD die from suicide (Paris and Zweig-Frank, Reference Paris and Zweig-Frank2001). Individuals with BPD are heavy utilizers of psychiatric treatment including therapy, hospitalizations, and pharmacotherapy (Bender et al., Reference Bender, Dolan, Skodol, Sanislow, Dyck, McGlashan, Shea, Zanarini, Oldham and Gunderson2001). Treatment for BPD can be beneficial, however, it is often extremely challenging (Aviram et al., Reference Aviram, Brodsky and Stanley2006), and quality of life following treatment often remains low despite improved functioning (Linehan et al., Reference Linehan, Comtois, Murray, Brown, Gallop, Heard, Korslund, Tutek, Reynolds and Lindenboim2006).
Research on the biology of BPD has generally focused on stable risk factors, whereas relatively little research examines the proximal causes of instability in BPD symptoms. Given that the ovarian steroid hormones estradiol (E2) and progesterone (P4) regulate nearly every brain system implicated in emotional disorders (Schiller et al., Reference Schiller, Johnson, Abate, Schmidt and Rubinow2016), the rapid changes in ovarian steroid hormones that occur across the monthly female menstrual cycle could represent one source of fluctuating biological vulnerability to BPD symptoms. The present study uses a longitudinal design and standardized diagnostic system to describe the impact of menstrual cycle phase on symptoms in unmedicated females with BPD.
The prototypical menstrual cycle is 28 days and has two parts: the follicular phase from menses to ovulation (low P4, increasing E2 that peaks prior to ovulation), and the luteal phase from ovulation to the next menses (high P4, secondary slower and generally lower peak in E2). E2 and P4 fall precipitously prior to and during the first few days of menses (described as perimenstrual steroid withdrawal), and the cycle begins again. Among patients with the primary cyclical mood disorder of DSM-5 premenstrual dysphoric disorder (PMDD), symptoms arise in the midluteal phase and peak in the perimenstrual window (Hartlage and Arduino, Reference Hartlage and Arduino2002; Epperson et al., Reference Epperson, Steiner, Hartlage, Eriksson, Schmidt, Jones and Yonkers2012), during perimenstrual steroid withdrawal. This dovetails with preclinical findings that ovarian steroid withdrawal precipitates anxious and depressive phenotypes (Stoffel and Craft, Reference Stoffel and Craft2004; Smith et al., Reference Smith, Ruderman, Frye, Homanics and Yuan2006).
Although most females do not show clinically significant menstrual cycle effects on emotional (Schwartz et al., Reference Schwartz, Romans, Meiyappan, De Souza and Einstein2012; Ben Dor et al., Reference Ben Dor, Harsh, Fortinsky, Koziol, Rubinow and Schmidt2013; Hengartner et al., Reference Hengartner, Kruger, Geraedts, Tronci, Mancini, Ille, Egli, Röblitz, Ehrig, Saleh, Spanaus, Schippert, Zhang and Leeners2017) or cognitive (Schmidt et al., Reference Schmidt, Keenan, Schenkel, Berlin, Gibson and Rubinow2013; Leeners et al., Reference Leeners, Kruger, Geraedts, Tronci, Mancini, Ille, Egli, Röblitz, Saleh, Spanaus, Schippert, Zhang and Hengartner2017) symptoms, experiments demonstrate that a subset of females suffer from abnormal sensitivity to normal ovarian steroid changes. This hormone sensitivity manifests as emotional, cognitive, and behavioral changes appearing only or primarily in the context of normative ovarian steroid changes, such as the midluteal and perimenstrual cycle phases, as in PMDD (Schmidt et al., Reference Schmidt, Nieman, Danaceau, Adams and Rubinow1998) or perimenstrual exacerbation (PME) of symptoms related to a chronic underlying disorder; during pregnancy and postpartum (Bloch et al., Reference Bloch, Schmidt, Danaceau, Murphy, Nieman and Rubinow2000); and during the menopause transition (Schmidt et al., Reference Schmidt, Ben Dor, Martinez, Guerrieri, Harsh, Thompson, Koziol, Nieman and Rubinow2015; Gordon et al., Reference Gordon, Rubinow, Eisenlohr-Moul, Leserman and Girdler2016). The content of emotional symptoms most commonly observed in these reproductive mood disorders overlaps to a greater degree with BPD than with major depressive disorder: Reproductive mood disorders are often characterized by high arousal negative affect and interpersonal symptoms like anger, irritability, and interpersonal sensitivity, whereas depressive symptoms are less common (Freeman et al., Reference Freeman, Halberstadt, Rickels, Legler, Lin and Sammel2011; Eisenlohr-Moul et al., Reference Eisenlohr-Moul, Girdler, Johnson, Schmidt and Rubinow2017a; Putnam et al., Reference Putnam, Wilcox, Robertson-Blackmore, Sharkey, Bergink, Munk-Olsen, Deligiannidis, Payne, Altemus, Newport, Apter, Devouche, Viktorin, Magnusson, Penninx, Buist, Bilszta, O'Hara, Stuart, Brock, Roza, Tiemeier, Guille, Epperson, Kim, Schmidt, Martinez, Di Florio, Wisner, Stowe, Jones, Sullivan, Rubinow, Wildenhaus and Meltzer-Brody2017). While being female with a mood or anxiety disorder may not in itself equate to a high risk of PME (Stein et al., Reference Stein, Schmidt, Rubinow and Uhde1989; Hartlage et al., Reference Hartlage, Brandenburg and Kravitz2004), PME may be more prevalent in a disorder such as BPD already characterized by instability of these symptoms.
Little prospective evidence is available to evaluate the hypothesis that females with BPD are at high risk of PME. Only one study has examined the impact of the menstrual cycle in patients with BPD (Ziv et al., Reference Ziv, Russ, Moline, Hurt and Zendell1995; N = 14). This study reported no evidence of reliable symptom changes from the premenstrual to postmenstrual phases of the cycle. However, participants took psychotropic medications, and ovulation was not confirmed; the latter point is critical since only ovulatory cycles are symptomatic in PMDD (Hammarbäck et al., Reference Hammarbäck, Ekholm and Bäckström1991). Another series of studies (DeSoto et al., Reference DeSoto, Geary, Hoard, Sheldon and Cooper2003) found that undergraduate females with high BPD features at baseline showed greater increases in symptoms after beginning oral contraceptives (OCs; N = 46), and during cyclical steroid changes (N = 57), both suggesting the risk of hormone sensitivity in BPD. In a final study of undergraduate females blinded to the purpose of the study (N = 40), individuals with elevated trait BPD features showed their highest BPD symptoms when P4 was higher-than-average and E2 was lower-than-average, a hormonal profile found primarily in the midluteal and perimenstrual phases (Eisenlohr-Moul et al., Reference Eisenlohr-Moul, DeWall, Girdler and Segerstrom2015). These findings converge to suggest the possibility of heightened risk for psychiatric hormone sensitivity and related PME in BPD.
The present study examines the prospective impact of cycle phase (in ovulatory, normal menstrual cycles) on daily self-reported symptoms in community-recruited, unmedicated females with BPD (N = 15) not recruited for perceived perimenstrual symptoms. Although participants collected saliva eight times to verify cycle phase, the present study was not powered to examine hormonal effects, instead focusing on cycle phase. In addition to examining the significance and size of phase differences, we utilize a standardized diagnostic system, the Carolina Premenstrual Assessment Scoring System (C-PASS; Eisenlohr-Moul et al., Reference Eisenlohr-Moul, Girdler, Schmalenberger, Dawson, Surana, Johnson and Rubinow2017b), to describe, in absolute terms, the clinical significance of PME in each participant.
Hypotheses
We tested the following hypotheses:
(1) PME (Relative to all other Phases): We predicted that participants with BPD would also show their greatest emotional symptom exacerbation during the perimenstrual phase relative to each other phases comparable with the symptom emergence pattern in PMDD.
(2) Midluteal Exacerbation (Relative to Follicular and Ovulatory Phases): Given that women with primary cyclical mood disorder (i.e. PMDD) generally begin to have emotional symptoms during the midluteal phase (when P4 rises and begins to fluctuate), we also predicted that participants with BPD would show symptom exacerbation in the midluteal phase relative to follicular and ovulatory phases, but that midluteal symptoms would still be lower than the perimenstrual phase.
(3) >50% Sample Diagnosis with C-PASS Premenstrual Symptom Elevation: Given overlapping risk factors and similar core symptom content in individuals with primary cyclical mood disorder (i.e. PMDD) and individuals with BPD, we predicted that a majority (>50%) of this BPD sample would meet the C-PASS diagnostic criterion of premenstrual elevation (worsening of at least 30% premenstrually v. postmenstrually) on at least one emotional symptom. However, given that BPD is a chronic emotional disorder that does not remit fully for weeks at a time (as is required in PMDD), we predicted that no participant would meet DSM-5 criteria for PMDD in the examined cycle.
Methods
Procedure
We recruited participants using listservs, flyers, and social media advertisements seeking ‘women with emotional and interpersonal problems that interfere with life’. These materials did not refer to the menstrual cycle to avoid self-selection. Interested individuals were directed to an online screening, including the Personality Diagnostic Questionnaire—Borderline Personality Disorder Subscale (PDQ; Hyler et al., Reference Hyler, Skodol, Oldham, Kellman and Doidge1992) and eligibility questions. Exclusionary criteria were: dysmenorrhea in the past 3 months, current pregnancy or breastfeeding, psychotropic medication or OC use, illicit drug use, and chronic medical conditions, and schizophrenia or manic episodes. Menstrual cycles between 25 and 35 days were required.
We contacted individuals for phone screening if they met inclusion criteria and had a PDQ score of 5 out of 9 BPD criteria. The phone screen included an interview version of the PDQ. Those providing symptom examples indicating significant ‘pathology, persistence, and pervasiveness’ (First et al., Reference First, Spitzer, Gibbons, Williams and Benjamin1996) on at least five of nine BPD symptoms were invited for an enrollment visit. This visit included a SCID-PD (BPD only) interview with a clinical psychologist. If they met BPD criteria, they completed questionnaires and oriented to study procedures (daily survey completion, urine ovulation testing, and salivary collection). Participants completed daily surveys for 35 days starting on their next menstrual period start day, daily urine ovulation testing based on cycle length, and passive drool saliva samples on 8 days across the cycle. Prospective charting of daily symptoms was critical due to the well-documented lack of correspondence between cross-sectional retrospective reports of premenstrual symptoms and prospective patterns of symptom change (e.g. Roy-Byrne et al., Reference Roy-Byrne, Rubinow, Hoban, Parry, Rosenthal, Nurnberger and Byrnes1986). Participants received $15 for the enrollment visit, and $100 for the larger study. All procedures were approved by the university institutional review board. Data were collected 2015–2016.
Participants
A total of 310 individuals completed online screening; 112 were eligible for phone screening (exclusion due to insufficient PDQ severity (n = 143), hormone use (n = 42), irregular cycles (n = 6), or medical conditions (n = 7)). Of those, 43 completed the phone screen; we invited 31 of those for an enrollment visit (exclusion due to subthreshold PDQ examples (n = 8), hormone use (n = 2), pregnancy/nursing (n = 2)). Of those, 18 completed the initial visit. Seventeen met SCID-BPD criteria and enrolled. One participant was noncompliant and one did not ovulate; the final sample included 15 medically healthy, unmedicated females with BPD. Demographics and clinical characteristics are provided in Table 1. All participants reported a lifetime history of psychotherapy; one was currently in psychotherapy.
Table 1. Sample descriptive information (N = 15)
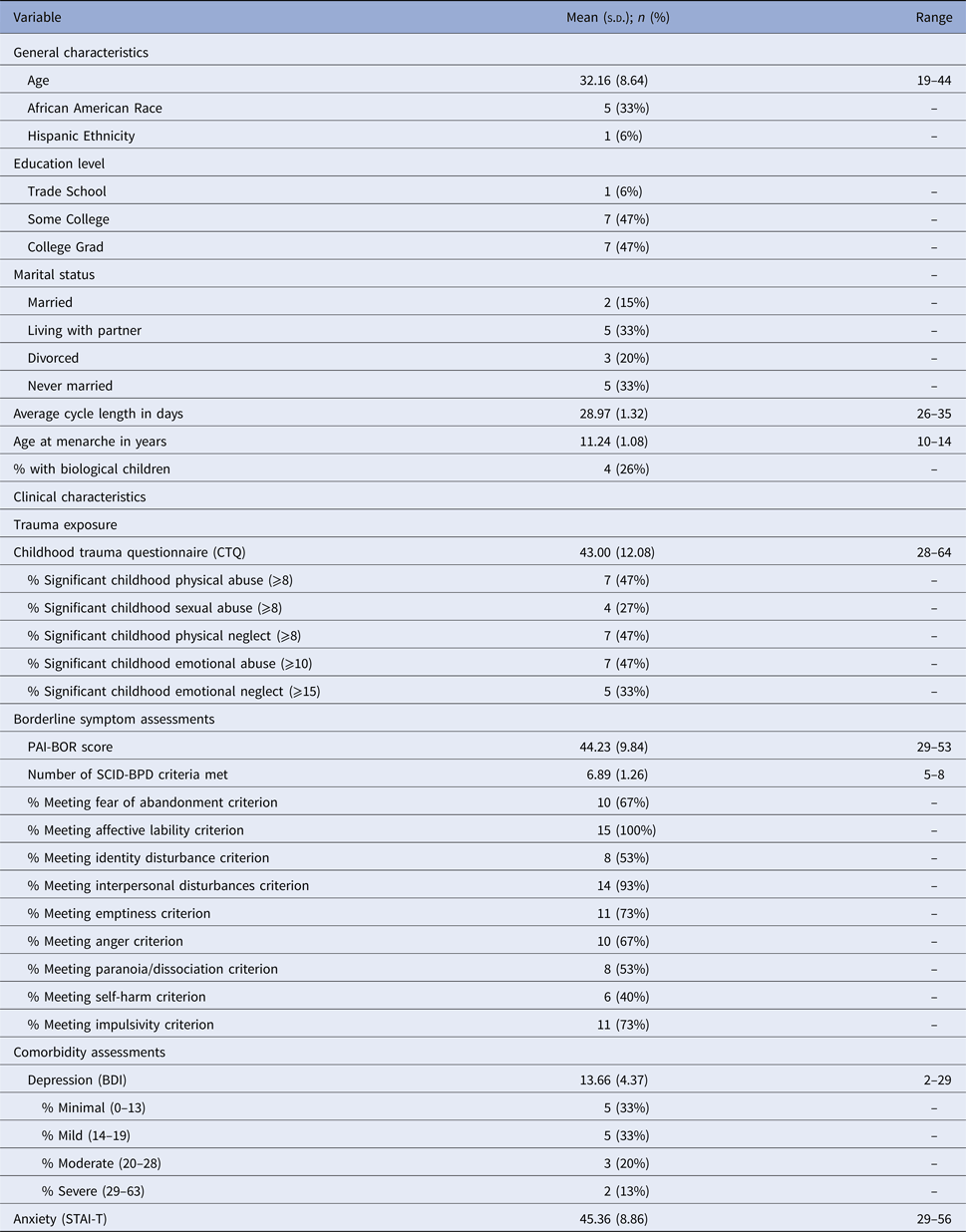
CTQ, Childhood Trauma Questionnaire; STAI, State-Trait Anxiety Inventory (Trait Form); BDI, Beck Depression Inventory; PAI-BOR, Personality Assessment Inventory – Borderline Subscale; SCID-BPD, Structured Clinical Interview for Personality Disorders – Borderline Personality Disorder Module.
Baseline measures
In addition to the SCID for BPD, participants completed several questionnaires during their baseline visit: (a) demographics, (b) the borderline subscale of the Personality Assessment Inventory (PAI-BOR; Morey, Reference Morey2007), (c) the Childhood Trauma Questionnaire (CTQ; Bernstein et al., Reference Bernstein, Fink, Handelsman, Foote, Lovejoy, Wenzel, Sapareto and Ruggiero1994), (d) the Beck Depression Inventory (BDI; Beck and Steer Reference Beck and Steer1987), and the trait form of the State-Trait Anxiety Inventory (STAI; Spielberger et al., Reference Spielberger, Gorsuch, Lushene, Vagg and Jacobs1983), and (e) items from the Premenstrual Assessment Form (Halbreich et al., Reference Halbreich, Endicott, Schacht and Nee1982) to assess perceptions of menstrually-related symptom change. Participants were asked to rate their typical degree of premenstrual symptom exacerbation for depressed mood, hopelessness, worthlessness or guilt, anxiety, anger, irritability, and interpersonal conflicts on a scale where 1 = no change, 2 = mild change, 3 = moderate change, and 4 = severe change.
Daily measures
Participants completed daily questionnaires via online survey; links were sent daily via text message and email.
Daily record of severity of problems (DRSP)
The DRSP (Endicott et al., Reference Endicott, Nee and Harrison2006) is a daily measure developed to reliably detect menstrually-related changes (e.g. PMDD, PME) in psychiatric and physical symptoms (depression, anxiety, anhedonia, hopelessness, emotional overwhelm, rejection sensitivity, anger/irritability, interpersonal conflict, and physical symptoms (i.e., average of breast tenderness, breast swelling, bloating, joint or muscle pain). Items are rated from 1 = Absent to 5 = Extreme.
PME diagnosis using the Carolina Premenstrual Assessment Scoring System (C-PASS) for the DRSP
The degree of premenstrual symptom elevation (and associated clinical significance cutoffs) can be determined with the C-PASS, a standardized scoring system used in conjunction with daily DRSP data to make the PMDD or PME diagnosis. The C-PASS operationalizes the DSM-5 PMDD diagnosis into diagnostic dimensions and numerical diagnostic thresholds validated against expert diagnosis based on daily ratings (Eisenlohr-Moul et al., Reference Eisenlohr-Moul, Girdler, Schmalenberger, Dawson, Surana, Johnson and Rubinow2017b). Here, the C-PASS provides standardized criteria for identifying whether or not a participant's daily ratings demonstrate clinically significant PME (⩾ 30% increase premenstrually v. postmenstrually) of any emotional symptom.
Additional BPD symptoms
Three additional BPD-relevant symptoms were measured daily: shame was measured using two items from the shame subscale of the State Shame and Guilt Scale (Marschall et al., Reference Marschall, Sanftner and Tangney1994); ‘I wanted to sink into the floor and disappear’, and ‘I felt humiliated, disgraced’). Daily felt invalidation was measured with two items created for the purposes of this study (‘I felt that someone wanted to me to stop feeling the way I was feeling’, ‘I felt that someone didn't take my feelings seriously’). Daily anger rumination was measured with two items from the Anger Rumination Scale (Sukhodolsky et al., Reference Sukhodolsky, Golub and Cromwell2001); ‘I kept thinking about events that have angered me’, and ‘I turned anger-provoking situations over and over again in my mind’). Each of these items was rated from 1 (Not at All) to 5 (Extremely).
Ovulation testing and salivary ovarian steroids
Urine luteinizing hormone tests (LH; Clearblue ovulation testing kit) confirmed ovulation. This test has been validated against ultrasound and has a 40 pg/mL LH threshold, reducing false positives. Ovulation can occur 12–36 h after the LH surge; here, the day after the positive test was considered ovulation. Testing started 7 days after menses onset until a positive result. Participants provided saliva, assayed for E2 and P4, on 8 days: days 2, 3, 8, and 9 following menstrual onset and days 2, 3, 8, and 9 following positive LH surge test. This method was used to increase sample reliability for cycle phase validation. Participants collected 5 mL of saliva in polypropylene vials (passive drool) each afternoon 4–6 pm, froze samples in their home freezer in a temperature stability pouch, and returned them to the laboratory after study completion. Afternoon sampling was chosen to increase participant compliance based on poor morning compliance in our pilot data. Hormones were assayed using RIA kits purchased from Salimetrics (Carlsbad, CA) at the UNC Chapel Hill School of Medicine Core Laboratory.
Reliability and validity of salivary E2 and P4
Intra-assay CVs were 6.3% and 4.0% for E2 and P4, respectively. Interassay CVs were 8.9% and 5.5% for E2 and P4, respectively. Per Salimetrics validation reports, the sensitivity of the Salimetrics RIA method is .1 pg/ml for E2, and 5 pg/ml for P4, and salivary E2 and P4 should show similar cyclical changes to serum E2 and P4 across the cycle. In our sample, P4 demonstrated the expected post-ovulatory rise in each participant. However, E2 demonstrated virtually no cyclical change for any participant. Given that ovulation was confirmed and P4 showed expected patterns, this lack of E2 clearly indicated sampling, storage, or assay issues. We suspect that at-home storage for 1 month, combined with the diurnal pattern reducing afternoon E2, may have caused this issue. Therefore, we proceeded to use the P4 values, and did not use the E2 values.
Menstrual cycle phase coding
Determination of cycle phases—ovulatory, midluteal, perimenstrual, and follicular—was based the following: (1) forward and backward count (Edler et al., Reference Edler, Lipson and Keel2007), (2) day of LH surge, and (3) exponential P4 rise following ovulation. First, the ovulatory phase was determined using backward count (days −15 to −12), and was validated using the LH surge ovulation test and a substantial postovulatory rise in P4. Next, the perimenstrual phase was determined using backward and forward count (days −4 to + 3, where day + 1 is menses onset and there is no day 0), and was validated by time since positive LH surge (14 ±2 days). The midluteal phase was defined as the days falling between the ovulatory and perimenstrual phases, and was validated by elevated P4. The follicular phase was defined as days falling between the perimenstrual and ovulatory phases, and was validated by low P4.
Data analysis and power
To test the first three predictions, multilevel models evaluated the impact of cycle phase (coded as a categorical variable, with alternating reference phases). SAS PROC MIXED accounted for the nested structure of observations (i.e. random intercept) as well as the autocorrelation of today's rating with yesterday's rating. Random effects of cycle phase contrasts were not included as they did not improve model fit; this indicates that the impact of the menstrual cycle on symptoms was roughly uniform in this sample. Five-day rolling averages of each person-standardized outcome were utilized for graphical depictions.
To determine power for phase contrasts, we calculated the design effect to determine the smallest detectible effect size (Snijders, Reference Snijders, Everitt and Howell2005). Each participant provided up to 35 daily surveys (lower-level N was 490), and symptoms generally showed low intraclass correlations (i.e. a high degree of flux within a person, average ICC for DRSP items = 0.18). Power for these models was adequate to detect conventionally small-to-medium sized contrasts between phases (average smallest detectable effect size was an f 2 of 0.10). That is, the study was powered to detect significant phase contrasts accounting for at least 10% of the variance in the daily outcome. Age was considered as a covariate but ultimately omitted as it was not associated with outcomes and did not alter our pattern of findings.
To test hypothesis 4, we evaluated whether each participant met the C-PASS diagnostic criterion of premenstrual elevation (over the postmenstrual baseline) on at least one emotional symptom (Eisenlohr-Moul et al., Reference Eisenlohr-Moul, Girdler, Schmalenberger, Dawson, Surana, Johnson and Rubinow2017b). In contrast to PMDD, which requires luteal phase elevation and full remission of symptoms in the postmenstrual week, clinically significant PME can be diagnosed when at least one emotional symptom demonstrates a ⩾30% premenstrual week elevation of symptoms relative to the postmenstrual week (with no requirement of a remission week as in PMDD; see Eisenlohr-Moul et al., Reference Eisenlohr-Moul, Girdler, Schmalenberger, Dawson, Surana, Johnson and Rubinow2017b for calculation details). This 30% change threshold for clinical significance was delineated by the NIMH work group for PMDD.
Results
Descriptives
Table 1 provides demographic and clinical characteristics. The sample was diverse with regard to age and race. Participants reported high BPD symptoms on the PAI-BOR, moderate depression on the BDI, moderate anxiety on the STAI, and significant experiences of childhood trauma; these characteristics are consistent with typical comorbidities and childhood experiences in BPD. Participants cross-sectionally reported an average perception of mild PME of emotional symptoms. 93% of surveys were completed, and 90% of salivary samples were completed.
Contrasting BPD symptom severity across menstrual cycle phases
Results of multilevel models testing phase effects on symptoms are presented in Table 2. All symptoms demonstrated strong cyclical changes; Figures 1–3 depict patterns of symptom change across cycle day and cycle phase for several exemplary symptoms. For each model reported below, additional robustness-check models controlled for person-standardized physical symptoms; we observed no substantive model changes, suggesting PME is not an epiphenomenon of perimenstrual physical pain (e.g. dysmenorrhea). Graphs by day and cycle phase are provided for each outcome in Supplement 1. Graphs include smoothed, person-standardized P4 values.

Fig. 1. Person-Standardized Progesterone (dashed line) and Interpersonal Conflict Across Menstrual Cycle Day (Panel A) and Person-Standardized Interpersonal Conflict Across Cycle Phase (Panel B) in 15 People with BPD.

Fig. 2. Person-Standardized Progesterone (dashed line) and Felt Invalidation Across Menstrual Cycle Day (Panel A) and Person-Standardized Felt Invalidation Across Cycle Phase (Panel B) in 15 People with BPD.

Fig. 3. Person-Standardized Progesterone (dashed line) and Hopelessness Across Menstrual Cycle Day (Panel A) and Person-Standardized Hopelessness Across Cycle Phase (Panel B) in 15 People with BPD.
Table 2. Multilevel model results: within-person menstrual cycle phase contrasts for intrapersonal and interpersonal symptoms

Note. *p < 0.05; **p < 0.01 *** p < 0.001. Bold values indicate statistical significance. Hormone profile labels for the cycle phases are descriptive of typical cycles since E was not assessed and P was assessed in only some of the cycle phases. For presentation in this table only, outcomes have been person-standardized so that estimates represent the number of standard deviations from one's mean (0); therefore, estimates reflect the average number of personal standard deviations by which the two phases differ. DRSP, Daily Record of Severity of Problems.
Testing hypothesis 1: perimenstrual exacerbation (v. all other phases)
We predicted that the perimenstrual phase would be associated with greater symptoms relative to each other cycle phase. Symptoms were greater in the perimenstrual phase than in the midluteal and ovulatory phases for all symptoms. Symptoms were also greater in the perimenstrual phase than in the follicular phase for the majority of symptoms, although no differences were observed between the perimenstrual and follicular phases for depression, hopelessness, anhedonia, or shame, indicating that the exacerbation of those four symptoms, which began perimenstrually, continued on into the follicular phase (see section for ‘Testing Hypothesis 3’ below).
Testing hypothesis 2: midluteal exacerbation (v. follicular and ovulatory phases)
We predicted that participants would begin to show greater emotional symptoms in the midluteal phase relative to the follicular and ovulatory phases. Relative to the ovulatory phase, the midluteal phase was associated with worsening of four symptoms: anxiety, anger/irritability, interpersonal conflict, and physical symptoms. Relative to the follicular phase, the midluteal phase showed a mixed pattern of contrasts, with anger/interpersonal conflict symptoms showing expected midluteal worsening relative to the follicular phase (anger/irritability, interpersonal conflict), and several depressive symptoms showing an unexpected worsening in the follicular phase relative to the midluteal phase (depression, hopelessness, and shame).
Exploratory contrasts between the follicular and ovulatory phases
In general, the ovulatory phase demonstrated the lowest symptoms. Exploratory contrasts indicated that, relative to the follicular phase, the ovulatory phase showed lower levels of depression, hopelessness, shame, and physical symptoms, indicating that the perimenstrual-to-follicular exacerbation of depressive symptoms observed above did return to the baseline level of symptoms in the ovulatory phase.
Testing hypothesis 4: >50% sample diagnosis with C-PASS premenstrual elevation
Given overlapping risk factors and core symptom content in PMDD and BPD, we predicted a majority (>50%) of the sample would meet the C-PASS diagnostic criterion of premenstrual elevation (⩾30% higher premenstrually v. postmenstrually) on at least one emotional symptom. Results of individual C-PASS scoring of daily DRSP ratings revealed 11 of 15 participants (73%) showed clinically significant PME (⩾30% premenstrual symptom elevation relative to the postmenstrual week) of at least one DRSP emotional symptom.
Exclusion of the first 3 days of menses may have omitted the most symptomatic days (Pearlstein et al., Reference Pearlstein, Yonkers, Fayyad and Gillespie2005); therefore, we also conducted analyses replacing the pre- and post-menstrual means with perimenstrual and follicular means (as defined in analyses for Hypotheses 1–2), respectively; this did not alter the 73% outcome. Given the observed follicular exacerbation of depressive symptoms, we also conducted analyses replacing the pre- and post-menstrual means with those from the perimenstrual and ovulatory phases, respectively. Fourteen out of 15 participants (93%) showed a clinically significant change of at least one emotional symptom from the ovulatory to the perimenstrual phase.
As predicted, no participant in the present study met C-PASS PMDD criteria for any symptom in the examined cycle. Since all participants continued to have moderate-or-greater symptoms in the postmenstrual week (consistent with BPD), they failed to meet the PMDD diagnostic criterion of absolute clearance (Eisenlohr-Moul et al., Reference Eisenlohr-Moul, Girdler, Schmalenberger, Dawson, Surana, Johnson and Rubinow2017b).
Post-hoc associations of P4 with daily symptoms
Despite the low statistical power, we examined preliminary within-person correlations between P4 and symptoms. Consistent with preclinical evidence that steroid withdrawal elicits relevant phenotypes (Stoffel and Craft, Reference Stoffel and Craft2004; Smith et al., Reference Smith, Ruderman, Frye, Homanics and Yuan2006), we observed negative (unstandardized) correlations of person-centered P4 with a variety of symptoms: depression (within-person correlation of P4 with depression on the same day, γ = −0.0021, p = 0.042), hopelessness (γ = −0.0015, p = 0.039), anxiety (γ = −0.0056, p = 0.012), and rejection sensitivity (γ = −0.0037, p = 0.027), anger/irritability (γ = −0.0021, p = 0.042), anhedonia (γ = −0.0031, p = 0.044), overwhelm (γ = −0.0045, p = 0.011), felt invalidation (γ = −0.005, p = 0.022), and physical symptoms (γ = −0.0033, p = 0.018). Upon inspection of individual symptom plots, these correlations appeared to be driven by heightened symptoms during perimenstrual and follicular withdrawal from P4.
Discussion
We sought to describe menstrual cycle patterns of symptom exacerbation in unmedicated females with BPD. Despite reporting only mild perceived PME in the baseline survey, daily ratings unequivocally demonstrated a high risk of perimenstrual symptom exacerbation in all symptoms. The observed symptom patterns can be described as follows. First, all symptoms generally show lowest levels in the ovulatory phase. Second, in the midluteal phase, high-arousal symptoms worsen but are not yet accompanied by exacerbation of other symptoms. Third, in the perimenstrual phase, all symptoms become exacerbated, with further exacerbation of high-arousal symptoms, but also increased the experience of physical discomfort and feelings of invalidation, shame, rejection sensitivity, anger rumination, emotional overwhelm, depression, anhedonia, and hopelessness. Finally, although high-arousal symptoms returned to baseline in the follicular phase, the lower-arousal depressive symptoms continued to be exacerbated in the follicular phase before returning to baseline at ovulation and the midluteal phase. Critically, physical symptoms did not account for PME of emotional symptoms. C-PASS diagnostic procedures indicated clinically significant PME in at least 11 of the 15 participants.
Although it is premature to comment on biological mechanisms of PME in BPD, we can compare and contrast observed patterns with those documented in PMDD, a disorder for which pathophysiology is better understood. Given their identical timing, PME of high-arousal symptoms in BPD may have a shared pathophysiology with PMDD—that is, a dysphoric response to luteal fluctuations in the GABAergic neurosteroid metabolites of P4 (Martinez et al., Reference Martinez, Rubinow, Nieman, Koziol, Leslie Morrow, Schiller, Cintron, Thompson, Khine and Schmidt2013; Schiller et al., Reference Schiller, Schmidt and Rubinow2014). However, regarding PME of depressive symptoms, the continued exacerbation in the follicular phase is not consistent with the follicular clearance observed in PMDD, suggesting a divergent pathophysiology. Females with BPD may have additional sensitivity to follicular withdrawal from—or low levels of—ovarian steroids. This withdrawal hypothesis receives preliminary support from our post-hoc finding of a negative within-person correlation between P4 and symptoms; however, these correlations should be interpreted with extreme caution due to low power. Finally, some have also postulated that females with BPD may have greater sensitivity of serotonergic systems to E2 fluctuations (DeSoto, Reference DeSoto and Czerbaska2007).
Several lines of research indicate that the predictors and characteristics of BPD overlap with those of hormone sensitivity. First, stress exposure and vulnerability are relevant to the etiology of BPD (Lynam and Widiger, Reference Lynam and Widiger2001; Ball and Links, Reference Ball and Links2009) and associated with hormone sensitivity (Gollenberg et al., Reference Gollenberg, Hediger, Mumford, Whitcomb, Hovey, Wactawski-Wende and Schisterman2010; Gordon et al., Reference Gordon, Girdler, Meltzer-Brody, Stika, Thurston, Clark, Prairie, Moses-Kolko, Joffe and Wisner2015; Nillni et al., Reference Nillni, Pineles, Patton, Rouse, Sawyer and Rasmusson2015; Eisenlohr-Moul et al., Reference Eisenlohr-Moul, Rubinow, Schiller, Johnson, Leserman and Girdler2016). Second, impulsivity, a core feature of BPD (Peters et al., Reference Peters, Upton and Baer2013), predicts sensitivity to hormone withdrawal in rodents (Löfgren et al., Reference Löfgren, Johansson, Meyerson, Turkmen and Bäckström2009) and humans (Martel et al., Reference Martel, Eisenlohr-Moul and Roberts2017). Finally, behavioral manifestations of emotion-related impulsivity show PME, including binge eating in women with bulimia nervosa and non-clinical samples of women from the community (Edler et al., Reference Edler, Lipson and Keel2007; Klump et al., Reference Klump, Keel, Racine, Burt, Burt, Neale, Sisk, Boker and Hu2013), substance use and abuse (Terner and de Wit, Reference Terner and de Wit2006), and suicide attempts (Saunders and Hawton, Reference Saunders and Hawton2006).
Clinical implications
Despite the development of useful psychotherapies for BPD, such as dialectical behavior therapy (DBT; Linehan, Reference Linehan2014) and mentalization-based therapy (Bateman and Fonagy, Reference Bateman and Fonagy2004), quality of life often remains low following treatment (Linehan et al., Reference Linehan, Comtois, Murray, Brown, Gallop, Heard, Korslund, Tutek, Reynolds and Lindenboim2006). Experimental work is needed to determine whether stabilization of ovarian steroid flux may relieve PME of BPD. Greater cycle awareness in the context of psychosocial treatment may support targeted skills use to improve symptom stability (e.g., integration of menses into a DBT diary card). Finally, the dramatic PME of hopelessness observed here combines with previous work (Saunders and Hawton, Reference Saunders and Hawton2006) to highlight cyclical changes in suicide risk.
Limitations and strengths
This work has important limitations. The clinical sample was small; although repeated measures mitigated power concerns somewhat, results need replication. There was no clinical or healthy control group; although use of the C-PASS (Eisenlohr-Moul et al., Reference Eisenlohr-Moul, Girdler, Schmalenberger, Dawson, Surana, Johnson and Rubinow2017b) ensures that the observed PMEs were clinically significant, the cyclical changes observed here may be due to transdiagnostic factors (e.g., trauma exposure; Eisenlohr-Moul et al., Reference Eisenlohr-Moul, Rubinow, Schiller, Johnson, Leserman and Girdler2016) rather than being specific to BPD. Women were excluded for OCs; since some people discontinue OCs following adverse reactions, those not taking OCs could be more likely to exhibit hormone sensitivity. Unfortunately, salivary E2 measurements did not meet quality control standards; future work may benefit from morning sampling and expediting transfer of samples to lower-temperature storage. Further, salivary steroid sampling was too infrequent for adequately-powered hormone analyses; future work should utilize more frequent sampling and eventually use experimental designs.
This work also has notable strengths relative to past work. The study purpose was blinded during recruitment to reduce self-selection issues. Participants were unmedicated, community-recruited, and demographically diverse. Use of online (rather than paper) surveys ensured proximal, time-verified symptom ratings. Both LH surge tests and salivary P4 were used to confirm ovulation and cycle phase. Finally, we examined all pairwise phase comparisons to precisely describe cyclical symptom change in BPD.
Conclusions
People with BPD suffer intense fluctuations in painful emotional and behavioral symptoms. The present work supports the hypothesis that females with BPD may be at high risk of PME, with secondary worsening in the midluteal (high arousal symptoms) and follicular (low arousal symptoms) phases. Patterns of symptom change suggest shared pathophysiology with PMDD with regard to midluteal and perimenstrual high-arousal symptoms, but also suggest potentially divergent pathophysiology with regard to depression. Experimental studies are needed for the dual purposes of investigating the therapeutic potential of hormone stabilization while probing the pathophysiologic relevance of steroid changes to cyclical worsening of BPD.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291718001253
Financial support
This research was supported by grants from the National Institute of Mental Health (K99MH109667; R01MH099076; T32MH019927; T32MH093315) and the National Center for Advancing Translational Sciences (NCATS) at the National Institutes of Health (UL1TR001111).
Conflicts of Interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.







