Introduction
Major depressive disorder (MDD) is a prevalent mental disorder ranked as the leading cause of mental health-related disease burden globally, affecting an estimated 300 million people worldwide (Malhi & Mann, Reference Malhi and Mann2018; Patel, Chisholm, & Parikh, Reference Patel, Chisholm and Parikh2016). Cardinal presentations of MDD, most notably anhedonia, are traditionally defined as ‘loss of pleasure’, which emphasises the consummatory or enjoyment aspect of the reward function. However, both the tenth edition of the International Statistical Classification of Diseases and Related Health Problems (ICD) and the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM) reflect a broader conceptualisation of MDD that includes reward motivation and consummatory pleasure (APA, 2013; WHO, 1992). Towards a more behaviour model, recent studies have emphasised the influence of decision-making on anhedonic symptoms (Coccurello, Reference Coccurello2019; Rizvi, Pizzagalli, Sproule, & Kennedy, Reference Rizvi, Pizzagalli, Sproule and Kennedy2016). Based on the different cognitive phases of the reward process, anhedonia can be divided into anticipatory and consummatory aspects of the reward cycle (Coccurello, Reference Coccurello2019; Rizvi et al., Reference Rizvi, Pizzagalli, Sproule and Kennedy2016; Treadway & Zald, Reference Treadway and Zald2011). These facets of anhedonia are intended to roughly correspond to the reward appetitive components of ‘liking’ and ‘wanting’ proposed in the previous literature (Berridge, Robinson, & Aldridge, Reference Berridge, Robinson and Aldridge2009). Patients with MDD often report either deficits in motivation to pursue rewards (i.e. anticipatory anhedonia or ‘wanting’) or difficulties in experiencing normally positive events as pleasurable (i.e. consummatory anhedonia or ‘liking’). Distinction between the motivational and hedonic aspects of anhedonia is critical, especially when attempting to elucidate the neurobiological pathways underlying the expression of this symptom.
Emerging findings imply that MDD is characterised by a reduced ability to modulate behaviour as a function of rewards. Numerous studies have found that depressed patients showed dopaminergic abnormalities in the mesocortico-limbic reward circuit with key structures including the ventral striatum (VS), dorsolateral prefrontal cortex (DLPFC), orbital prefrontal cortex (OFC), medial prefrontal cortex (mPFC), anterior cingulate cortex (ACC), amygdala, and hippocampus (Geugies et al., Reference Geugies, Mocking, Figueroa, Groot, Marsman, Servaas and Ruhe2019; Keren et al., Reference Keren, O'Callaghan, Vidal-Ribas, Buzzell, Brotman, Leibenluft and Stringaris2018; Ng, Alloy, & Smith, Reference Ng, Alloy and Smith2019; Russo & Nestler, Reference Russo and Nestler2013). This reduction in reward responsiveness appears to persist after remission (Dichter, Kozink, McClernon, & Smoski, Reference Dichter, Kozink, McClernon and Smoski2012; Schiller, Minkel, Smoski, & Dichter, Reference Schiller, Minkel, Smoski and Dichter2013) and has been found to induce the enhancement of corticostriatal activity and connectivity after receiving antidepressant treatment (Admon et al., Reference Admon, Kaiser, Dillon, Beltzer, Goer, Olson and Pizzagalli2017). This blunted reward processing pattern has been interpreted to reflect reduced appetitive motivation and is considered a promising endophenotype of depression (Hasler, Drevets, Manji, & Charney, Reference Hasler, Drevets, Manji and Charney2004). As a key clinical priority, aberrant reward processing may constitute part of an underlying neurobiological vulnerability to MDD and thus may have useful clinical implications.
Dysfunctions in anticipatory and consummatory reward processes in MDD as well as temporal differences in reward-related learning in depressed patients v. control subjects have been less often investigated and remain elusive, with frequent reports of contradictory findings. Whereas some studies have observed hypoactivation of VS (especially the nucleus accumbens, NAcc) (Arrondo et al., Reference Arrondo, Segarra, Metastasio, Ziauddeen, Spencer, Reinders and Murray2015; Pizzagalli et al., Reference Pizzagalli, Holmes, Dillon, Goetz, Birk, Bogdan and Fava2009; Schwarz et al., Reference Schwarz, Moessnang, Schweiger, Baumeister, Plichta, Brandeis and Meyer-Lindenberg2020; Stoy et al., Reference Stoy, Schlagenhauf, Sterzer, Bermpohl, Hägele, Suchotzki and Ströhle2012) or hyperactivation of VS (Dichter et al., Reference Dichter, Kozink, McClernon and Smoski2012) during the anticipation stage and the outcome stage (Knutson, Bhanji, Cooney, Atlas, & Gotlib, Reference Knutson, Bhanji, Cooney, Atlas and Gotlib2008; Pizzagalli et al., Reference Pizzagalli, Holmes, Dillon, Goetz, Birk, Bogdan and Fava2009), other studies have failed to find this during anticipation (Admon et al., Reference Admon, Kaiser, Dillon, Beltzer, Goer, Olson and Pizzagalli2017; Carl et al., Reference Carl, Walsh, Eisenlohr-Moul, Minkel, Crowther, Moore and Smoski2016; Dichter et al., Reference Dichter, Kozink, McClernon and Smoski2012; Schiller et al., Reference Schiller, Minkel, Smoski and Dichter2013) or for receipt of outcomes in MDD (Admon et al., Reference Admon, Kaiser, Dillon, Beltzer, Goer, Olson and Pizzagalli2017; Carl et al., Reference Carl, Walsh, Eisenlohr-Moul, Minkel, Crowther, Moore and Smoski2016). More inconsistencies are present in the prefrontal regions, which are considered to contribute to reward processing deficits in MDD. For instance, some studies have found that MDD exhibits greater activation in the OFC (Liu et al., Reference Liu, Admon, Mellem, Belleau, Kaiser, Clegg and Pizzagalli2020), DLPFC (Dichter et al., Reference Dichter, Kozink, McClernon and Smoski2012), mPFC (Liu et al., Reference Liu, Admon, Mellem, Belleau, Kaiser, Clegg and Pizzagalli2020), ACC (Knutson et al., Reference Knutson, Bhanji, Cooney, Atlas and Gotlib2008; Pizzagalli et al., Reference Pizzagalli, Holmes, Dillon, Goetz, Birk, Bogdan and Fava2009), subgenual cingulate (Pizzagalli et al., Reference Pizzagalli, Holmes, Dillon, Goetz, Birk, Bogdan and Fava2009), middle frontal gyrus (MFG) (Pizzagalli et al., Reference Pizzagalli, Holmes, Dillon, Goetz, Birk, Bogdan and Fava2009), inferior frontal gyrus (IFG) (Pizzagalli et al., Reference Pizzagalli, Holmes, Dillon, Goetz, Birk, Bogdan and Fava2009; Schiller et al., Reference Schiller, Minkel, Smoski and Dichter2013), and superior frontal cortex (Schiller et al., Reference Schiller, Minkel, Smoski and Dichter2013) during the anticipation of rewarding stimuli. In contrast, other studies have reported less activity in MDD in response to reward in the OFC (Dichter et al., Reference Dichter, Kozink, McClernon and Smoski2012), mPFC (Pizzagalli et al., Reference Pizzagalli, Holmes, Dillon, Goetz, Birk, Bogdan and Fava2009), ACC (Pizzagalli et al., Reference Pizzagalli, Holmes, Dillon, Goetz, Birk, Bogdan and Fava2009), superior frontal gyrus (Knutson et al., Reference Knutson, Bhanji, Cooney, Atlas and Gotlib2008), and frontal pole (Dichter et al., Reference Dichter, Kozink, McClernon and Smoski2012) during the outcome stage. Consequently, two recent quantitative meta-analyses have been performed to delineate brain regions that are consistently implicated in different reward stages (Keren et al., Reference Keren, O'Callaghan, Vidal-Ribas, Buzzell, Brotman, Leibenluft and Stringaris2018; Zhang, Chang, Guo, Zhang, & Wang, Reference Zhang, Chang, Guo, Zhang and Wang2013). However, these meta-analyses also yielded inconsistent findings. Both meta-analysis studies were performed across relatively large, different samples (currently depressed patients (cMDD), remitted depressed patients (rMDD), and a continuum of depression on severity), and different experimental paradigms [monetary incentive delay (MID) task, card guessing, Pavlovian prediction, and effort expenditure for rewards] (Keren et al., Reference Keren, O'Callaghan, Vidal-Ribas, Buzzell, Brotman, Leibenluft and Stringaris2018; Zhang et al., Reference Zhang, Chang, Guo, Zhang and Wang2013). The heterogeneity of the included samples and reward-related paradigms combined with the bias introduced by including region of interest (ROI) analyses may explain the inconsistencies among studies.
To address this issue, we performed a voxel-based meta-analysis to investigate the neural correlates of monetary response in MDD patients during the anticipation and outcome phases, relative to control groups. Given that cMDD and rMDD show different behavioural and psychopathological features (Caetano et al., Reference Caetano, Kaur, Brambilla, Nicoletti, Hatch, Sassi and Soares2006; Klauser et al., Reference Klauser, Fornito, Lorenzetti, Davey, Dwyer, Allen and Yucel2015; Pulcu et al., Reference Pulcu, Thomas, Trotter, McFarquhar, Juhasz, Sahakian and Elliott2015; Shankman, Mittal, & Walther, Reference Shankman, Mittal and Walther2020), we employed strict inclusion criteria that only included studies on cMDD patients. The MID task is a well-established reward paradigm that permits a precise examination of neural activations reflecting different subdomains of anhedonia, such as the reward anticipation (appetitive motivational processing), and consummatory (reward experiencing) phases of reward (Knutson, Fong, Adams, Varner, & Hommer, Reference Knutson, Fong, Adams, Varner and Hommer2001; Knutson, Westdorp, Kaiser, & Hommer, Reference Knutson, Westdorp, Kaiser and Hommer2000). To minimise the heterogeneity of functional imaging paradigms, we included only studies that employed the MID task in our present meta-analysis. We further examined the influence of demographic and clinical factors that contribute to the MID-related brain response with meta-regression analysis. Given the emerging findings from the vast functional magnetic resonance imaging (fMRI) literature in depression, we hypothesised that depressed patients would display blunted activation of the striatum and abnormal activation of the prefrontal regions (e.g. the ACC and mPFC) during the different stages of reward processing, and that striatal and prefrontal activations would correlate with clinical measures.
Methods
Search strategy
A systematic strategy was performed for relevant studies published in the PubMed, Embase, and Web of Science databases from 1 January 2000 to 1 June 2022. The key search words were as follows: (‘depression’ OR ‘major depressive disorder’ OR ‘MDD’ OR ‘affective disorder’) AND (‘MID’ OR ‘monetary’ OR ‘monetary incentive delay task’ OR ‘reward’) AND (‘functional magnetic resonance imaging’ OR ‘fMRI’ OR ‘neuroimaging’). Studies were also identified by consulting review articles and the references of retrieved articles.
Inclusion and exclusion criteria
The included studies had to meet the following criteria: (1) the depressed patients were diagnosed by structured interviews and clinical diagnosis based on the ICD or DSM diagnostic criteria: Patients with MDD fulfilled criteria for a current major depressive episode according to ICD or DSM criteria, (2) studies used MID task or modified MID task, (3) studies compared group differences between MDD patients and healthy controls (HC), and (4) peak activations were reported in Montreal Neurological Institute (MNI) or Talairach space.
Studies were excluded if they (1) were animal studies, book chapters, reviews or meta-analyses, (2) included subjects younger than 18 years old, or older than 60 years old, (3) reported ROI findings or volumes of interest (VOI) procedure, (4) reanalysed previously published data, (5) failed to include more than 10 individuals, or (6) recruited rMDD individuals. Two studies used overlapping samples (Admon et al., Reference Admon, Nickerson, Dillon, Holmes, Bogdan, Kumar and Pizzagalli2015; Pizzagalli et al., Reference Pizzagalli, Holmes, Dillon, Goetz, Birk, Bogdan and Fava2009); the earlier one that provided more reward processing aspects was included (Pizzagalli et al., Reference Pizzagalli, Holmes, Dillon, Goetz, Birk, Bogdan and Fava2009).
Our meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Moher, Liberati, Tetzlaff, Altman, & Grp, Reference Moher, Liberati, Tetzlaff, Altman and Grp2009). Two authors independently searched the literature and checked all articles according to the criteria.
Voxel-based meta-analysis
Two separate voxel-based meta-analyses of reward anticipation and outcome-related brain activity differences between MDD patients and HC were calculated using software seed-based d mapping (SDM version 5.15; https://www.sdmprojbect.com, online Supplementary Methods), which has been used successfully across several neuropsychiatric disorders (Dugre et al., Reference Dugre, Radua, Carignan-Allard, Dumais, Rubia and Potvin2020; Hart, Radua, Nakao, Mataix-Cols, & Rubia, Reference Hart, Radua, Nakao, Mataix-Cols and Rubia2013; Radua, Via, Catani, & Mataix-Cols, Reference Radua, Via, Catani and Mataix-Cols2011; Yang et al., Reference Yang, Tian, Zhang, Zeng, Chen, Wang and Gong2016). This method allowed us to use the reported peak coordinates to recreate maps of the effect size of group differences, and consider the different effect sizes (t values) reported in the original studies (Radua & Mataix-Cols, Reference Radua and Mataix-Cols2009; Radua et al., Reference Radua, Mataix-Cols, Phillips, El-Hage, Kronhaus, Cardoner and Surguladze2012).
Results
Included studies and sample characteristics
Table 1 summarises the clinical and demographic data of all the included studies. For anticipation article searching, based on the above strategy, a total of 11 studies met the inclusion criteria for the meta-analysis (Admon et al., Reference Admon, Kaiser, Dillon, Beltzer, Goer, Olson and Pizzagalli2017; Arrondo et al., Reference Arrondo, Segarra, Metastasio, Ziauddeen, Spencer, Reinders and Murray2015; Carl et al., Reference Carl, Walsh, Eisenlohr-Moul, Minkel, Crowther, Moore and Smoski2016; DelDonno et al., Reference DelDonno, Mickey, Pruitt, Stange, Hsu, Weldon and Langenecker2019; Hagele et al., Reference Hagele, Schlagenhauf, Rapp, Sterzer, Beck, Bermpohl and Heinz2015; Knutson et al., Reference Knutson, Bhanji, Cooney, Atlas and Gotlib2008; Pizzagalli et al., Reference Pizzagalli, Holmes, Dillon, Goetz, Birk, Bogdan and Fava2009; Schwarz et al., Reference Schwarz, Moessnang, Schweiger, Baumeister, Plichta, Brandeis and Meyer-Lindenberg2020; Stoy et al., Reference Stoy, Schlagenhauf, Sterzer, Bermpohl, Hägele, Suchotzki and Ströhle2012; Ubl et al., Reference Ubl, Kuehner, Kirsch, Ruttorf, Diener and Flor2015; Wakatsuki et al., Reference Wakatsuki, Ogura, Hashimoto, Toyomaki, Miyamoto, Nakagawa and Kusumi2022). This resulted in a sample of 279 MDD patients and 371 HC in the anticipation meta-analysis. There were no significant differences in age (t = −0.88, p = 0.38) or sex (χ2 = 1.70, p = 0.19) between the patient group (mean age = 35.53 years old; 50.20% female) and the control group (mean age = 36.29 years old; 46.59% female).
Table 1. Clinical and demographic characteristics of MDD patients in the meta-analysis

Abbreviations: N, number; NA, not applicable; MDD, major depressive disorder; rMDD, remitted major depressive disorder; HC, healthy controls.
During the outcome stage, a total of 8 studies were initially included for the meta-analysis (Admon et al., Reference Admon, Kaiser, Dillon, Beltzer, Goer, Olson and Pizzagalli2017; Carl et al., Reference Carl, Walsh, Eisenlohr-Moul, Minkel, Crowther, Moore and Smoski2016; Knutson et al., Reference Knutson, Bhanji, Cooney, Atlas and Gotlib2008; Pizzagalli et al., Reference Pizzagalli, Holmes, Dillon, Goetz, Birk, Bogdan and Fava2009; Reinen et al., Reference Reinen, Whitton, Pizzagalli, Slifstein, Abi-Dargham, McGrath and Schneier2021; Schwarz et al., Reference Schwarz, Moessnang, Schweiger, Baumeister, Plichta, Brandeis and Meyer-Lindenberg2020; Ubl et al., Reference Ubl, Kuehner, Kirsch, Ruttorf, Diener and Flor2015; Wakatsuki et al., Reference Wakatsuki, Ogura, Hashimoto, Toyomaki, Miyamoto, Nakagawa and Kusumi2022). This comprised a sample of 217 MDD patients and 278 HC. The mean age between MDD patients (35.44 years old) and HC (36.37 years old) was not significantly different (t = −1.69, p = 0.09). The percentage of females between MDD patients (60.83%) and HC (59.97%) was not significantly different (χ2 = 2.60, p = 0.11).
Main meta-analysis of brain activity during reward anticipation
In the anticipation meta-analysis, MDD patients displayed increased activation in the bilateral mPFC extending to the ACC, middle cingulate cortex (MCC) left postcentral gyrus, IFG and right MFG in comparison to the controls. Significant deactivations were located in the left striatum (extending to the insula and amygdala), right cerebellum, striatum and IFG (Fig. 1 and Table 2).
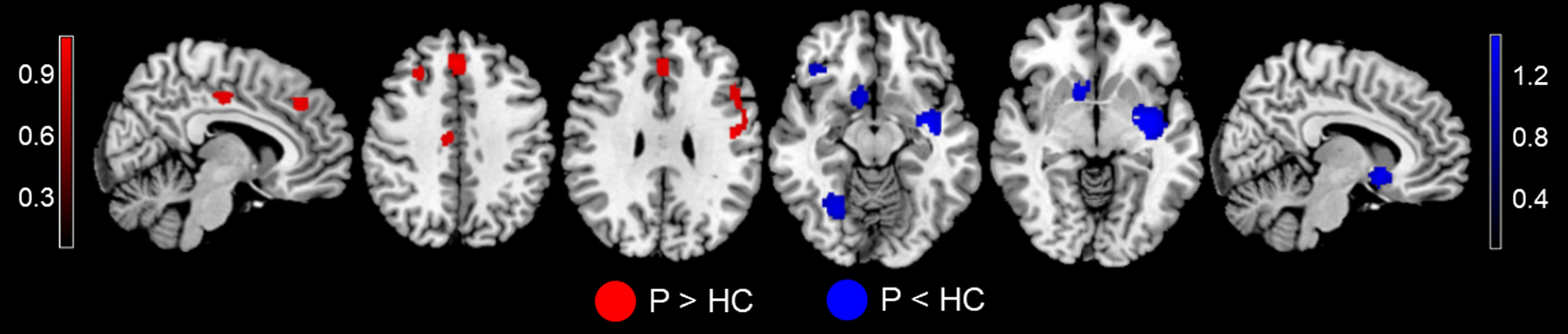
Fig. 1. MID-evoked activation differences during the reward anticipation phase between MDD patients and HC in the meta-analysis. Relative to HC, MDD patients showed hyperactivity in the bilateral mPFC (extending to ACC), MCC, left postcentral gyrus, IFG, and right DLPFC, and hypoactivity in the left striatum (extending to the insula and amygdala), right cerebellum, striatum, and IFG during the reward anticipation phase. Regions with increased activation in MDD patients compared to controls are displayed in red, while regions with decreased activation in MDD patients compared to controls are displayed in blue. Abbreviations: MID, monetary incentive delay task; MDD, major depressive disorder; p, MDD patients; HC, healthy controls; mPFC, medial prefrontal cortex; ACC, anterior cingulate cortex; MCC, median cingulate cortex; IFG, inferior frontal gyrus; DLPFC, dorsolateral prefrontal cortex.
Table 2. Meta-analyses of brain activation differences between MDD patients and HC during the anticipation stage
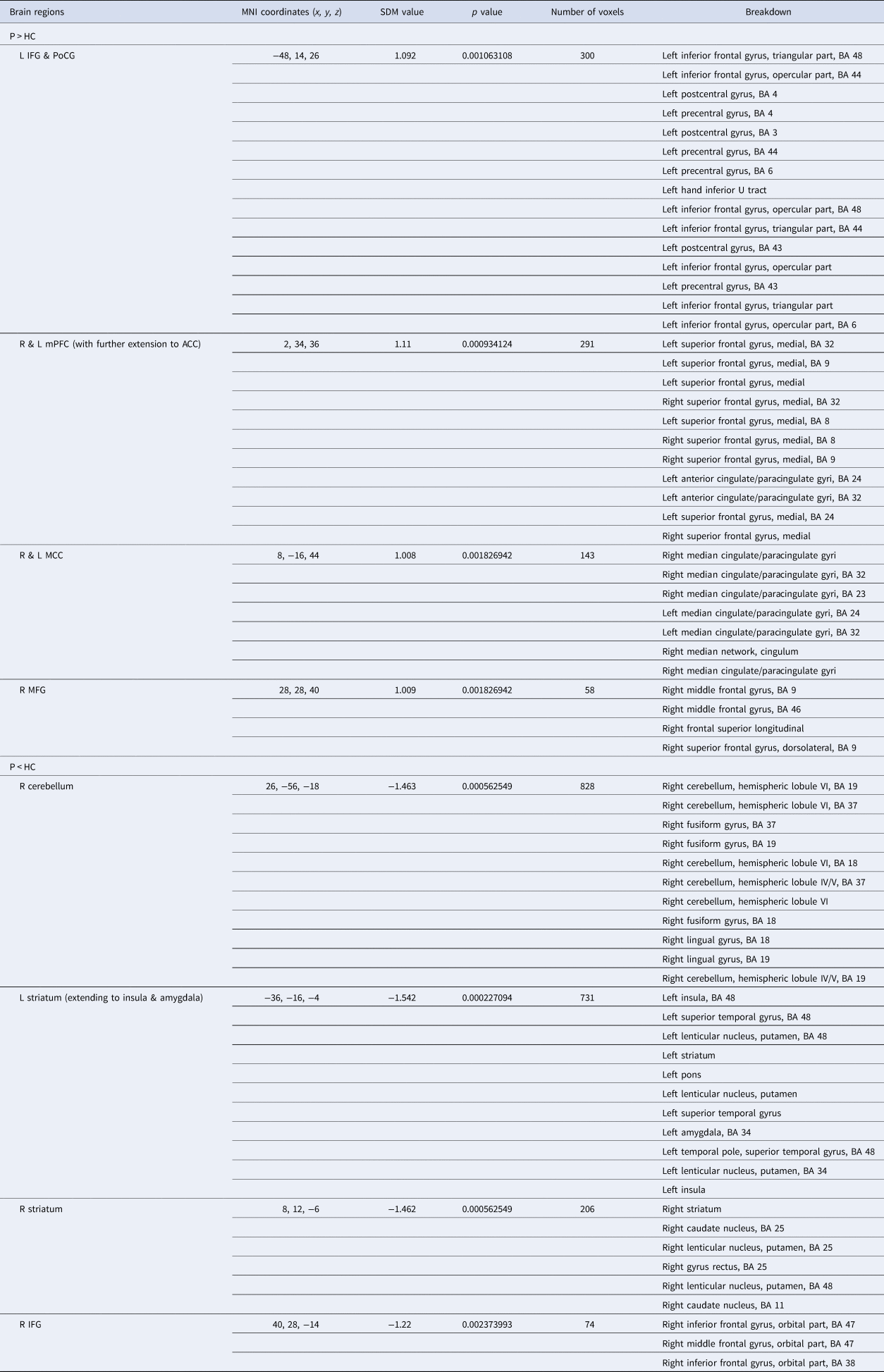
Abbreviations: BA, Brodmann area; MDD, major depression disorder; HC, healthy controls; SDM, seed-based d mapping; MNI, Montreal Neurological Institute; R, right; L, left; IFG, inferior frontal gyrus; PoCG, postcentral gyrus; mPFC, medial prefrontal cortex; ACC, anterior cingulate cortex; MCC, median cingulate cortex; DLPFC, dorsolateral prefrontal cortex.
Note: Clusters were identified at voxel-wise p < 0.005, SDM-Z > 1, and cluster size > 10 voxels.
Main meta-analysis of brain activity during reward outcome
In the outcome meta-analysis, patients with MDD showed increased activations in the left inferior temporal gyrus (ITG), and decreased activations in the bilateral MCC, left caudate nucleus, precentral gyrus, thalamus, and cerebellum, right striatum, insula, IFG, MFG, and temporal pole compared to controls (Fig. 2 and Table 3).
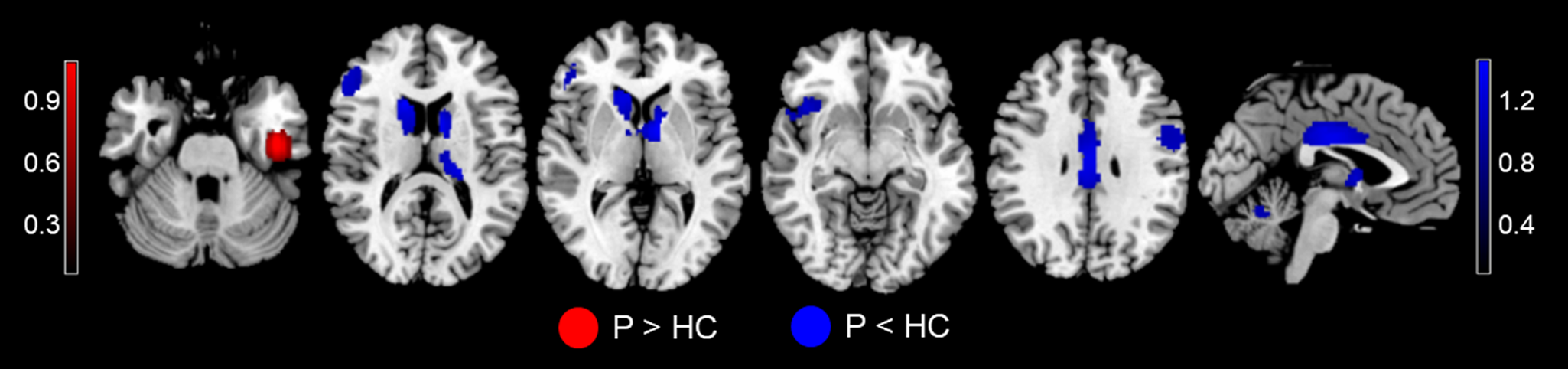
Fig. 2. MID-evoked activation differences during the reward outcome phase between MDD patients and HC in the meta-analysis. Relative to HC, MDD patients showed hyperactivity in the left ITG, and hypoactivity in the bilateral MCC, left caudate, precentral gyrus, cerebellum, and thalamus, right striatum, insula, IFG, MFG, and temporal pole. Regions with increased activation in MDD patients compared to controls are displayed in red, while regions with decreased activation in MDD patients compared to controls are displayed in blue. Abbreviations: MID, monetary incentive delay task; MDD, major depressive disorder; p, MDD patients; HC, healthy controls; ITG, inferior temporal gyrus; ACC, anterior cingulate cortex; MCC, median cingulate cortex; IFG, inferior frontal gyrus; MFG, middle frontal gyrus.
Table 3. Meta-analyses of brain activation differences between MDD patients and HC during the outcome stage

Abbreviations: BA, Brodmann area; MDD, major depression disorder; HC, healthy controls; SDM, seed-based d mapping; MNI, Montreal Neurological Institute; p, MDD patients; R, right; L, left; ITG, inferior temporal gyrus; ACC, anterior cingulate cortex; MCC, median cingulate cortex; IFG, inferior frontal gyrus; MFG, middle frontal gyrus.
Note: Clusters were identified at voxel-wise p < 0.005, SDM-Z > 1, and cluster size > 10 voxels.
The results of jack-knife sensitivity analyses, subgroup analyses and meta-regression analyses during the anticipation and outcome stages are presented in the online Supplementary Results.
Discussion
To compare the different neural substrates of MDD patients and healthy subjects in the MID task, a comprehensive meta-analysis of fMRI studies to detect the most consistent loci of brain activity towards monetary stimuli was conducted. As assumed, the results confirmed that MDD patients had different brain activation patterns at the anticipation and outcome stages of the MID task. During the anticipation stage, the depressed patients exhibited hyperactivity in the prefrontal regions, mainly in the bilateral mPFC, ACC and MCC, left postcentral gyrus, IFG, and right MFG, but reduced hyperactivity in the mesolimbic circuit, including the left striatum, insula, amygdala, right cerebellum, striatum, and IFG, compared to controls. During the outcome stage, the MDD group showed higher monetary activity in the left ITG, and lower activity in the mesocortical pathway, including the bilateral MCC, left caudate nucleus, precentral gyrus, cerebellum, thalamus, right striatum, insula, IFG, MFG, and temporal pole. Our meta-analytic findings revealed that reward responses within the prefrontal cortex are increased during anticipation but decreased in the outcome, while the response in the striatum is consistently blunted across studies in both the anticipation stage and the outcome stage in MDD patients relative to HC. The current findings suggest disparate neural substrates for reward anticipation and outcome in depressed patients, providing evidence for a more refined understanding of pathophysiological mechanisms in MDD.
Brain activation during the anticipation phase
In the current meta-analysis, the most important finding is that studies consistently reported that people with MDD exhibit hyper-responsivity to monetary reward in the mPFC, MFG, median and anterior cingulate gyri, and postcentral areas during anticipation. The medial walls of the frontal lobes, comprising the cingulate cortex and mPFC, are thought to be engaged in reward anticipation, and anhedonia, core features of MDD (Heilbronner & Hayden, Reference Heilbronner and Hayden2016). Specifically, as the cognitive part of the cingulate cortex, the ACC encodes reward evaluation and comparison and subserves monitoring throughout the decision-making process, such as the representation of the value of stimuli or actions and the monitoring of somatic states (Holroyd & Yeung, Reference Holroyd and Yeung2012). The frontal medial regions of the prefrontal cortex are key components of neural circuits involved in detecting the motivational significance of external stimuli. Altered functioning of the ACC in particular, which plays a central role in detecting the salience of external stimuli and in reward monitoring (Seeley et al., Reference Seeley, Menon, Schatzberg, Keller, Glover, Kenna and Greicius2007; Whitton et al., Reference Whitton, Kakani, Foti, Van't Veer, Haile, Crowley and Pizzagalli2016), has been observed in patients with MDD during reward processing tasks. Increased ACC activation has been observed in healthy individuals (Fan et al., Reference Fan, Kolster, Ghajar, Suh, Knight, Sarkar and McCandliss2007) and depressed individuals (Dichter et al., Reference Dichter, Kozink, McClernon and Smoski2012; Knutson et al., Reference Knutson, Bhanji, Cooney, Atlas and Gotlib2008) under conditions involving uncertainty and conflict, when errors are likely. Stronger activations are also observed in the MCC given that this region is a hub linking incoming affective information (Shackman et al., Reference Shackman, Salomons, Slagter, Fox, Winter and Davidson2011) and engages in reward processing (Vogt, Reference Vogt2016). The MCC plays an important role in decision-making, especially in relation to reward (Bush, Luu, & Posner, Reference Bush, Luu and Posner2000; van Heukelum et al., Reference van Heukelum, Mars, Guthrie, Buitelaar, Beckmann, Tiesinga and Havenith2020). The anticipation phase is characterised by heightened arousal and increased attention. These results support the notion that increased activation of the mPFC and cingulate cortex is due to reward expectation, salience processing, and cognitive response to the task. It should be noted that hyperactivation during reward anticipation is located in the dorsal ACC, which is also termed the anterior MCC (Holroyd & Yeung, Reference Holroyd and Yeung2012), rather than the pregenual aspect of the ACC in individuals with MDD. Some brain imaging evidence pointed to decreased pregenual ACC activation in depressed individuals (Ito et al., Reference Ito, Yokokawa, Yahata, Isato, Suhara and Yamada2017; Philippi et al., Reference Philippi, Cornejo, Frost, Walsh, Hoks, Birn and Abercrombie2018), but these findings might reflect activation in response to emotional stimulus rather than anticipatory reward processing. Such a finding of greater responses in the mPFC and anterior MCC in the MDD group may suggest greater monitoring of responses, increased motivational salience processing, and enhanced reward evaluation of the forthcoming reward.
During monetary anticipation, blunted brain activation was most prominent in the mesolimbic circuit, including the striatum, insula, and amygdala, when compared to healthy controls. In line with previous literature, a blunted striatal response to reward is indeed a hallmark of pathological reward anticipation in MDD. This region of the VS receives afferent projections from several cortical and limbic regions, including the prefrontal cortex, amygdala, and hippocampus, and, in turn, sends projections to the ventral pallidum, ventral tegmental area, and substantia nigra (Haber & Knutson, Reference Haber and Knutson2010). Many of the regions linked to the striatum, particularly prefrontal regions, have been associated with the computation and representation of reward value as well as the regulation of affect and reward-related behaviour in animals and healthy individuals (Grabenhorst & Rolls, Reference Grabenhorst and Rolls2011; Hiser & Koenigs, Reference Hiser and Koenigs2018). The assessment of reward value, or the motivation to obtain a reward, triggers emotions and leads to an understanding of motivation or ‘wanting’ (Grabenhorst & Rolls, Reference Grabenhorst and Rolls2011). The mesolimbic circuit is one of the most important anatomical substrates for natural rewards (e.g. food, sex) as well as for secondary rewards (e.g. money, social interactions). An accumulating body of evidence indicates that hypo-responsivity in the striatal regions plays an important role in the onset and course of MDD. For example, reduced striatal activation is present among currently depressed individuals (Rappaport, Kandala, Luby, & Barch, Reference Rappaport, Kandala, Luby and Barch2020) and in MDD during periods of remission (Geugies et al., Reference Geugies, Mocking, Figueroa, Groot, Marsman, Servaas and Ruhe2019). This reduction in reward responsiveness has been found to predict later depressive episode MDD and the emergence of depressive symptoms despite antidepressant treatment (Morgan, Olino, McMakin, Ryan, & Forbes, Reference Morgan, Olino, McMakin, Ryan and Forbes2013; Stringaris et al., Reference Stringaris, Vidal-Ribas Belil, Artiges, Lemaitre, Gollier-Briant, Wolke and Paillère-Martinot2015). The strength of these blunt responses is correlated with the severity of depression (Geugies et al., Reference Geugies, Mocking, Figueroa, Groot, Marsman, Servaas and Ruhe2019; Rappaport et al., Reference Rappaport, Kandala, Luby and Barch2020). In addition to VS, a reduced response in the putamen was found. In previous studies, the putamen has been demonstrated to be associated with reward sensitivity (Meyer et al., Reference Meyer, Kruger, Wilson, Christensen, Goulding, Schaffer and Kennedy2001; Russo & Nestler, Reference Russo and Nestler2013). Deficits in reward sensitivity are particularly evident in the melancholic subtype of depression (Foti, Carlson, Sauder, & Proudfit, Reference Foti, Carlson, Sauder and Proudfit2014). Some studies with depressed patients have found an increase in putamen volume after treatment, suggesting that patients can be treated by modulating their reward/motivation circuits (Wang et al., Reference Wang, Wang, Liu, Chen, Liu, Nie and Kong2017). Common findings of hypo-responsivity in the mesolimbic circuit highlight the role of dopamine signalling in the pursuit of reward, contributing to motivational impairment in depression.
Among regions outside the striatum, a notable finding was the reduced activity in the OFC, amygdala and insula. The OFC is thought to play a major role in many complex functions including recognition of reward stimuli and stimulus-reward association, which is considered a therapeutic target for depression (Lacerda et al., Reference Lacerda, Keshavan, Hardan, Yorbik, Brambilla, Sassi and Soares2004). Reduced activity in the OFC during reward anticipation may reflect diminished tagging of normally rewarding stimuli with positive affective value (Fettes, Schulze, & Downar, Reference Fettes, Schulze and Downar2017). The amygdala shares interconnections with the orbitofrontal cortex and supplies information to generate and use expectancies of reinforcers in the guidance of goal-directed behaviour (Holland & Gallagher, Reference Holland and Gallagher2004). Recent evidence has indicated the important roles of the amygdala in the representation of incentive value and the evaluation of salient stimuli (Holland & Gallagher, Reference Holland and Gallagher2004). The insula, together with the mPFC and posterior cingulate cortex, is an intricate part of the salience/reward network. The insula is involved in risk prediction and incentive motivational processes, possibly due to its role in detecting salient information and processing interoceptive information (Naqvi & Bechara, Reference Naqvi and Bechara2009). Our findings of blunted reward activity in limbic regions confirm previous fMRI studies in MDD. The hypo-reactivity of a broader set of regions within the mesolimbic circuit indicates that the amygdala and insula may be key brain regions implicated in the pathophysiology of depression (Price & Drevets, Reference Price and Drevets2010).
Brain activation during the outcome phase
With regard to receiving reward outcomes, MDD showed decreased activity within the cortices (medial/dorsolateral prefrontal cortex, precentral gyrus, and cingulate cortex) and subcortical structures (ventral and dorsal striatum, caudate nucleus, thalamus) and increased brain activity in the ITG; notably, these mesocortical structures were previously implicated as deficient in patients with MDD. Previous studies of reduced activity in the VS associated with an abnormal response to feedback were frequently reported in currently depressed (Forbes et al., Reference Forbes, Hariri, Martin, Silk, Moyles, Fisher and Dahl2009; Pizzagalli et al., Reference Pizzagalli, Holmes, Dillon, Goetz, Birk, Bogdan and Fava2009; Spati et al., Reference Spati, Chumbley, Doerig, Brakowski, Grosse Holtforth, Seifritz and Spinelli2015) and high-risk individuals (Sharp et al., Reference Sharp, Kim, Herman, Pane, Reuter and Strathearn2014). Decreased responses in the mesocortical pathway could indicate depression-related difficulties with several aspects of outcome-related reward processing. MCC hypoactivation presumably contributes to choice processes when receiving the outcome, as the MCC is an integral part of feedback-mediated decision-making and modification of rewarded behaviours to accommodate current and predicted contexts (Vogt, Reference Vogt2016). The thalamus is an interface for brain reward circuits and with input signals arising from structures such as the prefrontal cortex and hypothalamus that are broadcast to downstream limbic targets (Otis et al., Reference Otis, Zhu, Namboodiri, Cook, Kosyk, Matan and Stuber2019). It mediates appetitive associative learning, motivational drive, planning and cognition for the development of goal-directed behaviour (Krebs, Boehler, Roberts, Song, & Woldorff, Reference Krebs, Boehler, Roberts, Song and Woldorff2012; Zhu et al., Reference Zhu, Nachtrab, Keyes, Allen, Luo and Chen2018). Disruption of the response in the thalamus, could suggest difficulties with the execution of action in response to rewarding cues. Notably, the striatal response in depressed patients seems to be more involved in the dorsal part of the striatum during the outcome than in the ventral part of the striatum during anticipation. In reinforcement learning, the VS signals reward prediction error and incentive motivational salience (Berridge, Reference Berridge2012; Bromberg-Martin, Matsumoto, & Hikosaka, Reference Bromberg-Martin, Matsumoto and Hikosaka2010), while the DS supports habit, formation, actions, and stimulus-driven behaviour (Hilario & Costa, Reference Hilario and Costa2008). One possible interpretation is that the response of the DS is blunted rewards during the processing of reward delivery, which seems to reflect a ‘habit’ of reduced reward response, leading to more automatic hedonic responses. Compared to reward anticipation, blunted reactivity to reward after exposure to visual stimuli (Kaposvari, Kumar, & Vogels, Reference Kaposvari, Kumar and Vogels2018; Liu et al., Reference Liu, Admon, Mellem, Belleau, Kaiser, Clegg and Pizzagalli2020). The increased ITG activation evoked by reward cues is consistent with these studies and implies improved sensory processing for feedback information.
Clinical implications
Core depressive symptom dimensions, including persistent low mood and anhedonia, reflect predominant features of dysfunctional reward processing. From the translational potential of the insights, mapping the different domains of reward processing onto depressive symptoms could have important implications for informing illness diagnostics. Impaired reward learning has emerged as a key characteristic of MDD and may be a vulnerability marker in individuals with MDD (Coccurello, Reference Coccurello2019; Hasler et al., Reference Hasler, Drevets, Manji and Charney2004). MDD is characterised by decreased responsiveness of the frontostriatal brain regions to rewarding stimuli, including decreased anticipation of forthcoming rewards; reduced hedonic experience derived from reward presentation may contribute to the core symptom of anhedonia in MDD (Der-Avakian & Markou, Reference Der-Avakian and Markou2012; Zhang et al., Reference Zhang, Chang, Guo, Zhang and Wang2013, Reference Zhang, Lin, Shi, Ongur, Auerbach, Wang and Wang2016). These abnormalities in reward circuitry may be associated with behaviours linked to constructs in the positive valence systems domain, such as reward expectancy and anhedonia (Der-Avakian, Barnes, Markou, & Pizzagalli, Reference Der-Avakian, Barnes, Markou and Pizzagalli2016). Our meta-analysis found that MDD may be characterised by hyper-responsivity in the cortical prefrontal regions during the anticipation phase, and hypo-responsivity of the mesocortico-limbic circuit across the two phases of the reward response. Consistent with the NIMH Research Domain Criteria initiative (Insel et al., Reference Insel, Cuthbert, Garvey, Heinssen, Pine, Quinn and Wang2010), identifying brain responses to reward stimuli may be particularly useful for identifying brain-behaviour relationships and clinically and pathophysiologically meaningful MDD endophenotypes. Therefore, identification of the different neurobiological pathways underlying the different facets of reward processing may serve as potential neural markers for MDD diagnosis based on the framework of the appetitive reward system.
Of particular relevance here, targeting reward dysfunction also allows us to identify treatment outcomes and relapse propensity. A growing body of literature addresses attenuations of striatal activation, and frontostriatal connectivity can serve as a potential predictor of treatment response in depression (Phillips et al., Reference Phillips, Chase, Sheline, Etkin, Almeida, Deckersbach and Trivedi2015; Walsh et al., Reference Walsh, Carl, Eisenlohr-Moul, Minkel, Crowther, Moore and Dichter2017). Aberrant brain connectivity in the striatum is reported to be associated with the course of depressive episodes, suggesting that the reorganisation of striatal connectivity may interact with the course of episodes in depression, thereby contributing to depressive relapse risk (Meng et al., Reference Meng, Brandl, Tahmasian, Shao, Manoliu, Scherr and Sorg2014). Recently, a novel psychological treatment (called Positive Affect Treatment) for anxiety and depression was designed to specifically target deficits in reward sensitivity and appetitive responding (Craske, Meuret, Ritz, Treanor, & Dour, Reference Craske, Meuret, Ritz, Treanor and Dour2016). After receiving this new treatment, patients with unipolar depression showed a significant reduction in depression symptoms and a significant improvement in positive affect, although the effectiveness of treating anhedonia awaits careful testing in further studies (Craske et al., Reference Craske, Meuret, Ritz, Treanor and Dour2016). This may help to explain the success of behavioural activation treatments for MDD, which specifically target motivational symptoms (Dimidjian et al., Reference Dimidjian, Hollon, Dobson, Schmaling, Kohlenberg, Addis and Jacobson2006). Therefore, investigation of the precise reward processes that are affected in MDD will facilitate the diagnosis and treatment of these disorders that include reward and motivation deficits as key symptoms.
Limitations and future directions
Several limitations of the current study should be addressed. First, our voxel-wise meta-analysis was based on the coordinates from published studies instead of raw statistical brain maps, which limited the accuracy of our results. Second, we could not investigate the effect of some clinical characteristics, such as subtypes of depression, severity of anhedonia, comorbidity with anxiety, and mediation status in the depressed individuals on the MID-related brain response, due to limited data. We cannot directly investigate the neural correlates of the construct of anhedonia given the insufficient data for measuring self-reports of anhedonia (see online supplement for the in-depth discussion). We are also underpowered to examine the differences in monetary processing between depression with anxiety and without anxiety during the anticipation and outcome phases. Similarly, only a few studies reported the percentage of participants who had received antidepressant treatments in the past, preventing further analysis. Additionally, the complicated effects of treatment, such as drug types, clinical response, and side effect profiles, could not be examined in our present study due to insufficient data. These results should be interpreted with caution, and very careful clinical observation is required to examine treatment-related effects on reward response. Third, all fMRI studies have used monetary rewards, but the processing of primary and secondary rewards may differ in important respects. The results should be interpreted with caution when applying the findings to primary reward enforcers. With regard to methodological heterogeneity, diverse imaging acquisition techniques (e.g. different MRI field strengths, MRI scanners, and imaging parameters) might bias the results. Furthermore, the included studies applied different templates, smoothing kernel size, slice thickness and statistical threshold. These variations in data analysis parameters make it difficult to directly compare findings among studies. Thus, imaging acquisition and fMRI analysis parameters varied throughout the literature, potentially limiting our ability to detect robust group differences.
In the present study, we examined different stages of reward processing in cMDD patients using a voxel-wise meta-analysis. Because previous research suggests that neural activation during reward processing might vary with depression symptom profiles (Rappaport et al., Reference Rappaport, Kandala, Luby and Barch2020), our findings of functional alterations in MDD may provide as state markers of MDD that reflect the pathophysiological processes of the illness. Future studies investigating cMDD individuals and rMDD individuals may be able to identify the state or trait characteristic of the illness. Future longitudinal studies are also needed to further characterise the persistence of altered neural responses to monetary stimulus when patients enter or recover from depressive episodes. Regarding the task paradigm, the MID task is not designed to capture temporal learning or any reward prediction error signal. However, it would be interesting to examine aspects of reward coding, including learning rate, reward sensitivity, and prediction error, in the future using theories and models of reinforcement learning, such as goal-directed v. habitual decision-making.
Conclusions
Our meta-analysis revealed that MDD displayed different brain activation between reward anticipation and receipt in the MID task. The depressed patients showed hypo-responsivity in the mesolimbic circuit and hyper-responsivity in the prefrontal cortex during anticipation, whereas these responses were increased in the ITG and reduced in the mesocortical circuit during the outcome stage. Our findings suggested that MDD may be characterised by hyper-responsivity in the cortical regions associated with conflict monitoring during the anticipation phase, and hypo-responsivity of the mesocortico-limbic circuit across the two phases of the reward response. Our study showed dissociable neural circuit responses to monetary stimuli during reward anticipation and outcome underlying motivational v. hedonic deficits in depression.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291722002707.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Grant No. 31700964), Graduate Research and Innovation Foundation of Chongqing (Grant No.CYS21054), Fundamental Research Funds for the Central Universities of China (Grant No.2020CDJSK01XK02), the Venture & Innovation Support Program for Chongqing Overseas Returnees (Grant Nos. cx2019154 and cx2020119), the Social Science Foundation of Chongqing (Grant No. 2020YBGL80), and the Research on Teaching Reform Program of Chongqing University (Grant No. 2019Y04).
Conflict of interest
The authors declare no conflicts of interest.








