Introduction
One of the core symptoms of major depressive disorder (MDD) is anhedonia, a lack of interest and reduced responsiveness to rewarding stimuli that are usually found enjoyable (Treadway & Zald, Reference Treadway and Zald2011). Functional magnetic resonance imaging (fMRI) studies have reported blunted reward processing in fronto-striatal regions as characteristic for patients with current MDD (Hasler et al. Reference Hasler, Drevets, Manji and Charney2004; Knutson et al. Reference Knutson, Bhanji, Cooney, Atlas and Gotlib2008; Pizzagalli et al. Reference Pizzagalli, Holmes, Dillon, Goetz, Birk, Bogdan, Dougherty, Iosifescu, Rauch and Fava2009; Smoski et al. Reference Smoski, Felder, Bizzell, Green, Ernst, Lynch and Dichter2009; Pizzagalli, Reference Pizzagalli2011; Stoy et al. Reference Stoy, Schlagenhauf, Schlochtermeier, Wrase, Knutson, Lehmkuhl, Huss, Heinz and Strohle2011; Ubl et al. Reference Ubl, Kuehner, Kirsch, Ruttorf, Diener and Flor2015). Some studies focused on state-independent neurobiological mechanisms of MDD pathogenesis and showed that dysfunctional neural reward processing can be linked to observable anhedonic behaviour in remitted depressed individuals and in individuals at high-risk for depression (McCabe et al. Reference McCabe, Cowen and Harmer2009, Reference McCabe, Woffindale, Harmer and Cowen2012). In these studies, altered neural sensitivity to rewarding stimuli has been considered as a vulnerability marker of MDD, thought to be essential in the development and manifestation of MDD, and to represent a potential brain-based endophenotype of MDD (Hasler et al. Reference Hasler, Drevets, Manji and Charney2004; Dichter et al. Reference Dichter, Kozink, McClernon and Smoski2012; Simmons & Drevets, Reference Simmons and Drevets2012), allowing to link neural depressogenic markers with trait-like correlates of MDD, such as personality features (compare with Hasler et al. Reference Hasler, Drevets, Manji and Charney2004).
A promising approach to evaluate vulnerability markers of MDD is the investigation of individuals with remitted MDD (rMDD) with a history of depressive episodes (Alloy et al. Reference Alloy, Abramson, Whitehouse, Hogan, Tashman, Steinberg, Rose and Donovan1999). The analysis of rMDD enables the mapping of neural alterations that might be predictive for the recurrence of MDD but are only weakly confounded by psychopathological characteristics of depression in contrast to the analysis of persons with current MDD. Previously, McCabe et al. (Reference McCabe, Cowen and Harmer2009) employed an fMRI paradigm in which rMDD participants and healthy controls (HCs) received aversive and appetitive flavours and pictures and their combination. During reward reception, rMDD participants showed reduced responses in the ventral striatum (VS) to the appetitive flavour and in the anterior cingulate cortex (ACC) and orbitofrontal cortex (OFC) to the combined rewarding stimuli. Dichter et al. (Reference Dichter, Kozink, McClernon and Smoski2012) used a reward task which assesses neural correlates of both reward anticipation and reward outcome receipt. The use of such reward paradigms permits a more precise examination of neural activations reflecting abnormalities in appetitive motivational processing during reward anticipation and reward experiencing during outcome processing (Berridge & Kringelbach, Reference Berridge and Kringelbach2008; Dillon et al. Reference Dillon, Holmes, Jahn, Bogdan, Wald and Pizzagalli2008, Reference Dillon, Deveney and Pizzagalli2011; Kohls et al. Reference Kohls, Chevallier, Troiani and Schultz2012). Dichter et al. (Reference Dichter, Kozink, McClernon and Smoski2012) reported hyperactivation in rMDD individuals during reward anticipation in the ACC, midfrontal gyrus (MFG) and anterior cerebellum. Hyperactivation in the ACC and MFG was suggested to reflect greater recruitment of neural resources to represent the forthcoming value of rewards and to monitor the incentive-based motor response, which is necessary for obtaining rewards. rMDD individuals also showed reward-related hypoactivation in the OFC, frontal pole, insula and thalamus during reward outcomes. In summary, these studies show a homogeneous pattern of neural hypoactivation during the receipt of reward in brain regions related to the processing of reward outcome. For reward anticipation, the study by Dichter et al. (Reference Dichter, Kozink, McClernon and Smoski2012) suggests hyperactivation in regions of the prefrontal cortex (PFC) to be associated with greater allocation of executive functions and cognitive control in rMDD.
Since temperament has a strong impact on depressive disorders, the assessment of temperament dimensions and their associations with neuronal alterations in reward processing in depressed individuals might be a promising approach for identifying personality-related correlates of reward responsitivity and their role in the aetiology and recurrence of depression. To assess the relationship between depression and temperament, several studies applied the Tridimensional Personality Questionnaire (TPQ) (Cloninger et al. Reference Cloninger, Svrakic and Przybeck1993; Gusnard et al. Reference Gusnard, Ollinger, Shulman, Cloninger, Price, Van Essen and Raichle2003). The TPQ assesses novelty seeking, harm avoidance and reward dependence (Cloninger, Reference Cloninger1987). Harm avoidance is characterized by pessimistic and fearful concerns about the future, presumably generating avoidance behaviour. Reward dependence describes the tendency to depend on social relationships and external approval, and novelty seeking is defined by impulsivity and exploratory search for novel and pleasurable stimuli. There is consensus that harm avoidance is positively associated with depression (Nelson & Cloninger, Reference Nelson and Cloninger1995; Fava et al. Reference Fava, Farabaugh, Sickinger, Wright, Alpert, Sonawalla, Nierenberg and Worthington III2002; Ongur et al. Reference Ongur, Farabaugh, Iosifescu, Perlis and Fava2005; Celikel et al. Reference Celikel, Kose, Cumurcu, Erkorkmaz, Sayar, Borckardt and Cloninger2009; Nery et al. Reference Nery, Hatch, Nicoletti, Monkul, Najt, Matsuo, Cloninger and Soares2009; Mochcovitch et al. Reference Mochcovitch, Nardi and Cardoso2012; Zappitelli et al. Reference Zappitelli, Bordin, Hatch, Caetano, Zunta-Soares, Olvera and Soares2013) and predictive for the frequency of MDD episodes and alterations in depressive mood (Farmer & Seeley, Reference Farmer and Seeley2009). A study by Smith et al. (Reference Smith, Duffy, Stewart, Muir and Blackwood2005) revealed persistent high harm avoidance scores in depressed individuals during remission, while others have shown that euthymic patients exhibit harm avoidance scores comparable with those of HCs (Nery et al. Reference Nery, Hatch, Nicoletti, Monkul, Najt, Matsuo, Cloninger and Soares2009), suggesting that harm avoidance may have both trait and state characteristics in MDD (see Abrams et al. Reference Abrams, Yune, Kim, Jeon, Han, Hwang, Sung, Lee and Lyoo2004).
The present study aimed to investigate whether altered reward processing in depression is a vulnerability marker of MDD, which is also present during remission. Therefore, we examined neural correlates of reward processing in a sample of medication-free individuals remitted from depression compared with a HC sample. We were interested whether fronto-striatal-limbic regions (e.g. OFC, VS, hippocampus) show hyperactivation during the anticipation (compare with Dichter et al. Reference Dichter, Kozink, McClernon and Smoski2012) and hypoactivation during the outcome of reward (compare with McCabe et al. Reference McCabe, Cowen and Harmer2009; Dichter et al. Reference Dichter, Kozink, McClernon and Smoski2012). For this purpose, we used a modified reward paradigm with low and high monetary gains (compare with Kirsch et al. Reference Kirsch, Schienle, Stark, Sammer, Blecker, Walter, Ott, Burkart and Vaitl2003), which was found to show robust activation in the VS, a brain structure reported to be involved in motivational aspects of reward processing (Kirsch et al. Reference Kirsch, Schienle, Stark, Sammer, Blecker, Walter, Ott, Burkart and Vaitl2003; Plichta et al. Reference Plichta, Schwarz, Grimm, Morgen, Mier, Haddad, Gerdes, Sauer, Tost, Esslinger, Colman, Wilson, Kirsch and Meyer-Lindenberg2012). As shown in previous studies, we expected to find larger activation differences between groups for higher magnitudes of rewards (e.g. Knutson et al. Reference Knutson, Bhanji, Cooney, Atlas and Gotlib2008; Ubl et al. Reference Ubl, Kuehner, Kirsch, Ruttorf, Diener and Flor2015). Additionally, we were interested in the associations between reward-related neural activations and TPQ temperament dimensions since both have been suggested to represent important vulnerability markers of MDD. Based on findings on persisting harm avoidance scores in rMDD, we particularly expected harm avoidance to be negatively correlated with neural responses to reward cues and outcomes in remitted depressed individuals. We furthermore expected reward dependence and novelty seeking to be positively associated with neuronal reward-related activation in both groups.
Method
Participants
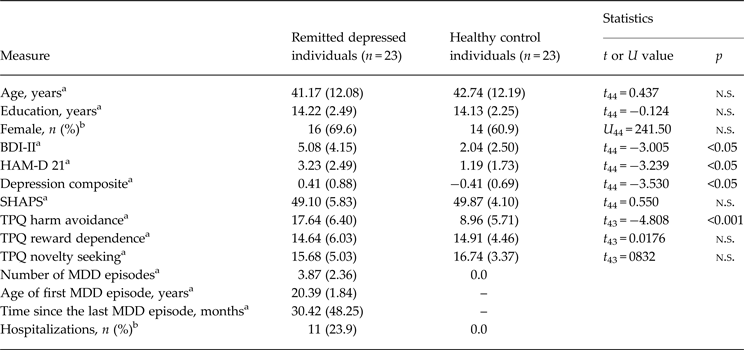
We recruited 23 medication-free individuals remitted from MDD (mean age 44.65, s.d. 12.08; age range 19–60 years) by public announcement; 23 age-, education- and sex-matched HCs were recruited by random selection from the local census bureau of the city of Mannheim, Germany. The participants were examined using the German version of the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID; Wittchen et al. Reference Wittchen, Zaudig and Fydrich1997). The remitted depressed individuals were required to meet the criteria for two or more past episodes of MDD as a primary diagnosis or at least one past episode of MDD as a primary diagnosis requiring admission to a psychiatric hospital due to symptom severity. Control participants were excluded if they met criteria for a current DSM-IV Axis I disorder or lifetime criteria for any affective disorder. General exclusion criteria were current major depressive episode and/or dysthymia, current alcohol or drug abuse, current use of psychotropic medication and current or lifetime psychotic symptoms or neurological disorders. The participants completed the German version of the Beck Depression Inventory II (BDI-II; Hautzinger et al. Reference Hautzinger, Keller and Kühner2006) within 2 weeks before the fMRI measurement and were evaluated for interviewer-rated severity of depression on the day of the fMRI measurement using the Hamilton Rating Scale for Depression (HAM-D; Hamilton, Reference Hamilton1960). Since the BDI-II and the HAM-D were significantly positively correlated (r = 0.60, p < 0.01) and to increase reliability, we collapsed the two symptom scores into a composite score of depressive symptoms by averaging the Z-standardized BDI-II and HAM-D scores (compare with Huffziger et al. Reference Huffziger, Ebner-Priemer, Zamoscik, Reinhard, Kirsch and Kuehner2013). Temperament was assessed using the TPQ with the three dimensions novelty seeking, harm avoidance and reward dependence (Cloninger et al. Reference Cloninger, Przybeck and Svrakic1991). All subjects were right-handed. One remitted depressed individual (4.3%) met criteria for a co-morbid anxiety disorder (social phobia disorder with mild anxiety symptoms) according to SCID-I (Wittchen et al. Reference Wittchen, Zaudig and Fydrich1997). Sample characteristics are provided in Table 1. The study was in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of the Medical Faculty Mannheim, Heidelberg University. All participants gave written informed consent to participate.
Table 1. Sample characteristics
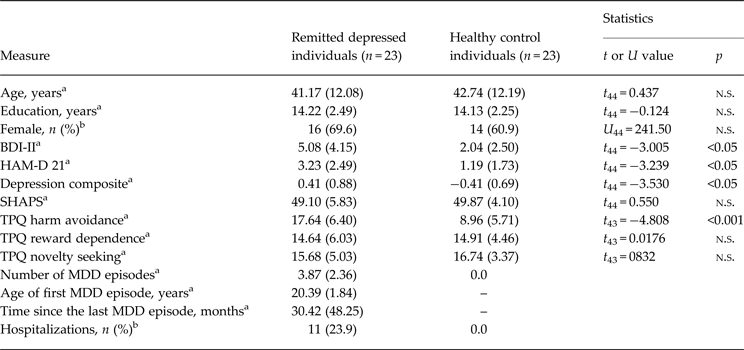
Data are given as mean (standard deviation) unless otherwise indicated.
n.s., Non-significant; BDI-II, Beck Depression Inventory II (German version); HAM-D 21, Hamilton Depression Scale 21 Items; SHAPS, Snaith–Hamilton Pleasure Scale; TPQ, Tridimensional Personality Questionnaire; MDD, major depressive disorder.
a p Value resulting from a two-sample t test.
b p Value resulting from a Mann–Whitney U test.
fMRI reward paradigm
Participants performed a modified version of the reward paradigm by Kirsch et al. (Reference Kirsch, Schienle, Stark, Sammer, Blecker, Walter, Ott, Burkart and Vaitl2003) (see Fig. 1) during fMRI. The trials began with the visual presentation of incentive cues (6 s) predicting potential monetary gains (upward arrows) or losses (downward arrows) with either low (±0.2€) or high (±2.0€) magnitudes. Horizontally oriented arrows indicated the control condition, which did not result in monetary outcomes. After the offset of the cue, a flash light was presented for 100 ms indicating that the participant had to press a button on the response device with the right index finger as quickly as possible to win the money or to not lose the money. Subsequently, participants received visual performance feedback (1.5 s) and were informed about their current balance for 1.5 s. During the control condition, only feedback about the button press was presented [‘button (not) pushed’]. Reaction time (RT) thresholds were adaptively determined depending on the subjects’ performance in the previous trial, varying from 300–1500 ms. The adaptive algorithm resulted in a decrease of 10% of the threshold after a fast response and an increase of 5% after a slow response. This was done in order to have comparable wins and losses across subjects and to maintain a sense of uncertainty in the participants. The experiment was run using the Presentation software package version 14.2 (Neurobehavioural Systems, USA; http://www.neurobs.com).

Fig. 1. Reward paradigm. Trials began with the visual presentation of different incentive cues which predicted potential monetary outcomes (gains/losses) with either low (±0.2€) or high (±2.0€) magnitudes. Trial outcome depended on the subject's response (button press) to a flash light that appeared after cue offset.
fMRI data acquisition
Before fMRI, all participants completed a practice session of the task. Whole-brain fMRI images were acquired using a 3 T Magnetom TRIO whole body MR-scanner (Siemens Medical Solutions, Germany) equipped with a standard 12-channel head coil. A gradient-echo echo planar imaging (EPI) sequence (protocol parameters: repetition time = 2700 ms; echo time = 27 ms; matrix size = 96 × 96; field of view = 220 × 220 mm2; flip angle = 90°, GRAPPA PAT 2) was used to record 658 functional volumes. Each volume consisted of 40 axial slices (slice thickness = 2.3 mm; gap = 0.7 mm) measured in descending slice order and positioned along the line from the anterior to the posterior commissure (AC-PC orientation). An automated high-order shimming technique was used to maximize magnetic field homogeneity.
Data analysis
RTs in the fMRI paradigm
RTs were analysed by SPSS (version 18; SPSS Inc., USA) using repeated-measures analyses of variance (RM-ANOVA) with group (rMDD v. HC) as between-subject and condition (high gains, low gains, and control condition) as within-subject factors. Significant main or interaction effects were analysed by post-hoc t tests. Statistical significance was accepted at p < 0.05 (two-tailed). In case of violation of sphericity, which was tested by Mauchly's test, we used the Greenhouse–Geisser correction. Levene's test was employed to assess the equality of variances between samples.
Imaging data analyses
fMRI volumes were analysed using statistical parametric mapping methods (Friston et al. Reference Friston, Holmes, Poline, Grasby, Williams, Frackowiak and Turner1995; Friston, Reference Friston, Toga and Mazziotta1996) with SPM5 (http://www.fil.ion.ucl.ac.uk/spm/software/spm5/) implemented in MATLAB R2006b (The MathWorks Inc., USA). After discarding the first four volumes to account for T1-saturation effects, the images were realigned to the fifth volume by minimizing the mean square error (rigid body transformation). None of the participants had motion estimates exceeding 3.0 mm and 2°, thus all were included in the analysis. The images were slice time corrected to reference slice 20 and normalized to the standard space of the Montreal Neurological Institute (MNI) using the EPI template provided by SPM5. The voxel size was set to 3.0 mm3. To reduce spatial noise (and allow for corrected statistical inference) the volumes were smoothed with a 6.0 mm3 Gaussian kernel.
The pre-processed data were subjected to a first-level fixed-effects analysis to separately determine gain-related neural responses for each participant. An event-related model-based analysis was implemented using the general linear model to estimate parameters for the different conditions. Blood oxygenation level-dependent responses were modelled as a canonical haemodynamic response function and convolved with the stimulus onset resulting in 17 regressors for all conditions. Additionally, one task reaction parameter and six realignment motion parameters (three translations/rotations) were included as condition-specific nuisance covariates, removing flash-light and movement-related signal changes that might be correlated with the experimental design. For statistical analyses, the fMRI time series were high-pass filtered (temporal cut-off: 128 s) to remove baseline drifts and corrected for serial autocorrelations using first-order autoregressive functions AR(1). Since we were specifically interested in reward-related processing and brain activity, we calculated contrast images for high and low reward anticipation and outcome (v. control condition) for each voxel.
A second-level random-effects analysis was conducted. Comparisons between groups were performed using two-sample t tests for contrast images. The results of the statistical analysis were first thresholded with an uncorrected voxel-wise threshold of p < 0.001, k = 10 (Lieberman & Cunningham, Reference Lieberman and Cunningham2009). We then applied family-wise error corrected (p < 0.05) voxel-level analyses to a set of hypothesis-driven regions of interest (ROIs), which match ROIs as established in previous studies investigating reward processing in depression (e.g. McCabe et al. Reference McCabe, Cowen and Harmer2009; Dichter et al. Reference Dichter, Kozink, McClernon and Smoski2012). Based on small volume correction, multiple comparison corrections were conducted using Gaussian random field theory within a VS mask (including parts of the caudate nucleus and putamen), limbic regions (hippocampus, amygdala and insula) and PFC regions [OFC, frontal pole, superior frontal gyrus (SFG) and MFG, and ACC]. The VS mask was defined as 8 mm spheres based on MNI coordinates (right: x = 9, y = 9, z = −8; left: x = −9, y = 9, z = −8) (e.g. Di Martino et al. Reference Di Martino, Scheres, Margulies, Kelly, Uddin, Shehzad, Biswal, Walters, Castellanos and Milham2008). All other ROIs were specified by mask files derived from the Wake Forest University PickAtlas v2.0 (Tzourio-Mazoyer et al. Reference Tzourio-Mazoyer, Landeau, Papathanassiou, Crivello, Etard, Delcroix, Mazoyer and Joliot2002; Maldjian et al. Reference Maldjian, Laurienti, Kraft and Burdette2003).
Brain activation, temperament and clinical characteristics
To test for relationships between reward-associated brain activation and temperament dimensions, partial correlations between individual peak activations (β weights) extracted from significant ROIs that predicted group differences during reward processing and the TPQ dimensions (novelty seeking, harm avoidance and reward dependence) were evaluated for each group separately in order to allow for statistical non-independence (p < 0.05; two-tailed) (Poldrack & Mumford, Reference Poldrack and Mumford2009). One individual with rMDD was excluded from this analysis due to missing TPQ data. Additionally, partial correlations between individual peak activations and variables indicating severity of illness, including number of MDD episodes, age of first MDD episode, number of hospitalizations, and number of months since the last MDD episode, were calculated for the rMDD group. All analyses were adjusted for residual depression.
Results
Behavioural data: RTs in the fMRI task
The RM-ANOVA revealed a significant effect for condition (F 1.53,67.47 = 27.80, p < 0.001, η2 = 0.39), but not group (F 1,44 = 0.25, p = 0.62) and a marginally significant group x condition effect (F 1.53,67.47 = 2.72, p = 0.087). Paired t tests showed that RTs were faster during both the high and low gain conditions compared with the control condition (t 45 = 5.88 and 5.20, p < 0.001).
fMRI data: neural activation in ROIs for rMDD v. HC
Anticipation: high/low reward v. control condition
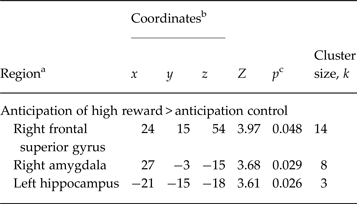
We identified a number of fronto-limbic brain areas reflecting significantly increased activity during anticipation of high reward in rMDD individuals compared with HCs, including the left hippocampus (x = −21, y = −15, z = −18, Z = 3.61, p = 0.026), the right amygdala (x = 27, y = −3, z = −15, Z = 3.68, p = 0.029), and the right SFG (x = 24, y = 15, z = 54, Z = 3.97, p = 0.037 (see Table 2 and Fig. 2). No significant group differences in any brain region were found during the anticipation of low gain.

Fig. 2. (a) Significantly enhanced activation of the right superior frontal gyrus (SFG, violet), right amygdala (AMYG, red) and left hippocampus (HPC, green) in individuals with remitted major depressive disorder (rMDD) compared with healthy controls (HCs) during high gain anticipation (slices from left to right). Sagittal and coronal slices are overlaid on the MRIcron ch2.bet template. Statistical images were thresholded using a voxel-wise-corrected significance threshold of p < 0.05. Coordinates are in Montreal Neurological Institute space. (b) Associated maximum peak activation (β values) of the right SFG, right AMYG and left HPC for rMDD and HC participants during the anticipatory phase (bars from left to right). Values are means, with standard errors represented by vertical bars. ROIs, Regions of interest.
Table 2. Between-group results of remitted depressed individuals (n = 23) and healthy controls (n = 23) showing regions associated with high reward anticipation
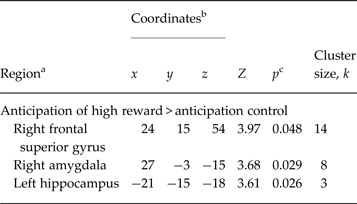
a Regions reflect the output of the local maxima labelling using Automated Anatomical Labelling of Activations in SPM.
b Montreal Neurological Institute coordinates.
c Significance at p < 0.05 (family-wise error-corrected for anatomical region of interest, voxel level).
Outcome: high/low reward v. control condition
For high reward outcome, we did not identify any region showing enhanced or decreased activation in rMDD individuals compared with HCs. Furthermore, no significant group differences in activations during low reward outcomes were found.
Brain activation, temperament and clinical characteristics
Individuals with rMDD showed significantly greater harm avoidance scores compared with HCs (t 43 = −4.81, p < 0.001). No significant group differences were found in reward dependence and novelty seeking. In HCs, but not in individuals with rMDD, the TPQ dimension reward dependence was positively correlated with activity in the left hippocampus (r 21 = 0.48, p = 0.023) during high reward anticipation. Harm avoidance was negatively correlated with activation of the right amygdala (r 21 = −0.42, p = 0.047) during reward anticipation in HCs and with activation of the left hippocampus (r 21 = −0.50, p = 0.021) in rMDD participants.
In rMDD individuals, individual neural peak activations during reward anticipation did not significantly correlate with the number of MDD episodes, age at first MDD episode, number of hospitalizations and number of months since the last MDD episode (all p's > 0.10).
Discussion
This study provides evidence for hyperactivation in the neural representation of reward anticipation in currently remitted depressed individuals with a history of depressive episodes. The design of the study allowed us to examine neural correlates of altered reward processing in individuals at high risk for depression relapse unconfounded by depression-related psychopathology and intake of psychotropic drugs (McCabe et al. Reference McCabe, Mishor, Cowen and Harmer2010; Peterson & Weissman, Reference Peterson and Weissman2011). The results indicate that altered reward processing seems to represent a persisting trait of MDD rather than a state effect, thereby pointing to its possible role as a vulnerability marker of MDD (McCabe et al. Reference McCabe, Cowen and Harmer2009, Reference McCabe, Woffindale, Harmer and Cowen2012).
In line with another study addressing reward anticipation and outcome (Dichter et al. Reference Dichter, Kozink, McClernon and Smoski2012), we found increased activation in the PFC (i.e. SFG) during high reward anticipation in remitted depressed individuals compared with controls. In addition, we showed that rMDD compared with HC participants exhibited enhanced responses to reward incentives in the hippocampus and the amygdala. Hippocampal and amygdala activations were negatively associated with harm avoidance. In contrast, no significant group differences in neural activation were identified during reward delivery. Hyperactivation during reward anticipation was apparent only for high reward magnitudes, potentially mirroring the effect that reward-related brain regions need substantial stimulation to respond (e.g. Knutson et al. Reference Knutson, Taylor, Kaufman, Peterson and Glover2005; Yacubian et al. Reference Yacubian, Glascher, Schroeder, Sommer, Braus and Buchel2006; Ubl et al. Reference Ubl, Kuehner, Kirsch, Ruttorf, Diener and Flor2015).
Remitted depressed individuals showed increased neural activity during reward anticipation in the hippocampus, amygdala and SFG. Increased activity in the SFG underpins previous findings of frontal hyperactivation during reward anticipation in rMDD (Dichter et al. Reference Dichter, Kozink, McClernon and Smoski2012). Activation in the SFG has been found to be associated with computational processes during uncertainty and working memory (Cowell et al. Reference Cowell, Egan, Code, Harasty and Watson2000; du Boisgueheneuc et al. Reference du Boisgueheneuc, Levy, Volle, Seassau, Duffau, Kinkingnehun, Samson, Zhang and Dubois2006). In healthy subjects, activation in regions of the SFG increases with increasing uncertainty (Volz et al. Reference Volz, Schubotz and von Cramon2005). The hippocampus interacts with midbrain areas during reward-based memory formation and receives projections from the amygdala and the OFC (Suzuki & Amaral, Reference Suzuki and Amaral1994; Adcock et al. Reference Adcock, Thangavel, Whitfield-Gabrieli, Knutson and Gabrieli2006). Hippocampal neurons are assumed to code the uncertainty of cue–outcome associations for reward prediction and seem to be involved in boosting attention towards relevant stimuli (Pearce & Hall, Reference Pearce and Hall1980; Strange et al. Reference Strange, Duggins, Penny, Dolan and Friston2005; Vanni-Mercier et al. Reference Vanni-Mercier, Mauguiere, Isnard and Dreher2009). Since the amygdala shares interconnections with the PFC, it supplies information to generate and use expectancies of reinforcers to guide goal-directed behaviour (Holland & Gallagher, Reference Holland and Gallagher2004). Furthermore, the amygdala is a prominent structure in the evaluation of salient stimuli, especially during the anticipation of monetary reward (compare with Zald, Reference Zald2003).
Previous studies demonstrated that in contrast to remitted depressed individuals, currently depressed individuals show blunted neural activation in frontal and striatal regions during reward anticipation, a finding rather consistently shown in studies on the neural processing of reward in depression (compare with Ubl et al. Reference Ubl, Kuehner, Kirsch, Ruttorf, Diener and Flor2015). Fronto-striatal hypoactivation in MDD has been suggested to reflect reduced motivational processing during an acute episode of depression. Taking this into consideration, there seems to be a change between acute and remitted states of depression from neural hypoactivation to hyperactivation in brain regions associated with reward processing. In our study, enhanced activity in rMDD might reflect increased neural efforts to activate greater neural resources which are needed to regulate reward-directed behaviour during motivational reward processing (compare with Dichter et al. Reference Dichter, Kozink, McClernon and Smoski2012). More precisely, the pattern of activation identified in this study can be interpreted as reflecting an increased effort to allocate neural activity in remitted depressed individuals for executive functioning, such as computational and attention-based processes, and for evaluative processes during anticipatory reward processing where uncertainty is high. Our findings support the assumption that hyperactivation in remitted depressed individuals may represent a compensatory effect, which seems necessary to reach and/or to maintain appropriate task-related performance (Kerestes et al. Reference Kerestes, Ladouceur, Meda, Nathan, Blumberg, Maloney, Ruf, Saricicek, Pearlson, Bhagwagar and Phillips2012).
Furthermore, harm avoidance scores of euthymic rMDD individuals were negatively correlated with neural responsivity in the hippocampus during reward anticipation. This finding may reflect that in rMDD greater provision of neural resources for computing the uncertainty of probabilistic serial events (Strange et al. Reference Strange, Duggins, Penny, Dolan and Friston2005) is associated with weaker avoidance behaviour and fewer pessimistic views towards future events (i.e. harm avoidance). Persistence of harm avoidance in individuals with rMDD furthermore underlines results of studies reporting significantly higher harm avoidance scores in rMDD and in non-depressed siblings of patients with MDD compared with HCs (Farmer et al. Reference Farmer, Mahmood, Redman, Harris, Sadler and McGuffin2003; Smith et al. Reference Smith, Duffy, Stewart, Muir and Blackwood2005). Both findings, the negative relationship between neural responses to anticipated future rewards and harm avoidance, and the persistence of high levels of harm avoidance in rMDD, suggest that alterations in the neural representation of reward anticipation and harm avoidance are related factors which may be linked to persisting trait characteristics of MDD vulnerability.
This study has several limitations. It is unclear from our data whether alterations in reward processing existed before the first episode of depression or may represent a post-morbid risk factor of MDD. Therefore, studies in high-risk individuals prior to the onset of MDD would be useful to investigate possible pre-morbid neural dysfunctions in reward processing. Furthermore, Mayberg (Reference Mayberg2003) suggested that psychotropic treatments may modify brain function in cortico-striatal regions in depression, including the PFC (see also Diener et al. Reference Diener, Kuehner, Brusniak, Ubl, Wessa and Flor2012). Although remitted depressed individuals in this study were medication-free, information about the type and duration of psychotropic therapy before remission and the time when they discontinued medication usage would have permitted the detection of potential predictive treatment-related factors for hyperactivation in anticipatory reward processing in rMDD.
In sum, our findings suggest that remitted depressed individuals show enhanced neural responses in fronto-limbic reward-related regions during reward anticipation. Activation in identified regions was negatively associated with harm avoidance. However, our study showed that reward outcome processing was unimpaired in rMDD. Taken together, these results suggest that altered anticipatory reward processing, but not the processing of reward delivery, appears to be a persisting trait marker of MDD vulnerability.
Acknowledgements
This work was supported by the Deutsche Forschungsgemeinschaft (SFB 636/D4 and SFB 636/Z3). We gratefully acknowledge the valuable help of our research assistants in data collection. We thank our participants for the generous cooperation and use of their time.
Declaration of Interest
None.