Introduction
Fear conditioning is vital for the detection of danger, the initiation of self-protection mechanisms and the survival of a species. The term ‘conditioning’ refers to the process of learning the association between two previously unrelated stimuli (Pavlov, Reference Pavlov1927). In a typical differential fear-conditioning design, a previously neutral stimulus (CS+) is associated with an aversive and fear-inducing unconditioned stimulus (US) and becomes intrinsically aversive, while another neutral stimulus remains unpaired (CS−) (Maren, Reference Maren2001). ‘Extinction’ is defined as the repeated exposure of the originally neutral stimulus without presenting the aversive stimulus, which gradually eliminates the learned fear reaction (Myers & Davis, Reference Myers and Davis2002).
Fear conditioning has proven to be an extremely robust and rapid experimental approach for studying the neurobiological substrates of fear and anxiety in animals and humans (Lissek et al. Reference Lissek, Powers, McClure, Phelps, Woldehawariat, Grillon and Pine2005; Anderson & Insel, Reference Anderson and Insel2006). Numerous neuroimaging studies have revealed a core neural network involved in conditioning and extinction, consisting of the amygdala, insula and anterior cingulate cortex (ACC) (for review see Sehlmeyer et al. Reference Sehlmeyer, Schoning, Zwitserlood, Pfleiderer, Kircher, Arolt and Konrad2009). Thus, amygdala activity is associated with variability in the individual fear-conditioning and fear-extinction response. Research on humans and animals has highlighted the medial prefrontal cortex (PFC) and especially the dorsal ACC (dACC) as the principal structures of the brain's extinction circuitry (Morgan et al. Reference Morgan, Romanski and LeDoux1993; Quirk et al. Reference Quirk, Likhtik, Pelletier and Pare2003; Phelps et al. Reference Phelps, Delgado, Nearing and LeDoux2004; Lang et al. Reference Lang, Kroll, Lipinski, Wessa, Ridder, Christmann, Schad and Flor2009). These areas regulate the expression of fear by inhibiting the amygdala, such that the fear-conditioned stimulus is prevented from causing a conditioned fear response (Gottfried & Dolan, Reference Gottfried and Dolan2004; Phelps et al. Reference Phelps, Delgado, Nearing and LeDoux2004; Quirk et al. Reference Quirk, Garcia and Gonzalez-Lima2006; Sotres-Bayon et al. Reference Sotres-Bayon, Cain and LeDoux2006).
Psychiatric disorders associated with increased anxiety and fear levels, such as post-traumatic stress disorder, phobias or panic disorder, exemplify how misguided fear conditioning might render originally innocuous stimuli fear-inducing and threatening. Facilitated fear conditioning and impaired extinction of acquired fear are also core symptoms of anxiety disorders (Blechert et al. Reference Blechert, Michael, Vriends, Margraf and Wilhelm2007; Michael et al. Reference Michael, Blechert, Vriends, Margraf and Wilhelm2007). Accordingly, fear extinction represents the main therapeutic ingredient of exposure-based psychotherapies (Myers & Davis, Reference Myers and Davis2002; Lissek et al. Reference Lissek, Powers, McClure, Phelps, Woldehawariat, Grillon and Pine2005; Anderson & Insel, Reference Anderson and Insel2006).
Anxiety-related personality traits, such as trait anxiety, which are regarded as stable and biological predispositions (Pujol et al. Reference Pujol, Lopez, Deus, Cardoner, Vallejo, Capdevila and Paus2002; Omura et al. Reference Omura, Todd Constable and Canli2005; Rauch et al. Reference Rauch, Milad, Orr, Quinn, Fischl and Pitman2005; Most et al. Reference Most, Chun, Johnson and Kiehl2006), are closely related to pathological anxiety (Schmidt et al. Reference Schmidt, Mitchell and Richey2008) and may influence the risk of psychiatric disorders (Bienvenu et al. Reference Bienvenu, Brown, Samuels, Liang, Costa, Eaton and Nestadt2001). Trait anxiety reflects an individual's general disposition to experience anxiety-relevant feelings or thoughts or to show anxiety-related behaviors (Spielberger, Reference Spielberger1979). Trait anxiety is a stable personality trait describing the tendency to respond fearfully to a wide variety of unspecific stressors (Spielberger, Reference Spielberger1972) and is regarded as a risk factor for anxiety disorders (Chambers et al. Reference Chambers, Power and Durham2004). Highly trait-anxious subjects tend to perceive more situations as threatening and experience more frequently intense and sustained anxiety states compared to subjects with low trait anxiety (Spielberger, Reference Spielberger1972, Reference Spielberger1979, Reference Spielberger1983; Mathews & MacLeod, Reference Mathews and MacLeod2005). It is assumed that anxiety-related personality factors are also associated with enhanced conditionability or impaired extinction of learned fear (Hooker et al. Reference Hooker, Verosky, Miyakawa, Knight and D'Esposito2008; Barrett & Armony, Reference Barrett and Armony2009). However, the literature on this topic is equivocal (Pineles et al. Reference Pineles, Vogt and Orr2009).
An overactive neuronal fear circuitry and reduced recruitment of prefrontal control have been proposed as neural correlates of facilitated fear conditioning and reduced extinction (Bishop et al. Reference Bishop, Duncan and Lawrence2004; Bishop, Reference Bishop2007, Reference Bishop2009; Haas et al. Reference Haas, Omura, Constable and Canli2007; Hooker et al. Reference Hooker, Verosky, Miyakawa, Knight and D'Esposito2008). It has been shown, for example, that high trait anxiety is related to amygdala dysregulation during the processing of aversive and neutral stimuli in healthy volunteers (Bishop et al. Reference Bishop, Duncan and Lawrence2004; Etkin et al. Reference Etkin, Klemenhagen, Dudman, Rogan, Hen, Kandel and Hirsch2004; Dickie & Armony, Reference Dickie and Armony2008; Kienast et al. Reference Kienast, Hariri, Schlagenhauf, Wrase, Sterzer, Buchholz, Smolka, Grunder, Cumming, Kumakura, Bartenstein, Dolan and Heinz2008; Mujica-Parodi et al. Reference Mujica-Parodi, Korgaonkar, Ravindranath, Greenberg, Tomasi, Wagshul, Ardekani, Guilfoyle, Khan, Zhong, Chon and Malaspina2009) or even during the extinction of conditioned fear (Barrett & Armony, Reference Barrett and Armony2009). Moreover, anxiety disorders are associated with hypo-activation of the dACC during the regulation of emotions (Etkin & Wager, Reference Etkin and Wager2007). As the relationships between anxiety-related personality traits and increased neurobiological vulnerability for fear acquisition and extinction are not fully understood, they are of specific interest in the current study.
We used event-related functional magnetic resonance imaging (fMRI) to clarify the relationship between amygdala reactivity, the involvement of the medial PFC, especially the dACC, and trait anxiety during the acquisition and extinction of conditioned fear in healthy subjects. We hypothesized that highly trait-anxious subjects exhibit enhanced fear conditioning and reduced extinction of conditioned responses, reflected by the amounts of amygdala and dACC activation.
Method
Subjects
Thirty-two healthy volunteers (12 males, 20 females; mean age 23.6 years, s.d.=4.41, range 19–39 years) participated in the study. All were right-handed (Edinburgh Handedness Inventory; Oldfield, Reference Oldfield1971) and were recruited by notice or advertisement in the local press. Exclusion criteria were medical, neurological and psychiatric diseases or MRI contra-indications. No family history of mental illness or hereditary neurological disorders in first-degree relatives was reported. All participants gave written informed consent in accordance with the guidelines of the ethical standards of the Declaration of Helsinki. All procedures were approved by the local Ethical Review Board. On the day of scanning, participants completed the trait version of the German State-Trait Anxiety Inventory (STAI-T; Laux et al. Reference Laux, Glanzmann, Schaffner and Spielberger1981), a self-report scale to determine the level of trait anxiety. To ensure that the top of the range of trait anxiety scores did not represent individuals with undiagnosed anxiety disorders, standardized clinical assessment with the German version of the Structured Clinical Interview (SCID-I) was performed according to DSM-IV criteria (Wittchen et al. Reference Wittchen, Wunderlich, Gruschwitz and Zaudig1997). No evidence of anxiety disorders or any other current or previous axis I psychiatric disorder was found.
Experimental paradigm
In a differential conditioning paradigm, pictures of two different neutral male faces selected from the MacArthur–McDonnell face library (NimStim; Tottenham et al. Reference Tottenham, Borscheid, Ellertsen, Marcus and Nelson2002) served as conditioned stimuli (CS−, CS+). Stimuli were gray in color, presented for 2 s in the centre of a black screen. A pseudo-randomized order was used with the restrictions that (a) no more than two successive presentations of the same CS would occur and (b) the CSs were equally distributed within each half of the acquisition period. The two faces were counterbalanced as CS+ between participants. The US consisted of an acoustic white noise burst (duration 100 ms, 95 dB). The experiment was divided into four phases. During habituation, each CS was shown five times without US. Each of the two acquisition phases consisted of 15 CS−, 15 CS+ without US (CS+unpaired) and five CS+ with US (CS+paired) trails. In this 25% partial reinforcement schedule, the US co-terminated with the presentation of the CS+paired (delay conditioning). Subjects were not informed about this CS–US contingency. In the following extinction phase, 25 CS+unpaired and 25 CS– trials were presented. Interstimulus intervals ranged from 8.5 to 14.5 s, during which subjects had to look at a white fixation cross on a black screen. After each experimental phase, participants rated the valence and arousal of the CSs by means of a five-point Lickert scale, the Self-Assessment Manikin (SAM; Bradley & Lang, Reference Bradley and Lang1994), ranging from 0=‘very unpleasant’ to 4=‘very pleasant’ and 0=‘not arousing’ to 4=‘very arousing’. They responded by pressing the response buttons of an MRI-compatible response box with the right index and middle fingers. Prior to scanning, detailed task instructions were given and participants were familiarized with the task. Post-experimentally, participants were debriefed.
Image acquisition
MRI data were acquired in a 3-T whole-body scanner (Gyroscan Intera T 3.0, Philips, The Netherlands), equipped with master gradients (nominal gradient strength 30 mT/m, maximal slew rate 150 mT/m/ms). A circularly polarized transmit/receive birdcage head coil with a high-frequency (HF) reflecting screen at the cranial end of the scanner was used for spin excitation and resonance signal acquisition. Functional images were acquired during one fMRI run using a T2*-weighted single shot echo planar (EPI) sequence [echo time (TE) 32 ms, repetition time (TR) 2000 ms, flip angle 90°, slice thickness 3.6 mm without gap, matrix 64×64, field of view (FOV) 230 mm×230 mm, in-plane resolution 3.6 mm×3.6 mm]. In total, 825 image volumes of 30 transversal slices orientated parallel to the AC–PC line were acquired, resulting in a total scan time of 27 min. After the completion of the functional scans, a high-resolution three-dimensional (3D) T1-weighted structural scan (TE 3.4 ms, TR 7.4 ms, flip angle 9°, 320 0.5 mm sagittal slices, FOV 256 mm×256 mm, matrix 512×512 resulting in isotropic voxels with an edge length of 0.5 mm, scan duration 11:09 min) was acquired for anatomical localization.
Data analysis
Behavioral data
During fMRI scanning, responses of the CS arousal and valence ratings were recorded from all 32 subjects. A repeated-measures analysis of variance (ANOVA) with two within-subject factors phase (four levels: habituation, acquisition I, acquisition II, extinction) and stimulus (two levels: CS+unpaired, CS−) within the general linear model as implemented in SPSS version 15 (SPSS Inc., USA) was performed to validate the conditioning effect.
fMRI data
Analysis was performed using the Statistical Parametric Mapping software package version 5 (SPM5; Wellcome Department of Cognitive Neurology, London, UK; www.fil.ion.ucl.ac.uk/spm). Images for each subject were realigned, slice time acquisition corrected, normalized to the Montreal Neurological Institute (MNI) template, and resliced to a voxel size of 2 mm×2 mm×2 mm. Data were smoothed with an 8 mm kernel and subsequently filtered with a high-pass filter (cut-off period of 128 s). Event-related blood oxygen level-dependent (BOLD) responses were analyzed in SPM5 using the general linear model with a canonical hemodynamic response function as the basic function, and separately one regressor for each condition in each phase: CS+unpaired, CS+paired and CS−. Paired CS+ was modeled as one event and included as predictor of no interest in the regression analysis. The six movement parameters of the rigid body transformation determined during realignment were introduced as covariates into the model. To investigate differences between the phases of the conditioning and extinction procedure, the time courses of both conditioning and extinction were split into two subphases each. The first and second conditioning phases were separated by the SAM rating. Each phase consists of 35 trials and has the same length. The extinction section was also divided into two subphases of same length each lasting 5 min and containing 25 trials, referred to here as early and late extinction phases. In a first-level fixed-effects analysis, one statistical parametric map and corresponding contrast images for each subject reflecting the contrasts of interest were derived. The following contrasts of interest were computed: CS+unpaired>CS−, CS−>CS+unpaired for early and late acquisition and extinction phases. The individual contrast images were entered into a second-level random-effects t test to obtain activation maps across subjects. Based on our prior knowledge about the core areas involved in fear conditioning and extinction, summarized in our review article (Sehlmeyer et al. Reference Sehlmeyer, Schoning, Zwitserlood, Pfleiderer, Kircher, Arolt and Konrad2009), region of interest (ROI) analyses focused on the right and left amygdalae and the dACC. The dACC was defined as a sphere with a radius of 8 mm placed in the most posterior part of the ACC (center x=0, y=2, z=30) (Dannlowski et al. Reference Dannlowski, Ohrmann, Konrad, Domschke, Bauer, Kugel, Hohoff, Schoning, Kersting, Baune, Mortensen, Arolt, Zwitserlood, Deckert, Heindel and Suslow2009). The left and right amygdala ROIs were defined according to the AAL Atlas (Tzourio-Mazoyer et al. Reference Tzourio-Mazoyer, Landeau, Papathanassiou, Crivello, Etard, Delcroix, Mazoyer and Joliot2002). Mean contrast estimates were extracted for left and right amygdalae in each experimental phase. Linear regression analyses were calculated to determine the effect of trait anxiety on amygdala and dACC activations, during the acquisition and extinction phases. These anxiety scores were entered separately as a parametric variable in the analyses (MacCallum et al. Reference MacCallum, Zhang, Preacher and Rucker2002). To control for multiple statistical testing, we maintained a cluster-level false-positive detection rate at p<0.05 using a voxel threshold of p<0.05 with a cluster (k) extent determined empirically by Monte Carlo simulations. These were implemented in AlphaSim, which accounted for spatial correlations between BOLD signal changes in neighboring voxels (Forman et al. Reference Forman, Cohen, Fitzgerald, Eddy, Mintun and Noll1995) (48 voxels for each amygdala and 53 voxels for the dACC ROI). As an add-on, an analysis of whole-brain activation during conditioning and extinction was carried out to demonstrate that the ROI analysis did not ignore significant activations outside the ROIs. As the aim was to demonstrate that no relevant activations were ignored, a more liberal threshold of p<0.001 and k>10 voxels spatial extent was used.
Results
Behavioral data
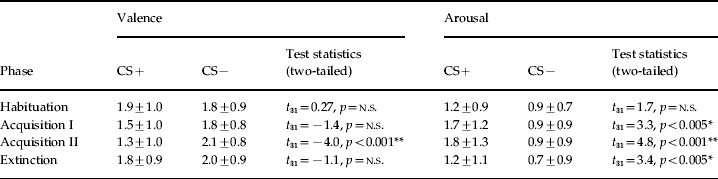
The mean trait anxiety score was 35.2 (s.d.=7.5), ranging from 22 to 52. The repeated-measures ANOVA yielded significant main effects for stimulus [F(3, 93)=6.99, p<0.001, ηp2=0.18] and phase [F(1, 31)=22.3, p<0.001, ηp2=0.42] and a significant stimulus×phase interaction [F(2.3, 71.6)=3.61, p<0.05, ηp2=0.1] for arousal ratings (for details see Table 1). Post-hoc tests revealed that arousal ratings were significantly higher for CS+unpaired than CS− after both acquisition phases (early: t 31=3.30, p<0.005; late: t 31=4.77, p<0.001) and after extinction (t 31=3.42, p<0.005). The repeated-measures ANOVA yielded a significant stimulus×phase interaction [F(2.3, 72.3)=5.7, p<0.005, ηp2=0.15] for valence ratings. As expected, valence ratings differed significantly between CS+unpaired and CS− after the late acquisition phase (t 31=−4.0, p<0.001). Correlation analysis revealed no significant interactions between trait anxiety and CS ratings.
Table 1. Mean ratings and statistical results for valence and arousal ratings of the CS+ and CS− after the experimental phases
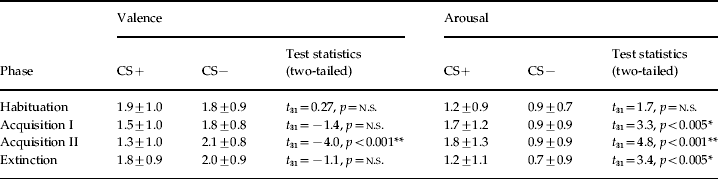
CS, Conditioned stimulus; n.s., not significant.
CS+ and CS− ratings given as means ± standard deviation.
* p<0.005, ** p<0.001.
fMRI data
Fear acquisition
During habituation, the BOLD signal did not differ significantly between CS+unpaired and CS−. During the late acquisition phase, ROI analyses yielded larger activation of the left (x=−30, y=−4, z=−22, t 31=3.27, k=96 voxels, p=0.0057 corrected) and right amygdalae (x=18, y=4, z=−18, t 31=2.79, k=48 voxels, p=0.0497 corrected) comparing the presentation of CS+unpaired to CS−. A significant increase in BOLD signal was observed in the dACC during early (x=2, y=−2, z=36, t 31=2.59, k=97 voxels, p=0.0073 corrected) and late conditioning (x=−4, y=−2, z=28, t 31=3.54, k=202 voxels, p<0.0001 corrected). No significant correlations with trait anxiety were found within the ROIs corresponding to the ACC or the amygdalae. Whole-brain analysis revealed conditioning-related (CS+unpaired>CS−) neural responses throughout the two acquisition phases outside the ROIs in the following regions: rostral ACC, bilateral insula, thalamus and striatum (p<0.001 uncorrected, k>10 voxels) (see Supplementary Table 1 and Supplementary Fig. 1, available online).

Fig. 1. ANOVA results for the amygdala regions of interest (ROIs). Mean contrast estimates (± standard error of the mean) (CS+unpaired >CS−) for left (L) and right (R) amygdala ROI for early and late acquisition and extinction phases are presented. The ANOVA revealed a significant phase (acquisition, extinction)×order (first, second half) interaction for right and left amygdalae (p's <0.05 corrected for multiple comparisons). Activation increased from the first to the second acquisition phase and decreased during the extinction phases.
Fear extinction
No significant activation of the amygdala or the dACC could be detected on comparing the presentation of CS+unpaired to CS− trails in any of the extinction phases. By contrast, CS− yielded significantly stronger activation of the left amygdala than CS+unpaired during the late extinction phase (x=−26, y=−2, z=−12, t 31=2.47, k=81 voxels, p=0.012 corrected), suggesting a deactivation of the amygdala during extinction. An additional full-factorial ANOVA with phase (two levels: acquisition, extinction) and order (two levels: first, second) as within-subject factors revealed a significant phase×order interaction (left amygdala: x=−28, y=−4, z=−12, t 124=2.59, k=85 voxels, p=0.0103 corrected; right amygdala: x=20, y=6, z=−18, t 124=2.32, k=63 voxels, p=0.0272 corrected). Amygdala activation increased from the first to the second acquisition phase, and decreased during the extinction phases. Contrast estimates of the amygdala activation interaction are shown in Fig. 1. Regression analysis revealed significant positive effects of trait anxiety on amygdala reactivity during the early extinction phase (left amygdala: x=−22, y=−8, z=−14, t 30=3.17, r=0.50, k=44 voxels, p=0.059 corrected; right amygdala: x=20, y=0, z=−16, t 30=3.25, r=0.51, k=114 voxels, p=0.003 corrected) (Figs 2 a, b and 3 a, b). A significant correlation was also found for the late extinction phase (left amygdala: x=−28, y=−4, z=−16, t 30=2.69, r=0.44, k=74 voxels, p=0.0173 corrected; right amygdala: x=24, y=−4, z=−18, t 30=2.19, r=0.37, k=18 voxels, p=0.2 corrected). In addition, significant negative effects of trait anxiety on dACC activity were observed during late extinction (x=4, y=−2, z=28, t 30=3.41, r=−0.53, k=143 voxels, p=0.001 corrected) (Fig. 3 c). Trait-anxious subjects showed reduced prefrontal activation during late extinction of conditioned responses. The whole-brain analysis of the extinction phase revealed additional bilateral activation outside the ROIs in the insular cortex and the supplementary motor area for CS+unpaired in contrast to CS− (p<0.001 uncorrected, k>10 voxels) (see Supplementary Table 1 and Supplementary Fig. 2, online).

Fig. 2. Coronal sections illustrating statistical parametric maps of significant positive relationships between trait anxiety and (a) activity within the left (L) amygdala (x=−22, y=−8, z=−14, t 30=3.17, r=0.50, p=0.002 uncorrected, k=44 voxels, p=0.059 corrected) and (b) activation of the right (R) amygdala during the early extinction of conditioned fear (x=20, y=0, z=−16, t 30=3.25, r=0.51, k=114 voxels, p=0.001 uncorrected, p=0.0030 corrected).

Fig. 3. Scatter plots showing correlations between individual's trait anxiety score and contrast estimates for the CS+unpaired presentation compared to CS− within the (a) left (L) amygdala (r=0.50) and (b) right (R) amygdala (r=0.51) during early extinction, (c) and the dorsal anterior cingulate cortex (dACC) (r=−0.53) during late extinction of conditioned fear.
Discussion
In the present study, we used fMRI during a cued fear-conditioning design to identify the neural mechanisms of fear learning, and to investigate whether these neural mechanisms are associated with an important personality trait: trait anxiety (Spielberger, Reference Spielberger1972). Analysis of the fMRI data revealed significant activation of the amygdala during the late acquisition phase and activation of the dACC during both acquisition phases. During extinction, deactivation of the amygdala was observed. In addition, whole-brain analysis yielded activation of fear-related brain areas such as the insula, striatum and rostral ACC during fear conditioning, and activation of the bilateral insula during extinction, as expected. Of interest, significant correlations between high trait anxiety, enhanced amygdala reactivity and decreased activation of the dACC were apparent during the extinction phase, suggesting that subjects with high trait anxiety show delayed and reduced extinction of conditioned responses.
Behavioral data
Behavioral data indicated successful cued conditioning. During acquisition, ratings of negative valence and arousal were increased significantly for CS+unpaired compared to CS−. Specifically, valence ratings were significantly different during the late acquisition phase whereas arousal ratings had already differed after the initial acquisition phase. We assume that the evaluation of arousal is more sensitive for conditioning effects than valence rating, which might be confounded by subjects' personal preference, or higher cognitive processes. After extinction, CS− and CS+unpaired ratings became equal.
Neuronal networks involved in fear conditioning and extinction
As expected, ROI analyses revealed larger bilateral activation of the amygdalae during the acquisition phase (CS+unpaired>CS−), in accordance with previous studies (LaBar et al. Reference LaBar, Gatenby, Gore, LeDoux and Phelps1998; Fischer et al. Reference Fischer, Andersson, Furmark and Fredrikson2000; Phelps et al. Reference Phelps, O'Connor, Gatenby, Gore, Grillon and Davis2001; Morris & Dolan, Reference Morris and Dolan2004; Schiller et al. Reference Schiller, Levy, Niv, LeDoux and Phelps2008). The only difference to these studies is the occurrence of amygdala activation during the late conditioning phase. The time course of amygdala activation observed here may be due to the small CS–US contingency rate of 25% used in this study, suggesting that the association between CS and US had only been established after a certain number of pairings (Phelps et al. Reference Phelps, Delgado, Nearing and LeDoux2004; Barrett & Armony, Reference Barrett and Armony2009). This pairing rate was used in our study to deliberately avoid habituation effects occurring before the extinction phase. Furthermore, the lower aversiveness of the tone compared to the electrical stimulation presented in most studies may also have contributed to the delayed amygdala response. Consistent with prior studies on human fear conditioning and extinction, the presentation of CS+unpaired compared to CS– during fear conditioning elicited whole-brain activation of fear-related brain areas, such as the insula, ACC and striatum. These areas are known to be involved in emotional processing and are regarded as key areas of pain processing and classical fear conditioning (for review see Sehlmeyer et al. Reference Sehlmeyer, Schoning, Zwitserlood, Pfleiderer, Kircher, Arolt and Konrad2009).
During extinction, the main conditioning effect (CS+unpaired>CS–) was associated with a deactivation of the amygdala. In particular, we found a reversal response in the amygdala, such that the activation was greater for CS– than for CS+unpaired during the late extinction phase. Outside the ROI, activations of the bilateral insulae and the supplementary motor cortex were detected. These results are also in line with those of other neuroimaging studies (Buchel & Dolan, Reference Buchel and Dolan2000; Phelps et al. Reference Phelps, Delgado, Nearing and LeDoux2004). The supplementary motor area (SMA) is a key structure for both preparation and execution of movements (Remy et al. Reference Remy, Zilbovicius, Leroy-Willig, Syrota and Samson1994; Johnson et al. Reference Johnson, Cunnington, Iansek, Bradshaw, Georgiou and Chiu2001; Wang et al. Reference Wang, Zhu, Li and Weng2007). Activation within the extinction phase may reflect a preparatory action in response to the formerly conditioned stimulus, in terms of avoiding or escaping from the threatening stimulation. The insular cortex is assumed to process emotional contents, such as fear (Phelps et al. Reference Phelps, O'Connor, Gatenby, Gore, Grillon and Davis2001) or pain (Ploghaus et al. Reference Ploghaus, Tracey, Gati, Clare, Menon, Matthews and Rawlins1999; Ostrowsky et al. Reference Ostrowsky, Magnin, Ryvlin, Isnard, Guenot and Mauguiere2002; Lopez-Sola et al. Reference Lopez-Sola, Pujol, Hernandez-Ribas, Harrison, Ortiz, Soriano-Mas, Deus, Menchon, Vallejo and Cardoner2010). Moreover, the insula is recruited in the context of uncertainty and in anticipation of aversive events (Carlson et al. Reference Carlson, Greenberg, Rubin and Mujica-Parodi2010; Sarinopoulos et al. Reference Sarinopoulos, Grupe, Mackiewicz, Herrington, Lor, Steege and Nitschke2010), which is particularly the case during the extinction phase. To conclude, our data support the development of activity within the amygdala over the course of conditioning and the decline of amygdala activation during extinction. Surprisingly, we did not observe significant activation within the ROI of the dACC for the main conditioning effect (CS+unpaired>CS–) in any of the extinction phases, although it is preferentially engaged during the inhibition of conditioned responses (Phelps et al. Reference Phelps, Delgado, Nearing and LeDoux2004; Lang et al. Reference Lang, Kroll, Lipinski, Wessa, Ridder, Christmann, Schad and Flor2009).
Influence of trait anxiety
Significant correlations of trait-anxiety scores with amygdala and dACC activation were revealed. Although the acquisition phases were unaffected by this personality trait, higher levels of trait anxiety were associated with greater sustained conditioned amygdala activation, or rather with less amygdala deactivation (r=0.50), mainly during early extinction. These results are remarkably consistent with earlier studies on healthy subjects and anxiety patients that reported a positive interaction of amygdala responses with anxiety during the processing of fearful stimuli (Bishop et al. Reference Bishop, Duncan and Lawrence2004; Etkin et al. Reference Etkin, Klemenhagen, Dudman, Rogan, Hen, Kandel and Hirsch2004; Dickie & Armony, Reference Dickie and Armony2008), during fear learning (Bremner et al. Reference Bremner, Vermetten, Schmahl, Vaccarino, Vythilingam, Afzal, Grillon and Charney2005; Hooker et al. Reference Hooker, Verosky, Miyakawa, Knight and D'Esposito2008) and fear extinction (Barrett & Armony, Reference Barrett and Armony2009).
Moreover, we were able to show that high levels of trait anxiety are also strongly associated with decreased activation of the dACC, the cognitive part of the ACC (Bush et al. Reference Bush, Luu and Posner2000), during late extinction (r=−0.53). At first glance, this finding seems counter-intuitive as we did not observe activation of the dACC in any of the extinction phases. However, regression analysis yielded a significant correlation between dACC activation and trait anxiety. This finding implies that, without controlling for personality, the dACC is not equally engaged across all subjects to extinguish fear responses. The dACC is instead engaged as a function of trait anxiety. Hence, we conclude that involvement of the dACC during the extinction of fear is modulated by differences in trait anxiety. We assume that the hypo-activation of the dACC reported for anxious subjects results in a deficient inhibition of conditioned amygdala responses, additionally prolonging extinction and exaggerating fear responses. These findings are consistent with previous neuroimaging studies that reported an association of anxiety traits, pathological anxiety and activation of the PFC/ACC during fear extinction (Bremner et al. Reference Bremner, Vermetten, Schmahl, Vaccarino, Vythilingam, Afzal, Grillon and Charney2005; Rauch et al. Reference Rauch, Milad, Orr, Quinn, Fischl and Pitman2005, Reference Rauch, Shin and Phelps2006). Nevertheless, our study extends the current literature showing that high levels of trait anxiety are associated with both increased amygdala activity and reduced activation of the ACC during the process of extinction. In particular, our results partly confirm and expand the recent findings of Barrett & Armony (Reference Barrett and Armony2009). Even though different conditioning designs were used, the findings seem to converge on trait anxiety correlating with the amygdala and modulating fear extinction rather than fear conditioning. However, the results vary in the areas of the ACC examined. Whereas Barrett & Armony (Reference Barrett and Armony2009) observed a relationship between trait anxiety and enhanced subgenual ACC activation, we were able to show an association of trait anxiety and reduced dACC activation, as expected. Our data show that this strong and significant relationship was found exclusively during the late extinction phase. The correlation observed during early extinction failed to show significance. Besides this problem with statistical significance, we assume that activation of the dACC is decreased during the extinction process in anxious subjects, leading to the strong negative correlation during late extinction. This suggests that high and low anxious persons do not differ with respect to the dACC activation at early stages of extinction, but at a later point of time. We suppose that trait-anxious subjects are not able to maintain activation of the inhibitory dACC during the extinction process, which may lead to enhanced vulnerability and risk for relapse.
Taken together, subjects characterized by enhanced trait anxiety show deficits in the extinction of acquired fear. This is reflected not only by sustained amygdala activation during early extinction but also by additional decreased dACC activation during late extinction. Highly trait-anxious subjects fail to adapt to altering circumstances and maintain their anticipatory anxiety even when threat-related stimuli (US) are absent (Chan & Lovibond, Reference Chan and Lovibond1996), as is the case during extinction. Therefore, we assume that the most prominent feature separating high and low anxious subjects may not be conditionability, but the ability to extinguish conditioned responses.
Our simple and robust paradigm has revealed important findings for the understanding of the relationship between personality and neurobiological vulnerability in the development of anxiety disorders. We have shown that anxious subjects are characterized by both amygdala hyper-activation and dACC hypo-activation. This double impact of increased amygdala reactivity and deficient cognitive control may represent enhanced risk for spontaneous recovery of the extinguished response and pathophysiologically for relapse in anxiety disorders.
Limitations
A few limitations should be pointed out in the current study. First, in accordance with previous studies (Lissek & Powers, Reference Lissek and Powers2003; LaRowe et al. Reference LaRowe, Patrick, Curtin and Kline2006), we used an acoustic, brief and loud noise as unconditioned stimulus to startle subjects. As we did not conduct a priori aversiveness rating of the US, we cannot rule out that the tone was low aversive and hence caused the observed delayed amygdala response during the acquisition phase. Second, consistent with most studies, we used the subjective measure of valence and arousal only at the end of the experimental blocks (Gottfried & Dolan, Reference Gottfried and Dolan2004; Straube et al. Reference Straube, Weiss, Mentzel and Miltner2007). The application of a continuous measure would have improved the investigation of conditioning-related changes in CS− ratings. However, we did use only neutral male faces as conditioned stimuli instead of both female and male faces.
Conclusions
Our study reveals, for the first time, that deficits in the extinction of conditioned fear responses in highly trait-anxious subjects are not only associated with enhanced amygdala reactivity but also related to reduced dACC activation, providing a neurobiological correlate for trait anxiety. Therefore, our findings help to elucidate the enhanced neurobiological vulnerability of anxious subjects to develop and maintain an anxiety disorder.
Note
Supplementary material accompanies this paper on the Journal's website (http://journals.cambridge.org/psm).
Acknowledgements
This work was supported by a young investigator grant to C.K. by the Interdisciplinary Center for Clinical Research of the University of Muenster, Germany (IZKF). C.S. was supported by the German Ministry of Science (Bundesministerium für Bildung und Forschung, BMBF Paniknetz, ‘Improving CBT for panic by identifying the active ingredients and understanding the mechanisms of action: a multicenter trial’) and by a grant from the University of Muenster.
Declaration of Interest
None.