Introduction
Self-relevant stimuli are usually better remembered than other-relevant stimuli; this is known as the self-referential memory (SRM) effect (Rogers et al. Reference Rogers, Kuiper and Kirker1977). Research has demonstrated that the occurrence of the SRM effect may depend on the intact ability of self-reflection (Kelley et al. Reference Kelley, Macrae, Wyland, Caglar, Inati and Heatherton2002). Self-reflection (or self-referential processing) refers to a conscious process in which an individual makes a decision regarding themselves (van der Meer et al. Reference van der Meer, Costafreda, Aleman and David2010). Self-reflection is the core component of self-awareness and social cognition (Harvey et al. Reference Harvey, Lee, Horan, Ochsner and Green2011; Shad et al. Reference Shad, Brent and Keshavan2011) and is crucial for the consolidation of memory (Rogers et al. Reference Rogers, Kuiper and Kirker1977) and adaptive functioning in the social realm (Philippi et al. Reference Philippi, Duff, Denburg, Tranel and Rudrauf2012). Furthermore, functional magnetic resonance imaging (fMRI) studies in normal subjects have demonstrated that self-referential processing is associated with cortical midline structures, which are mainly composed of the medial prefrontal cortex (MPFC), anterior cingulated cortex and posterior cingulated cortex (Macrae et al. Reference Macrae, Moran, Heatherton, Banfield and Kelley2004; van der Meer et al. Reference van der Meer, Costafreda, Aleman and David2010; Philippi et al. Reference Philippi, Duff, Denburg, Tranel and Rudrauf2012).
Studies on normal human adults have consistently found that trait adjectives are better remembered in the self-reflection condition than in the other-reflection condition (Symons & Johnson, Reference Symons and Johnson1997). However, a recent study by Harvey et al. (Reference Harvey, Lee, Horan, Ochsner and Green2011) found that schizophrenia patients do not benefit from the SRM bias. Impairments in self-reflection and related neural networks are relevant to the expression of psychosis (Modinos et al. Reference Modinos, Renken, Ormel and Aleman2011) and lack of illness awareness (van der Meer et al. Reference van der Meer, Costafreda, Aleman and David2010). In addition, the performance of self-referential processing can be used to predict the quality of life in schizophrenia (Lysaker et al. Reference Lysaker, Dimaggio, Carcione, Procacci, Buck, Davis and Nicolo2010). However, to our knowledge, very few brain imaging studies have investigated the neuronal substrates underlying self-reflection abnormalities in schizophrenia and no consistent results have been obtained (Blackwood et al. Reference Blackwood, Bentall, Ffytche, Simmons, Murray and Howard2004; Murphy et al. Reference Murphy, Brent, Benton, Pruitt, Diwadkar, Rajarethinam and Keshavan2010; Holt et al. Reference Holt, Cassidy, Andrews-Hanna, Lee, Coombs, Goff, Gabrieli and Moran2011; Shad et al. Reference Shad, Keshavan, Steinberg, Mihalakos, Thomas, Motes, Soares and Tamminga2012). Some fMRI studies found group activation differences (schizophrenia versus control) in both the anterior and posterior cortical midline structures in response to self-reflection stimuli (Blackwood et al. Reference Blackwood, Bentall, Ffytche, Simmons, Murray and Howard2004; Holt et al. Reference Holt, Cassidy, Andrews-Hanna, Lee, Coombs, Goff, Gabrieli and Moran2011) whereas others only found abnormal activation of posterior cortical midline structures (Shad et al. Reference Shad, Keshavan, Steinberg, Mihalakos, Thomas, Motes, Soares and Tamminga2012) or did not observe any abnormal activation of cortical midline structures during self-reflection processing (Murphy et al. Reference Murphy, Brent, Benton, Pruitt, Diwadkar, Rajarethinam and Keshavan2010).
A few studies have focused on the SRM effect of schizophrenia using an electrophysiological method. Compared with fMRI, an event-related potential (ERP) technique is an excellent method to evaluate the time course of cognitive processing with a high time resolution. Many studies on normal subjects have found ERP correlates of self-related processing. In general, self-referential stimuli (e.g. own face, own name) elicited more positive-going ERP components of N2, P2, P3, and the late positive component (LPC) (Keyes et al. Reference Keyes, Brady, Reilly and Foxe2010; Tacikowski & Nowicka, Reference Tacikowski and Nowicka2010; Chen et al. Reference Chen, Yuan, Feng, Chen, Gu and Li2011). For example, Chen et al. (Reference Chen, Weng, Yuan, Lei, Qiu, Yao and Li2008) explored the temporal features of self-referential processing evoked by handwriting; significant ERP differences were found between own and other handwriting conditions in the time windows 200–500 ms (N2 and P3 components) and 1000–2000 ms (LPC). Similarly, Su et al. (2010) observed that own hand elicited more positive components than did other hand in the time window 350–600 ms (P3 and LPC).
Previous ERP studies on normal subjects have provided a good basis for exploring the temporal features of impaired self-referential processing in schizophrenia patients. However, as far as we know, only one study (Silva et al. Reference Silva, Torres and Ortiz2008) has examined the electrophysiological differences between paranoid schizophrenia (n = 8) and normal controls (n = 7). The study of Silva et al. (Reference Silva, Torres and Ortiz2008) suggested that the early modulation of word-related meaning creation in self-reflective processing was impaired in schizophrenia. In particular, they found that, compared with normal controls, schizophrenia patients had lower P2 amplitudes in the self-reflection condition. They suggested that the abnormal electrophysiological activity occurring at approximately 200 ms post-stimulus might contribute to the impairment of self-reflective processing in schizophrenia patients. However, extrapolation of these results may be limited because the study only focused on paranoid schizophrenia patients and involved a relatively small number of subjects.
The present study used a high-time-resolution ERP technique to compare the electrophysiological differences in activation between 20 schizophrenia patients and 22 controls during self-reflection processing and investigated the neural basis of the abnormal SRM effect in schizophrenia. We hypothesized that schizophrenia patients would fail to show the SRM effect in behavior measures and that the group differences in ERP data would be observed in both early (P2/N2) and later stages (P3/LPC) of self-referential processing.
Method
Subjects
Twenty-one in-patients of Beijing Huilongguan Hospital and 22 normal controls from surrounding community were recruited as paid participants.
Patients were diagnosed according to DSM-IV (APA, 1994) criteria for schizophrenia. The diagnosis was made by one psychiatrist and confirmed by another senior psychiatrist. Patients with schizo-affective disorder, schizotypal or schizoid personality disorder were excluded. None of the patients were in a major depressive or manic episode at the time of testing. Additional exclusion criteria for patients included: (1) history of significant brain trauma, (2) neurological disorder, (3) substance abuse or dependence in the past 6 months, (4) mental retardation (IQ < 70 based on medical records), (5) insufficient fluency in Chinese, and (6) electroconvulsive therapy in the past 6 months. All patients were receiving stable medication treatments (no medication changes) for at least 1 month before the experiment. Patients' psychopathology was assessed using the Positive and Negative Syndrome Scale (PANSS; Kay et al. Reference Kay, Fiszbein and Opler1987). Patients went to the laboratory to take part in the experiment at the appointment time. Twenty patients (eight with paranoid schizophrenia and 12 with undifferentiated schizophrenia) completed the experiment successfully; one patient could not tolerate the ERP cap and declined the experiment.
Control participants were recruited from the surrounding community through poster advertisements. Healthy control participants were screened with SCID-I/NP (First et al. Reference First, Gibbon, Spitzer and Williams2002) and SCID-II (First et al. Reference First, Spitzer, Gibbon, Williams and Benjamin1996). Exclusion criteria for control participants were (1) any lifetime Axis I psychotic or mood disorders, (2) recurrent depression, (3) paranoid, schizotypal or schizoid personality disorder, (4) seizure disorder, (5) history of head injury with possible neurological sequelae, (6) the presence of a first-degree relative with schizophrenia, and (7) substance abuse or dependence in the past 6 months.
The interview and the clinical symptom rating were performed by two senior psychiatrists (C.S. and J.C.). The two raters were trained in the Center for Psychiatric Research of Beijing Huilongguan Hospital and showed high reliability (κ = 0.82).
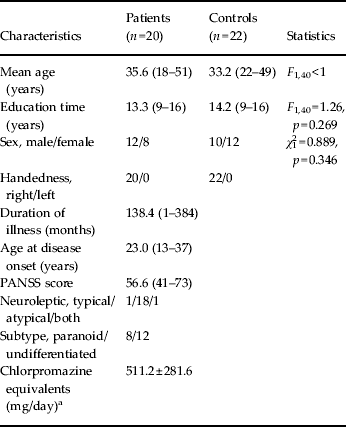
There was no significant difference between the two groups with respect to age, handedness and education (Table 1). All subjects had normal or corrected-to-normal vision. Participants were told about the objectives and content of the experiment by the ward director. Written informed consent was obtained prior to the experiment. The experimental protocol was approved by the local ethics committee (Beijing Huilongguan Hospital) and was in compliance with the ethical guidelines of the American Psychological Association (APA).
Table 1. Demographic and clinical data for patient and control groups

PANSS, Positive and Negative Syndrome Scale.
Descriptive data are presented as mean (range) or mean ± standard deviation.
a According to Woods (Reference Woods2003).
Stimuli
The experiment was performed as described in Mu & Han (Reference Mu and Han2010) with slight modifications. The SRM task was divided into two phases: an encoding phase and a recognition phase. During the encoding/learning phase, subjects performed judgment tasks in three blocks (associated with three experimental conditions). In the self-reflection block, participants judged whether each personality-trait adjective was appropriate to describe themselves. In the other-reflection block, subjects judged whether each word was appropriate to describe a familiar person (i.e. Jintao Hu, Chairman of the People's Republic of China from 2003 to 2012). In the font block, subjects were asked to judge the font of the trait adjectives (bold or not).
A total of 310 personality-trait adjectives (155 positive and 155 negative adjectives) were selected from Yang & Wang's Personality Trait Adjective List (Yang & Wang, Reference Yang, Wang, Wang and Cui1999). All the words were composed of two Chinese characters. A total of 210 words were used in the encoding phase (behavioral and ERP data were recorded), and these 210 words plus an additional 100 new words were used in the recognition phase (studied using behavioral measurements). There were 70 words (35 positive and 35 negative words) in each condition of the encoding phase. There were no significant differences in familiarity (F 5,204 < 1) and strokes (F 5,204 < 1) across conditions (positive/negative self-reflection, positive/negative other-reflection, and positive/negative font-judgment). The font of the characters was Song Ti No. 48. All stimuli were presented in the center of the screen and in black color on a gray background with the same contrast and brightness.
Experimental procedure
Encoding phase
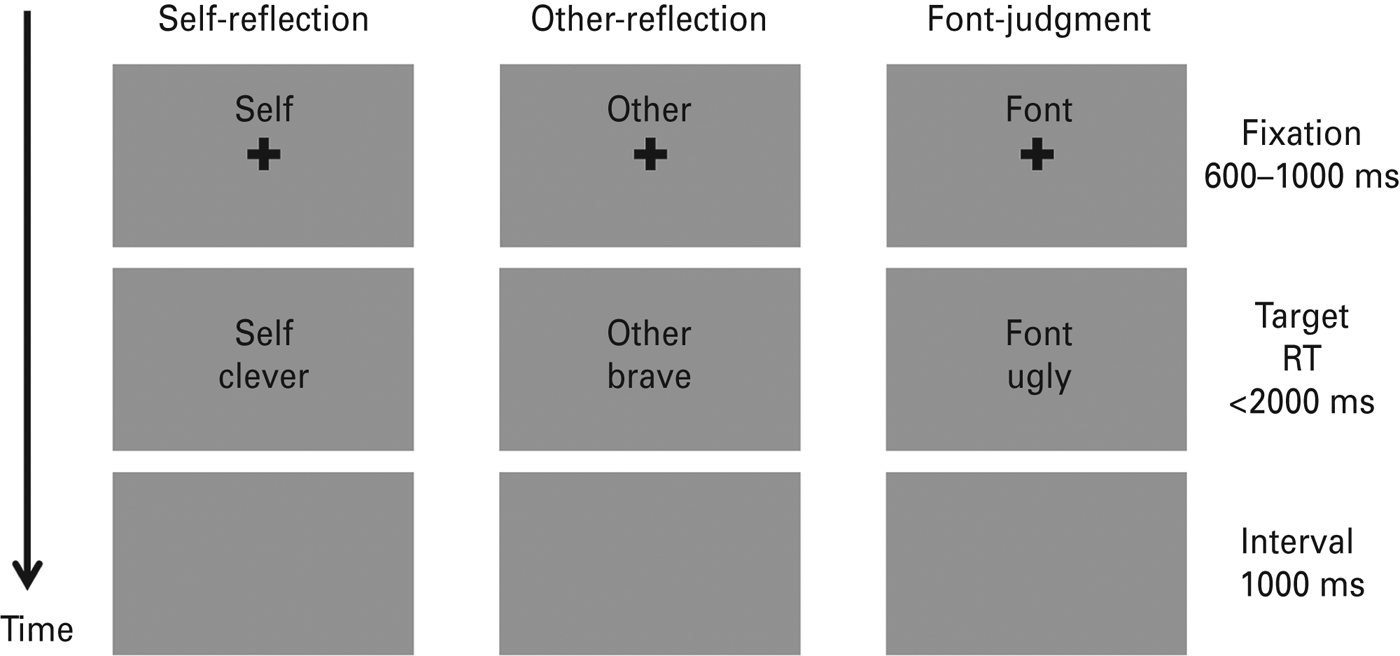
The experimental procedure of the encoding phase is illustrated in Fig. 1. Subjects were seated in a sound-proof room with their eyes approximately 90 cm from a 17-in screen. An instruction was given before each block (e.g. ‘In the following task, please judge whether the word is appropriate to describe yourself’). As shown in Fig. 1, each trial started with a 600–1000 ms fixation cross (0.67° × 0.67° visual angle) followed by a trait adjective (2.39° × 1.43° visual angle) with a maximum duration of 2000 ms. Subjects were required to respond to the associated question as quickly and as accurately as possible by pressing the button on the response box with their left and right index fingers (‘yes’ – ‘left’ and ‘no’ – ‘right’, or reversed). The left-/rightness of the responses was counterbalanced across subjects. The target word disappeared when the subject indicated their response. Subjects would then be led into the next stimulus series after a 1000-ms period during which the screen remained gray and blank. During each trial a small cue word (‘self’, ‘other’ or ‘font’ in Chinese; 1.15° × 0.48° visual angle) remained in the upper part of the screen to remind the participant of the task in the block. The sequence of 70 trials in each block during the encoding phase was randomized. The order of task conditions was counterbalanced across subjects using a Latin Square design.
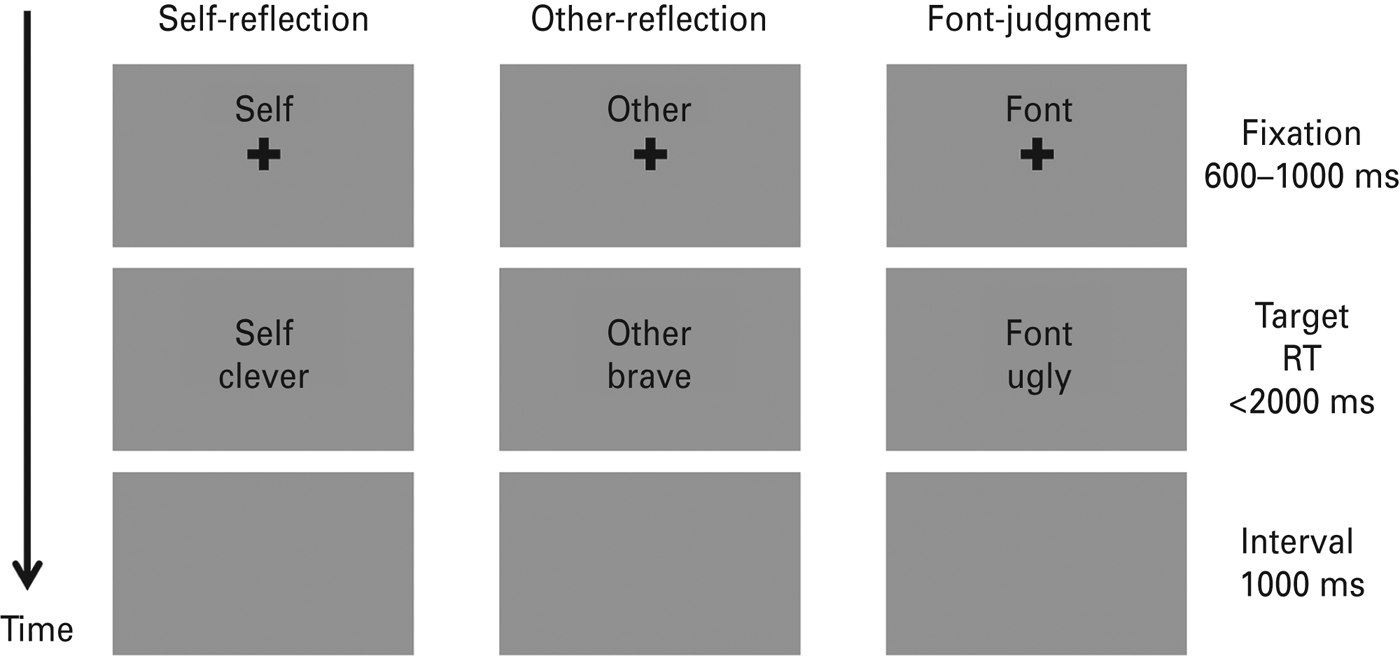
Fig. 1. Schematic diagram of three experimental trials in the encoding phase. Each column represents a trial in one experimental block. RT, Response time. Both the cues and trait adjectives were in Chinese.
Recognition phase
Participants watched an irrelevant movie for 40 min after the encoding phase. Then, they were asked to finish an unexpected recognition task. All of the 310 words (2.39° × 1.43° visual angle) were used in this phase. The trial sequence was randomized. Participants were required to answer whether the word was presented in the encoding phase. There was no time limit for the response. The word disappeared until subjects made a choice. Then next trial began after an interval of 800–1600 ms.
Behavioral measures and statistics
Stimulus display and behavioral data acquisition were conducted using E-Prime software (Version 2.0, Psychology Software Tools, Inc., USA). The encoding phase was mainly measured by the reaction time (RT). The recognition phase was mainly measured by the recognition score, which was defined as the proportion of hits minus the proportion of false alarms in each condition (Kelley et al. Reference Kelley, Macrae, Wyland, Caglar, Inati and Heatherton2002; Mu & Han, Reference Mu and Han2010; Philippi et al. Reference Philippi, Duff, Denburg, Tranel and Rudrauf2012). To obtain a comprehensive measurement of the SRM effect, we defined the SRM bias score as the differential recognition score between self- and other-reflection conditions.
For all the analyses in this study, the significance level was set at 0.05. An analysis of variance (ANOVA) and an independent-samples t test were conducted for behavioral measurements. Eta-squared (η 2) was reported to demonstrate the effect size in ANOVAs, where 0.01 represents a small effect, 0.06 a medium effect and 0.14 a large effect. For the sake of brevity, effects that did not reach significance have been omitted.
Electroencephalogram (EEG) recording and preprocessing
Brain electrical activity during the encoding phase was recorded referentially against the left mastoid and re-referenced offline to averaged mastoids, using a 64-channel amplifier with a standard 10–20 system (Brain Products, Germany). In addition to referential and electro-oculogram electrodes, data from a 57-channel EEG system were collected with electrode impedances kept below 5 kΩ. EEG signals were sampled continuously at 500 Hz and filtered within 0.01–100 Hz.
The data analyses in this study were performed using Brain Products Analyzer 2.0 (Brain Products), Matlab R2011a (MathWorks, USA) and SPSS Statistics 17.0 (IBM, USA). The recorded EEG data were downsampled to 250 Hz and bandpass filtered with a 0.1–30-Hz finite impulse response filter with zero phase distortion. EEG segments containing large line noises and myoelectricity were manually rejected. Eye blinks and lateral eye movements were removed using independent component analysis, performed with EEGLAB 11.0.2.1b, a freely available Matlab toolbox developed by Delorme & Makeig (Reference Delorme and Makeig2004). The runica algorithm of EEGLAB was used on a subject-to-subject basis as an implementation of extended infomax independent component analysis to obtain 57 independent components from each of 42 datasets. Independent components accounting for eye blinks and lateral eye movements were identified visually according to their scalp maps, component activations and power spectra. These independent components were removed from each dataset. The non-rejection independent components were back-projected to reconstruct channel EEG data without an electro-oculogram.
An ERP is the evoked electrical brain activity elicited by external or internal stimuli. It reflects electrocortical activity at a high temporal resolution and is commonly used in cognitive neuroscience as a powerful tool for tracking and timing the dynamic neural activity during psychophysiological processes. When analyzing ERP signals, the spontaneous ongoing EEG is usually treated as background activity or noise. Under this assumption, stimulus-locked averaging is applied prior to further analysis to increase the signal-to-noise ratio of the ERP data. Refer to Luck (Reference Luck2005) and Picton et al. (Reference Picton, Bentin, Berg, Donchin, Hillyard, Johnson, Miller, Ritter, Ruchkin, Rugg and Taylor2000) for more details of the ERP technique. In this study, cleared data were segmented in association with the personality-trait adjectives in each trial, beginning 200 ms prior to the stimulus onset and lasting for 1700 ms. Epochs were baseline corrected with respect to the mean voltage over the 200 ms preceding stimulus onset.
ERP analysis and statistics
The ERP epochs were averaged separately in three conditions and two groups. As a result, four components (P2, N2, P3 and the positive slow wave) showed different amplitudes across task conditions and/or groups. In this study we analyzed the four ERP components across different sets of electrodes according to both the ERP topographies and relevant literature (Chen et al. Reference Chen, Weng, Yuan, Lei, Qiu, Yao and Li2008; Silva et al. Reference Silva, Torres and Ortiz2008; Su et al. Reference Su, Chen, Yin, Qiu, Lv, Wei, Tian, Tu and Wang2010). The peak amplitude and the peak latency of the P2 component were calculated at F5 and F6 electrode sites (peak detection window 160–260 ms). The peak amplitude and the peak latency of the N2 component were calculated at AF3, AF4, F3 and F4 electrode sites (peak detection window 270–380 ms). The P3, Pz and P4 electrode sites were selected for statistical analysis of the average amplitude of the P3 component (component window 350–600 ms). The average amplitude of the positive slow wave was analyzed at AF3, AF4, F3 and F4 electrode sites in a time window of 800–1200 ms.
A three-way ANOVA was conducted for each ERP component with group (patient/control) as the between-subjects factor, and task condition (self-reflection, other-reflection and font judgment) and electrode sites as within-subjects factors. Statistical results were corrected using the Greenhouse–Geisser correction. In addition, correlation analysis between ERP measurements and the SRM bias score was performed using a two-tailed Pearson correlation test. Correction for multiple comparisons was based on Holm's stepwise correction. For the sake of brevity, effects that did not reach significance were omitted.
Results
Behavior results
Encoding phase
A two-way ANOVA for the participants' RT was conducted with group (patients/controls) as the between-subjects factor and task condition (self/other/font) as the within-subjects factor. The RT exhibited a significant main effect of task condition (F 2,80 = 124, p < 0.001, η 2 = 0.527). For all subjects, the RT at the font-judgment condition (mean ± s.d. = 645 ± 13.3 ms) was shorter than that at the other two conditions (self-reflection = 1011 ± 21.8 ms; other-reflection = 1028 ± 27.8 ms) (p's <0.001). Moreover, RTs showed a significant main effect of group effect (F 1,40 = 23.7, p < 0.001, η 2 = 0.372). Patients responded more slowly than controls throughout the experiment (967 ± 21.6 v. 822 ± 20.6 ms).
The independent-samples t test showed that (1) the proportion of ‘yes’ answers in the self-reflection condition was not significantly different between the two groups (t 40 = 1.32, p = 0.196; patients = 55.0 ± 13.6%, controls = 50.6 ± 5.5%) and (2) there was no significant difference in accuracy between patients (96.4 ± 7.8%) and controls (98.6 ± 2.5%) in the font-judgment task (t 40 = –1.23, p = 0.227).
Recognition phase
A two-way ANOVA with the factors of task condition (self/other/font) and group for the recognition scores revealed a significant interaction effect (F 2,80 = 9.81, p < 0.001, η 2 = 0.142) (Fig. 2 a). Simple effects analyses found that, compared with the controls (0.47 ± 0.15), the patients had lower recognition scores in the self-reflection condition (0.35 ± 0.18, p = 0.021) whereas there were no significant difference between the two groups in the other-reflection (p = 0.807) or font-judgment condition (p = 0.801). Moreover, the control group had higher recognition scores in the self-reflection condition than in the other-reflection condition (0.34 ± 0.13, p < 0.001), reflecting a reliable SRM effect. The same SRM effect was not found in the patient group (p = 0.451). Moreover, the independent-samples t test showed that the control group (0.13 ± 0.08) had higher SRM bias scores than the patient group (0.03 ± 0.08, t 40 = –4.32, p < 0.001) (Fig. 2 b).

Fig. 2. Behavior results of the recognition phase. (a) Recognition scores of patients and controls in the self-reflection, other-reflection and font-judgment conditions. (b) The self-referential memory (SRM) bias scores of patients and controls. Error bars correspond to one standard error.
ERP results
P2
The latency and the amplitude of the P2 component were submitted separately to a 2 × 2 × 3 ANOVA with group as the between-subjects factor and with electrode site (F5/F6) and task condition (self/other/font) as within-subjects factors (Fig. 3 a). The P2 latency demonstrated a significant main effect of group (F 1,40 = 4.13, p = 0.049, η 2 = 0.094). The P2 latencies were longer in the controls (224 ± 5.8 ms) than in the patients (207 ± 6.1 ms). The P2 amplitude revealed a significant main effect of group (F 1,40 = 6.94, p = 0.012, η 2 = 0.148); it was smaller in patients (6.63 ± 0.62 μV) than in controls (8.88 ± 0.59 μV).

Fig. 3. Comparison of grand mean event-related potentials (ERPs) between patients and controls. (a) The P2 component at electrode site F5. (b) The positive slow wave at electrode site AF4.
N2
The latency and the amplitude of the N2 component were submitted separately to a 2 × 4 × 3 ANOVA with group as between-subjects factor and with electrode site (AF3/AF4/F3/F4) and task condition as within-subjects factors. A significant main effect of group was observed in N2 latencies (F 1,40 = 7.53, p = 0.009, η 2 = 0.158), with a longer latency in controls (339 ± 5.0 ms) than in patients (319 ± 5.3 ms). The ANOVA for the N2 amplitudes showed a significant main effect of task condition (F 2,80 = 6.96, p = 0.003, η 2 = 0.087) and a significant interaction effect of task by group (F 2,80 = 4.56, p = 0.018, η 2 = 0.102) (Fig. 4 a). Simple effects analysis demonstrated that self-reflection elicited a more negative N2 component than other-reflection did in patients (p = 0.009) whereas there was no significant N2 amplitude difference between the font-judgment and self-reflection conditions (p = 0.169) or between the font-judgment and other-reflection conditions (p = 1.000) in patients. However, the N2 amplitudes in the control group showed a different pattern: the N2 amplitude elicited by self-reflection was comparable to that elicited by other-reflection (p = 1.000) whereas font-judgment elicited a less negative N2 than did the other two conditions (p = 0.001 for self-reflection; p = 0.048 for other-reflection).

Fig. 4. Comparison of grand mean event-related potentials (ERPs) between font-judgment and self/other-reflection conditions. (a) The N2 component at electrode site AF4. (b) The P3 component at electrode site Pz.
P3
The mean amplitude of P3 was analyzed at the electrode sites of P3, Pz and P4. The 2 × 3 × 3 ANOVA revealed a significant main effect of task condition (F 2,80 = 48.5, p < 0.001, η 2 = 0.393) (Fig. 4 b). Compared with the other-reflection task (3.11 ± 0.42 μV), self-reflection (3.67 ± 0.40 μV, p = 0.003) evoked larger P3 amplitudes; compared with the self- and other-reflection conditions, the font-judgment task (5.98 ± 0.50 μV) evoked lager P3 amplitudes (p's <0.001). The main effect of group was not significant (F 1,40 = 1.04, p = 0.315, η 2 = 0.025; patient group = 3.84 ± 3.58 μV, control group = 4.66 ± 1.78 μV).
Positive slow wave
The mean amplitude of the positive slow wave was analyzed at the electrode sites AF3, AF4, F3 and F4. The three-way ANOVA indicated a significant main effect of group (F 1,40 = 7.88, p = 0.008, η 2 = 0.165), which showed that the component amplitudes were smaller in patients (1.04 ± 0.53 μV) than in normal controls (3.08 ± 0.50 μV) (Fig. 3 b).
Correlation between ERP components and SRM bias
In the present study, we used the SRM bias score (i.e. the difference in recognition scores between self- and other-reflection conditions) to quantify the SRM effect at the behavioral level. In this subsection, correlations between ERP measurements and the SRM bias score were explored. The results show that the SRM bias score in the patient group correlated significantly with the P3 amplitudes elicited by the self-reflection task in the parietal cortex (more prominent in the right hemisphere) (r 20 = –0.688, p = 0.006 at electrode site P4). No significant correlation was found between the ERP components and the SRM bias score in the control group.
Diagnostic subtype, medications, age of illness onset and duration of illness
Diagnostic subtype
To examine the self-reflective impairments in patients with different schizophrenia subtypes, the behavioral/ERP data for paranoid schizophrenia, undifferentiated schizophrenia and the controls were analyzed comparatively. There were no significant differences across the three groups with respect to age (F 2,39 = 1.40, p = 0.258), duration of education (F 2,39 < 1) and gender (χ 2 2 = 1.42, p = 0.491).
A significant interaction effect of group by task condition was found in the recognition scores (F 4,78 = 5.33, p < 0.001). A simple effects analysis indicated that patients with paranoid schizophrenia performed worse than patients with undifferentiated schizophrenia in the self-reflection (0.24 ± 0.14 v. 0.42 ± 0.16) and the other-reflection conditions (0.22 ± 0.14 v. 0.40 ± 0.18, p's < 0.05).
The P2 amplitudes showed a significant main effect of group (F 2,39 = 4.28, p = 0.021, η 2 = 0.180). The P2 component was smaller in the undifferentiated patients (6.01 ± 0.79 μV) compared with the controls (8.88 ± 0.59 μV, p = 0.018) whereas there was no difference between the paranoid patients and controls (7.56 ± 0.97 μV, p = 0.753). The N2 latency showed a significant main effect of group (F 2,39 = 5.89, p = 0.006, η 2 = 0.232); it was longer in controls (339 ± 4.9 ms) than in the paranoid patients (307 ± 8.1 ms, p = 0.005) whereas there was no difference between the undifferentiated patients and controls (327 ± 6.6 ms, p = 0.458).
Age of illness onset and duration of illness
A correlation analysis was performed to explore the effects of age of illness onset and duration of illness on the behavioral/ERP measurements. We found that the N2 latency in the other-reflection condition at the F3 electrode site was correlated with illness duration (r 20 = 0.454, p = 0.044); the P2 latency in the self-reflection condition at the F6 electrode site was correlated with the age of illness onset (r 20 = –0.498, p = 0.025).
Medications
There was no significant correlation between the dose of medications and behavioral/ERP measurements.
Discussion
Abolished SRM effect in schizophrenia
In the controls, the recognition score of self-reflection was higher than that of other-reflection (Fig. 2 a). However, in the schizophrenia patients there was no significant difference between the recognition scores of self- and other-reflection. Furthermore, the SRM bias score was smaller in the schizophrenia patients than in the controls (Fig. 2 b), corroborating the evidence that normal subjects exhibit a typical SRM effect whereas the SRM effect is abolished in schizophrenia patients (Harvey et al. Reference Harvey, Lee, Horan, Ochsner and Green2011). Staresina et al. (Reference Staresina, Gray and Davachi2009) and Harvey et al. (Reference Harvey, Lee, Horan, Ochsner and Green2011) suggested that the different SRM results between patients and controls may be due to ‘event congruency’. The term ‘event congruency’ refers to the phenomenon that the events eliciting ‘yes’ answers (i.e. congruous events) are better remembered than the events eliciting ‘no’ answers (i.e. incongruous events). However, our data do not support this interpretation because the proportion of ‘yes’ answers in the self-reflection condition during the encoding phase show no significant differences between the two groups.
It should be noted that our patients showed a lower recognition score than the controls only in the self-reflection condition. In other words, there was no significant recognition difference between the two groups in the other-reflection and font-judgment conditions during the recognition phase. Furthermore, there was no significant accuracy difference between patients and controls in the font-judgment task during the encoding phase. These data indicate that the basic or low-level cognitive function of schizophrenia patients was comparable to the normal controls in this study.
Abnormal pre/frontal electrophysiological activity in schizophrenia
The present study found several ERP differences between the two groups during the encoding phase. First, the schizophrenia patients had smaller P2 amplitudes over the frontal electrodes compared with the controls (Fig. 3 a). Similarly, reduced P2 amplitudes were observed in paranoid schizophrenia by Silva et al. (Reference Silva, Torres and Ortiz2008). However, data in their study showed a significant group difference only in the self-reflection condition (p = 0.03 in self-reflection; p = 0.07 in other-reflection). This discrepancy between their results and ours is probably due to the relatively small population involved in the earlier study (eight patients and seven controls). The P2 component is usually regarded as an attention-related biomarker at the early processing stage (∼200 ms) (Karayanidis & Michie, Reference Karayanidis and Michie1996; Chen et al. Reference Chen, Yuan, Feng, Chen, Gu and Li2011, Reference Chen, Zhang, Zhong, Hu and Li2013; Hu et al. Reference Hu, Wu and Fu2011). In the current study, the smaller P2 in the schizophrenia patients may reflect a decreased recruitment of attentional resources toward self- and other-reflection stimuli. In addition, the different P2 amplitudes between patients and controls may also be caused by the abnormality of an early modulation of the word-related meaning creation in schizophrenia (Silva et al. Reference Silva, Torres and Ortiz2008).
Second, the pre/frontal N2 component of the schizophrenia patients was more negative in the self-reflection than in the other-reflection condition whereas it did not distinguish between the self- and other-reflection conditions in the controls (Fig. 4 a). Previous studies have shown that the N2 component is an index of top-down information encoding and retrieving (Chen et al. Reference Chen, Yuan, Feng, Chen, Gu and Li2011, Reference Chen, Zhang, Zhong, Hu and Li2013). We observed that larger N2 amplitudes were elicited by the self-reflection task in patients, indicating that it is more difficult for schizophrenia patients to encode/retrieve self-related information and that this processing may consume more top-down cognitive resources in patients than in normal subjects. Moreover, the peak latencies of the P2 and N2 components were longer in the controls than the patients, suggesting that self- and other-referential stimuli were psychologically salient and biologically important for the controls so they elicited prolonged attention engagement and cognitive processing (Chen et al. Reference Chen, Yuan, Feng, Chen, Gu and Li2011). However, this adaptive function seems to be reduced in schizophrenia.
Third, the amplitudes of the positive slow wave (the LPC) during 800 to 1200 ms post-stimulus were larger in the controls than the patients at the frontal electrodes (Fig. 3 b). It has been suggested that the LPC is related to emotional evaluation of stimuli and reflects an interaction of cognitive and emotional processing (Huang & Luo; Reference Huang and Luo2006; Chen et al. Reference Chen, Weng, Yuan, Lei, Qiu, Yao and Li2008). In this study, larger LPC amplitudes in the controls may indicate that self- and other-related information was processed more fully and may elicit a stronger emotional experience in controls compared to schizophrenia patients. The LPC amplitude difference between the groups provided, for the first time, direct electrophysiological evidence for abnormal frontal functioning at the late processing stage (800–1200 ms) of self- and other-reflection in schizophrenia patients.
The abnormal findings of pre/frontal electrophysiological activity in schizophrenia are consistent with fMRI studies showing that activation of anterior cortical midline structures in schizophrenia is reduced in response to self- (Shad et al. Reference Shad, Keshavan, Steinberg, Mihalakos, Thomas, Motes, Soares and Tamminga2012) and other-related stimuli (Murphy et al. Reference Murphy, Brent, Benton, Pruitt, Diwadkar, Rajarethinam and Keshavan2010). Our results at the pre/frontal cortex indicate that the MPFC plays an important role in self-referential processing (Philippi et al. Reference Philippi, Duff, Denburg, Tranel and Rudrauf2012). A previous study in autism patients has also reported an association between abnormal MPFC activity and self-referential processing deficits (Lombardo et al. Reference Lombardo, Chakrabarti, Bullmore, Sadek, Pasco, Wheelwright, Suckling and Baron-Cohen2010).
Correlation between SRM bias and ERP components
The present study found that, in the patient group, the SRM bias score correlated significantly with the P3 amplitudes elicited by the self-reflection task in the parietal cortex. This result is consistent with the transcranial magnetic stimulation study by Lou et al. (Reference Lou, Luber, Stanford and Lisanby2010), who found that a disruption of parietal activity during self-reflective processing suppressed the SRM effect in normal subjects. It has been suggested that besides the pre/frontal region, parietal cortices also contribute largely to self-referential processing (Lou et al. Reference Lou, Luber, Stanford and Lisanby2010; Philippi et al. Reference Philippi, Duff, Denburg, Tranel and Rudrauf2012). Although we did not find a significant P3 component difference between the two groups in this study, Fig. 4 b does show that the average P3 amplitude is larger in normal controls than in patients. It may be that the lack of P3 difference between groups is due to large individual differences in P3 amplitudes in schizophrenia patients (see also Zhang et al. Reference Zhang, Wang, Luo and Luo2012). The correlation found between P3 amplitudes and SRM bias scores suggests a potential application of ERP indexes for SRM effect evaluation in schizophrenia patients.
Limitations
Before starting the formal experiment, we performed a survey in another 36 subjects (18 schizophrenia patients and 18 controls), whose characteristics were very similar to the subjects included in this study. In the pre-experiment survey, we selected five famous persons (including the one used in this experiment) and asked the 36 participants to evaluate their familiarity with these persons on a nine-point scale. A relatively high familiarity score was obtained for the person ‘Jintao Hu’ (Chairman of China) and no significant difference was observed between patients and controls (t 34 = –0.25, p = 0.802; patients = 6.1 ± 1.3, controls = 6.2 ± 1.3). Thus, this study used Jintao Hu as the stimulus in the other-reflection condition. We acknowledge that we cannot exclude the possibility that the delusional content of some of our patients may have affected the experimental results. It might have been better if had we included a post-hoc questionnaire or interview in the experiment.
We found that the valence (positive versus negative) of trait words did not show any significant main effect or interaction effect, so this within-subjects factor was not entered in the final statistical analyses in this study. Inconsistent with our result, Watson et al. (Reference Watson, Dritschel, Obonsawin and Jentzsch2007) did find a significant interaction between self-reference and emotional valence of the stimuli. It is possible that the divergent results of these two studies are due to the participants included in the experiments. We focused on both schizophrenia patients and normal controls whereas Watson et al. (Reference Watson, Dritschel, Obonsawin and Jentzsch2007) only focused on normal participants. As there was a relatively large heterogeneity in the patients and also in the matched control participants, it is possible that the effect of emotional valence was not significant in our study.
Further work
Continuing efforts should be made in the near future to enhance the information derived from the present study. First, the patients in our experiment were taking antipsychotics. Although there was no significant recognition score difference between the two groups in the other-reflection and font-judgment conditions and no significant accuracy difference between the two groups in the font-judgment condition, the SRM effect should be further investigated in antipsychotic-naïve patients (e.g. first-episode schizophrenia) to exclude the possibility that medication may affect the performance of the patients. Second, the SRM effect and self-reflective processing may be related to social cognition (Harvey et al. Reference Harvey, Lee, Horan, Ochsner and Green2011) and insight (van der Meer et al. Reference van der Meer, Costafreda, Aleman and David2010). More experiments are needed to explore these issues and to discuss the clinical significance of the impaired SRM effect and self-reflection.
Conclusions
In conclusion, the present study investigated the impaired self-reflection processing in schizophrenia patients at behavioral and neurophysiological levels. We found that SRM abnormity occurred as early as 160–260 ms post-stimuli, with smaller P2 amplitudes in patients than in controls. In addition, the N2 component (270–380 ms) showed different amplitudes between the self- and other-reflection conditions in patients but not in controls. Finally, the P3 amplitudes (350–600 ms) in the parietal cortex correlated significantly with the SRM bias score in patients. These results further our understanding of the neural time course of impaired self-reflective processing in schizophrenia patients.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (H0921), Beijing Natural Science Foundation (7102086), Fund of Capital Medical Development and Research (2011-2013-02), Beijing Municipal Training Programme Foundation for the Talents (2007id0301400101), and Beijing Municipal Science & Technology Commission Grant(D121100005012005). We thank J. Zhang and D. Li for their help with the EEG recording, and Y. Mu for her suggestions in the task design.
Declaration of Interest
None.