Introduction
Attention deficit hyperactivity disorder (ADHD) is a common, early-onset, clinically and genetically heterogeneous neuropsychiatric disorder. Individuals with ADHD may have life-long deficits in executive functions (Gau et al. Reference Gau and Shang2010a ) and visual memory (Shang & Gau, Reference Shang and Gau2011). Among executive function deficits, impaired inhibitory control is the most prominent cognitive deficit in ADHD (Barkley & Routh, Reference Barkley and Routh1974; as reviewed in Durston, Reference Durston2003). Moreover, impairments with greater reductions in visual processing than in verbal processing are noted in ADHD (Rhodes et al. Reference Rhodes, Coghill and Matthews2005). In the present study, we used functional magnetic resonance imaging (fMRI) to explore the neural correlates of these two processes (i.e. inhibitory control and visual processing), which allowed us to examine putative compensatory mechanisms in ADHD. It is important to examine these two processes within a study to understand deficits in the fronto-parietal network (Fassbender & Schweitzer, Reference Fassbender and Schweitzer2006; Nakao et al. Reference Nakao, Radua, Rubia and Mataix-Cols2011; Cortese et al. Reference Cortese, Kelly, Chabernaud, Proal, Martino, Milham and Castellanos2012).
Evidence of disturbed structural and functional connectivity in fronto-parietal networks in ADHD was further provided by a recent meta-analysis of task-based fMRI studies (Cortese et al. Reference Cortese, Kelly, Chabernaud, Proal, Martino, Milham and Castellanos2012) and structural MRI studies (Nakao et al. Reference Nakao, Radua, Rubia and Mataix-Cols2011). Cortese et al. (Reference Cortese, Kelly, Chabernaud, Proal, Martino, Milham and Castellanos2012) reported that hypoactivation in fronto-parietal networks is the most frequently observed functional imaging finding in children with ADHD; Nakao et al. (Reference Nakao, Radua, Rubia and Mataix-Cols2011) reported abnormal gray matter volumes in prefrontal, anterior cingulate cortex (ACC), and parietal regions in children with ADHD. According to a meta-analysis of patients with ADHD (Hart et al. Reference Hart, Radua, Nakao, Mataix-Cols and Rubia2013), most imaging studies have shown abnormal neural activity for inhibitory control in the right inferior frontal gyrus (IFG), ACC, and parietal regions. First, impaired IFG functions reduced the ability to optimally recruit subsidiary brain regions and strategies to perform cognitive tasks in ADHD (Fassbender & Schweitzer, Reference Fassbender and Schweitzer2006). The IFG has been implicated in higher-level cognitive functioning, including attentional processes, inhibitory control, working memory and planning (Fassbender & Schweitzer, Reference Fassbender and Schweitzer2006; Bush, Reference Bush2011). Particularly, the right IFG is thought to be involved in response inhibitory control or target detection (Durston, Reference Durston2003; Hampshire et al. Reference Hampshire, Chamberlain, Monti, Duncan and Owen2010; Cortese et al. Reference Cortese, Kelly, Chabernaud, Proal, Martino, Milham and Castellanos2012). Second, the ACC is also believed to play critical roles in complex and effortful cognitive processing, target detection, response selection and inhibitory control, error detection, performance monitoring, and motivation (as reviewed in Bush, Reference Bush2011). A number of studies have implicated that the ACC is involved in top-down attentional control and inhibitory control of competing responses to various stimuli (Pardo et al. Reference Pardo, Pardo, Janer and Raichle1990). Studies have shown hypoactivity in the ACC and robust activity in the right IFG in adults with ADHD using the counting Stroop task (Bush et al. Reference Bush, Frazier, Rauch, Seidman, Whalen, Jenike, Rosen and Biederman1999). Third, parietal activations have been reported to be involved in attention and visual processing (Fassbender & Schweitzer, Reference Fassbender and Schweitzer2006; Schneider et al. Reference Schneider, Krick, Retz, Hengesch, Retz-Junginger, Reith and Rösler2010). Specifically, the superior parietal lobule (SPL) in the left hemisphere has been suggested to be associated with number processing on screen in healthy adults (Dehaene et al. Reference Dehaene, Piazza, Pinel and Cohen2003; Cavanna & Trimble, Reference Cavanna and Trimble2006). Bush et al. (Reference Bush, Whalen, Rosen, Jenike, Mcinerney and Rauch1998) and Lévesque et al. (Reference Lévesque, Beauregard and Mensour2006) found robust activity in the left SPL/precuneus [Brodmann area (BA) 7] during a counting Stroop task.
Neuropsychological studies have combined several constructs, namely executive functions and visual processing, into a single study to examine drug-related improvement among children with ADHD (Coghill et al. Reference Coghill, Rhodes and Matthews2007, Shang & Gau, Reference Shang and Gau2012). Previous imaging studies have also combined inhibitory control and sustained attention into a single task using the Go/No-Go task in ADHD (Trommer et al. Reference Trommer, Hoeppner, Lorber and Armstrong1988; Schulz et al. Reference Schulz, Fan, Tang, Newcorn, Buchsbaum, Cheung and Halperin2004; Fassbender & Schweitzer, Reference Fassbender and Schweitzer2006). They found that commission errors (failure to inhibit to the no-go response) were related to inhibitory control and impulsivity, and omission errors (failure to respond to the go stimulus) were associated with inattention. The counting Stroop task is designed to evaluate the underlying mechanism of cognitive interference between number and meaning (Bush et al. Reference Bush, Whalen, Rosen, Jenike, Mcinerney and Rauch1998), and the visual properties of the numbers to evaluate visual processing (Rasmussen & Bisanz, Reference Rasmussen and Bisanz2005). In the current study, we used the counting Stroop task to explore the differential brain activity related to visual processing load between the ADHD and neurotypical groups, in addition to inhibitory control that has been explored in fMRI studies.
The Cambridge Neuropsychological Test Automated Battery (CANTAB) was also used to examine these two processes. The CANTAB has been used to assess executive functions, including both inhibitory control and visual processing. Previous studies have shown that children with ADHD perform worse in response inhibitory control (Slaats-Willemse et al. 2007; Gau et al. Reference Gau, Chiu, Shang, Cheng and Soong2009; Gau & Shang, Reference Gau and Shang2010a , Reference Gau and Shang b ; Gau & Huang, Reference Gau and Huang2014) and visual memory (Rhodes et al. Reference Rhodes, Coghill and Matthews2005; Gau et al. Reference Gau, Chiu, Shang, Cheng and Soong2009; Gau & Shang, Reference Gau and Shang2010a ; Shang & Gau, Reference Shang and Gau2011), as compared with neurotypical children. Previous studies have also suggested that the deficits in inhibitory control and visual processing are major endophenotypes for ADHD genetic studies (Gau & Shang, Reference Gau and Shang2010a ; Shang & Gau, Reference Shang and Gau2011). Hence, combining the CANTAB and counting Stroop fMRI offers a better understanding of the relationships among these two processes. We used the CANTAB to assess participants' cognitive mechanisms of inhibitory control and visual processing load outside the scanner because the CANTAB has been reported to be used to measure the endophenotypes for ADHD (Doyle et al. 2005; Gau & Shang, Reference Gau and Shang2010a ; Shang & Gau, Reference Shang and Gau2011; Gau & Huang, Reference Gau and Huang2014). In addition, previous studies have shown that the deficits in inhibitory control and visual processing are major endophenotypes for ADHD genetic studies (Gau & Shang, Reference Gau and Shang2010a ; Shang & Gau, Reference Shang and Gau2011). Despite the striking nature of the inhibitory control and visual processing impairment in ADHD, knowledge about its corresponding alterations in the brain is still evolving. Previous studies have used the approach of correlating the structural integrity of fiber tracts based on a group difference with the CANTAB to investigate executive function and inhibitory control in children with ADHD (Shang et al. Reference Shang, Wu, Gau and Tseng2012; Wu et al. Reference Wu, Gau, Lo and Tseng2014). In the findings of Konrad et al. (Reference Konrad, Dielentheis, El Masri, Dellani, Stoeter, Vucurevic and Winterer2012), adults with ADHD had significantly lower structural integrity in the left inferior longitudinal fasciculus based on a group difference, which was negatively correlated with attentional performance of the test of variables of attention. Therefore, the present study was designed to use the approach of correlating brain activity and the CANTAB to assess the differential inhibitory control and visual processing between the ADHD and neurotypical groups. Two tests of inhibitory control and visual processing were chosen from the CANTAB.
Studies on brain activity in the right IFG and parietal regions have revealed inconsistent results in terms of increased or decreased activation in ADHD (Fassbender & Schweitzer, Reference Fassbender and Schweitzer2006; Silk et al. Reference Silk, Vance, Rinehart, Bradshaw and Cunnington2008; Bush, Reference Bush2011; Hart et al. Reference Hart, Radua, Nakao, Mataix-Cols and Rubia2013). These studies are mainly limited by separate investigations of these two brain regions and the study of adult populations. We therefore used counting Stroop fMRI to examine the neural correlates of inhibitory control and visual processing, and correlated the brain activity with the CANTAB in youths with ADHD and neurotypical youths. According to a priori hypotheses, we hypothesized that the involvement of the right IFG and ACC may be related to the inhibitory control that can be measured by the CANTAB in youths with ADHD, and that the involvement of the parietal regions may be related to the visual processing that can be measured by the CANTAB in neurotypical youths.
Method
Participants and procedures
We assessed 25 youths (mean age = 10.9 years, s.d. = 2.2 years, age range 8–16 years, two females) with a clinical diagnosis of ADHD according to the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV) diagnostic criteria and 23 neurotypical youths without ADHD (mean age = 11.2 years, s.d. = 2.9 years, age range 8–16 years, two females) who were matched to the distribution of age, gender, handedness and intelligence quotient (IQ) of the ADHD group. Youths with ADHD were recruited from the Department of Psychiatry (National Taiwan University Hospital, Taipei, Taiwan). The participants and their parents were interviewed to confirm their diagnosis of ADHD and to exclude any other psychiatric disorders by one of the corresponding authors (S. S.-F. Gau) using the Chinese Kiddie epidemiological version of the Schedule for Affective Disorders and Schizophrenia (K-SADS-E) interview (Gau et al.2005). The neurotypical group was recruited from similar school districts as the ADHD group with the help of principals and schoolteachers rather than by advertisement outside schools. Similar to the ADHD group, they and their parents were interviewed using the Chinese K-SADS-E to ensure that they did not meet DSM-IV diagnosis of ADHD from early childhood until now and any of the other psychiatric disorders.
Both groups were native Mandarin–Chinese speakers, had standard scores of the full-scale IQ greater than 80 as assessed by the Wechsler Intelligence Scale for Children, third edition (WISC-III; Wechsler, Reference Wechsler1991), and had normal hearing and normal or corrected-to-normal vision. Participants who had a clinical diagnosis of any other psychiatric disorders were excluded from the study. In addition, participants with ADHD who currently took medication affecting the central nervous system, and neurotypical participants who ever or currently took any psychotropic drug and had a history of attention or verbal-language deficits were excluded from the study.
The study was approved by the Research Ethics Committee at the National Taiwan University Hospital, Taiwan and all the participants and their parents provided written informed consent before study implementation (IRB no. 200903062R; ClinicalTrials.gov no. NCT00916851). All the participants were assessed using the Chinese K-SADS-E interviews, followed by the WISC-III and the CANTAB. The six participants with ADHD, who took psychotropic medication before, did not take any medication for at least 1 week before the assessments.
Functional activation task
In the counting Stroop task, experimental stimuli were divided into congruent, incongruent and control conditions (see Fig. 1), with 24 trials in each condition. In the ‘congruent’ condition, the number of words was consistent with the meaning of the word such as ‘one’, ‘two’, ‘three’ or ‘four’ (i.e. ![]() ,
, ![]() ,
, ![]() or
or ![]() in Chinese). In the ‘incongruent’ condition, the number of words was inconsistent with the meaning of the word. In the ‘control’ condition, the Chinese words did not give any clue to number [i.e.
in Chinese). In the ‘incongruent’ condition, the number of words was inconsistent with the meaning of the word. In the ‘control’ condition, the Chinese words did not give any clue to number [i.e. ![]() (it; pronoun),
(it; pronoun), ![]() (human; noun),
(human; noun), ![]() (understand; verb) or
(understand; verb) or ![]() (no; adverb)]. All the words of the three conditions were matched for the number of syllables, visual complexity (strokes per word) and frequency.
(no; adverb)]. All the words of the three conditions were matched for the number of syllables, visual complexity (strokes per word) and frequency.
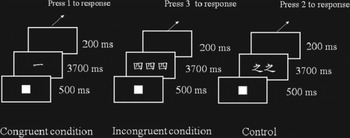
Fig. 1. The counting Stroop task in Chinese. Experimental stimuli were divided into congruent, incongruent and control conditions. In the ‘congruent’ condition, the number of words (i.e. one) was consistent with the meaning of the word (i.e. one). In the ‘incongruent’ condition, the number of words (i.e. three) was inconsistent with the meaning of the word (i.e. four). In the ‘control’ condition, the Chinese words did not give any clue to number.
Trials consisted of a solid square (500 ms), followed by sets of between one and four identical words (3200 ms). There was a 200-ms blank between trials. Participants were instructed to report the number of words in each set via button-press with one, two, three and four buttons from left to right on the keypad. They used their index and middle fingers of each hand to respond accordingly.
WISC-III
The WISC-III (Wechsler, Reference Wechsler1991) has been widely used to assess full-scale intelligence levels of children aged 6 years to 16 years, 11 months. Among the 13 subtests, forward and backward digit spans were used to represent the index of sustained attention and verbal working memory, respectively.
Neuropsychological measures
The CANTAB is a standard, computerized, non-linguistic and culturally blind test to assess a wide range of executive functions and visual processing. Rapid visual information processing (RVP) was chosen to assess inhibitory control (Sahakian et al. Reference Sahakian, Jones, Levy, Gray and Warburton1989; Gau & Huang, Reference Gau and Huang2014), while pattern recognition memory (PRM) was used to assess visual processing (Shang & Gau, Reference Shang and Gau2011, Reference Shang and Gau2012).
In the RVP test, participants were asked to respond to the specific sequence of digits, when a white box was presented in the center of the screen with digits (ranging from 2 to 9) appearing one at a time (100 digits/min) in the center of the screen in a pseudo-random order. Participants were instructed to respond to three specific number sequences of digits (i.e. 2–4–6, 3–5–7, 4–6–8) by pressing the touch pad. Two measures were recorded: A’, a signal detection measure of sensitivity to the target, regardless of response tendency (Sahgal, Reference Sahgal1987); and probability of false alarms (of the participant responding inappropriately), i.e. total false alarms divided by the sum of total false alarms and total correct rejections.
The PRM test is designed to measure the capacity of visual processing in a two-choice forced discrimination paradigm (Sahakian et al. Reference Sahakian, Morris, Evenden, Heald, Levy, Philpot and Robbins1988). Participants were presented with a series of visual geometric patterns, one at a time, in the center of the screen. In the recognition phase, participants were presented with two geometric patterns and the test patterns were presented in the reverse order to the original order of presentation. The participants were instructed to choose the correct pattern between an already-seen pattern and a novel pattern. The percentage of correct responses was recorded.
MRI image acquisition
Participants lay in the scanner with their head position secured. The head coil was positioned over the participants' head. Participants viewed visual stimuli projected onto a screen via a mirror attached to the inside of the head coil. Each participant performed two functional runs. Each run took 2.8 min. Images were acquired using a 3-T Siemens Tim-Trio scanner with the 32-channel head coil (Siemens Medical Solutions, Germany). Each functional run had 85 image volumes that were acquired with the echo planar imaging method to detect the blood oxygenation level-dependent (BOLD) signal.
The scanning parameters were the following: repetition time (TR) = 2000 ms; echo time (TE) = 24 ms; flip angle = 90°; matrix size = 64 × 64; field of view = 25.6 cm; slice thickness = 3 mm; number of slices = 34. A high-resolution, T1-weighted three-dimensional image was also acquired (magnetization prepared rapid gradient-echo; TR = 2300 ms; TE = 2.98 ms; flip angle = 9°; matrix size = 256 × 256; field of view = 25.6 cm; slice thickness = 1 mm). The orientation of the three-dimensional image was identical to the functional slices. The task was administered in a pseudo-random order for all participants for event-related design (Burock et al. Reference Burock, Buckner, Woldorff, Rosen and Dale1998). We used the Optseq script for randomized event-related design (http://surfer.nmr.mgh.harvard.edu/optseq, written by D. Greve, Charlestown, MA, USA) that implemented the Burock et al. (Reference Burock, Buckner, Woldorff, Rosen and Dale1998) approach.
Image and statistical analysis
Data analysis was performed using SPM5 (Statistical Parametric Mapping). The functional images were corrected for the differences in slice-acquisition time to the middle volume and were realigned to the first volume in the scanning session using affine transformations. The exclusion criteria for motion were 3 mm for displacement and 3° for rotations. No participant had more than 3 mm of movement in any plane. Co-registered images were normalized to the Montreal Neurological Institute (MNI) average template. Statistical analyses were calculated on the smoothed data (10 mm isotropic Gaussian kernel, the concept of kernel was defined the shape of function to calculate weighted average of each data point with its neighboring data points), with a high-pass filter (128 s cut-off period) in order to remove low frequency artifacts.
Data from each participant were entered into a general linear model using an event-related analysis procedure (Josephs & Henson, Reference Josephs and Henson1999). Stimuli were treated as individual events for analysis and modeled using a canonical hemodynamic response function (HRF). Parameter estimates from contrasts of the canonical HRF in single-subject models were entered into random-effects analysis using the one-sample t test across all participants to determine whether activation during a contrast was significant (i.e. parameter estimates were reliably greater than 0) in a whole brain analysis. There were three event types: congruent, incongruent, and control. To observe the neural correlates of inhibitory control, we compared the incongruent with the congruent condition. To further observe the neural correlates of visual processing, we compared the larger number of words (i.e. ‘three’ and ‘four’) with the fewer number of words (i.e. ‘one’ and ‘two’) in the incongruent condition and in the congruent condition. For the contrast within each group, all reported areas of activation were significant using p < 0.05 for family-wise error (FWE) corrected at the voxel level with a cluster size greater than 50 voxels in a whole brain analysis. For the contrasts between groups, all reported areas of activation were significant using p < 0.05 for FWE corrected at the voxel level with a cluster size greater than 10 voxels, with the anatomical masks of right IFG, ACC, and left SPL due to our a priori hypothesis. The three anatomical masks were defined from the WFU PickAtlas, with the option of right IFG, ACC, and left SPL (http://fmri.wfubmc.edu/software/PickAtlas). We then extracted the β values from peak voxels of significant brain regions between groups.
We used SPSS to conduct statistical analysis (IBM, USA). The descriptive results were displayed as frequency and percentage for categorical variables, and mean and s.d. for continuous variables. We also conducted two-way analysis of variance (ANOVA) with group and condition interactions for behavioral results of the counting Stroop test. Moreover, we performed Pearson's correlations between β values of peak voxels of significant brain regions and the scores of the parameters derived from the RVP and PRM tests. All reported results were significant using p < 0.05.
Results
Behavioral results
There were no group differences in IQ profiles, or digit span forward. Compared with neurotypical youths, youths with ADHD had significantly fewer digits recalled backward, and lower scores on A’ (target sensitivity), higher probability of false alarm in the RVP test, and lower percentage of correct responses in the PRM test (Table 1).
Table 1. Age, IQ scores, and performance in the RVP and PRM tests for all participants
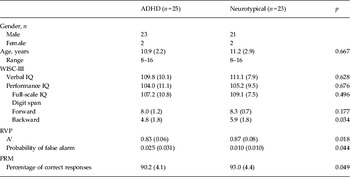
IQ, Intelligence quotient; RVP, rapid visual information processing; PRM, pattern recognition memory; ADHD, attention deficit hyperactivity disorder; WISC-III, Wechsler Intelligence Scale for Children, Third Edition; A’, signal detection measure of sensitivity to the target.
Data are given as mean (standard deviation).
Online Supplementary Table S1 presents the accuracy and reaction time of the counting Stroop test in the three conditions by the two groups. A two-way ANOVA on accuracy revealed significant main effects of condition (F 2,92 = 14.58, p < 0.01), with being more accurate in the congruent and control conditions than the incongruent condition (t 47 = 4.91, p < 0.01; t 47 = 3.77, p < 0.01, respectively), but no significant effect of group (F 1,46 = 1.59, p = 0.214). The interaction of group and condition was not significant (F 2,92 = 0.37, p = 0.692).
Regarding reaction time, a two-way ANOVA showed a significant main effect of condition (F 2,92 = 20.37, p < 0.01), with shorter reaction time in the congruent and control conditions than in the incongruent condition (t 47 = −5.33, p < 0.01; t 47 = −5.40, p < 0.01, respectively) but a marginally significant effect of group (F 1,46 = 4.00, p = 0.052). The interaction of group and condition was not significant (F 2,92 = 2.85, p = 0.063).
To further observe visual processing in the congruent and incongruent conditions, the larger number of words (i.e. ‘three’ and ‘four’) showed significant longer reaction time than the fewer number of words (i.e. ‘one’ and ‘two’) (t 95 = −2.13, p < 0.05), suggesting that all participants spent more time in making judgments as the number of words increased.
fMRI results
Table 2 presents activation of brain regions for the incongruent versus congruent condition for the ADHD and neurotypical groups. For the contrast of ‘incongruent versus congruent condition’ within group, the ADHD group showed greater activation in the right IFG (BA 45) and ACC (online Supplementary Fig. S1, Table 2). For group comparisons, youths with ADHD had greater activation in the right IFG (BA 45) and ACC than neurotypical youths (Fig. 2, Table 2). There was no significant activation for neurotypical youths as compared with youths with ADHD.
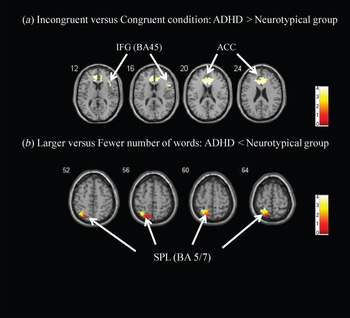
Fig. 2. (a) Inhibitory control. Greater activation was found in the right inferior frontal gyrus [IFG; Brodmann area (BA) 45] and anterior cingulate cortex (ACC) with region of interest (ROI)-masked analysis for the attention deficit hyperactivity disorder (ADHD) group as compared with the neurotypical group during the contrast of ‘incongruent versus congruent condition’. (b) Visual processing. Greater activation was found in the left superior parietal lobule (SPL; BA 5/7) with ROI-masked analysis for the neurotypical group as compared with the ADHD group during the contrast of ‘larger versus fewer numbers of words’. The color bar presents the strength of the activation.
Table 2. Inhibitory control: greater activation for the incongruent compared with congruent condition within each group, and between the two groups a
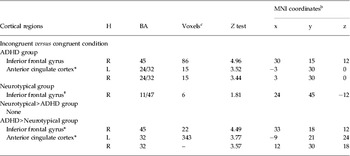
MNI, Montreal Neurological Institute; H, hemisphere; BA, Brodmann area; ADHD, attention deficit hyperactivity disorder; R, right; L, left; FWE, family-wise error.
a Region of interest masks (anatomical masks of right inferior frontal gyrus, anterior cingulate cortex, and left superior parietal lobule) were only used in between-group analysis.
b Coordinates of activation peak(s) within a region based on a Z test are given in the MNI stereotactic space (x, y, z).
c Number of voxels in cluster at p < 0.05 FWE corrected, only clusters greater than or equal to 50 are presented.
* p < 0.05 for FWE corrected with the use of an anatomical mask.
† p < 0.05 for uncorrected.
For the contrast of ‘larger versus fewer numbers of words’ within group, the ADHD group showed greater activation in the left postcentral gyrus (BA 3), while the neurotypical group showed greater activation in the left precentral gyrus (BA 6) and left SPL (BA 5/7) (online Supplementary Fig. S2, Table 3). For group comparisons, neurotypical youths had greater activation in the left SPL as compared with youths with ADHD (Table 3).
Table 3. Visual processing: greater activation for the larger number of words compared with the fewer number of words within each group, and between the two groups a

MNI, Montreal Neurological Institute; H, hemisphere; BA, Brodmann area; ADHD, attention deficit hyperactivity disorder; L, left; FWE, family-wise error.
a Region of interest masks (anatomical masks of right inferior frontal gyrus, anterior cingulate cortex, and left superior parietal lobule) were only used in between group analysis.
b Coordinates of activation peak(s) within a region based on a Z test are given in the MNI stereotactic space (x, y, z).
c Number of voxels in cluster at p < 0.05 FWE corrected, only clusters greater than or equal to 50 are presented.
d Subcluster.
* p < 0.05 for FWE corrected with the use of an anatomical mask.
† p < 0.05 for uncorrected.
Correlations between brain activation and neuropsychological measures
Pearson correlations showed that in the ADHD group, increasing activation in the right IFG (for inhibitory control) was positively correlated with probability of false alarm in the RVP test (r = 0.52, p < 0.05), and increasing activation in the right ACC (for inhibitory control) was positively correlated with probability of false alarm in the RVP test (r = 0.51, p < 0.05) (removing an outlier with outside 2.5 s.d. from the group mean). In the neurotypical group, increasing activation in the left SPL (for visual processing) was positively correlated with the percentage of correct responses in the PRM test (r = 0.52, p < 0.05), suggesting that neurotypical youths might have better visual processing than youths with ADHD.
Discussion
Several features of this study constitute its strengths: combination of neuropsychological and functional neuroimaging assessments to investigate inhibitory control and visual processing in ADHD; neuropsychological assessments of the two processes as proposed potential ADHD cognitive endophenotypes using the CANTAB; and assessment of neural mechanisms of two functions using counting Stroop fMRI. The major findings were that youths with ADHD showed greater activation in the right IFG and ACC during the incongruent versus congruent condition but less activation in the left SPL for the larger versus fewer numbers of words than neurotypical youths. Moreover, positive correlations of activation of the right IFG and ACC with probability of false alarm in the RVP test were only noted in youths with ADHD; however, a positive correlation of activation of the left SPL with the percentage of correct responses in the PRM test was only noted in neurotypical youths.
Our finding of more brain activation in the right IFG and ACC among youths with ADHD than neurotypical youths was consistent with the findings related to inhibitory control (Fassbender & Schweitzer, Reference Fassbender and Schweitzer2006; Bush, Reference Bush2011). Previous fMRI studies have suggested that children and adults with ADHD might engage in alternative, compensatory brain regions with concomitant cognitive strategies due to a selectively weakened neural system (for a review, see Fassbender & Schweitzer, Reference Fassbender and Schweitzer2006). They suggested that hyperactivity in a brain region can be considered as ‘inefficiency’ because an individual needs to use more energy than should be to perform a given task. Moreover, extra activity in the clinical group may be viewed as compensatory activity of brain regions that the clinical group is enrolling in order to compensate for under-activity in the ‘appropriate’ brain network (Fassbender & Schweitzer, Reference Fassbender and Schweitzer2006). With regards to ADHD studies, Schulz et al. (Reference Schulz, Fan, Tang, Newcorn, Buchsbaum, Cheung and Halperin2004) have suggested the recruitment of additionally compensatory prefrontal regions to perform the Go/No-Go task in ADHD. Moreover, an earlier study showed that individuals with ADHD might have an immature prefrontal cortex, so they may compensate with the recruitment of additional cortical areas in order to perform at the same level as that of neurotypical subjects (Rubia et al. Reference Rubia, Overmeyer, Taylor, Brammer, Williams, Simmons and Bullmore1999). In our findings, greater activations in the right IFG and ACC among youths with ADHD than neurotypical youths were consistent with previous findings related to the inhibitory control process (Hampshire et al. Reference Hampshire, Chamberlain, Monti, Duncan and Owen2010; Whelan et al. Reference Whelan, Conrod, Poline, Lourdusamy, Banaschewski, Barker, Bellgrove, Büchel, Byrne, Cummins, Fauth-Bühler, Flor, Gallinat, Heinz, Ittermann, Mann, Martinot, Lalor, Lathrop, Loth, Nees, Paus, Rietschel, Smolka, Spanagel, Stephens, Struve, Thyreau, Vollstaedt-Klein, Robbins, Schumann and Garavan2012), suggesting that youths with ADHD might need more activation in the right IFG and ACC to compensate their deficits in inhibitory control. According to the meta-analysis in ADHD (Hart et al. Reference Hart, Radua, Nakao, Mataix-Cols and Rubia2013), adult patients with ADHD relative to controls showed decreased activation in the right frontal region for inhibitory control. However, we found that youth participants with ADHD showed greater activations than neurotypical youths in the right IFG and ACC. The current study has added value to the effect of age.
In contrast, youths with ADHD showed less activation in the left SPL than neurotypical youths. Previous studies point out that ADHD is associated with impaired visual processing (Shang & Gau, Reference Shang and Gau2011, Reference Shang and Gau2012) and less activation in the left parietal region may be attributed to impaired visual processing in children with ADHD (Silk et al. Reference Silk, Vance, Rinehart, Bradshaw and Cunnington2008). Our finding of a positive correlation between left SPL activation and the percentage of correct responses in the PRM test in neurotypical youths suggests that neurotypical youths might have better visual processing than youths with ADHD. Taken together, our findings lend important evidence to support that youths with ADHD might have worse visual processing of numbers as compared with neurotypical youths while performing the counting Stroop task.
Although previous studies have suggested that the counting Stroop task was designed to evaluate the neural correlates of inhibitory control (Bush et al. Reference Bush, Whalen, Rosen, Jenike, Mcinerney and Rauch1998, Reference Bush, Frazier, Rauch, Seidman, Whalen, Jenike, Rosen and Biederman1999; Lévesque et al. Reference Lévesque, Beauregard and Mensour2006), visual processing could also be involved in this task. For example, Bush et al. (Reference Bush, Whalen, Rosen, Jenike, Mcinerney and Rauch1998) and Lévesque et al. (Reference Lévesque, Beauregard and Mensour2006) found robust activity in the left SPL/precuneus (BA 7) during a counting Stroop task. In the present study, we used the contrast of the larger versus the fewer numbers of words to examine whether visual processing was involved in the counting Stroop task. Longer reaction time and greater left SPL activation were related to visual processing for the larger number of words compared with the fewer number of words across participants. Taken together, our findings lend evidence to support that left SPL is related to visual processing.
More importantly, our further examination of the relationship between frontal and parietal functions showed that hyperactivation in the right IFG was correlated with hypoactivation in the left SPL (r = −0.29, p < 0.05) across all the participants, given that the correlations did not vary between groups (Fisher exact test, z = 0.17, p > 0.05). Our findings suggest that less activation of parietal regions may be compensated by increased brain activation in the right IFG and ACC. As compared with neurotypical youths, youths with ADHD might have poor visual processing associated with parietal regions that may be related to compensatory inhibitory control associated with the right IFG. These findings suggest a deficit on association between frontal and parietal function in youths with ADHD. In addition to the significant correlation between frontal and parietal activations (r = −0.29, p < 0.05), we presented a significant correlation between congruency cost (incongruent v. congruent) and visual processing (larger v. fewer words) across all the participants (r = −0.29, p < 0.05) on reaction time after partialing for medication status.
Limitations
Relatively small sample size and potential effect of methylphenidate on six participants are the two methodological limitations of this study. Although our results were consistent and statistically reliable, the sample size was small (statistical power 0.544, effect size 0.611). Therefore, the findings should be interpreted with caution until replicated in a larger sample size. Moreover, six of the participants with ADHD had ever been treated with methylphenidate some time at least 1 week before MRI assessment; the effect of methylphenidate on cognitive performance cannot be excluded.
Implications
Our results clearly contribute to our knowledge about cognitive deficits in ADHD, which are not only demonstrated by deficits in inhibition controls and visual processing assessed by neuropsychological tests but also by functional imaging research. Most importantly, our findings of compensatory neuronal mechanisms revealed by imaging data are not clinically observable; hence, imaging assessment may be recommended to be included in the assessment of ADHD, particularly for the diagnosis of complicated cases.
Conclusion
As the first study combining the CANTAB and the counting Stroop task during fMRI scanning to examine inhibitory control and visual processing in ADHD, the findings of greater activation in the right IFG and ACC in ADHD imply increased inhibitory control to suppress interference between number and meaning in ADHD. In contrast, the finding of less activation in the left SPL in ADHD suggests a deficit in visual processing to process the numbers in the counting Stroop task in ADHD. Taken together, in view of that the fronto-parietal network is considered to be impaired in ADHD (Makris et al. Reference Makris, Buka, Biederman, Papadimitriou, Hodge, Valera, Brown, Bush, Monuteaux, Caviness, Kennedy and Seidman2008), our findings add some evidence to suggest that less activation of parietal regions may be compensated by increased brain activation in the right IFG.
Supplementary material
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0033291714000038.
Acknowledgements
This work was supported by the National Science Council of Taiwan (NSC) (NSC98-3112-B-002-004, NSC99-2627-B-002-016 and NSC100-2627-B-002-013 to T.-L.C.; NSC96-2628-B-002-069-MY3, NSC99-2627-B-002-015, NSC100-2627-B-002-014 and NSC101-2627-B-002-002 to S.S.-F.G.) and the National Health Research Institute (NHRI) (NHRI-EX98-9407PC and NHRI-EX100-0008PI to S.S.-F.G.) and in part by the Department of Medical Imaging and the 3 T MRI Laboratory in the National Taiwan University Hospital.
Declaration of Interest
None.







