Introduction
Schizophrenia (SCZ) is a psychiatric disorder characterized by serious disruptions in thinking, perception, and the sense of self (American Psychiatric Association, 2013). Neurobiological alterations in patients with SCZ have been extensively investigated with neuroimaging methods, contributing to a better understanding of the pathophysiology of the disease (Galderisi, DeLisi, & Borgwardt, Reference Galderisi, DeLisi, Borgwardt, Galderisi, DeLisi and Borgwardt2019). On the one hand, structural magnetic resonance imaging (MRI) studies have reported gray matter volume reduction and cortical mantle thinning across thalamocortical circuitry, including the prefrontal cortex (PFC), in patients with first-episode psychosis (FEP) and chronic SCZ, together with increased ventricular volume (Chan, Di, McAlonan, & Gong, Reference Chan, Di, McAlonan and Gong2011; Kuo & Pogue-Geile, Reference Kuo and Pogue-Geile2019; Radua et al., Reference Radua, Vieta, Shinohara, Kochunov, Quidé, Green and Pineda-Zapata2020). Gray matter abnormalities in SCZ seem to be partially hereditary, as shown in twin and candidate gene studies (Luo et al., Reference Luo, Chen, Wang, Desrivières, Quinlan and Jia2019), and partially modulated by intrauterine risk exposures such as fetal hypoxia (Cannon et al., Reference Cannon, van Erp, Bearden, Loewy, Thompson, Toga and Tsuang2003). Postmortem studies indicate that cortical gray matter reduction does not reflect loss of cell bodies but, rather, reduced dendritic complexity and synaptic density, which may impact interneuronal communication and integration (Glantz & Lewis, Reference Glantz and Lewis2000). Moreover, accumulating longitudinal neuroimaging data point to the presence of specific brain abnormalities at the onset of the disease and even in clinical high-risk youth (Fusar-Poli et al., Reference Fusar-Poli, Broome, Woolley, Johns, Tabraham, Bramon and McGuire2011; Satterthwaite et al., Reference Satterthwaite, Wolf, Calkins, Vandekar, Erus, Ruparel and Gur2016; Vita, De Peri, Silenzi, & Dieci, Reference Vita, De Peri, Silenzi and Dieci2006), suggesting that these changes represent a primary pathological process that would play a causal role in the onset of symptoms (Karlsgodt, Sun, & Cannon, Reference Karlsgodt, Sun and Cannon2010).
On the other hand, functional MRI (fMRI) has been used to identify brain functional abnormalities evoked by specific processes known to be altered in SCZ. For example, different aspects of cognitive dysfunction have been examined, including working memory (WM), response inhibition, conflict processing, and problem-solving, finding deficits across several PFC regions (Barch, Csernansky, Conturo, & Snyder, Reference Barch, Csernansky, Conturo and Snyder2002). Moreover, emotional and social processing abnormalities have been found to be related to hypoactivation in the right inferior occipital gyrus (IOG), right fusiform gyrus, amygdala, hippocampus and anterior cingulate cortex (ACC) (Anticevic et al., Reference Anticevic, Van Snellenberg, Cohen, Repovs, Dowd and Barch2012; Li, Chan, McAlonan, & Gong, Reference Li, Chan, McAlonan and Gong2010; Taylor et al., Reference Taylor, Kang, Brege, Tso, Hosanagar and Johnson2012). At the network level, previous functional imaging studies have reported dysfunction of frontotemporal and frontoparietal networks in patients with SCZ at rest and during cognitive and emotional processing (EP) (Lui et al., Reference Lui, Yao, Xiao, Keedy, Reilly, Keefe and Sweeney2015; Wolf et al., Reference Wolf, Gur, Valdez, Loughead, Elliott, Gur and Ragland2007).
Despite this broad literature, the results across imaging modalities and functional imaging paradigms are generally difficult to reconcile. Previous meta-analyses have typically examined structural and different functional domains in isolation (Anticevic et al., Reference Anticevic, Van Snellenberg, Cohen, Repovs, Dowd and Barch2012; Chan et al., Reference Chan, Di, McAlonan and Gong2011; Jáni & Kašpárek, Reference Jáni and Kašpárek2018; Kuo & Pogue-Geile, Reference Kuo and Pogue-Geile2019; Li et al., Reference Li, Chan, McAlonan and Gong2010; Minzenberg, Laird, Thelen, Carter, & Glahn, Reference Minzenberg, Laird, Thelen, Carter and Glahn2009; Taylor et al., Reference Taylor, Kang, Brege, Tso, Hosanagar and Johnson2012; Zmigrod, Garrison, Carr, & Simons, Reference Zmigrod, Garrison, Carr and Simons2016), or combining the analysis of one particular process (e.g. cognitive control) with structural alterations (Fornara, Papagno, & Berlingeri, Reference Fornara, Papagno and Berlingeri2017; Radua et al., Reference Radua, Borgwardt, Crescini, Mataix-Cols, Meyer-Lindenberg, McGuire and Fusar-Poli2012). Moreover, previous multimodal meta-analytical studies have also focused on patients with specific characteristics such as early-onset SCZ or FEP (Gao et al., Reference Gao, Zhang, Yao, Xiao, Liu, Liu and Lui2018; Ioakeimidis, Haenschel, Yarrow, Kyriakopoulos, & Dima, Reference Ioakeimidis, Haenschel, Yarrow, Kyriakopoulos and Dima2020; Radua et al., Reference Radua, Borgwardt, Crescini, Mataix-Cols, Meyer-Lindenberg, McGuire and Fusar-Poli2012), or specific regions of the brain (Ding et al., Reference Ding, Ou, Pan, Shan, Chen, Liu and Guo2019). Despite this, to date, no meta-analysis has examined whole-brain gray matter volume (GMV) changes in combination with functional studies including a broad array of cognitive tasks in the general SCZ population. By combining results from different techniques, we can take advantage of cross-modal information, which could reveal information that would be difficult to detect through the use of single modalities, and can also help compensate for imperfect imaging studies (Calhoun & Sui, Reference Calhoun and Sui2016). This approach is particularly useful in the context of psychiatric disorders, and more specifically in finding the relationship of brain pathologies in psychosis, as many pathophysiological questions can only be answered with cross-modal information (Schultz et al., Reference Schultz, Fusar-Poli, Wagner, Koch, Schachtzabel, Gruber and Schlösser2012).
This study aimed to conduct a voxel-based meta-analytic comparison between patients with SCZ and comparison controls without SCZ to summarize and integrate findings from whole-brain structural and functional brain activation studies. Regarding functional studies, these were categorized according to the domains of cognitive impairment in SCZ identified by the National Institute of Mental Health (NIMH)-Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) consensus (Green et al., Reference Green, Nuechterlein, Gold, Barch, Cohen, Essock and Marder2004), as has been done in previous meta-analyses (Fett et al., Reference Fett, Viechtbauer, Dominguez, Penn, van Os and Krabbendam2011; Fusar-Poli et al., Reference Fusar-Poli, Deste, Smieskova, Barlati, Yung, Howes and Borgwardt2012). According to the MATRICS, the domain of neurocognitive functioning would include the following subdomains: processing speed (PS), attention/vigilance (AV), working memory, verbal learning and memory (VeLM), visual learning and memory (ViLM), executive function/reasoning and problem solving (EF), verbal fluency (VF), and verbal comprehension (VC) (Nuechterlein et al., Reference Nuechterlein, Barch, Gold, Goldberg, Green and Heaton2004). On the other hand, the domain of social cognition is referred to as the mental operations underlying social behavior, such as the interpretation of another person's intentions or emotions, and is a multi-dimensional construct comprising: EP, social perception and knowledge (SP), theory of mind (TM), and attributional bias (AB) (Green et al., Reference Green, Penn, Bentall, Carpenter, Gaebel, Gur and Heinssen2008). We aimed to determine whether the alterations in these functional domains have a structural correlate, and whether this correlate is shared or domain-specific. Moreover, in order to take into account part of the clinical heterogeneity found in SCZ, we aimed to explore the potential modulating role of several clinical variables, such as symptom severity, medication use, and illness duration.
Methods
The meta-analysis was conducted according to PRISMA guidelines (http://www.prismastatement.org/). Details of the meta-analysis were registered at PROSPERO, an international prospective register of systematic reviews/meta-analysis (https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=184604&VersionID=1355076). We note that part of the methods was modified after peer-review and thus differ from the original preregistration. The main differences are the task fMRI domains included (initially including only executive functioning/EP, and after peer-review including more broadly the neurocognitive/social cognitive domains), and some details regarding specifics of the meta-analytical software (due to the use of the newest version). We performed all procedures following the recent recommendations for neuroimaging meta-analyses (Müller et al., Reference Müller, Cieslik, Laird, Fox, Radua, Mataix-Cols and Eickhoff2018).
Literature search and study selection
A comprehensive literature search was conducted for peer-reviewed human studies in PubMed, ScienceDirect and Scopus databases published until the end of December 2020. Keywords related to the disorder (‘schizophrenia’ OR ‘SCZ’ OR ‘psychosis’) plus terms related with structural or functional imaging (‘MRI’ OR ‘magnetic resonance imaging’ OR ‘fMRI’ OR ‘functional magnetic resonance imaging’) plus terms related with the type of analysis/process of interest (‘voxel-based morphometry’ OR ‘VBM’ OR ‘task’ OR ‘cognition’ OR ‘neurocognitive’ OR ‘social cognitive’) were used. In addition, manual searches were conducted within review articles and via the reference lists of individual studies. After duplicate removal, 3786 articles studies were identified (Fig. 1).

Fig. 1. PRISMA flow diagram of the inclusion of studies in the meta-analysis.
Note: PRISMA = Preferred reporting items for systematic reviews and meta-analyses (http://www.prismastatement.org/).
Studies were considered for inclusion if they included adult samples, a comparison with a control group and reported whole-brain results and stereotactic coordinates of the peaks of clusters of group differences. We excluded studies with less than 10 participants per group, including patients with psychotic-related disorders other than SCZ (e.g. schizoaffective disorder, schizophreniform disorder, affective psychosis), not specifically assessing our domains of interest, from which peak information could not be retrieved, or that did not report whole-brain statistical results, and/or in which statistical thresholds varied across the assessment of different brain regions as it may be the case of small volume corrections (Fig. 1). Regarding fMRI, studies were included from two different cognitive domains: neurocognitive (see online Supplementary Table S4 for the type of tasks included and their corresponding subdomains), and social cognitive (online Supplementary Table S5). Importantly, to decide to which domain and subdomain a study belonged, the specific contrasts analyzed were examined, and not just the general task. In this regard, for example, an emotional N-back task may be included within the neurocognitive working memory domain if the contrast used analyzes the working memory component of the task regardless of emotion (see Guimond et al., Reference Guimond, Padani, Lutz, Eack, Thermenos and Keshavan2018). Moreover, our criteria for task and contrast selection was to include those contrasts which better represented the process of interest measured in each task (typically validated for such purpose). For example, those with a higher cognitive load for neurocognitive tasks, and those with a higher emotional/social component for social cognitive tasks. The specific contrasts used can also be found in online Supplementary Tables S4 and S5. As for the final number of included studies, two independent datasets could be included from the same article for the neurocognitive meta-analysis (Jiménez, Mancini-Marïe, Lakis, Rinaldi, & Mendrek, Reference Jiménez, Mancini-Marïe, Lakis, Rinaldi and Mendrek2010).
Peak coordinates and effect sizes were extracted and coded from the original publication. The literature search, decisions on inclusion and data extraction were all performed independently by two investigators (MPP and RV), and any discrepancy was resolved by consensus with a third investigator (MFR). For each dataset, several sociodemographic and clinical variables were extracted (online Supplementary Tables S1, S2 and S3). Symptom severity was measured with the Positive and Negative Symptoms Scale (PANSS) (Kay, Fiszbein, & Opler, Reference Kay, Fiszbein and Opler1987), while medication use was coded as the percentage of patients currently taking medication in each study.
Statistical methods
The Seed-based d Mapping with Permutation of Subject Images (SDM-PSI) software, version 6.21 (www.sdmproject.com) was used to generate voxel-wise (random effects) effect size maps corresponding to the analyses and contrasts of interest. Details of the SDM-PSI method have been published previously (Albajes-Eizagirre et al., Reference Albajes-Eizagirre, Solanes, Fullana, Ioannidis, Fusar-Poli, Torrent and Radua2019a; Albajes-Eizagirre, Solanes, Vieta, & Radua, Reference Albajes-Eizagirre, Solanes, Vieta and Radua2019c). SDM-PSI conducts a standard permutation of subject images (PSI). In addition, it uses unbiased estimation of effect sizes based on MetaNSUE algorithms (a method for univariate meta-analysis developed to include studies from which the meta-analytic researcher knows that the analysis was not statistically significant, but he/she cannot know the actual effect size; Albajes-Eizagirre, Solanes, and Radua, Reference Albajes-Eizagirre, Solanes and Radua2019b), random-effects models, Freedman-Lane-based permutations, and threshold-free cluster enhancement (TFCE) statistics.
We first performed a separate meta-analysis for each modality (structural, neurocognitive, and social-cognitive). Then, areas of overlapping functional and structural abnormalities were assessed by conjunction analysis through the multimodal meta-analysis in SDM-PSI. This analysis is conceptually the same as conducting the simple overlap of the meta-analytical maps from individual meta-analyses (i.e. to find the regions presenting differences both at the structural and functional level). However, it considers the error in the p-values (Picó-Pérez et al., Reference Picó-Pérez, Silva, De Melo, Radua, Mataix-Cols, Sousa and Morgado2020; Radua, Romeo, Mataix-Cols, & Fusar-Poli, Reference Radua, Romeo, Mataix-Cols and Fusar-Poli2013).
Next, exploratory subgroup meta-analyses were performed in order to explore the potential impact of relevant clinical variables, as well as the type of cognitive domain studied (for the fMRI meta-analyses). Specifically, for all three modalities (structural, neurocognitive, and social-cognitive), meta-analyses were performed dividing the sample based on: medication use (one meta-analysis including studies with 0% of participants under medication, and another one with 100% of participants under medication), illness duration (one meta-analysis for studies with mean illness duration between 0 and 2 years, another one between 3 and 10 years, and another one with more than 10 years; Kay, Fiszbein, Lindenmayer, and Opler, Reference Kay, Fiszbein, Lindenmayer and Opler1986), and PANSS symptom severity (for each of the three subscales of the PANSS, as well as for the Total score, the studies were categorized as high severity or low severity depending on whether they were above or below the median of all studies included). Regarding the fMRI meta-analyses, further subgroup analyses were performed based on the subdomains previously mentioned (PS, AV, WM, VeLM, ViLM, EF, VF, and VC for the neurocognitive meta-analysis, and EP, SP, TM, and AB for the social cognitive meta-analysis). Importantly, in order to avoid spurious findings, these exploratory analyses were only performed when 10 or more datasets were available. Online Supplementary Table S6 shows the number of datasets available for each subgroup for all three modalities.
Finally, multimodal meta-analyses were also performed using the output of the subgroup exploratory analyses (for example, exploring the overlap between each pair of modalities when including only those studies with 100% of participants medicated, etc.). Regarding the fMRI tasks subdomains, multimodal meta-analyses were performed between the general structural results and each of these functional subdomains (both for neurocognitive and social cognitive domains).
The I 2 index and Q values were used to explore the heterogeneity of effect sizes, and publication bias was assessed using a meta-regression by the standard error, analog to the Egger test. Statistical significance was set at a p < 0.05 using TFCE correction, and a minimum cluster extent of ten contiguous voxels was required. Reported peak coordinates are in Montreal Neurological Institute (MNI) space.
Results
Included studies and sample characteristics
We included 114 independent datasets for VBM, with a total of 4789 SCZ patients (63.52% males, mean age of 33.52 years, s.d. = 11.1) and 5216 controls (56.65% males, mean age of 32.52 years, s.d. = 11.59) (see online Supplementary Table S1 for details), 143 independent datasets for the neurocognitive meta-analysis, including a total of 3446 SCZ patients (70.23% males, mean age of 33.26 years, s.d. = 10.28) and 3723 controls (62.72% males, mean age of 32.32 years, s.d. = 10.68) (see online Supplementary Table S2), and 86 independent datasets for the social cognitive meta-analysis, including a total of 1755 SCZ patients (64.16% males, mean age of 33.7 years, s.d. = 10.05) and 1819 controls (58.5% males, mean age of 33.07 years, s.d. = 9.6) (see online Supplementary Table S3).
Individual meta-analytic results
Regional differences in GMV
In comparison to control individuals, SCZ patients showed decreased GMV in bilateral fronto-temporal clusters including the inferior, middle and superior frontal gyri (IFG, MFG and SFG respectively), the inferior, middle and superior temporal gyri (ITG, MTG, STG), the parahippocampal gyri, and the posterior and anterior insulae, in a medial prefrontal cortex (mPFC) cluster including both the ventral and dorsal parts, as well as the dorsal anterior cingulate (dACC) and the posterior cingulate (PCC), in the parietal cortex including the bilateral angular and supramarginal gyri, and in subcortical structures such as the bilateral thalamus and caudate (Table 1, Fig. 2). There were no regions of increased GMV in patients with SCZ.
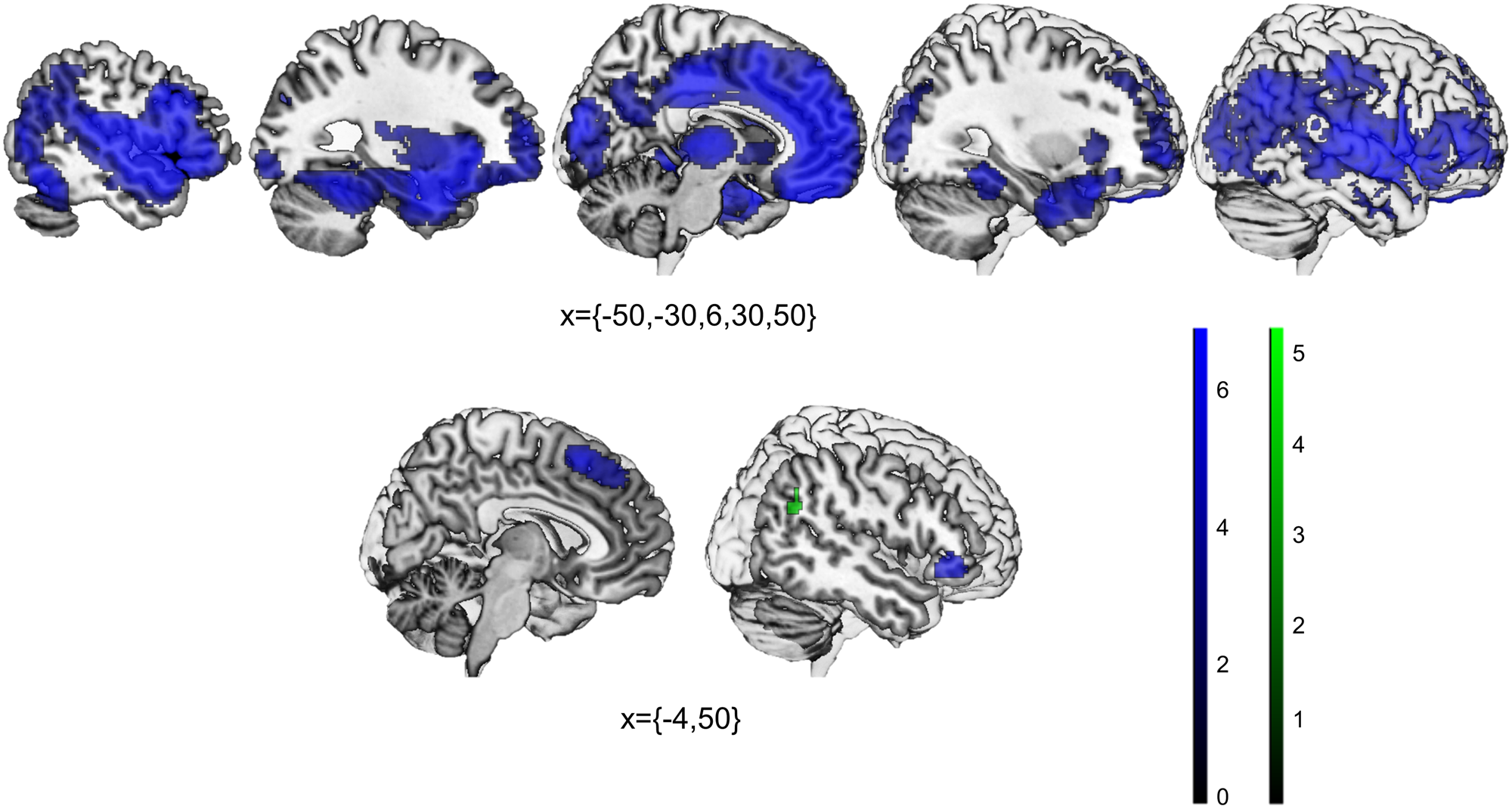
Fig. 2. Statistically significant decreases in patients with schizophrenia when compared to controls in gray matter volume (top), and task-fMRI activations (bottom; blue = neurocognitive tasks, green = social cognitive tasks).
Table 1. Results of the meta-analysis with VBM studies for the Controls > Patients contrast

Ke, cluster extent; MNI, Montreal Neurological Institute; SDM, Signed Differential Mapping; P, p-value; I 2, percentage of variance attributable to study heterogeneity.
*P-values based on Z-converted Q values.
Only one local peak per gray matter brain region is displayed.
fMRI activation differences in neurocognitive tasks
Patients with SCZ presented statistically significant decreased activation in the dorsomedial prefrontal cortex (dmPFC)/supplementary motor area (SMA) and the right IFG (Table 2, Fig. 2). There were no regions of increased activation in patients with SCZ.
Table 2. Results of the task-based meta-analyses with neurocognitive and social cognitive fMRI studies for the Controls > Patients contrast

Ke, cluster extent; MNI, Montreal Neurological Institute; SDM, Signed Differential Mapping; P, p-value; I 2, percentage of variance attributable to study heterogeneity.
*P-values based on Z-converted Q values.
fMRI activation differences in social cognitive tasks
In comparison to control individuals, SCZ patients showed decreased activation in the right angular gyrus (Table 2, Fig. 2). There were no regions of increased activation in patients with SCZ.
As shown in Tables 1 and 2, most findings showed very low heterogeneity and the Egger test was not statistically significant in any results from the three meta-analyses, suggesting an absence of publication bias. The heterogeneity found in some of the regions from the VBM meta-analysis could be due to between-study differences in clinical variables, which were explored in the exploratory subgroup meta-analyses.
Multimodal results
Conjunction of GMV and fMRI abnormalities during neurocognitive tasks
Multimodal analyses showed shared GMV and neurocognitive related-activation decreases in SCZ patients in the dmPFC/SMA (MNI coordinates = 4, 16, 54, cluster extent = 695 voxels, SDM-Z = −5.29) and the right IFG (MNI coordinates = 48, 36, −8, cluster extent = 157 voxels, SDM-Z = −5.102). Anatomically, these regions are the same already presented in Fig. 2 (bottom).
Conjunction of GMV and fMRI abnormalities during social cognitive tasks
Multimodal analyses showed shared GMV and social cognitive related-activation decreases in SCZ patients in the right angular gyrus (MNI coordinates = 56, −54, 30, cluster extent = 98 voxels, SDM-Z = −4.903). Anatomically, this region is the same already presented in Fig. 2 (bottom).
Conjunction of fMRI abnormalities during neurocognitive and social cognitive tasks
There were no significant shared abnormal decreased or increased activations between neurocognitive and social cognitive results at the selected TFCE-corrected level.
Exploratory sub-group results
Individual meta-analytic results
The results of the subgroup exploratory analyses are presented in online Supplementary Tables S7 (VBM), 8 (neurocognitive) and 9 (social cognitive). In general, all VBM subgroup analyses showed significant TFCE-corrected results which replicated to a certain extent the main results (being all regions of GMV decreases in patients with SCZ), although the regions of GMV decreases were more extended for the 100% medication and the illness duration 3–10 years subgroups. Regarding the task fMRI meta-analyses, only some of the subgroups showed significant TFCE-corrected results. Focusing specifically at the task subdomains, only AV showed significantly decreased activations for SCZ patients in the neurocognitive meta-analysis, and TM was the only subdomain with significantly decreased activations for the social cognitive meta-analysis. Interestingly, for some of the subgroup analyses from the neurocognitive meta-analysis, regions of increased activation in SCZ patients appeared that were not present in the main analysis (for example, the bilateral vmPFC in studies including patients with an illness duration of more than 10 years).
Multimodal results
Regarding the multimodal results, the conjunction of GMV and fMRI abnormalities during neurocognitive tasks for the 100% medicated and the AV subgroups replicated to a certain extent the main findings, while for the subgroups of illness duration >10 years, and lower PANSS positive and negative symptoms severity, a pattern of decreased GMV coupled with increased activation was found (see online Supplementary Table S10). Finally, new regions of decreased activation for social cognitive tasks were coupled with decreased GMV in the higher PANSS positive symptoms severity and the TM subgroups (online Supplementary Table S10).
Discussion
Nowadays, SCZ, which is characterized by a lack of integration between thought, emotion, and behavior, is considered to be a brain-based disease due to increasing evidence pointing to both structural and functional brain alterations in this disorder (Fusar-Poli et al., Reference Fusar-Poli, Broome, Woolley, Johns, Tabraham, Bramon and McGuire2011). In this context, multimodal imaging represents a promising strategy in order to help disentangle the complex neuropathological associations found in SCZ. To the best of our knowledge, this is the first meta-analytic study assessing structural changes and functional alterations during neurocognitive and social cognitive tasks in patients with SCZ.
In agreement with previous research (Gao et al., Reference Gao, Zhang, Yao, Xiao, Liu, Liu and Lui2018; Radua et al., Reference Radua, Borgwardt, Crescini, Mataix-Cols, Meyer-Lindenberg, McGuire and Fusar-Poli2012, Reference Radua, Vieta, Shinohara, Kochunov, Quidé, Green and Pineda-Zapata2020), the meta-analysis of structural-only data indicated that patients with SCZ showed widespread decreased GMV in cortical and subcortical regions (i.e. dACC/dmPFC, bilateral insula, frontal, temporal and parietal gyri, and thalamus, among others). These findings largely concur with previous research and prevailing SCZ models, and have been extensively discussed in previous meta-analyses (Gao et al., Reference Gao, Zhang, Yao, Xiao, Liu, Liu and Lui2018; Jáni & Kašpárek, Reference Jáni and Kašpárek2018; Minzenberg et al., Reference Minzenberg, Laird, Thelen, Carter and Glahn2009; Radua et al., Reference Radua, Borgwardt, Crescini, Mataix-Cols, Meyer-Lindenberg, McGuire and Fusar-Poli2012). Interestingly, the subgroup exploratory meta-analyses replicated to a certain extent the main findings, but with subtle differences that may give further insights into the clinical specificity of these alterations. Regarding medication use, in both the 0% and the 100% medicated meta-analyses the main regions of GMV decreases in SCZ patients remained significant, but the clusters were much more extended for the 100% medicated subgroup, especially for the right STG/insula cluster. Moreover, regarding illness duration, GMV decreases were only significant in the left STG/insula for the 0–2 years subgroup (which included some FEP studies), covered most of the other regions of GMV decreases for the 3–10 years subgroup, and these results were slightly reduced for the >10 years subgroup. Taken together, these results seem to give support to the idea of specific brain abnormalities being present at disease onset, rather than all being a consequence of disease duration or medication (Fusar-Poli et al., Reference Fusar-Poli, Broome, Woolley, Johns, Tabraham, Bramon and McGuire2011; Karlsgodt et al., Reference Karlsgodt, Sun and Cannon2010). Moreover, there seems to be a lateralized pattern of findings, with left-hemisphere GMV decreases being already present at early stages of the disease and in unmedicated patients, and right-hemisphere GMV decreases appearing later on and in a more extended way in medicated samples. On the other hand, high and low PANSS symptom severity subgroups showed fairly similar results for all subscales and for the total scale, being the only noticeable difference the significant GMV decrease in the cerebellum found in patients with lower severity. In the previous meta-analysis by Gao et al. (Reference Gao, Zhang, Yao, Xiao, Liu, Liu and Lui2018) they did not have any significant findings regarding PANSS scores, suggesting a relative stability of the identified brain alterations across different severity levels.
As for neurocognitive related differences in brain activity, across the different processes included in our analysis, patients with SCZ showed decreased activations in the dmPFC/SMA and in the right IFG. These regions partially overlap with the salience network, which includes the cingulate cortex and the bilateral anterior insula/IFG, and whose activity is thought to guide behavior in front of emotionally relevant stimuli by regulating attention and cognitive resources allocation (Menon, Reference Menon and Toga2015; Menon & Uddin, Reference Menon and Uddin2010). This network's connectivity has previously been found to be altered in first-episode SCZ (Huang et al., Reference Huang, Botao, Jiang, Tang, Zhang, Tang and Wang2019). Moreover, a reduction in anterior cingulate volume and activation has previously been observed in psychotic disorders in association with impairments in EP and higher executive functions (Baiano et al., Reference Baiano, David, Versace, Churchill, Balestrieri and Brambilla2007; Ioakeimidis et al., Reference Ioakeimidis, Haenschel, Yarrow, Kyriakopoulos and Dima2020), and alterations in this area may partly explain the difficulties in cognitive and emotional integration that characterize the clinical manifestations of psychosis (Fornito, Yücel, Dean, Wood, & Pantelis, Reference Fornito, Yücel, Dean, Wood and Pantelis2009). Regarding the subgroup exploratory analyses, the most relevant findings were the regions of increased activation in SCZ patients for some of these subgroups, namely for the duration of illness >10 years, and low Total, Positive, and Negative PANSS symptoms severity. These findings comprised the vmPFC for the duration of illness and the right rolandic operculum and the posterior cingulate cortex for the PANSS, and will be later discussed in the context of the multimodal subgroup findings. Moveover, SCZ patients showed significantly decreased activations in only one of the neurocognitive subdomains, AV. This does not seem to be driven by higher statistical power for this subdomain, since other subdomains had a similar or higher amount of included studies (see online Supplementary Table S6). Taking into account that SCZ patients present cognitive deficits in all these subdomains (Green et al., Reference Green, Nuechterlein, Gold, Barch, Cohen, Essock and Marder2004), this finding could be due to the tasks included in AV being more homogeneous, facilitating encountering significant differences in bran activations. Importantly, we decided to maintain the TFCE-corrected threshold for these analyses in order to facilitate comparison, which could be too conservative for exploratory analyses and prevented findings in other subdomains.
Regarding the social-cognitive domain, only the right angular gyrus showed a significantly decreased activation in SCZ patients compared to controls. The angular gyrus is a relevant region for the allocation of attentional resources and monitoring emotional experiences (Pessoa, Kastner, & Ungerleider, Reference Pessoa, Kastner and Ungerleider2003). Moreover, from a network perspective, the angular gyrus is part of the default mode network (DMN). In healthy controls, the DMN is typically deactivated during the initiation of a task and active during rest and self-referential and social processing (Li, Mai, & Liu, Reference Li, Mai and Liu2014). Alterations in this network have been extensively reported in neuropsychiatric disorders, and particularly in SCZ (Hu et al., Reference Hu, Zong, Mann, Zheng, Liao, Li and Tang2017; Lee, Lee, Park, Kim, & Ryu, Reference Lee, Lee, Park, Kim and Ryu2019), and hypoactivity during this type of tasks may represent a failure to properly engage with emotional and social information (Pelletier-Baldelli, Bernard, & Mittal, Reference Pelletier-Baldelli, Bernard and Mittal2015). When looking at the exploratory analyses, TM was the only social cognitive subdomain where SCZ patients presented decreased activations, namely in the right MTG, which goes in line with previous literature (Jáni & Kašpárek, Reference Jáni and Kašpárek2018). The same considerations made for the neurocognitive exploratory analyses may be applied here. Interestingly, in general, there seem to be more regions of significant abnormal activations in SCZ patients for the neurocognitive domain, perhaps due to the more pervasive deficits found in this functional domain, in comparison to emotional and social processing, and thus impacting a broader range of brain regions (Liddle, Reference Liddle2000; Reed, Harrow, Herbener, & Martin, Reference Reed, Harrow, Herbener and Martin2002).
Among our multimodal findings, the dmPFC/SMA and the right IFG presented shared decreases between GMV and neurocognitive-related activations, while the right angular gyrus presented shared decreases between GMV and social cognitive-related activation. These were the same regions as the ones found for the fMRI meta-analyses in isolation, and globally, our findings would point toward widespread GMV alterations across the whole brain that are only impacted at a functional level in specific locations. According to the findings from the exploratory analyses and to previous literature (Fusar-Poli et al., Reference Fusar-Poli, Broome, Woolley, Johns, Tabraham, Bramon and McGuire2011; Karlsgodt et al., Reference Karlsgodt, Sun and Cannon2010; Vita et al., Reference Vita, De Peri, Silenzi and Dieci2006), we could speculate that GMV abnormalities appear first, leading then to cognitive deficits and abnormal activations in relevant hubs for attention and social processing. Despite this, it is important to keep in mind that the included studies were all cross-sectional, and this hypothesis can only be tested in longitudinal imaging studies. In a previous multimodal study looking at SCZ patients v. controls (Plis et al., Reference Plis, Amin, Chekroud, Hjelm, Damaraju, Lee and Calhoun2018), associations were found between brain structure and functional dynamics within the cingulo-temporal network, as well as a relationship to cognitive scores. They found a different pattern of correlations between groups, suggesting distinct structural-functional mechanisms, and demonstrating an interplay between deficits and brain dysfunction in the patients.
On the other hand, there were overlapping regions showing opposite patterns of decreases and increases for some of the neurocognitive subgroup analyses. Of note, a reduction of gray matter can be accompanied by a compensatory hyper-functionality of the remaining gray matter, which could involve a higher vascularization and thus show as hyper-activation. In our findings, GMV was found to be decreased in the vmPFC and the right rolandic operculum, while these regions were hyperactive during neurocognitive tasks. Interestingly, a previous meta-analysis specifically focusing on FEP found a similar pattern than ours (Radua et al., Reference Radua, Borgwardt, Crescini, Mataix-Cols, Meyer-Lindenberg, McGuire and Fusar-Poli2012), while our vmPFC finding appeared in the subgroup of patients with illness duration >10 years. Thus, the specificity of this finding regarding illness duration must be taken with caution, since other sources of variability from the included studies might also be influencing this result. Somewhat similar findings were also described in Gao et al. (Reference Gao, Zhang, Yao, Xiao, Liu, Liu and Lui2018), who hypothesized that this profile of activity increases and decreases may constitute a disease-specific variation in the topographic basis for cognitive control. In this scenario, the topography of activity engaged during the performance of these tasks is displaced for patients, giving rise to areas of relative hypoactivity adjacent to those with relative hyperactivity. Importantly, these regions appear to affect different neural networks outside the salience network discussed earlier. Instead, the vmPFC cluster found would be part of the DMN, and the right rolandic operculum of the auditory network (AN). The AN includes primary and secondary auditory areas, and deficits in these regions have been associated with auditory hallucinations and thought disorders in patients with SCZ (Barta, Pearlson, Powers, Richards, & Tune, Reference Barta, Pearlson, Powers, Richards and Tune1990; Shenton et al., Reference Shenton, Kikinis, Jolesz, Pollak, LeMay, Wible and McCarley1992).
There are several limitations to this study, including the cross-sectional and observational nature of the included studies, precluding any inferences about directionality or causality of the findings. Additional limitations are those inherently linked to meta-analysis, such as the inclusion of studies with different statistical thresholds. Moreover, although we tried to keep heterogeneity at a minimum, in this study we aimed at identifying the common functional alterations across different paradigms to assess their combined overlap with structural changes, what may have some drawbacks. Further research is therefore warranted to expand on our exploratory analyses and specifically assess overlap with structural changes of brain activations associated with the different paradigms and experimental settings combined here into single functional domains. Moreover, findings from the exploratory analyses should be taken with caution due to the relatively small number of studies included in some of them, such as the subgroup VBM analysis with only unmedicated patients. Moreover, we were unable to perform this subgroup analysis for the fMRI meta-analyses, due to having only one study with unmedicated participants for the neurocognitive domain, and another for the social-cognitive domain. Thus, this remains an open question for future meta-analyses. Finally, the amount of time patients was under medication was not considered and could be influencing the findings for the duration of illness subgroups. Thus, it might be of interest for future studies to try to disentangle these two effects.
To summarize, in a first integrative meta-analysis of structural and functional brain imaging studies in patients with SCZ, we found task-specific correlates of brain structure and function in SCZ which help summarize and integrate a growing literature. From our findings, it may be derived that the widespread GMV abnormalities found in SCZ patients have a functional overlap in regions of the salience network for the neurocognitive tasks, and in a region part of the DMN for the social cognitive tasks. Moreover, the exploratory VBM analyses point toward a lateralized pattern (from left to right) of increased alterations with a duration of illness and medication but not symptom severity, while regions of compensatory hyperactivation were found for patients with lower severity and higher duration of illness in neurocognitive tasks.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291721005523
Financial support
This work has been funded by the European Regional Development Fund (FEDER), through the Competitiveness Factors Operational Programme (COMPETE); by National funds, through the Foundation for Science and Technology (FCT), under the scope of the project POCI-01-0145-FEDER-007038, the project NORTE-01-0145-FEDER-000013, supported by the Northern Portugal Regional Operational Programme (NORTE 2020), under the Portugal 2020 Partnership Agreement, by 2CA-Braga, Braga, Portugal; and the project PI19/00394, supported by Carlos III Health Institute. JR is supported by a ‘Miguel Servet’ contract from the Carlos III Health Institute (CPII19/00009).
Conflict of interest
None.







