Introduction
Recurrence and treatment refractoriness in major depressive disorder (MDD) represent two of the major challenges that mental health professionals have to face in their daily clinical practice. In the best of cases, less than a half of depressed patients will completely recover and will have no further episodes and about 15% of cases will present a chronic unremitting course, failing to respond to multiple treatment trials (Mueller et al. Reference Mueller, Leon, Keller, Solomon, Endicott, Coryell, Warshaw and Maser1999; Eaton et al. Reference Eaton, Shao, Nestadt, Lee, Bienvenu and Zandi2008; Vuorilehto et al. Reference Vuorilehto, Melartin and Isometsä2009). Incomplete remission has been extensively associated with higher risk of relapse and higher rates of disability, psychosocial impairment, co-morbidity, suicidality and medical costs (Trivedi et al. Reference Trivedi, Hollander, Nutt and Blier2008; Fekadu et al. Reference Fekadu, Wooderson, Markopoulo, Donaldson, Papadopoulos and Cleare2009).
Some brain abnormalities might predispose to relapse or to treatment failure in MDD whereas others might also accumulate over time and hinder full recovery. This neural substrate is far from being well established. Structural and functional neuroimaging studies have identified alterations located in fronto-limbic networks that seem to be critical for illness remission (Mayberg, Reference Mayberg2003) and the ventromedial prefrontal cortex plays a crucial role in the neural circuitry of MDD (Johansen-Berg et al. Reference Johansen-Berg, Gutman, Behrens, Matthews, Rushworth, Katz, Lozano and Mayberg2008). White-matter tracts are key components of these networks and abnormalities in their microstructure can potentially be studied in vivo by means of diffusion tensor imaging (DTI), a magnetic resonance imaging (MRI) technique that is becoming increasingly popular. Fractional anisotropy (FA) quantifies how strongly directional the fibre tract organization is (Smith et al. Reference Smith, Jenkinson, Johansen-Berg, Rueckert, Nichols, Mackay, Watkins, Ciccarelli, Cader, Matthews and Behrens2006) and it is the most commonly used measure in the assessment of white-matter microstructure.
Prior DTI reports have described altered fronto-temporal FA values mostly in geriatric, but also in young and middle-aged population with MDD (for detailed reviews, see Sexton et al. Reference Sexton, Mackay and Ebmeier2009; Murphy & Frodl, Reference Murphy and Frodl2011). However, most of them have focused on identifying differences between a single group of depressed patients versus healthy controls, and the study of the influence of relevant variables related to clinical burden of illness has received modest attention. Moreover, only a few have evaluated middle-aged patients with chronic or treatment-resistant depression (Zhou et al. Reference Zhou, Qin, Chen, Qian, Tao, Fang and Xu2011; Guo et al. Reference Guo, Liu, Chen, Xu, Wu, Ma, Gao, Tan, Sun, Xiao, Chen and Zhao2012; Hoogenboom et al. Reference Hoogenboom, Perlis, Smoller, Zeng-Treitler, Gainer, Murphy, Churchill, Kohane, Shenton and Iosifescu2012). Two prospective reports, with a completely different study design, examined baseline FA differences between depressed patients in which response to treatment was subsequently evaluated. Zhou et al. (Reference Zhou, Qin, Chen, Qian, Tao, Fang and Xu2011), using a voxel-based morphometry approach, showed low baseline FA values within the hippocampal region among patients who developed a treatment-resistant depression. Hoogenboom et al. (Reference Hoogenboom, Perlis, Smoller, Zeng-Treitler, Gainer, Murphy, Churchill, Kohane, Shenton and Iosifescu2012), using a region of interest (ROI) analysis based on legacy data, revealed low baseline FA values in the medial fornix among MDD patients who failed to achieve remission. Guo et al. (Reference Guo, Liu, Chen, Xu, Wu, Ma, Gao, Tan, Sun, Xiao, Chen and Zhao2012), for their part, performed a cross-sectional DTI study in patients with treatment-resistant depression using tract-based spatial statistics (TBSS) methodology, a recent approach that increases the sensitivity and the interpretability of the results compared with conventional voxel-based approaches (Smith et al. Reference Smith, Jenkinson, Johansen-Berg, Rueckert, Nichols, Mackay, Watkins, Ciccarelli, Cader, Matthews and Behrens2006). These patients showed lower FA values than healthy controls in the right anterior limb of the internal capsule, the body of the corpus callosum and the bilateral external capsule, but the absence of a group of non-treatment-resistant patients precluded further interpretation of their findings.
In this study, we aimed to investigate whole-brain white-matter microstructure using TBSS in a sample of patients with treatment-resistant/chronic MDD as compared with patients with remitted-recurrent MDD, patients with a first-episode MDD and healthy control subjects. Given the prominent role of the ventromedial prefrontal cortex in treatment refractoriness (Johansen-Berg et al. Reference Johansen-Berg, Gutman, Behrens, Matthews, Rushworth, Katz, Lozano and Mayberg2008), an additional regional analysis centred on this area was carried out. We also examined the relationship between clinical variables and white-matter abnormalities, hypothesizing that FA values would be diminished in those patients with treatment-resistant/chronic depression and higher current and past illness burden (i.e. greater severity of symptoms, longer duration of illness, earlier age at onset and/or more previous episodes).
Method
Participants and assessments
A total of 52 right-handed adult patients with MDD [Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria] and 17 right-handed healthy controls were recruited to undergo a MRI protocol specifically designed for the present study. All patients were recruited from the out-patient clinic of the Department of Psychiatry of the University Hospital de la Santa Creu i Sant Pau. Since it is well known that age-related structural changes can occur in the brain, all participating individuals were similarly aged. Depressed participants were split into three different groups. The first group consisted of patients with chronic depressive disorder, whose last episode had a duration of more than 2 years with no response to multiple antidepressant strategies, a Thase–Rush index of treatment resistance ⩾3 and a score above 14 in the Hamilton Depression Rating Scale (HAMD) (n = 18, ‘treatment-resistant/chronic’ group). The second comprised patients who had experienced three or more major depressive episodes and were currently euthymic (score <8 in the HAMD) for at least 6 months before the enrolment (n = 15, ‘remitted-recurrent’ group). Euthymia in the 6 previous months was determined during the clinical interview and checked out with clinical records. The third group comprised currently depressed patients who were suffering from their first episode: (n = 19, ‘first-episode’ group). The latter was representative of depressed patients with lower clinical burden of illness (i.e. no previous episodes, shorter illness duration and later age at onset). Enrolled healthy individuals received a small monetary compensation for their participation. To satisfy the inclusion criteria, controls had to have no history of psychiatric diagnoses, no first-degree relatives with psychiatric diagnoses and no clinically significant physical or neurological illnesses. The exclusion criteria for all subjects included: mental retardation, history of head injury, neurological illness, clinically significant physical illness, and other psychiatric disorders such as bipolar disorder, schizophrenia, any other psychotic disorder, or alcohol and other substance abuse/dependence. Presence of co-morbid anxiety symptoms did not constitute an exclusion criterion if MDD was the primary diagnosis.
Semi-structured interviews were carried out for all participants to collect demographics and clinical information according to DSM-IV text revision (DSM-IV-TR) criteria. Current depressive symptoms were assessed using the HAMD. We estimated a composite measure of medication load for each patient according to a previously established method (Sackeim, Reference Sackeim2001; Almeida et al. Reference Almeida, Akkal, Hassel, Travis, Banihashemi, Kerr, Kupfer and Phillips2009), attempting to control for the effects of psychotropic drugs. In brief, doses of each antidepressant, mood stabilizer, antipsychotic and anxiolytic medication were coded as 0 = absent, 1 = low or 2 = high, following the rating system described by Sackeim (Reference Sackeim2001) and adapted by Almeida et al. (Reference Almeida, Akkal, Hassel, Travis, Banihashemi, Kerr, Kupfer and Phillips2009); a composite measure of medication load for each patient was obtained by summing all individual medication codes for each medication category.
The study was approved by the Research Ethics Committee of the Hospital de la Santa Creu i Sant Pau and was carried out in accordance with the Declaration of Helsinki. Written consent was obtained from each participant. This work is part of a wider project that aims to investigate in vivo neuroimaging markers of the clinical burden of depression.
MRI acquisition
All brain MRI data were acquired on a 3 T Philips Achieva MR Scanner (software version 2.1.3.2; Philips Healthcare, The Netherlands) with an eight receive-channel head-coil at the Department of Neuroradiology, Hospital de la Santa Creu i Sant Pau, Barcelona. Acquisition parameters for DTI data were the following: sensitivity-encoded (SENSE) single-shot echo-planar imaging, SENSE factor of 2, repetition time (TR) = 8166 ms, echo time (TE) = 60 ms, slice thickness = 2 mm, field of view (FOV) = 224 × 224 × 120 mm, reconstruction matrix = 128 × 128 with 60 contiguous axial slices, voxel dimensions = 1.75 × 1.75 × 2 mm. A diffusion sensitizing gradient was applied along 15 directions (b-value = 800 s/mm2) and one volume without diffusion weighting (b = 0 s/mm2, b0). For each subject, high-resolution three-dimensional magnetization-prepared rapid gradient-echo imaging (3D-MPRAGE) images were also acquired (whole-brain coverage; TR = 6.7 ms, TE = 3.2 ms, 170 slices, voxel size = 0.89 × 0.89 × 1.2 mm, reconstruction matrix = 288 × 288 × 170; FOV = 256 × 256 × 204 mm, slice thickness = 1.2 mm), with a sagittal slice orientation, T1 contrast enhancement, flip angle 8°, grey matter as a reference tissue, acquisition matrix (ACQ matrix M × P) = 256 × 240 and turbo-field echo (TFE) shots = 218.
Image processing
DTI data processing was performed at the Port d'Informació Científica (PIC) in Barcelona through the so-called PICNIC (PIC NeuroImaging Center) platform with version 4.1.4 of the Functional MRI of the Brain (FMRIB) Software Library (FSL) (Smith et al. Reference Smith, Jenkinson, Woolrich, Beckmann, Behrens, Johansen-Berg, Bannister, De Luca, Drobnjak, Flitney, Niazy, Saunders, Vickers, Zhang, De Stefano, Brady and Matthews2004). An experienced neuroradiologist (B.G.-A.) and a brain neuroimaging researcher (P.P.) conducted visual inspection of all DTI image data to ensure quality. Unweighted b0 images were extracted and used for head motion correction by an affine registration to the same subjects' weighted images, using Eddy Current Correction of the FMRIB Diffusion Toolbox (FDT). A brain extraction tool (Smith et al. Reference Smith, Zhang, Jenkinson, Chen, Matthews, Federico and De Stefano2002) with a fractional intensity threshold of f = 0.3 was used to create a binary brain mask for further analyses. FA maps were generated by fitting a diffusion tensor for each voxel using FDT dtifit. Voxel-wise statistical analysis of the FA data was performed with the TBSS package (Smith et al. Reference Smith, Jenkinson, Johansen-Berg, Rueckert, Nichols, Mackay, Watkins, Ciccarelli, Cader, Matthews and Behrens2006) implemented in FSL. First, a non-linear registration was used in order to align all participants' FA images into a standard space (FMRIB58-FA) through the FMRIB's Non-Linear Image Registration Tool (FNIRT). The mean FA image was created and thinned (0.2 threshold value) to obtain the mean FA skeleton that represents the centres of all tracts common to the group. Each individual's FA map was projected onto the threshold mean FA skeleton and these data were entered into voxel-wise statistics. In addition, a ROI was defined using the medial frontal cortex mask provided by FSL within the TBSS-generated FA skeleton overlaid on the mean FA map. To determine approximately which white-matter tracts showed significant differences, two different atlases were used: an MRI atlas of human white matter (Oishi et al. Reference Oishi, Faria, van Zijl and Mori2011) and the JHU White-Matter Tractography Atlas included in the FSL program. Additionally, high-resolution 3D-MPRAGE images were segmented into grey matter, white matter and cerebrospinal fluid using the standard segmentation model in SPM8 (http://www.fil.ion.ucl.ac.uk/spm).
Statistical analysis
Demographic, clinical and brain-tissue segmentation analyses were performed with the statistical package SPSS version 18 (IBM, USA) using analyses of variance (ANOVAs) and the χ 2 test for quantitative and categorical variables, respectively. The level of statistical significance was set at p < 0.05. One-way ANOVA was performed to detect differences among groups in mean FA values of whole-brain and ventromedial prefrontal ROIs. FA data were also analysed using voxel-wise statistics by the FSL randomise procedure included in FSL 4.1.4 (Nichols & Holmes, Reference Nichols and Holmes2002), with 5000 permutations. One-way ANOVA was used to detect group differences in FA across the skeletonized brain maps. Correction for multiple comparisons was carried out with a familywise error (FWE) rate at a p < 0.05 threshold, after threshold-free cluster enhancement (TFCE). Likewise, the same process was applied for the ROI analysis by projecting each subject's aligned FA data onto the ventromedial prefrontal region mask at a p < 0.05 threshold significance (FWE) after TFCE. Finally, backwards stepwise multiple regression analyses were computed across the three groups of patients to identify clinical variables that were independently associated with white-matter microstructure.
Results
Demographic and clinical data
Demographics, clinical variables and brain volumetric data are summarized in Table 1. Healthy controls and patients were comparable in age, gender and years of education. According to the design of the study, there were expected significant differences among distinct MDD groups relative to relevant clinical variables such as severity at recruitment (see HAMD scores), duration of illness, number of episodes or age at illness onset. The remitted-recurrent and treatment-resistant/chronic groups were comparable in terms of illness duration and age at debut but totally opposed in terms of symptomatic state. Patients with a first episode, for their part, showed intermediate HAMD scores, lesser duration of illness and older age at onset than the other patients, consistent with a low clinical burden profile. Information regarding current medication is also presented in Table 1. Treatment-resistant/chronic patients received more second-line antidepressants and combined regimens with stabilizers, antipsychotics or other antidepressants, resulting in a higher medication load. No significant differences at this level were found between first-episode and remitted-recurrent groups, although medication exposure in the latter was obviously longer than in first-episode patients. On the other hand, there were no significant differences among groups in any of the whole-brain or prefrontal ROI volumetric measurements (data not shown for the latter).
Table 1. Demographics, clinical variables and brain volumetric data
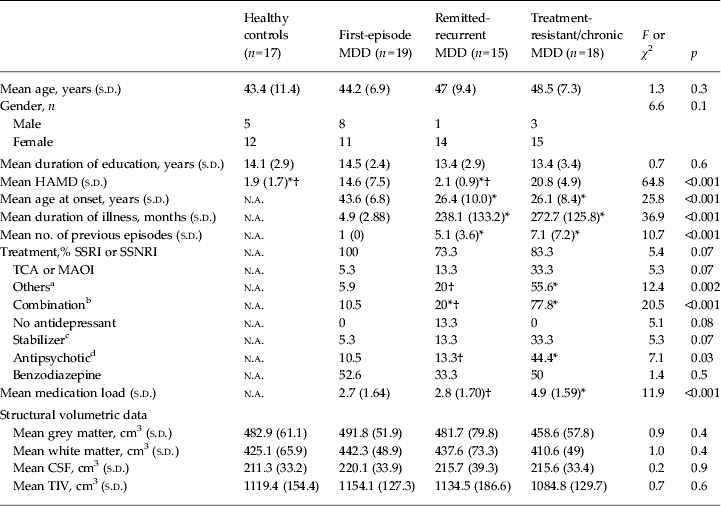
MDD, Major depressive disorder; s.d., standard deviation; n.a., not applicable; HAMD, Hamilton Depression Rating Scale; SSRI, selective serotonin reuptake inhibitors; SSNRI, selective serotonin and noradrenaline reuptake inhibitors; TCA, tricyclic antidepressant; MAOI, monoamine oxidase inhibitors; CSF, cerebrospinal fluid; TIV, total intracranial volume.
a ‘Others’ includes noradrenaline reuptake inhibitors, noradrenaline and dopamine reuptake inhibitors, tetracyclic antidepressants, Mirtazapine, Metilfenidate or Trazodone.
b Combination indicates concomitant use of antidepressants with different mechanisms of action (e.g. SSRI with reboxetine).
c Stabilizer includes anticonvulsants and mostly lithium.
d Antipsychotic includes mainly atypical antipsychotics associated with antidepressants.
* Value was significantly different from that of the first-episode patients (p < 0.05).
† Value was significantly different from that of the treatment-resistant/chronic patients (p < 0.05).
Differences in whole-brain FA
Whole-brain mean FA values of patients and controls are displayed in Fig. 1 a. The one-way ANOVA showed a significant main effect of group (F 3,65 = 3.14, p = 0.03). This effect was maintained when medication load was included as a covariate (F 3,72 = 3.64, p = 0.016). Post-hoc comparisons showed that treatment-resistant/chronic patients exhibited a significant decrease of mean FA values compared with healthy controls (p = 0.01) and with first-episode patients (p = 0.02). Voxel-wise whole-brain analyses revealed a generalized significant reduction in FA in treatment-resistant/chronic patients compared with healthy controls (p < 0.05, FWE-corrected; cluster size: 30 176 mm2), mostly affecting the following white-matter tracts: bilateral inferior fronto-occipital fasciculus, bilateral inferior longitudinal fasciculus (ILF), bilateral superior longitudinal fasciculus (SLF), forceps major and forceps minor, body of corpus callosum and bilateral cingulum (see Fig. 2 a). A significant decrease in FA was also observed in treatment-resistant chronic patients compared with first-episode patients (p < 0.05, FWE-corrected; cluster size: 10 458 mm2), affecting the body of the corpus callosum, bilateral SLF, forceps minor, forceps major, bilateral cingulum, and bilateral ILF (see Fig. 2 b).
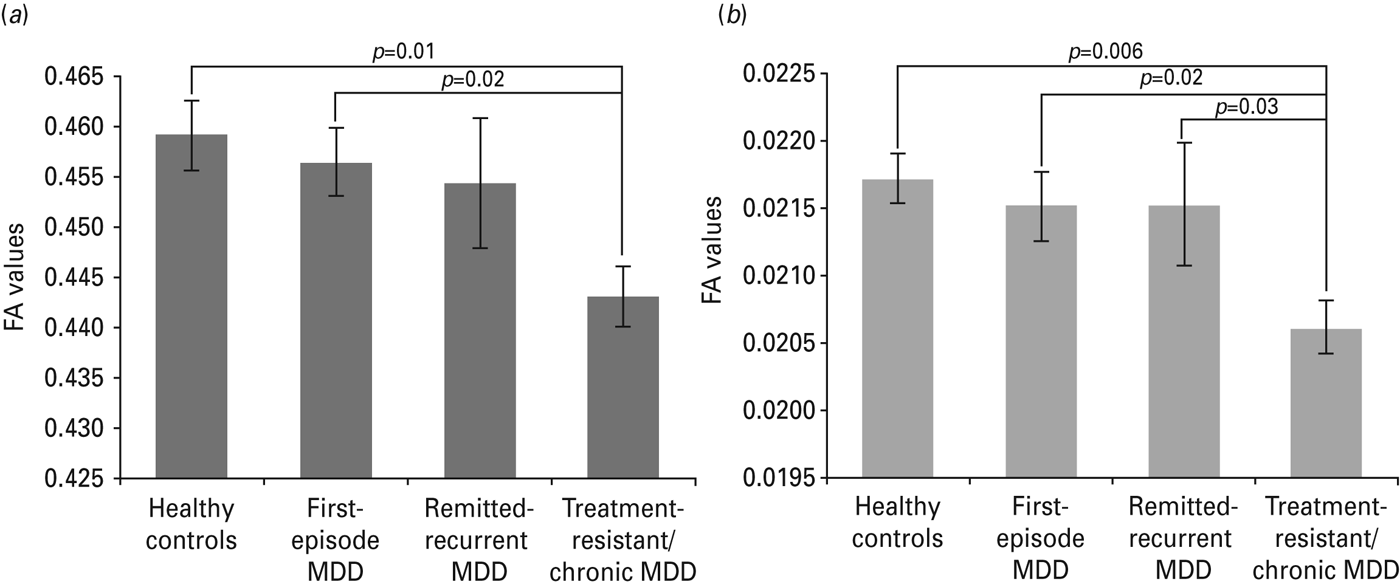
Fig. 1. Whole-brain (a) and ventromedial prefrontal region of interest (b) values of fractional anisotropy (FA) for healthy control and major depressive disorder (MDD) groups. Values are means, with standard deviations represented by vertical bars.

Fig. 2. Diffusion tensor imaging voxel-wise analysis of whole brains. Rows show selected axial, sagittal and coronal slices in which tracts with a significant decrease of fractional anisotropy (FA) is observed for healthy controls versus treatment-resistant/chronic major depressive disorder (MDD) patients (a) and for first-episode MDD patients versus treatment-resistant/chronic MDD patients (b). The background image is the Montreal Neurological Institute (MNI152) standard template 1 × 1×1 mm brain template (MNI coordinates). Green voxels represent the FA white-matter skeleton. Red voxels represent regions in which FA was significantly decreased. IFO, Inferior frontal-occipital; R, right; L, left; ILF, inferior longitudinal fasciculus; CC, corpus callosum; SLF, superior longitudinal fasciculus.
Differences in regional FA (ventromedial prefrontal cortex)
Mean FA values within medial prefrontal ROI are presented in Fig. 1 b. The one-way ANOVA showed a significant group effect (F 3,65 = 3.28, p = 0.03), which was maintained when medication load was controlled for (F 3,64 = 2.97, p = 0.04). Post-hoc tests showed that treatment-resistant/chronic MDD patients had significantly lower FA values than healthy controls (p = 0.006), first-episode MDD (p = 0.02) and also remitted-recurrent MDD patients (p = 0.03). As can be observed in Fig. 3, voxel-wise analyses confirmed a significant decrease of FA in treatment-resistant chronic patients compared with healthy controls and with the other patients (p < 0.05, FWE-corrected; cluster size: 164 mm2). Subregions of the uncinate fasciculus and corpus callosum were affected within this area.

Fig. 3. Diffusion tensor imaging voxel-wise analysis of the ventromedial prefrontal cortex (MFC). Blue squares indicate the place of the MFC mask provided by FSL (Smith et al. Reference Smith, Zhang, Jenkinson, Chen, Matthews, Federico and De Stefano2004) (left side). Red blobs correspond to significant reduced fractional anisotropy values (p < 0.05, family-wise error-corrected) in treatment-resistant/chronic major depressive disorder (MDD) patients compared with: (a) healthy controls (cluster size: 164 mm2), (b) first-episode MDD patients (cluster size: 116 mm2) and (c) remitted-recurrent MDD patients (cluster size: 74 mm2). Images represent coronal, axial and sagittal views, respectively. R, Right; L, left.
Influence of clinical variables on FA
HAMD scores, duration of illness, age at onset, number of previous episodes and medication load were entered in the stepwise multiple regression models. Duration of illness was the unique predictor of whole-brain mean FA, with a significant negative linear relationship (β = –0.49, p = 0.04). HAMD scores showed a marginal significant effect (β = –0.26, p = 0.06). The regression equation accounted for 15% of variance (R 2 = 0.15, F = 2.83, p = 0.05). Within ventromedial prefrontal ROI, HAMD scores (β = –0.29, p = 0.03) and number of previous episodes (β = –0.28, p = 0.04) were significant predictors of FA values, showing again a negative linear relationship; the regression model explained 12% of variance (R 2 = 0.12, F = 3.17, p = 0.05). No other clinical variable contributed significantly to the prediction of FA values in any of the regression models.
Discussion
We have observed widespread alterations in white-matter microstructure of patients with treatment-resistant/chronic MDD when compared with patients with a first-episode MDD and with healthy controls. Moreover, longer duration of illness and higher severity (this latter at a trend level) were associated with greater white-matter disruptions. Focusing on the ventromedial prefrontal region, the treatment-resistant/chronic group showed multiple affected fibres with significant FA reductions even when compared with patients with remitted-recurrent MDD. In this region, more previous episodes and higher severity of symptoms predicted white-matter abnormalities. Interestingly, findings remained unchanged after controlling for medication status. To our knowledge, this is the first DTI study that has investigated white-matter microstructure in different stages of non-geriatric MDD using TBSS methodology. Our results extend previous findings showing that microstructural white-matter anomalies may be present in middle-aged subjects with MDD but these are particularly heightened in patients with a chronic course and higher current and past burden of illness.
The reported abnormal FA values in patients with treatment-resistant/chronic depression involved multiple white-matter tracts. Among them, the SLF, cingulum, corpus callosum (including body, forceps major and forceps minor) and ILF deserve special attention given that significant differences within these tracts were found in comparison with both healthy controls and patients with a first-episode MDD. The SLF is a big bundle of association fibres that connects the dorsolateral prefrontal cortex and other frontal regions with the temporal, parietal and occipital lobes (Schmahmann & Pandya, Reference Schmahmann and Pandya2006). The cingulum bundle lies within the cingulate gyrus and is an important association pathway linking prefrontal and parahippocampal cortices (Schmahmann & Pandya, Reference Schmahmann and Pandya2006). Therefore, both of these tracts constitute key components of the fronto-temporal and fronto-limbic connections whose dysfunction is thought to underlie many of the emotional, cognitive and behavioural deficits associated with depression (Mayberg et al. Reference Mayberg, Liotti, Brannan, McGinnis, Mahurin, Jerabek, Silva, Tekell, Martin, Lancaster and Fox1999). On the other hand, diverse alterations in the shape and size of the corpus callosum – the largest interhemispheric bundle of the human brain – have been previously (although not consistently) described in depression (Walterfang et al. Reference Walterfang, Yücel, Barton, Reutens, Wood, Chen, Lorenzetti, Velakoulis, Pantelis and Allen2009; Sun et al. Reference Sun, Maller, Daskalakis, Furtado and Fitzgerald2009; MacMaster et al. Reference MacMaster, Carrey and Marie Langevin2013). The ILF is an association fibre tract that connects the occipital and temporal lobes, including the hippocampus and amygdala (Schmahmann & Pandya, Reference Schmahmann and Pandya2006), and could be related to emotion and visual processing information deficits of depression (Leyman et al. Reference Leyman, De Raedt, Schacht and Koster2007; Desseilles et al. Reference Desseilles, Balteau, Sterpenich, Dang-Vu, Darsaud, Vandewalle, Albouy, Salmon, Peters, Schmidt, Schabus, Gais, Degueldre, Phillips, Luxen, Ansseau, Maquet and Schwartz2009). Significant white-matter FA reductions observed herein along all these tracts agree well with preliminary DTI studies based on middle-aged patients with treatment-resistant depression (Zhou et al. Reference Zhou, Qin, Chen, Qian, Tao, Fang and Xu2011; Hoogenboom et al. Reference Hoogenboom, Perlis, Smoller, Zeng-Treitler, Gainer, Murphy, Churchill, Kohane, Shenton and Iosifescu2012; Guo et al. Reference Guo, Liu, Chen, Xu, Wu, Ma, Gao, Tan, Sun, Xiao, Chen and Zhao2012). Cole et al. (Reference Cole, Chaddock, Farmer, Aitchison, Simmons, McGuffin and Fu2012) have also reported extensive white-matter alterations heightened with increasing severity of symptoms in patients with long-lasting recurrent MDD. Our findings strengthen the view that a generalized altered neurocircuitry is present in MDD, especially in the most chronic and severe treatment-resistant forms of depression.
Longer duration of illness was predictive of greater white-matter microstructural abnormalities. Abe et al. (Reference Abe, Yamasue, Kasai, Yamada, Aoki, Inoue, Takei, Suga, Matsuo, Kato, Masutani and Ohtomo2010) observed a negative correlation between total days depressed and FA values in the right anterior cingulate and left frontal cortex. However, other DTI studies have failed to detect a relationship with duration of illness (Ma et al. Reference Ma, Li, Shu, Liu, Gong, He, Li, Tan, Stone, Zhang, Xu and Jiang2007; Zou et al. Reference Zou, Huang, Li, Gong, Li, Ou-yang, Deng, Chen, Li, Ding and Sun2008; Zhu et al. Reference Zhu, Wang, Xiao, Zhong, Liao and Yao2011; Guo et al. Reference Guo, Liu, Chen, Xu, Wu, Ma, Gao, Tan, Sun, Xiao, Chen and Zhao2012). This discrepancy could be due in part to differences in sample characteristics, such as low number of subjects or limited subsets of patients (including only those with either low or high past burden of illness). Previous evidence linking lifetime duration of illness with structural brain changes in depression is predominantly based on grey-matter volume data, in particular of the hippocampus and fronto-limbic regions (Frodl et al. Reference Frodl, Koutsouleris, Bottlender, Born, Jäger, Scupin, Reiser, Möller and Meisenzahl2008; McKinnon et al. Reference McKinnon, Yucel, Nazarov and MacQueen2009; Bora et al. Reference Bora, Harrison, Davey, Yücel and Pantelis2012). Although the cross-sectional study design demands a cautious interpretation, our findings expand these observations to white-matter microstructure, warning of further potential risks associated with long-term depression.
Defects observed within the ventromedial prefrontal cortex were provocative, since this brain region is known to play a crucial role on refractoriness to antidepressant treatment. Patients with treatment-resistant/chronic MDD showed abnormalities in subregions of the uncinate fasciculus and corpus callosum compared with the rest of the subjects, including patients with a remitted-recurrent MDD. These two groups shared a similar past clinical background except in the response to antidepressants. Consistently, current severity of symptoms as measured by the HAMD predicted lower FA values within the ventromedial region. This observation complements a previous study in which another altered measure of the uncinate fasciculus microstructure was associated with greater severity in patients with MDD (Zhang et al. Reference Zhang, Leow, Ajilore, Lamar, Yang, Joseph, Medina, Zhan and Kumar2012). The uncinate fasciculus connects the medial prefrontal cortex, including the subgenual region, with temporo-limbic structures crucial for mood disorders, such as the amygdala or hippocampus (Schmahmann & Pandya, Reference Schmahmann and Pandya2006). Modulation of neural activity in this distributed fronto-limbic network has been suggested to underlie the promising therapeutic effects of deep brain stimulation in the subgenual area of patients with severe forms of chronic treatment-resistant depression (Johansen-Berg et al. Reference Johansen-Berg, Gutman, Behrens, Matthews, Rushworth, Katz, Lozano and Mayberg2008). Our findings bring up the question whether deep brain stimulation achieves its effects by resolving or bypassing the white-matter anomalies observed in this subset of patients. Number of previous episodes was also associated with FA reductions within the ventromedial prefrontal area, which, taken as a whole, suggests that white-matter microstructure of this area may be related to recurrence and persistence of depression.
Changes in FA could represent multiple anatomopathological processes, such as changes in axonal density or axonal diameter, abnormal myelination or altered coherence of the fibre tracts (Sexton et al. Reference Sexton, Mackay and Ebmeier2009). The few post-mortem studies that have focused on middle-aged patients support an increased prevalence of deep white-matter lesions in unipolar depression (for a review, see Tham et al. Reference Tham, Woon, Sum, Lee and Sim2011). In particular, they have reported decreases in oligodendrocyte density (Uranova et al. Reference Uranova, Vostrikov, Orlovskaya and Rachmanova2004) and in deep white-matter myelin staining intensity within prefrontal areas (Regenold et al. Reference Regenold, Phatak, Marano, Gearhart, Viens and Hisley2007). Additional indirect evidence of white-matter damage in MDD come from nuclear magnetic spectroscopic studies that have described abnormal decreased N-acetylaspartate and increased choline-containing compound levels in the prefrontal cortex and hippocampus, particularly in patients with treatment-resistant/chronic depression and high past illness burden (Portella et al. Reference Portella, de Diego-Adeliño, Gómez-Ansón, Morgan-Ferrando, Vives, Puigdemont, Pérez-Egea, Ruscalleda, Álvarez and Pérez2011; de Diego-Adeliño et al. Reference de Diego-Adeliño, Portella, Gómez-Ansón, López-Moruelo, Serra-Blasco, Vives, Puigdemont, Pérez-Egea, Álvarez and Pérez2013). These alterations in N-acetylaspartate and choline signals presumably reflect neuronal/axonal integrity and aberrant cell membrane turnover, respectively (Wijtenburg et al. Reference Wijtenburg, McGuire, Rowland, Sherman, Lancaster, Tate, Hardies, Patel, Glahn, Hong, Fox and Kochunov2013).
Contrary to some prior studies (Ma et al. Reference Ma, Li, Shu, Liu, Gong, He, Li, Tan, Stone, Zhang, Xu and Jiang2007; Zhu et al. Reference Zhu, Wang, Xiao, Zhong, Liao and Yao2011), we did not detect any significant FA difference between patients with a first-episode MDD and healthy controls. These patients had started medication shortly before the scanning; hence, the likelihood that restorative effects of antidepressant treatment could have already emerged seems to be low. On the other hand, current analyses were specifically designed to test differences between patients with a low and high illness burden. We performed an ANOVA with four groups (rather than simply two) and applied a significance threshold based on FWE correction, the most rigorous method for avoiding type I errors and probably the one that unequivocally reflects true population-level differences (Lieberman & Cunningham, Reference Lieberman and Cunningham2009). Therefore, we cannot rule out the existence of white-matter anomalies in the first stages of MDD, but, in light of our results, these could be more subtle and perhaps limited to those patients who will subsequently develop a treatment-resistant disorder. In this regard, Zhou et al. (Reference Zhou, Qin, Chen, Qian, Tao, Fang and Xu2011) reported an association between low baseline FA values in the hippocampus and poor subsequent antidepressant response in a sample of non-geriatric patients.
Limitations
Several methodological issues of the present study deserve a comment. We employed an established protocol for the Philips 3 T scanner with a SENSE factor of 2, which provides a good balance between scan time and signal-to-noise ratio. Nevertheless, according to the current state of the art (e.g. Jones et al. Reference Jones, Knösche and Turner2013), there are now better acquisition parameters to ensure improved image quality for DTI research. For example, current protocols recommend a minimum of 20 gradient directions, b-values of at least 1000 s/mm2 or higher numbers of b = 0 volumes. The ‘crossing fibre problem’ is an acknowledged concern in DTI research (Jbabdi et al. Reference Jbabdi, Behrens and Smith2010). Some of the observed differences in FA among groups may actually correspond to differences in the number of crossing fibres. With regard to the analyses of data, we applied a TBSS approach using FWE correction and TFCE, which confers a meaningful advantage in ensuring accuracy and robustness of the findings. The sample was large and included a representative, well-characterized group of MDD out-patients, covering a broad spectrum of clinical illness burden, although our conclusions should be considered in the context of a cross-sectional study design. All patients were medicated at the time of scanning, which could represent a major limitation of the study. The inclusion of severely chronic ill patients prevented us from establishing a drug wash-out period because of obvious ethical concerns. The effects of treatment on white-matter microstructure have not been well established yet; several studies have not reported a clear influence (McIntosh et al. Reference McIntosh, Muñoz Maniega, Lymer, McKirdy, Hall, Sussmann, Bastin, Clayden, Johnstone and Lawrie2008; Wang et al. Reference Wang, Jackowski, Kalmar, Chepenik, Tie, Qiu, Gong, Pittman, Jones, Shah, Spencer, Papademetris, Constable and Blumberg2008; Sussmann et al. Reference Sussmann, Lymer, McKirdy, Moorhead, Muñoz Maniega, Job, Hall, Bastin, Johnstone, Lawrie and McIntosh2009) but some evidence suggests that medication might even attenuate FA abnormalities in certain patients (Yoo et al. Reference Yoo, Jang, Shin, Kim, Park, Moon, Chung, Lee, Kim, Kim and Kwon2007; Versace et al. Reference Versace, Almeida, Hassel, Walsh, Novelli, Klein, Kupfer and Phillips2008). Even though, we included an established index of medication load (Sackeim, Reference Sackeim2001; Almeida et al. Reference Almeida, Akkal, Hassel, Travis, Banihashemi, Kerr, Kupfer and Phillips2009) in the analyses to control for the potential confounding effects, and the results remained unaltered.
Conclusions
In summary, the present study aimed to investigate microstructural white-matter differences between patients with MDD in distinct stages of the illness and healthy controls, applying TBSS on DTI data. Our findings showed a generalized decrease of FA in patients with the poorest clinical response and the highest current and past illness burden. Longer duration of illness predicted lower FA at the whole-brain level whereas more previous episodes and greater severity of the symptoms were the negative predictors when focused on the ventromedial prefrontal area. These observations, in sum, support the notion that a disruption in cortical–subcortical networks, particularly the fronto-limbic connection, is associated with persistence of symptoms and treatment refractoriness. Whether abnormalities in white-matter microstructure are actually cumulative over time because of prolonged exposure to depression should be confirmed with longitudinal studies.
Acknowledgements
This study was funded by two grants of the Fondo de Investigación Sanitaria (FIS: PI 10/00372; FIS: 07/00770) from the Instituto de Salud Carlos III, by the Centro de Investigación Biomédica en Red de Salud Mental (CIBERSAM). J.d.D.-A. is funded by the Instituto de Salud Carlos III through a ‘Río Hortega’ research fellowship. M.S.-B. is funded by the Agència de Gestió d'Ajuts Universitaris i de Recerca of the Catalan Government through a pre-doctorate fellowship (FI-DGR 2012). M.J.P. is funded by the Ministerio de Ciencia e Innovación of the Spanish Government and by the Instituto de Salud Carlos III through a ‘Miguel Servet’ research contract (CP10-00393), co-financed by the European Regional Development Fund (ERDF) (2007–2013).
We thank Erick J. Canales-Rodríguez for his valuable comments. We also thank the staff of the Department of Psychiatry and of Neuroradiology of the Hospital de la Santa Creu i Sant Pau, and the staff of the medical imaging group at PIC for their assistance in this study. We are deeply grateful to all the individuals who participated in the present study for their kind cooperation.
Declaration of Interest
V.P. declares having received educational honoraria from: Servier, Lundbeck, Bristol-Myers, Pfizer, AstraZeneca and Eli Lilly, and he has participated as main local investigator in clinical trials for AstraZeneca, Eli Lilly and Bristol-Myers. E.Á. has received consulting and educational honoraria from several pharmaceutical companies including Servier, Eli Lilly, Lundbeck and Pfizer, and he has participated as main local investigator in clinical trials for Eli Lilly, Bristol-Myers and also as national coordinator of clinical trials for Servier and Lundbeck.






