Introduction
Obsessive-compulsive disorder (OCD) is a debilitating neuropsychiatric disorder that comprises two main symptom dimensions: obsessions, recurrent, persistent and intrusive thoughts, images or urges which cause anxiety and distress; compulsions, repetitive physical or mental acts aiming to reduce distress. OCD is a heritable condition with a lifetime prevalence of 2–3% (Karno, Golding, Sorenson, & Burnam, Reference Karno, Golding, Sorenson and Burnam1988; Weissman et al., Reference Weissman, Bland, Canino, Greenwald, Hwu, Lee and Wickramaratne1994). The family and twin studies have clearly established the heritability of OCD with genetic influences in the range of 45%–65% in children and 27%–47% in adults (Mathews, Delucchi, Cath, Willemsen, & Boomsma, Reference Mathews, Delucchi, Cath, Willemsen and Boomsma2014; van Grootheest, Cath, Beekman, & Boomsma, Reference van Grootheest, Cath, Beekman and Boomsma2005). However, specific genetic underpinnings of OCD remain unclear. To date, several genome-wide association studies (GWAS) have been conducted in OCD (Arnold et al., Reference Arnold, Askland, Barlassina, Bellodi, Bienvenu, Black and Zai2018; den Braber et al., Reference den Braber, Zilhão, Fedko, Hottenga, Pool, Smit and Boomsba2016), however, the findings of these studies regarding OCD-related single-nucleotide polymorphisms (SNPs) are highly inconsistent. In addition to relatively small sample sizes of available GWAS studies, clinical and etiological heterogeneity of OCD are most likely factors underlying our limited success in identifying contributory genes of OCD. Further GWAS studies including improved efforts for clinical phenotyping is necessary. However, one may argue that OCD cannot be differentiated into genetically homogenous subgroups by only using symptoms and signs of the illness (phenotypic markers). Some neurobiological and neurocognitive measures, which might potentially be closer to underlying genetic etiology than symptoms (intermediate markers) might have a utility in aiding genetic research. This approach is also consistent with Research Domain Criteria (RDoC) initiative (Insel et al., Reference Insel, Cuthbert, Garvey, Heinssen, Pine, Quinn and Wang2010), a framework based on the premise that clinical constructs should be defined (and therefore investigated) on the basis of their neurobiological validity. In this context, abnormalities in fronto-striatal circuits such as imbalances between ventral and dorsal fronto-striatal recruitment and fronto-striatal reward prediction errors have been advocated as potential neurobiological underpinnings of symptoms of OCD and associated cognitive deficits (Chamberlain, Blackwell, Fineberg, Robbins, & Sahakian, Reference Chamberlain, Blackwell, Fineberg, Robbins and Sahakian2005; Figee et al., Reference Figee, Pattij, Willuhn, Luigjes, van den Brink, Goudriaan and Denys2016; Snyder, Kaiser, Warren, & Heller, Reference Snyder, Kaiser, Warren and Heller2015).
Considerable evidence suggests that OCD is associated with neurocognitive deficits that can be related to abnormalities in fronto-striatal networks (Abramovitch, Abramowitz, & Mittelman, Reference Abramovitch, Abramowitz and Mittelman2013; Chamberlain et al., Reference Chamberlain, Blackwell, Fineberg, Robbins and Sahakian2005; Harrison et al., Reference Harrison, Soriano-Mas, Pujol, Ortiz, López-Solà, Hernández-Ribas and Cardoner2009; Menzies et al., Reference Menzies, Chamberlain, Laird, Thelen, Sahakian and Bullmore2008; Purcell, Maruff, Kyrios, & Pantelis, Reference Purcell, Maruff, Kyrios and Pantelis1998; Shin, Lee, Kim, & Kwon, Reference Shin, Lee, Kim and Kwon2014; Snyder et al., Reference Snyder, Kaiser, Warren and Heller2015). A meta-analysis of neuropsychological studies conducted in OCD reported cognitive deficits in several domains including executive functions (EF), memory, processing speed and fluency (Shin et al., Reference Shin, Lee, Kim and Kwon2014). While OCD was associated with deficits in a wide range of tests, effect sizes of these deficits were not very large (Cohen d = 0.4–0.6) for most cognitive tests. In the same meta-analysis (Shin et al., Reference Shin, Lee, Kim and Kwon2014), relatively larger effect sizes were found for deficits in visual memory and planning ability as measured by Tower of London/Tower of Hanoi tests (d > 0.7). However, it is not certain whether these findings are trait features of OCD as cognitive impairment in OCD might indicate state-dependent phenomena and can fluctuate based on the change in the severity of the obsessive and compulsive symptoms. Moreover, confounding factors including co-morbidity (i.e. depression) and medications can also contribute to cognitive impairment in OCD. Studies in unaffected first-degree relatives of patients with OCD (OCDrel) might be particularly relevant to identify cognitive markers of underlying core neurobiological abnormalities in this disorder and such cognitive markers might aid to define biologically valid subgroups of OCD.
In other areas of psychiatry such as schizophrenia-spectrum disorders and bipolar disorders, there is a large number of published studies and meta-analyses investigating cognitive deficits in unaffected first-degree relatives (Bora, Reference Bora2017; Bortolato, Miskowiak, Köhler, Vieta, & Carvalho, Reference Bortolato, Miskowiak, Köhler, Vieta and Carvalho2015). In contrast, cognitive deficits in OCDrel is a relatively understudied area. Still, a number of studies have investigated neurocognitive deficits in OCD over the years. However, the findings of these studies are contradictory. While some available studies reported cognitive deficits in OCDrel (Bey et al., Reference Bey, Kaufmann, Lennertz, Riesel, Klawohn, Heinzel and Wagner2018; Cavedini, Zorzi, Piccinni, Cavallini, & Bellodi, Reference Cavedini, Zorzi, Piccinni, Cavallini and Bellodi2010), others found no group difference between OCDrel and healthy controls (Lennertz et al., Reference Lennertz, Rampacher, Vogeley, Schulze-Rauschenbach, Pukrop, Ruhrmann and Wagner2012; Lochner, Chamberlain, Kidd, Fineberg, & Stein, Reference Lochner, Chamberlain, Kidd, Fineberg and Stein2016). No previous meta-analysis has explored cognitive functioning in OCDrel in comparison to healthy controls and patients with OCD (OCDpt).
The primary aim of the current systematic review was to conduct a meta-analysis of cognitive functioning in OCDrel. The secondary aim of the current meta-analysis was to explore cognitive differences between OCDrel and OCDpt.
Methods
Data source and study selection
PRISMA guidelines were used in conducting this meta-analysis (Moher, Liberati, Tetzlaff, & Altman, Reference Moher, Liberati, Tetzlaff and Altman2009). A literature search was conducted using the databases PubMed and Scopus to identify the relevant studies (01 January 1980 to 28 February 2019). The combination of the following keywords was used in searching the literature; ‘obsessive-compulsive disorder’ AND (relatives OR siblings OR parents OR offspring) AND (Cogn* OR Neuropsycho*). Reference lists of published reports and reviews were also searched for additional studies. Inclusion criteria for the studies were: (1) cognitive differences between OCDrel and healthy controls were investigated; (2) reported sufficient data to calculate the effect size and standard error for group difference between OCDrel and healthy controls. Studies sharing participants with another study that was already selected were excluded unless they report a different cognitive measure.
All stages of literature search including initial screening stage to final data extraction phase were performed separately by E.B and B.Y (See Acknowledgement section). Meta-analyses were conducted for cognitive domains that were investigated in at least three independent OCDrel samples. Five EF domains investigated were planning/problem solving, set-shifting, inhibition, decision-making and working memory. Other cognitive domains included were verbal memory, visual memory, processing speed, and fluency. The individual tests summarized under each cognitive domain is provided in online Supplementary eTable 1s (supplement). When there were a sufficient number of studies, in addition to meta-analyses of cognitive domains, meta-analyses for individual cognitive tasks were also conducted under each cognitive domain. This is a common practice in meta-analyses to partially tackle the issues related to the inclusion of similar tasks with different psychometric properties under a cognitive domain. In studies including an OCDpt group, relevant data for the comparison of OCDpt v. OCDrel and OCDpt v. healthy controls were also coded. Yale-Brown Obsessive-Compulsive Scale (YBOCS) ratings and depression rating scores [Montgomery-Åsberg Depression Rating Scale (MADRS), Beck Depression Inventory (BDI) and Hamilton Depression Rating Scale (HDRS)] were also coded.
Statistical analyses
Meta-analyses were performed using ‘metafor’ package (version 2.1-0) in R (version 3.6.0) environment (Viechtbauer, Reference Viechtbauer2010). Effect sizes (Cohen's d) were calculated for OCDrel v. healthy controls, OCDpt v. OCDrel and OCDpt v. healthy control contrasts. Calculated Cohen's d values were multiplied by −1 to have positive effect sizes for the results indicating the expected direction (Healthy controls|>OCDrel>OCD). Effect sizes were weighted using the inverse variance method and a random-effects model. Homogeneity of the distribution of weighted effect sizes was tested with the Q-test, and I 2 (I 2 values <50% indicate low heterogeneity, I 2 > 50% indicate moderate heterogeneity and I 2 > 75% indicate large heterogeneity). The possibility of publication bias was assessed by regression analysis of the funnel plot of Cohen's d and standard error (Egger's test).
Meta-regression analyses were conducted to investigate the effect of demographic [age, sex (ratio of males in OCD/OCDrel sample), effect size of the difference of duration of education between OCD/OCDrel and healthy controls)], clinical (depression severity, OCD symptom severity in OCD/OCDrel) variables on cognitive differences between OCD/OCDrel and healthy controls. Another set of meta-regressions were conducted to investigate the effect of severity of symptoms (YBOCS, depression ratings) in OCDpt and the level of matching for age, duration of education, sex (OCDpt v. OCDrel) on cognitive differences between OCDpt and OCDrel. Cognitive variables included were average effect sizes for EF and non-EF (nEF). In addition, individual cognitive domains were used in meta-regression analyses only if eight or more studies reported both particular cognitive domain and variable of interest. For the meta-regression analysis of depression scores BDI and HDRS scores were converted to MADRS based on published equations (Furukawa et al., Reference Furukawa, Reijnders, Kishimoto, Sakata, DeRubeis, Dimidjian and Cuijpers2019; Leucht, Fennema, Engel, Kaspers-Janssen, & Szegedi, Reference Leucht, Fennema, Engel, Kaspers-Janssen and Szegedi2018).
Results
The selection process is summarized in online Supplementary eFigure 1. Current meta-analysis included 16 studies (Table 1).
Table 1. Characteristics of the studies included in the meta-analysis

IGT, Iowa gambling task; TMT, Trail making test; WCST, Wisconsin card sorting test; ToL, Towel of London; ToH, Tower of Hanoi; CPT, continuous performance test; LNS, letter number sequencing; CGT, Cambridge gambling task; DAT, delayed alternation task; OCD, obsessive-compulsive disorder; OCDrel, unaffected relatives of patients with OCD; HC, healthy controls, OCDpt, patients with OCD.
OCDrel v. healthy controls
The OCDrel-healthy control meta-analysis included 16 studies consisting of 527 (58.0% females) OCDrel and 639 healthy controls (56.9% females) (Table 1). OCDrel group was significantly older than healthy controls (d = 0.32, 0.18–0.45, p < 0.001). The duration of education was modestly but significantly shorter in OCDrel compared to healthy controls (d = 0.16, CI = 0.01–0.32, p = 0.04). Co-morbidity of depression and other psychiatric disorders in OCDrel were excluded in all but three studies. Mean depression ratings in these three studies were below criteria for euthymia (MADRS score <10).
OCDrel underperformed healthy controls in inhibition (d = 0.58, CI = 0.29–0.86) (Fig. 1a), planning (d = 0.44, CI = 0.25–0.64) (Fig. 1b), decision-making (d = 0.58, CI = 0.19–0.98) (online Supplementary eFigure2), set-shifting (d = 0.37, CI = 0.04–0.69) (online Supplementary eFigure3) and visual memory (d = 0.28, CI = 0.08–0.49) (online Supplementary eFigure4) but not in verbal memory (d = 0.20, CI = −0.12 to 0.53), fluency (d = 0.09, CI = −0.09 to 0.27), processing speed (d = 0.09, CI = −0.12 to 0.30) and working memory (d = 0.19, CI = −0.02 to 0.41). In meta-analyses of individual cognitive tasks, OCDrel significantly underperformed healthy controls in ToH/ToL, Stop signal, Stroop interference tests (Table 2). There was significant evidence for heterogeneity of distribution of effect sizes for inhibition (I 2 = 66%), set-shifting (I 2 = 71%), decision-making (I 2 = 64%) and verbal memory (I 2 = 66%) but not for other cognitive domains. There was no evidence for publication bias for any of the cognitive measures.

Fig. 1. Forest plot of the meta-analysis of group differences in inhibition and planning/problem solving between OCDrel and healthy controls; (a) inhibition; (b) planning/problem solving.
Table 2. Mean weighted effect sizes for differences between unaffected first-degree relatives of individuals with OCD and healthy controls

OCDrel, unaffected relatives of individuals with OCD, HC, healthy controls, d, Cohen's d, SSRT, stop signal reaction time; WCST, Wisconsin card sorting test, per errors, perseverative errors. Bold values: Significant underperformance of OCDrel compared to healthy controls.
a Individual task analysis for set-shifting.
b Individual; task analysis for inhibition.
c Individual task analysis for processing speed.
d Individual task analysis for fluency.
Meta-regression analyses found no significant effect of age (Z = 0.3–1.3, p = 0.20–0.63), sex (Z = −0.6 to 0.1, p = 0.56–0.71), YBOCS (Z = 0.2, p = 0.87) and depression (Z = −0.7 to 0.7, p = 0.45–0.46) ratings on the degree of cognitive differences between OCDrel and healthy controls. Shorter duration of education in OCDrel compared to healthy controls was associated with larger effect sizes for group comparison for EF (Z = 2.3, p = 0.02) but not nEF (Z = 0.9, p = 0.35) between OCDrel and healthy controls.
OCDpt v. healthy controls
The OCDpt-healthy control meta-analysis included 13 studies consisting of 445 OCDpt (49.4% females) and 537 healthy controls (49.3% females) (Table 1). There were no significant between-group differences for age (d = −0.10, −0.23 to 0.03, p = 0.11) and duration of education (d = 0.12, CI = −0.04 to 0.27, p = 0.14).
As expected, healthy controls overperformed OCDpt in all cognitive domains (d = 0.36–0.86) (Table 3). The largest effect sizes were found for visual memory (d = 0.86, CI = 0.57–1.16) and inhibition (d = 0.86, CI = 0.45–1.26) domains. The distributions of effect sizes were homogenous for visual memory, planning, set-shifting, working memory and fluency. However, the distributions of effect sizes were significantly heterogeneous for inhibition, decision-making, verbal memory and processing speed (I 2 = 66–80%). There was no evidence of publication bias.
Table 3. Mean weighted effect sizes for differences between OCDpt and healthy controls

OCDpt, patients with OCD, HC, healthy controls, d, Cohen's d. Bold values: Significant underperformance of OCDpt compared to healthy controls.
Meta-regression analyses found no significant effect of age (Z = 0.2–0.6, p = 0.56–0.82), duration of education (Z = 0.5 to 1.3, p = 0.18–0.58), sex (Z = 0.3, p = 0.74–0.79), YBOCS (Z = −0.2 to 1.1, p = 0.28–0.87) and depression (Z = −0.8 to 0.2, p = 0.41–0.80) ratings on the degree of cognitive differences between OCDpt and healthy controls.
OCDpt v. OCDrel
The OCDpt-OCDrel meta-analysis included 13 studies consisting of 445 OCDpt (49.4% females) and 408 OCDrel (51.2% females) (Table 1). The groups were well matched for age (d = −0.05, CI = −0.20 to 0.10, p = 0.48) and duration of education (d = 0, CI = −0.16 to 0.16, p = 0.96).
In meta-analyses of cognitive domains, OCDpt underperformed OCDrel in visual memory (d = 0.45, CI = 0.22–0.67) and set-shifting (d = 0.23, CI = 0.04–0.42) (Table 4). OCDpt also tended to underperform OCDrel in verbal memory and working memory (d = 0.29–0.36, p = 0.08–0.10). There were no significant group differences between OCDrel and OCDpt for planning (d = 0.05, CI = −0.20 to 0.31), inhibition (d = 0.10, CI = −0.09 to 0.29), fluency (d = 0.14, CI = −0.07 to 0.37), decision-making (d = 0.13, CI = −0.12 to 0.38 and processing speed (d = 0.18, CI = −0.05 to 0.40). The distributions of effect sizes were homogeneous except verbal memory (Q = 13.0, p = 0.01, I 2 = 69%) and working memory (Q = 9.9, p = 0.04, I 2 = 60%). There was no evidence for publication bias for any of the cognitive measures.
Table 4. Mean weighted effect sizes for differences between OCDpt and unaffected first-degree relatives of individuals with OCD
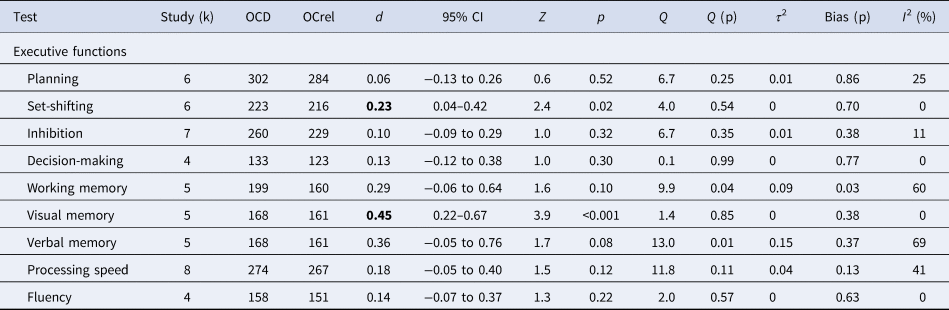
OCDpt, OCD patients, OCDrel, unaffected relatives of individuals with OCD, d, Cohen's d. Bold values: Significant underperformance of OCDpt compared to OCDrel.
Meta-regression analyses found no significant effect of age (Z = 0.5–1.3, p = 0.18–0.62), duration of education (Z = 0.4 to 1.4, p = 0.15–0.66), sex (Z = −0.2 to −0.9, p = 0.33–0.83), YBOCS (Z = −1.1, p = 0.26–0.28) and depression (Z = −0.8 to 1.7, p = 0.08–0.40) ratings on the degree of cognitive differences between OCDpt and OCDrel.
Discussion
The findings of this meta-analysis suggest that both OCDrel and OCDpt have significant neurocognitive abnormalities in inhibition, planning/problem solving and decision-making. In addition to shared cognitive deficits, OCDpt had widespread cognitive abnormalities. OCDpt underperformed OCDrel in several cognitive domains and between-group difference was relatively pronounced for visual memory.
Current findings suggest that abnormalities in inhibition (OCDrel v. controls: d = 0.58; OCDpt v. controls: d = 0.86), planning/problem solving (OCDrel v. controls: d = 0.44; OCDpt v. controls: d = 0.54) and reward-based decision-making (OCDrel v. controls: d = 0.58; OCDpt v. controls: d = 0.63) are trait markers related to vulnerability for developing OCD. Both OCDpt and OCDrel were characterized by a significant and a similar level of impairment in these functions (except relatively pronounced impairment in inhibition in OCDpt). There were no significant differences in these domains in direct comparisons of OCDpt and OCRrel. These deficits are likely related to functional abnormalities in fronto-striatal circuits, which is a consistent finding in OCD (Chamberlain et al., Reference Chamberlain, Blackwell, Fineberg, Robbins and Sahakian2005; Norman et al., Reference Norman, Taylor, Liu, Radua, Chye, De Wit and Fitzgerald2019; Vaghi et al., Reference Vaghi, Hampshire, Fineberg, Kaser, Brühl, Sahakian and Robbins2017). These findings are consistent with a number of hypotheses linking OCD to abnormalities in performance monitoring, goal-directed planning and reward prediction errors (Gillan, Fineberg, & Robbins, Reference Gillan, Fineberg and Robbins2017; Norman et al., Reference Norman, Taylor, Liu, Radua, Chye, De Wit and Fitzgerald2019; Riesel et al., Reference Riesel, Klawohn, Grützmann, Kaufmann, Heinzel, Bey and Kathmann2019). Impairment in inhibitory control in OCDrel is not surprising as enhanced error-related negativity is the most consistent biomarker of OCD (Gillan et al., Reference Gillan, Fineberg and Robbins2017; Norman et al., Reference Norman, Taylor, Liu, Radua, Chye, De Wit and Fitzgerald2019; Riesel, Endrass, Kaufmann, & Kathmann, Reference Riesel, Endrass, Kaufmann and Kathmann2011). Recent evidence, extending enhanced error signals to reward-related stimuli, might be particularly important to understand decision-making abnormalities in OCD. OCD might be associated with underactivation in the ventral striatum and ventromedial orbitofrontal cortex in decision-making task and increased reward prediction errors in ACC and putamen (Hauser et al., Reference Hauser, Iannaccone, Dolan, Ball, Hättenschwiler, Drechsler and Brem2017; Norman et al., Reference Norman, Carlisi, Christakou, Murphy, Chantiluke, Giampietro and Rubia2018). Similarly, increased activation in regions related to performance monitoring along with decreased dorsolateral prefrontal-striatal responsiveness and connectivity in OCD might underlie deficits in goal-directed planning (Vaghi et al., Reference Vaghi, Hampshire, Fineberg, Kaser, Brühl, Sahakian and Robbins2017; van den Heuvel et al., Reference van den Heuvel, Veltman, Groenewegen, Cath, van Balkom, van Hartskamp and van Dyck2005). As a result, cognitive deficits related to fronto-striatal networks are likely shared features of OCDpt and OCDrel.
Selective deficits in inhibition, planning/problem solving and reward-based decision-making might be relevant to RDoC approach as these deficits might be directly related to symptoms of OCD. For example, inhibition abnormalities may underlie deficits in stopping unwanted compulsive behaviors in the disorder and reward prediction errors/decision-making difficulties might explain uncertainty, indecisiveness and feeling of ‘not just right’ in OCD. Current findings also suggest that deficits in inhibition, planning/problem solving and reward-based decision-making are important candidates for being endophenotypes of OCD. Most of the previous studies investigating neuropsychological findings as endophenotypes of psychiatric disorders were conducted in schizophrenia (Bortolato et al., Reference Bortolato, Miskowiak, Köhler, Vieta and Carvalho2015). However, the endophenotype paradigm in schizophrenia had limited success so far. This is not surprising as cognitive deficits in schizophrenia seem to relate mostly to premorbid global cognitive development not directly linked to the emergence of symptoms of this disorder and effect sizes of cognitive deficits in unaffected relatives of schizophrenia are modest compared to their probands (Bora, Reference Bora2015). In contrast, unlike in schizophrenia, some cognitive deficits in OCD might have better potential in aiding risk genes as they are more selective and the severity of deficits in inhibition, planning/problem solving and reward-based decision-making are similar in OCDrel and their probands (in schizophrenia, they are quite modest in relatives compared to patients) and might be directly related to neurobiological abnormalities causing symptoms of OCD.
In addition to shared cognitive deficits in OCDpt and OCDrel, OCDpt seems to have cognitive deficits. In the current meta-analysis, OCDrel significantly overperformed OCDpt in visual memory and set-shifting abilities in which OCDrel also had small-sized abnormalities. Consistent with previous meta-analyses, nonverbal memory deficits were particularly robust in OCDpt (Abramovitch et al., Reference Abramovitch, Abramowitz and Mittelman2013; Shin et al., Reference Shin, Lee, Kim and Kwon2014). Relative robustness of visual compared to verbal memory deficits in OCD suggests that deficits in non-memory components (i.e. visual imagery, visual organization) of nonverbal memory tasks might contribute to such findings. Beyond the traditional view on fronto-striatal abnormalities, OCD is associated with structural and functional abnormalities in the inferior parietal lobe, which is important for visual processing and imagery. OCD is also associated with abnormal resting-state connectivity in fronto-parietal networks and failure to deactivate default mode network during cognitive tasks (Fouche et al., Reference Fouche, du Plessis, Hattingh, Roos, Lochner, Soriano-Mas and van den Heuvel2017; Gonçalves et al., Reference Gonçalves, Soares, Carvalho, Leite, Ganho-Ávila, Fernandes-Gonçalves and Sampaio2017; Gürsel, Avram, Sorg, Brandl, & Koch, Reference Gürsel, Avram, Sorg, Brandl and Koch2018; Koçak, Özpolat, Atbaşoğlu, & Çiçek, Reference Koçak, Özpolat, Atbaşoğlu and Çiçek2011; Stern, Fitzgerald, Welsh, Abelson, & Taylor, Reference Stern, Fitzgerald, Welsh, Abelson and Taylor2012). In addition, regions involved in self-referential processing including precuneus and posterior cingulate cortex seem to be overactive in OCD during cognitive tasks (Rasgon et al., Reference Rasgon, Lee, Leibu, Laird, Glahn, Goodman and Frangou2017). Set-shifting abnormalities in OCDpt might also be related to underactivation in fronto-parietal networks (Morein-Zamir et al., Reference Morein-Zamir, Voon, Dodds, Sule, van Niekerk, Sahakian and Robbins2016). Current findings suggest that OCDpt experience cognitive difficulties beyond fronto-striatal network-related neuropsychological abnormalities that they share with their unaffected relatives and abnormalities in fronto-parietal and default mode networks are likely playing an important role in these additional cognitive difficulties observed in OCDpt. It is also important to note that visual memory and set-shifting abnormalities might potentially be biomarkers of incipient illness or subclinical presentation in the minority of OCDrel who present with these abnormalities. However, longitudinal studies at young individuals with familial risk for OCD are needed to test this hypothesis.
Cognitive differences between OCDpt and OCDrel might also be related to state related to factors and the emergence of symptoms in OCDpt can exacerbate cognitive deficits. OCD prone individuals who have an underlying difficulty in goal-directed learning, can have further difficulty as the environment becomes more complex, responsibilities of the individual and expectations of the society increase over time. Underlying perfectionism (Frost & Steketee, Reference Frost and Steketee1997) and fear of being wrong can impair processing speed and set-shifting abilities. Stress and anxiety associated with OCD symptoms can further promote habitual behavior and impair memory in OCDpt. Memory deficits and processing speed abnormalities can have a negative impact on self-esteem and level of confidence about facts and in turn, might lead to increased OCD symptoms such as checking. In return, repetitive checking can further delay the amount of time it takes to complete an assignment at work or in school. More severe deficits in OCDpt compared to OCDrel in visual memory (d = 0.86 v. 0.28), set-shifting (d = 0.48 v. 0.37), verbal memory (d = 0.52 v. 0.20) and inhibition (d = 0.86 v. 0.56) might be partly related to such factors. A recent meta-analysis investigated the relationship between cognitive functions and symptom severity in OCD and found only a small-to-moderate degree of association between OCD symptom severity and cognitive functions (Abramovitch, McCormack, Brunner, Johnson, & Wofford, Reference Abramovitch, McCormack, Brunner, Johnson and Wofford2019). Current meta-regression analyses found no significant relationship between symptoms and cognition in OCD which might be partly related to insufficient power of meta-regression analyses to detect small effects as they were based on a relatively small number of studies. These findings suggest that cognitive deficits in OCD are mostly independent of the severity of the symptoms. However, some aspects of the relationship between symptoms and cognitive functions might be non-linear and not completely detectable with correlational analyses.
It is also important to note that there was significant heterogeneity in some cognitive domains in both OCDpt and OCDrel reflecting the contradictory findings in the published literature. Of note, there were relatively large levels of heterogeneity for inhibition, decision-making and verbal memory both in OCDpt and OCDrel groups. Meta-regression analyses were not able to explain the source of heterogeneity in this meta-analysis except some evidence for the effect of differences in the level of education. Due to the lack of relevant data in the available reports, it was not possible to investigate a number of alternative hypotheses which can explain the heterogeneity of cognitive functions in OCDpt and OCDrel. One potential explanation might be the potential impact of cognitive deficits related to different dimensions of OCD. There might be cognitive differences between clinical subgroups of OCD (i.e; obsessing/checking (O/C) v. symmetry/ordering (S/O); autogenous v. reactive) (Abramovitch & Cooperman, Reference Abramovitch and Cooperman2015; Bragdon, Gibb, & Coles, Reference Bragdon, Gibb and Coles2018; Fan et al., Reference Fan, Liu, Lei, Cai, Zhong, Dong and Zhu2016; Lee, Yost, & Telch, Reference Lee, Yost and Telch2009) and none of the studies included in this review explored the effect of symptoms dimensions in the probands of the OCDrel. Neurobiological subtypes including ‘neurodevelopmental’ and ‘Tic-related OCD’ subtypes might potentially be associated with distinctive cognitive deficits (Blanes & McGuire, Reference Blanes, McGuire, Keshavan and Murray1997; Castle & Phillips, Reference Castle and Phillips2006; Kloft, Steinel, & Kathmann, Reference Kloft, Steinel and Kathmann2018; Leckman, Bloch, & King, Reference Leckman, Bloch and King2009; Saxena, Brody, Schwartz, & Baxter, Reference Saxena, Brody, Schwartz and Baxter1998). Unfortunately, the studies included in this meta-analysis did not include relevant data to investigate the relationship between cognitive functioning and neurobiological subtypes of OCD in the patient sample or in probands of OCDrel sample.
The novel contribution of the current systematic review was that a meta-analytic comparison of OCDrel with healthy controls and OCDpt was conducted. Current meta-analysis has several limitations. As discussed above, no information was given about putative subtypes of OCD (in the patients or family) and studies included had a cross-sectional design. In addition, the number of available studies was small for some cognitive dimensions. Also, one cannot rule out the possibility that some findings in OCDrel might reflect proneness for psychopathology in general rather than being related to core OCD dimensions. Finally, it was not possible to investigate some cognitive domains including sustained attention and social cognition.
In conclusion, the current meta-analysis indicated that OCDrel and OCDpt shared deficits in inhibition, planning/problem solving and reward-based decision-making abilities which can be considered as endophenotypes of OCD. In addition, OCD patients presented with cognitive deficits in other domains. OCD patients significantly underperformed OCDrel in visual memory and set-shifting abilities. Exploration of cognitive subgroups in OCDrel and investigating the effects of subtype of OCD in probands on cognitive impairment in OCDrel are needed.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291720001634.
Acknowledgements
I would like to thank Berna Yalınçetin for her support in coding the data and literature search.
Conflict of interest
None







