Introduction
Bipolar disorder (BP) is associated with cognitive deficits in a number of domains including executive functions, processing speed, attention, memory and social cognition (Arts et al. Reference Arts, Jabben, Krabbendam and van Os2008; Bora et al. Reference Bora, Yucel and Pantelis2009a , Reference Bora, Bartholomeusz and Pantelis2016a , Reference Bora, Hıdıroğlu, Özerdem, Kaçar, Sarısoy, Civil Arslan, Aydemir, Cubukcuoglu Tas, Vahip, Atalay, Atasoy, Ateşci and Tümkaya b , Reference Bora, Veznedaroğlu and Vahip c , Reference Bora, Akdede and Alptekin2017; Cardenas et al. Reference Cardenas, Kassem, Brotman, Leibenluft and McMahon2016). The cognitive impairment in BP is relatively less severe than deficits observed in schizophrenia, but cognitive profile of both disorders are similar (Krabbendam et al. Reference Krabbendam, Arts, van Os and Aleman2005; Bora et al. Reference Bora, Yucel and Pantelis2009b ). However, it has been argued that cognitive deficits in BP and schizophrenia might have very different trajectories (Demjaha et al. Reference Demjaha, MacCabe and Murray2012). In schizophrenia, neurodevelopmental abnormalities play a major role in cognitive deficits (Weinberger, Reference Weinberger, Nasrallah and Weinberger1986; Murray & Lewis, Reference Murray and Lewis1987; Bora, Reference Bora2015a ). In contrast to findings in schizophrenia, a number of studies have suggested normal, at times superior, cognitive abilities and school achievement in children and adolescents who develop adult BP (Kumar & Frangou, Reference Kumar and Frangou2010; Bora, Reference Bora2015b ). It has been proposed that developmental cognitive abnormalities might be specific to schizophrenia (Murray et al. Reference Murray, Sham, Van Os, Zanelli, Cannon and McDonald2004; Kahn & Keefe, Reference Kahn and Keefe2013), and BP only develops cognitive deficits during the course of illness as a result of neurodegenerative changes (Goodwin et al. Reference Goodwin, Martinez-Aran, Glahn and Vieta2008).
The findings of some cross-sectional studies reporting a relationship between a larger number of previous episodes and severity of cognitive deficits have been interpreted as an evidence for illness-related progressive cognitive decline in BP (Hellvin et al. Reference Hellvin, Sundet, Simonsen, Aminoff, Lagerberg, Andreassen and Melle2012; Cardoso et al. Reference Cardoso, Bauer, Meyer, Kapczinski and Soares2015; Passos et al. Reference Passos, Mwangi, Vieta, Berk and Kapczinski2016). For example, López-Jaramillo et al. (Reference López-Jaramillo, Lopera-Vásquez, Gallo, Ospina-Duque, Bell, Torrent, Martínez-Arán and Vieta2010) found that those patients who had experienced three or more manic episodes were more impaired in cognitive functions when compared with patients who had a history of only one or two episodes. However, the cognitive dysfunction in BP might be a severity marker rather than being the consequence of cumulative effect of mood episodes. In the study of López-Jaramillo et al. (Reference López-Jaramillo, Lopera-Vásquez, Gallo, Ospina-Duque, Bell, Torrent, Martínez-Arán and Vieta2010), duration of illness was similar between groups, but the first group had an average of six manic episodes, unlike others who had one or two. Therefore, it is quite likely that, underlying illness and its neurodevelopmental markers are much more severe in the former group characterized by a larger number of manic episodes. It is not possible to understand the nature of relationship between illness course and neurocognition based on cross-sectional studies.
Only longitudinal neuropsychological studies might provide evidence for neurodegeneration and progressive cognitive decline in BP. In schizophrenia, in accordance with neurodevelopmental view, longitudinal studies have found that cognitive deficits in chronic and first-episode patients remain stable or modestly improve overtime (Rund, Reference Rund1998; Szöke et al. Reference Szöke, Trandafir, Dupont, Méary, Schürhoff and Leboyer2008; Bora & Murray, Reference Bora and Murray2014; Heilbronner et al. Reference Heilbronner, Samara, Leucht, Falkai and Schulze2016). Practice effects, clinical improvement and treatment effects might play a role in cognitive change in schizophrenia and BP. However, the course of cognitive deficits in BP in comparison with schizophrenia and healthy controls remains a controversial subject. A preliminary meta-analysis of Samamé et al. (Reference Samamé, Martino and Strejilevich2014) found that cognitive deficits in BP remained stable after the follow-up period. However, the number of available studies for each cognitive measure was small and no comparison with longitudinal course of schizophrenia was possible. Recently, increasing number of studies have investigated the course of cognitive deficits in BP (Santos et al. Reference Santos, Aparicio, Bagney, Sánchez-Morla, Rodríguez-Jiménez, Mateo and Jiménez-Arriero2014; Torres et al. Reference Torres, Kozicky, Popuri, Bond, Honer, Lam and Yatham2014; Daglas et al. Reference Daglas, Allott, Yücel, Pantelis, Macneil, Berk and Cotton2015; Lee et al. Reference Lee, Hermens, Naismith, Lagopoulos, Jones, Scott, Chitty, White, Robillard, Scott and Hickie2015; Lera-Miguel et al. Reference Lera-Miguel, Andrés-Perpiñá, Fatjó-Vilas, Fañanás and Lázaro2015; Ryan et al. Reference Ryan, Assari, Pester, Hinrichs, Angers, Baker, Marshall, Stringer, Saunders, Kamali, McInnis and Langenecker2016; Schouws et al. Reference Schouws, Comijs, Dols, Beekman and Stek2016).
Most of the available cognitive follow-up studies have small sample sizes and they might be underpowered to detect subtle cognitive decline (or improvements) in BP and to reveal differences between schizophrenia and BP regarding longitudinal patterns of cognitive abilities. Our aim was to conduct a meta-analysis of longitudinal course of cognition in BP. We also aimed to compare the longitudinal course of cognition in BP with healthy controls and schizophrenia.
Methods
Study selection
We followed PRISMA guidelines in conducting this meta-analysis (Moher et al. Reference Moher, Liberati, Tetzlaff and Altman2009). A literature search was conducted using the databases PubMed, PsycINFO, ProQuest and Scopus to identify the relevant studies (January 1987–November 2016) using the combination of keywords as follows: (‘Cognition’ OR ‘neuropsychol*’) AND ‘bipolar disorder’ AND (‘longitudinal’ OR ‘follow-up’). Reference lists of published reports were also reviewed for additional studies. Inclusion criteria were studies that: (1) published in English; (2) reported longitudinal neurocognitive data in BP (minimum follow-up duration of 1 year); (3) reported sufficient data to calculate the effect size and standard error of the cognitive measure. The effect size for cognitive change in healthy and schizophrenia control groups were also coded when available in included studies. The studies were also coded as being short-term (mean duration < 3 years) and long-term (mean duration ⩾ 3 years) follow-up studies. In the case of multiple studies based on an overlapping sample, the study with the longest follow-up period was selected for the main analysis. However, as a number of groups have reported short-term and long-term follow-up results in separate publications, a second report from each of these groups was selected for subgroup meta-analysis of short-term studies only. Vast majority of studies have investigated longitudinal cognitive changes in BP in adult/late adolescent samples (mean age > 16). We decided not to include pediatric samples, which are very rare, to minimize confounding effects of neurodevelopmental processes on the findings of the current meta-analysis.
Statistical analyses
Effect sizes for cognitive domains were calculated by averaging effect size of individual cognitive tests in each domain. Cognitive domains included in the current review were the global cognition, verbal memory, visual memory, processing speed, sustained attention, executive functions, verbal fluency and working memory (see online Supplementary Table S1 for cognitive tests under each domain). An average effect size for global cognition was calculated by averaging all available cognitive domains. We calculated this measure as nearly half of the variance of cognitive performances in BP, schizophrenia and healthy controls are explainable by a general cognitive factor (Jensen, Reference Jensen, Sternberg and Grigorenko2002; Dickinson & Harvey, Reference Dickinson and Harvey2009; Bora & Vahip, Reference Bora and Vahip2011). It was also possible to conduct individual task meta-analyses for several measures including letter fluency, list learning, Trail making A and B (TMT-A and TMT-B), Stroop interference, Wisconsin card sorting test (WCST) perseverative errors.
Meta-analyses were performed using packages in R environment (OpenMetaAnalyst, Metafor) (Viechtbauer, Reference Viechtbauer2010; Wallace et al. Reference Wallace, Dahabreh, Trikalinos, Lau, Trow and Schmid2012). Effect sizes were weighted using the inverse variance method. A random-effects model (DerSimonian–Laird estimate) was used as the distributions of effect sizes are expected to be heterogeneous in neurocognitive studies in major psychoses. Homogeneity of the distribution of weighted effect sizes were tested with the Q test, and degree of heterogeneity was quantified using the I 2 test. I 2 estimates the percentage of total variation across studies that are due to heterogeneity rather than chance. I 2 values between 0 and 0.25 suggest small magnitudes of heterogeneity, while I 2 values in the range 0.25 and 0.50 suggest medium magnitudes and those >0.50 indicate large magnitudes. Theτ 2, an estimate of between-study variance, was used as a measure of the magnitude of heterogeneity in the random-effects model. Publication bias was assessed by inspection of funnel plots. Funnel plot asymmetry was also analyzed with Egger's test. The assessment of publication bias test relies on the theory that small studies with significant rather than negative findings would be more likely to be reported, while large-scale studies would be more likely to be published regardless of significance of the findings.
We also calculated homogeneity statistics using Q bet to test the differences between cognitive changes in diagnostic groups (BP, schizophrenia and controls) and the effect of follow-up duration in BP (long-term v. short-term). Meta-regression analyses were conducted for age, gender (male ratio), duration of education, change in manic and depressive symptoms (effect size for change in scales measuring manic and depressive symptoms). Meta-regression analyses performed with a random-effects model were conducted using the restricted-information maximum likelihood method with a significance level set at p < 0.05.
Results
Selection and characteristics of studies
The selection process is summarized in Fig. 1. Three reports based on overlapping samples with other studies in the meta-analysis were excluded. Two studies that included drug-induced mania and one pediatric study (Pavuluri et al. Reference Pavuluri, West, Hill, Jindal and Sweeney2009) were also excluded. In total 22 reports were included in the meta-analysis and two of these reports included data for both short-term and long-term follow-ups (Table 1). A total of 19 studies consisting of 643 BP (54.1% females, mean age = 40.6) were included in the main analysis (mean duration of follow-up = 3.7 years). In all but two studies, Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria were used for diagnosis. One study used Research Diagnostic Criteria (Burdick et al. Reference Burdick, Goldberg, Harrow, Faull and Malhotra2006) and other used DSM-III (Engelsmann et al. Reference Engelsmann, Katz, Ghadirian and Schachter1988). Four reports that were based on overlapping samples with three of the studies included in the main analysis were used for subgroup analysis of short-term studies only. A total of 15 studies included outcome of short-term follow-up (561 BP, mean duration = 1.5 years) and nine studies reported outcome of long-term follow-up (351 BP, mean duration = 5.5 years). Twelve of the studies included a healthy control v. BP comparison (459 BP and 367 healthy controls) and five of these studies included a schizophrenia v. BP comparison (172 BP and 168 schizophrenia). There were no statistical differences for age between BP and other groups (p > 0.50). BP patients had a higher percentage of females compared with schizophrenia comparison group.

Fig. 1. Flow diagram for meta-analysis of longitudinal neurocognitive studies of BP. BP, bipolar disorder.
Table 1. Longitudinal neurocognitive studies in BP

BP, bipolar disorder; HC, healthy controls; Sch, schizophrenia; WMS, Wechsler memory scale; WCST, Wisconsin card sorting; TMT, trail making test; EF, executive functions; CPT, continuous performance test; LNS, letter number sequencing; CVLT, California verbal learning test; SWM, spatial working memory; IED, intra-extra dimensional shifting
Longitudinal changes in neurocognition in BP
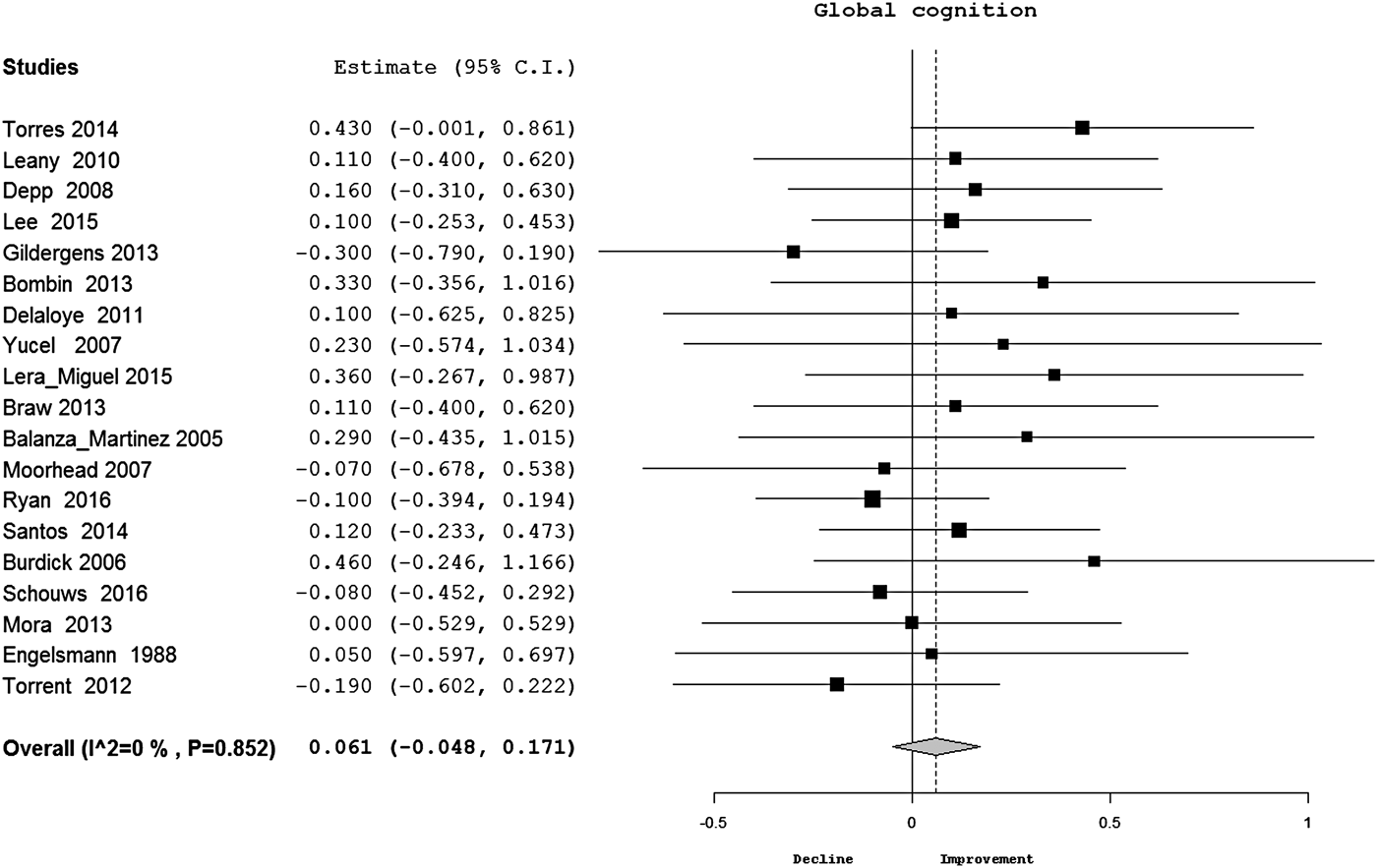
There was no significant change in global cognition overtime in BP (d = 0.06, CI −0.05 to 0.17) (Table 2) (Fig. 2). When meta-analysis was restricted to studies using DSM-IV criteria, there was no evidence of significant cognitive change either (d = 0.05, CI −0.06 to 0.16, p = 0.37). In meta-analyses of individual cognitive domains, there was no significant change in processing speed, sustained attention, executive functions at follow-up in BP. Meta-analysis of individual cognitive tests under processing speed (TMT-A), executive functions (TMT-B, Stroop interference, WCST) and verbal fluency (letter fluency) domains had also found no evidence of cognitive change at follow-up. However, there was significant improvement in verbal (Fig. 3) and visual (see online Supplementary Fig. S1) memory and working memory (online Supplementary Fig. S2) performances of patients with BP overtime (d = 0.16–0.20). The distribution of effect sizes was significantly homogeneous for all cognitive measures except TMT-B. Inspection of funnel plots and Egger's tests found no evidence of publication bias for any cognitive measure. Meta-regression analyses found that age (Z = 1.5, p = 0.13), gender (Z = 0.98, p = 0.33), duration of education (Z = 0.11, p = 0.91), change in depressive symptoms (Z = 1.31, p = 0.19) and manic symptoms (Z = 0.86, p = 0.39) had no significant effect on cognitive change in BP at follow-up.
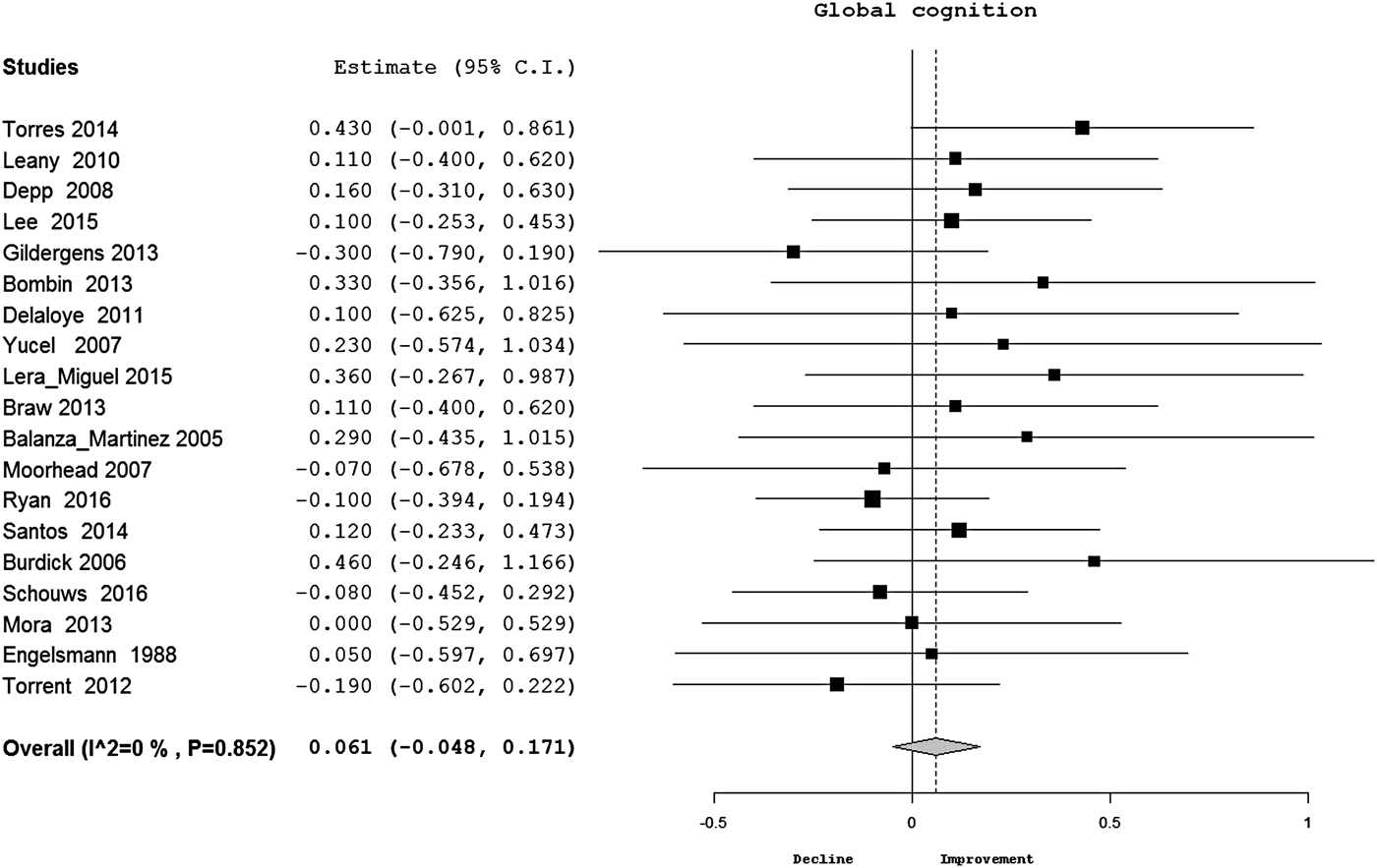
Fig. 2. Forest plot for change in global cognition in BP (estimate = Cohen's d; p value is for Q test; diamond shape = overall estimate). BP, bipolar disorder.

Fig. 3. Forest plot for change in verbal memory in BP (estimate = Cohen's d; p value is for Q test; diamond shape = overall estimate). BP, bipolar disorder.
Table 2. Mean weighted effect sizes for cognitive changes in BP

d, Cohen's d; CI, confidence interval; TMT, trail making test; EF, executive functions; WM, working memory; WCST, Wisconsin cart sorting test; BP, bipolar disorder
Cognitive change in short-term v. long-term follow-up
In general, evidence for improvement in performance in cognitive tasks over time was more evident in short-term rather than long-term follow-up. BP was associated with significant improvements in task performance in memory and executive functions at short-term but not long-term follow up (Table 3). However, the magnitude of cognitive change observed at short-term follow-up was not statistically different from long-term follow-up for global cognition and any of the cognitive domains in BP. There was no evidence for heterogeneity of distribution of effect sizes in any cognitive domains in long-term and short-term follow-up.
Table 3. Mean weighted effect sizes for cognitive change at short-term and long-term follow-up in BP

BP, bipolar disorder; d, Cohen's d; EF, executive functions; CI, confidence interval; WM, working memory
Cognitive change in BP in comparison with schizophrenia and healthy controls
In the meta-analysis of 12 studies investigating longitudinal cognitive changes in both BP and healthy controls, the profile of cognitive changes over time was very similar across both groups. The improvement in verbal and visual memory performances was also evident in healthy controls. The level of improvement in task performance was not significantly different in any of the cognitive measures across BP and healthy controls (p = 0.39–0.84) (Table 4). There was no evidence for heterogeneity of distribution of effect sizes in any cognitive domains in BP and healthy controls.
Table 4. Mean weighted effect sizes for cognitive change in BP and healthy controls

BP, bipolar disorder; HC, healthy controls; d, Cohen's d; EF, executive functions; CI, confidence interval
In the meta-analysis of five studies investigating longitudinal cognitive changes in both BP and schizophrenia, it was possible to calculate effect sizes for change in global cognition, verbal memory and executive functions overtime. There was improvement of cognitive performances in both BP (d = 0.19–0.31) and schizophrenia (d = 0.22–0.29) at follow-up in these studies (Fig. 4). Magnitude of improvement was not significantly different in any of these three cognitive measures across BP and schizophrenia groups (p = 0.75–0.89) (Table 5). There was no evidence for heterogeneity of distribution of effect sizes in any cognitive domains in BP and schizophrenia.

Fig. 4. Forest plot for change in global cognition in schizophrenia and BP (estimate = Cohen's d; p value is for Q test; diamond shape = overall estimate). BP, bipolar disorder.
Table 5. Mean weighted effect sizes for cognitive change in BP and schizophrenia

BP, bipolar disorder; Sch, schizophrenia; d, Cohen's d; EF, executive functions; CI, confidence interval
Discussion
The current meta-analysis investigated the longitudinal cognitive changes in BP and compared trajectory of cognitive changes in BP with schizophrenia and healthy controls. Our findings provided no evidence of cognitive deterioration at follow-up in BP. In contrast, there was evidence of improvement in task performance in some cognitive domains. The trajectories of cognitive functioning of BP, schizophrenia and healthy control groups were not significantly different during follow-up period.
Current findings are not supportive of the notion of progressive cognitive deficits in BP. There was no evidence of cognitive decline in any of the cognitive domains in BP. In contrast, there were modest but significant gains in task performance of BP patients in verbal and visual memory and working memory (d = 0.16–0.20). Improvement in cognitive task performance was evident only in short-term follow-up studies (mean duration = 1.5 year). In fact, there were also significant improvements for task performance in executive functions when short-term follow-up studies were considered. Practice effects are likely to play a significant role in improvements in cognitive task performance in BP, healthy control and schizophrenia groups. This might be particularly true for some tests including verbal and visual memory tasks, which are more vulnerable to learning effects. It is also important to consider the possibility of masking of potential cognitive decline by practice effects in BP. The small learning effects on verbal and visual memory might potentially minimize the actual overall decline in these measures. To address this issue, we compared cognitive change in BP and healthy controls. However, there was no significant difference in level of cognitive improvement in any cognitive measure between BP and healthy controls. The lack of difference of cognitive trajectories of BP and healthy controls does not support this explanation in the current meta-analysis.
It is also important to investigate relationship between change in symptoms and cognition in BP. While fluctuations in symptoms might play a role in variability in cognitive performances at follow-up studies at the level of individuals (Arts et al. Reference Arts, Jabben, Krabbendam and van Os2011), current findings cannot be explained by symptomatic improvements as most of the patients with BP were stable at the initial and follow-up assessments and modest changes in manic and depressive symptoms had no significant effects on change in cognitive functions at follow-up. However, clinical outcome during follow-up period might have a stronger association with the level of cognitive change than current subclinical symptoms. In the study of Kozicky et al. (Reference Kozicky, Torres, Silveira, Bond, Lam and Yatham2014), cognitive functions were improved in the patients with BP who remained well during follow-up period but not in patients who experienced a relapse (even though they were in remission at the second assessment).
These findings were similar to outcome of previous longitudinal neurocognitive studies in schizophrenia and our meta-analysis had found no significant difference of the trajectories of cognitive functioning between BP and schizophrenia (Szöke et al. Reference Szöke, Trandafir, Dupont, Méary, Schürhoff and Leboyer2008; Bora & Murray, Reference Bora and Murray2014). Current findings, together with outcome of first-episode and high-risk studies, suggest that cognitive deficits are already evident early in BP (Klimes-Dougan et al. Reference Klimes-Dougan, Ronsaville, Wiggs and Martinez2006; Doyle et al. Reference Doyle, Wozniak, Wilens, Henin, Seidman, Petty, Fried, Gross, Faraone and Biederman2009; Lee et al. Reference Lee, Hermens, Scott, Redoblado-Hodge, Naismith, Lagopoulos, Griffiths, Porter and Hickie2014; Bora & Pantelis, Reference Bora and Pantelis2015). Neurodevelopmental abnormalities, like in schizophrenia, might be considered as being the most important reason underlying cognitive deficits in BP (Bora, Reference Bora2015b ). This is not surprising as both BP and schizophrenia are associated with a number of common susceptibility genes, which have important roles in neurodevelopment (Craddock & Owen, Reference Craddock and Owen2010; Gatt et al. Reference Gatt, Burton, Williams and Schofield2015). Other studies suggesting a link between minor physical abnormalities, prenatal/perinatal abnormalities, abnormal cortical folding and BP also support the notion of developmental abnormalities, at least in a subset of patients (Fornito et al. Reference Fornito, Malhi, Lagopoulos, Ivanovski, Wood, Saling, Pantelis and Yücel2008; McIntosh et al. Reference McIntosh, Moorhead, McKirdy, Hall, Sussmann, Stanfield, Harris, Johnstone and Lawrie2009; Parboosing et al. Reference Parboosing, Bao, Shen, Schaefer and Brown2013; Sivkov et al. Reference Sivkov, Akabaliev, Mantarkov, Ahmed-Popova and Akabalieva2013; Vonk et al. Reference Vonk, van der Schot, van Baal, van Oel, Nolen and Kahn2014; Freedman et al. Reference Freedman, Brown, Shen and Schaefer2015).
However, it is not entirely possible to exclude the possibility of a subset of patients with BP being characterized by progressive cognitive decline. Cross-sectional studies suggested that there is a considerable heterogeneity of cognitive functions in BP. Using data-driven methods, number of authors found evidence of several cognitive subgroups including a cluster with very severe impairment, another cluster with preserved cognitive abilities and other subgroups with selective or modest impairment (Burdick et al. Reference Burdick, Russo, Frangou, Mahon, Braga, Shanahan and Malhotra2014; Lewandowski et al. Reference Lewandowski, Sperry, Cohen and Ongür2014; Bora, Reference Bora2016; Bora et al. Reference Bora, Hıdıroğlu, Özerdem, Kaçar, Sarısoy, Civil Arslan, Aydemir, Cubukcuoglu Tas, Vahip, Atalay, Atasoy, Ateşci and Tümkaya2016b , Reference Bora, Veznedaroğlu and Vahip c ; Clementz et al. Reference Clementz, Sweeney, Hamm, Ivleva, Ethridge, Pearlson, Keshavan and Tamminga2016). Currently, it is not known whether different cognitive subgroups might be associated with different longitudinal outcomes in BP. For example, it is important to investigate whether cognitive decline might be evident in a subgroup of patients having frequent episodes during follow-up period. Large sample sized longitudinal studies with first-episode patients and use of statistical methods to investigate potential subgroups with different long-term trajectories of cognitive functioning in BP would be important to test the hypothesis of cognitive decline in a subset of patients with BP. However, even future studies provide evidence of a subgroup of patients with BP characterized by cognitive decline, which cannot be simply explained by normal heterogeneity of cognitive trajectories in healthy controls or effect of persisting mood symptoms; it would be essential to show that illness process but not other factors explain such a difference. For example, components of metabolic syndrome such as diabetes and hyperlipidemia, which are associated with cognitive deficits in the normal population, have a higher prevalence in BP compared with healthy controls (Vancampfort et al. Reference Vancampfort, Vansteelandt, Correll, Mitchell, De Herdt, Sienaert, Probst and De Hert2013). Available, cross-sectional evidence suggests that components of metabolic syndrome might be associated with cognitive deficits in BP and schizophrenia (Hubenak et al. Reference Hubenak, Tuma and Bazant2015; Bora et al. Reference Bora, Akdede and Alptekin2017; Naiberg et al. Reference Naiberg, Newton, Collins, Dickstein, Bowie and Goldstein2016). Treatment with antipsychotics and substance and alcohol abuse can also have negative effect on cognitive functions in BP (Balanzá-Martínez et al. Reference Balanzá-Martínez, Selva, Martínez-Arán, Prickaerts, Salazar, González-Pinto, Vieta and Tabarés-Seisdedos2010, Reference Balanzá-Martínez, Crespo-Facorro, González-Pinto and Vieta2015; Flowers et al. Reference Flowers, Ryan, Lai, McInnis and Ellingrod2016; Steen et al. Reference Steen, Aas, Simonsen, Dieset, Tesli, Nerhus, Gardsjord, Mørch, Agartz, Melle, Ueland, Spigset and Andreassen2016).
It is also important to consider the possibility of time-limited cognitive deterioration before and around the onset of first-episode mania as the vast majority of the available neurocognitive follow-up studies have been conducted in chronic BP. A similar notion had been proposed for schizophrenia but has not been supported by a meta-analysis of longitudinal neurocognitive studies in individuals at clinical risk for psychosis and first-episode schizophrenia (Bora & Murray, Reference Bora and Murray2014). To date, very few studies have investigated trajectory of cognitive functions in individuals at high-risk for BP and in first-episode mania (Torres et al. Reference Torres, Kozicky, Popuri, Bond, Honer, Lam and Yatham2014; Daglas et al. Reference Daglas, Allott, Yücel, Pantelis, Macneil, Berk and Cotton2015; Lee et al. Reference Lee, Hermens, Naismith, Lagopoulos, Jones, Scott, Chitty, White, Robillard, Scott and Hickie2015; Papmeyer et al. Reference Papmeyer, Sussmann, Hall, McKirdy, Peel, Macdonald, Lawrie, Whalley and McIntosh2015). So far, the outcome of available studies has not provided evidence for cognitive decline in early phases of BP. However, further studies investigating trajectory of cognitive functions in early BP are needed.
There are number of limitations of the current meta-analysis. Relevant information regarding a number of important variables, including number of episodes during follow-up, psychotic symptoms, treatment used (including antipsychotics) and illicit substance and alcohol use during follow-up period in most studies, was not reported. Another consideration was the relatively small number of studies that compared cognitive trajectory of BP with schizophrenia. Global cognition measure in this meta-analysis also might be biased toward cognitive domains, which are more frequently used across studies. Another limitation was the absence of the social cognition domain in the current meta-analysis. Longitudinal trajectory of social cognitive abilities has not been the focus of cognitive studies in BP. However, a single study that investigated facial emotion recognition found that social cognition might be stable during a follow-up period of 7 years (Martino et al. Reference Martino, Samamé and Strejilevich2016). Maximum duration of follow-up was also relatively shorter in BP studies compared with schizophrenia studies. In schizophrenia, number of studies found no evidence of cognitive decline after 20 years (Bonner-Jackson et al. Reference Bonner-Jackson, Grossman, Harrow and Rosen2010); it is not known whether it might be possible to find evidence of progressive cognitive decline in BP after longer follow-up periods. Therefore, it is important to conduct studies investigating cognitive change in BP during a period of 10–20 years follow-up.
As a conclusion, current findings suggest that cognitive impairment is stable, at least in majority of patients with BP. The trajectory of cognitive functions in BP is similar to schizophrenia. Cognitive deficits in BP are likely to be mainly neurodevelopmental rather than being neurodegenerative in nature.
Supplementary Material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291717001490.
Acknowledgements
Dr Bora is supported by The Scientific and Technological Research Council of Turkey (TÜBİTAK) BİDEB2232 fellowship.
Declaration of Interest
None.