Introduction
Placental abruption, a severe condition that affects up to 1% of pregnancies, is an important cause of increased burden of perinatal morbidity and mortality (Oyelese & Ananth, Reference Oyelese and Ananth2006). The pathophysiology of the disorder is not well understood, but evidence suggests that inadequate placentation plays an important role (Oyelese & Ananth, Reference Oyelese and Ananth2006; Brosens et al. Reference Brosens, Pijnenborg, Vercruysse and Romero2011). Pregnancies with a subsequent placental abruption are often characterized by increased pro-thrombotic activity, placental infarcts and uteroplacental ischemia (Oyelese & Ananth, Reference Oyelese and Ananth2006; de Paz et al. Reference de Paz, Sanchez, Huaman, Chang, Pacora, Garcia, Ananth, Qiu and Williams2011). Several of the pathophysiologic features of the disorder are thus similar to those observed in pre-eclampsia and fetal growth restriction (Oyelese & Ananth, Reference Oyelese and Ananth2006; Ananth & Vintzileos, Reference Ananth and Vintzileos2011; Brosens et al. Reference Brosens, Pijnenborg, Vercruysse and Romero2011), and risk factors for the three conditions have also shown some overlap (Ananth & Vintzileos, Reference Ananth and Vintzileos2011).
Some studies indicate that maternal stress during pregnancy is associated with a modestly increased risk of fetal growth restriction (Precht et al. Reference Precht, Andersen and Olsen2007; Khashan et al. Reference Khashan, McNamee, Abel, Pedersen, Webb, Kenny, Mortensen and Baker2008; Class et al. Reference Class, Lichtenstein, Långström and D'Onofrio2011) and pre-eclampsia (Marcoux et al. Reference Marcoux, Bérubé, Brisson and Mondor1999; László et al. Reference László, Liu, Svensson, Wikström, Li, Olsen, Obel, Vestergaard and Cnattingius2013a ). In light of the vasoconstrictive (Khatun et al. Reference Khatun, Kobayashi, Belayet, Sumimoto, Iwaki and Kanayama2001) and pro-thrombotic (von Känel et al. Reference von Känel, Mills, Fainman and Dimsdale2001) effects of stress, the similarity in pathophysiology (Oyelese & Ananth, Reference Oyelese and Ananth2006; Brosens et al. Reference Brosens, Pijnenborg, Vercruysse and Romero2011) and in some risk factors (Ananth & Vintzileos, Reference Ananth and Vintzileos2011) amongst fetal growth restriction, pre-eclampsia and placental abruption, it is possible that maternal stress during pregnancy is associated with increased placental abruption risk.
To our knowledge, the link between prenatal stress and placental abruption has been investigated only by a recent case–control study. This study found that women with placental abruption reported exposure to stress during pregnancy twice as often as women in the control group (de Paz et al. 2011). The extent to which biases due to recall and reverse causation may have affected these associations remains unknown. Prospective studies are thus the next logical step to further investigate this potential association.
In a large population-based cohort study, we examined whether maternal exposure to the death of a close relative shortly before or during pregnancy, a major life event that is likely to be perceived as stressful independently of coping mechanisms [Diagnostic and Statistical Manual of Mental Disorders, third edition, revised (DSM-III-R); APA, 1987], is associated with an increased risk of placental abruption.
Method
Study population and design
We conducted a population-based cohort study by linking individual-level data from several Danish (Munk-Jorgensen & Mortensen, Reference Munk-Jorgensen and Mortensen1997; Knudsen & Olsen, Reference Knudsen and Olsen1998; Andersen et al. Reference Andersen, Madsen, Jorgensen, Mellemkjoer and Olsen1999; Pedersen et al. Reference Pedersen, Gotzsche, Moller and Mortensen2006; Timmermans, Reference Timmermans2010) and Swedish (National Board of Health and Welfare, 2003, 2009, 2011; Statistics Sweden, 2006, 2010) national registers (Li et al. Reference Li, Vestergaard, Obel, Cnattingus, Gissler and Olsen2010; László et al. Reference László, Liu, Svensson, Wikström, Li, Olsen, Obel, Vestergaard and Cnattingius2013a , Reference László, Svensson, Li, Obel, Vestergaard, Olsen and Cnattingius b ). Linkage of individual records within registers was based on the unique personal number assigned in each country to all legal residents. In Denmark, data from the Medical Birth Register (Knudsen & Olsen, Reference Knudsen and Olsen1998), the Danish Civil Registration System (Pedersen et al. Reference Pedersen, Gotzsche, Moller and Mortensen2006), the National Hospital Register (Andersen et al. Reference Andersen, Madsen, Jorgensen, Mellemkjoer and Olsen1999), the Psychiatric Central Register (Munk-Jorgensen & Mortensen, Reference Munk-Jorgensen and Mortensen1997) and the Integrated Database for Longitudinal Labour Market Research (Timmermans, Reference Timmermans2010) were linked. In Sweden, we linked the Medical Birth Register (National Board of Health and Welfare, 2003) to the Multi-Generation Register (Statistics Sweden, 2010), the Cause of Death Register (National Board of Health and Welfare, 2011), the Patient Register (National Board of Health and Welfare, 2009) and the Education Register (Statistics Sweden, 2006; László et al. Reference László, Liu, Svensson, Wikström, Li, Olsen, Obel, Vestergaard and Cnattingius2013a , Reference László, Svensson, Li, Obel, Vestergaard, Olsen and Cnattingius b ).
The study population included all live births during 1978–2008 from the Danish Medical Birth Register (DMBR; n = 2 085 521) and all births during 1973–2006 from the Swedish Medical Birth Register (SMBR; n = 3 413 812) (Fig. 1). The DMBR has been computerized since 1973, and has collected data on gestational age since 1978 (Knudsen & Olsen, Reference Knudsen and Olsen1998), while the SMBR started in 1973 (National Board of Health and Welfare, 2003). Both registries include prospectively ascertained data on pregnancies, deliveries and the neonates in the case of almost 99% of births in both countries (Knudsen & Olsen, Reference Knudsen and Olsen1998; National Board of Health and Welfare, Reference Nath, Ananth, Smulian, Shen-Schwarz and Kaminsky2003). Analyses were restricted to women that delivered a singleton infant between 22 and 45 (in Denmark) or 46 (in Sweden) weeks of gestation and without a record of placental abruption in a previous pregnancy (Fig. 1). A total of 5 103 272 births were thus included in the study. Compared with excluded women, mothers included in the study were more likely to be from Sweden, primiparous, to have higher education and to originate from the country where they gave birth to their child (data not shown). The prevalence rates of abruption were 0.6% (0.8% in Denmark and 0.5% in Sweden) and 1.0% (0.9% in Denmark and 1.4% in Sweden) in the included and excluded groups, respectively.

Fig. 1. Flow diagram of study participants.
Ethical approval for the study was obtained from the Data Protection Agency in Copenhagen (no. 2008-41-2680) and the Scientific Ethics Committee of Central Region Jylland (no. M-20100252) in Denmark, and from the Research Ethics Committee at Karolinska Institutet, Stockholm (no. 2008/4:6) in Sweden.
Study variables
Exposure
We considered women who lost a child, a parent, a sibling or a partner the year before or during pregnancy as exposed to bereavement during pregnancy. We retrieved information on the relatives' date and cause of death from the Danish Civil Registration System and from the Swedish Cause of Death Register. Deaths were categorized based on their cause as natural versus unexpected (i.e. deaths due to accidents, violence, suicide, complications of medical and surgical care and other sudden unnatural deaths) (Precht et al. Reference Precht, Andersen and Olsen2007; László et al. Reference László, Liu, Svensson, Wikström, Li, Olsen, Obel, Vestergaard and Cnattingius2013a , Reference László, Svensson, Li, Obel, Vestergaard, Olsen and Cnattingius b ) using the diagnostic codes presented in the Appendix. Causes of death and diseases have been coded in both countries based on the International Classification of Diseases (ICD). During the study period, Denmark used the eighth revision (ICD-8) up to 1993 and the tenth revision (ICD-10) was used from 1994 onwards. In Sweden, ICD-8 was used up to 1986, the ninth revision (ICD-9) from 1987 to 1996, and ICD-10 since 1997.
Mothers were linked to their first-degree relatives (children, siblings and parents) and to the father of the index child by means of the Danish Civil Registration System and the Swedish Multi-Generation Register. Register linkage to relatives was possible if both the mother and her family members were Danish or Swedish residents (Pedersen et al. Reference Pedersen, Gotzsche, Moller and Mortensen2006; Statistics Sweden, 2010). In Denmark during 1968–1978, additional criteria for linkage to parents were (i) age younger than 15 years, (ii) living with parents, and (iii) being unmarried and not having children (Pedersen et al. Reference Pedersen, Gotzsche, Moller and Mortensen2006). Furthermore, since the Swedish Multi-Generation Register included only live-born persons (born 1932 or later) (Statistics Sweden, 2010), identification of partners was not possible in the case of stillbirths (László et al. Reference László, Liu, Svensson, Wikström, Li, Olsen, Obel, Vestergaard and Cnattingius2013a , Reference László, Svensson, Li, Obel, Vestergaard, Olsen and Cnattingius b ).
Outcome
Data on placental abruption were retrieved from the Danish National Hospital Register and from the SMBR. A Danish validation study found that the sensitivity was 70% and the positive predictive value was 69% in the case of the placental abruption diagnosis coded according to ICD-8 in the Danish National Hospital Register (Kristensen et al. Reference Kristensen, Langhoff-Roos, Skovgaard and Kristensen1996). A Swedish study analysing the hospital records of 80 women diagnosed with placental abruption in a university hospital during 1972–1982 found that in the case of 78% of these patients the delivery record contained information about (i) a blood clot on the maternal part of the placenta after vaginal delivery or in the uterus in the case of a caesarean, (ii) bleeding during parturition and/or (iii) evidence of premature placental detachment (Kåregård & Gennser, Reference Kåregård and Gennser1986). Information on placental abruption was found only in the pregnancy record in the case of 17 of the remaining 18 patients (Kåregård & Gennser, Reference Kåregård and Gennser1986). A quality study of the SMBR showed that rates of placental abruption were comparable across Swedish hospitals, suggesting no substantial variability in diagnostic practices across birth centers (Cnattingius et al. Reference Cnattingius, Ericson, Gunnarskog and Källén1990). Nevertheless, as some placental abruption cases may have been coded as unspecified late pregnancy bleeding, we defined the combination of placental abruption and unspecified late pregnancy bleeding as a secondary endpoint. Corresponding ICD codes are presented in the Appendix.
Covariates
Data on chronic hypertension, pre-eclampsia and pre-gestational diabetes were obtained from the Danish National Hospital Register and from the SMBR. Medical complications during pregnancy were recorded by the attending physician or midwife at the time of diagnosis in Denmark and after delivery in Sweden. The Danish Psychiatric Central Register and the Swedish Patient Register provided data on maternal psychiatric illnesses. Data on cardiovascular diseases of the mothers' parents and siblings were available only from the National Hospital Register in Denmark. The diagnostic codes used to identify these diseases are provided in the Appendix. The Danish National Hospital Register contains information on all hospitalizations since 1977, while the Danish Central Psychiatric Register includes data on all hospitalizations for psychiatric disorders since 1969; information on out-patient diagnoses are included in both registers since 1995 (Munk-Jorgensen & Mortensen, Reference Munk-Jorgensen and Mortensen1997; Andersen et al. Reference Andersen, Madsen, Jorgensen, Mellemkjoer and Olsen1999). The Swedish Patient Register includes data on all hospitalizations in the country since 1987, whereas for the period 1969–1986 hospitalization data were incomplete (National Board of Health and Welfare, 2009).
Data on date of delivery, parity, infant sex and birth weight, gestational age at delivery and maternal smoking habits in early pregnancy (available since 1991 in Denmark and since 1982 in Sweden) were obtained from the DMBR and SMBR (Knudsen & Olsen, Reference Knudsen and Olsen1998; National Board of Health and Welfare, 2003). Length of gestation was estimated based on ultrasound measurements generally performed early in the second trimester; when such measurements were not available – largely before the early 1990s – the date of last menstruation was used instead (Kristensen et al. Reference Kristensen, Langhoff-Roos, Skovgaard and Kristensen1996; National Board of Health and Welfare, 2003). Data on maternal height and weight in early pregnancy have been collected in Sweden since 1992; women with a body mass index of 30 kg/m2 or above were considered obese.
Data on the mother's highest attained education were obtained from the Danish Integrated Database for Longitudinal Labour Market Research and from the Swedish Education Register, whereas the Danish Integrated Database for Longitudinal Labour Market Research and the SMBR provided data on the mothers' country of origin.
Statistical analysis
Logistic regression models were fitted to assess the association between bereavement during pregnancy and the risk of placental abruption. We first analysed whether the association was modified by country, year of delivery, ICD version in use at the time of birth, maternal education, age at delivery, country of origin and chronic hypertension. Effect measure modification was investigated by stratified analysis and by introducing cross-product terms between exposure and the hypothesized effect modifiers in the multiplicative regression models.
To examine differences in placental abruption risk according to the time of the relative's death, the exposure period was divided into five intervals: (i) 7–12 months and (ii) 0–6 months before pregnancy; and during the (iii) first, (iv) second, and (v) third trimester of pregnancy. When analysing whether the risk of placental abruption increased with severity of stress, the exposure was categorized based on (i) the mother's relationship to the deceased (parent, sibling, partner or child); and (ii) the relative's cause of death (natural versus unexpected). In the case of more than one loss during the exposure window, we gave priority to the first death. Losses of parents and siblings were studied among women who had register linkage to parents, and who 1 year before pregnancy had at least one live parent and at least one live sibling, respectively. The association between the death of a child and placental abruption was investigated among women with at least one living older child. Analyses concerning the partner were conducted among women whose index child had register linkage to the father. Similar analyses to those presented above were also performed with the secondary endpoint, the combination of placental abruption and unspecified late pregnancy bleedings.
The following possible confounders were included in our primary multivariable models: country of childbirth, year of delivery, parity, maternal education, country of origin, age at delivery and psychiatric disorder before the exposure period. In secondary analysis, we further adjusted the association between the loss of any relative and placental abruption for (i) family history of cardiovascular disease before the exposure period (in the Danish cohort), (ii) pre-pregnancy obesity (among women who gave birth in Sweden from 1992 onwards) and (iii) pre-gestational diabetes (among women from the Danish cohort and women from the Swedish cohort who gave birth during the period of ICD-9 or ICD-10, i.e. since 1987). As the investigated association could be confounded by the woman's involvement in a traffic accident leading to the death of a close relative, we repeated the main analysis after excluding women whose family member died due to a land traffic accident (see Appendix for ICD codes) during the last 2 weeks of pregnancy; this short time period was chosen because we considered that injury in a severe traffic accident would carry an acute rather than a long-lasting risk for placental abruption. Furthermore, since information on the date of placental abruption was unavailable in Sweden and thus some deaths occurring in very late pregnancy could have been preceded by the abruption, we replicated the main model after excluding women who lost a relative during the last week of pregnancy. To ensure that our findings were not substantially affected by studying uteroplacental dysfunction disorders recurring across pregnancies, we repeated our analyses regarding the loss of a child after excluding infants whose mother in a previous pregnancy had pre-eclampsia, a stillbirth or a small-for-gestational-age infant (i.e. a live-born infant with a birth weight for gestational age more than two standard deviations below the sex-specific Swedish fetal growth curve; Marsál et al. Reference Marcoux, Bérubé, Brisson and Mondor1996). We also estimated the effect of maternal bereavement on placental abruption among women without a diagnosis of pre-eclampsia and among women who were non-smokers in early pregnancy.
Results
The distribution of the study variables according to maternal exposure to bereavement during pregnancy is shown in Table 1. Compared with unexposed women, bereaved women were older, more likely to be multiparous, to have only basic education, to smoke in early pregnancy, to have a family history of cardiovascular disease, and to have had a uteroplacental dysfunction disorder in a previous pregnancy.
Table 1. Distribution of study variables according to maternal exposure to the death of a close family member the year before or during pregnancy a

a Analysis was performed among women with register linkage to parents and to the father of the index child.
b Data have been collected since 1982 in Sweden and since 1991 in Denmark.
c Data have been collected in Sweden only, since 1992.
d Data were available to us only for the Danish cohort.
e Defined as pre-eclampsia, a stillborn or a small-for-gestational-age infant.
f The diagnosis was identifiable in Sweden from 1987 onwards.
Since we observed an interaction between the loss of any relative and chronic hypertension (p = 0.01), the data were stratified on chronic hypertension. The death of any close relative was not associated with increased odds of abruption among women without chronic hypertension, whereas the odds were two-fold higher in women with chronic hypertension (Table 2).
Table 2. Association between maternal bereavement during pregnancy and placental abruption, stratified by chronic hypertension a

CI, Confidence interval.
a Analysis was performed among women with register linkage to parents and to the father of the index child.
b Adjustment was made for country, year of delivery, maternal country of origin, education, age at delivery, parity and psychiatric disorder before the exposure period.
The association between bereavement and placental abruption varied by type of relative and time of death (Table 3). Among normotensive women, death of a child was associated with a 54% increased odds of abruption [95% confidence interval (CI) 1.30–1.82]; only losses occurring the year before pregnancy or in the first trimester were related to the outcome (Table 3). Among women with chronic hypertension, the death of a child was associated with an eight-fold increased odds of placental abruption (odds ratio 8.17, 95% CI 3.17–21.10), but only losses occurring the year before pregnancy or in the first trimester were related to the outcome. Losses of other relatives were not associated with abruption risk (Table 3). Loss of a child among hypertensive women had an adjusted odds ratio of 7.39 (95% CI 2.60–21.10) in the case of natural deaths and 14.29 (95% CI 1.65–124.08) in the case of unexpected deaths. For other types of relatives, there were no differences in the odds of abruption between deaths from natural versus unexpected causes (data not shown).
Table 3. Association between maternal bereavement during pregnancy and placental abruption, by type of deceased relative and time of exposure

OR, Odds ratio; CI, confidence interval.
a Adjustment was made for country, year of delivery, maternal country of origin, education, age at delivery, parity and psychiatric disorder before the exposure period.
b Analyses were restricted to women who had at least a live older child.
c Analyses were restricted to women whose index child had register linkage to the father.
d Analyses were restricted to women who had register linkage to parents and at least a live parent at the beginning of the exposure window.
e Analyses were restricted to women who had register linkage to parents and at least a live sibling at the beginning of the exposure window.
The relationship between loss of any relative and abruption was not substantially modified after including (i) pre-gestational diabetes, (ii) obesity, or (iii) a family history of cardiovascular disease in our multivariable model. Similarly, we did not find effect measure modification after excluding women (i) who lost a relative the week before delivery, (ii) who lost a family member due to a land traffic accident during the last 2 weeks of pregnancy, (iii) who smoked in the first trimester, or (iv) who developed pre-eclampsia during the index pregnancy (data not shown). The association between loss of a child and abruption was slightly attenuated after excluding mothers who had a recorded diagnosis of pre-eclampsia, stillbirth or small-for-gestational-age infant in a previous pregnancy; the odds ratio for abruption following the death of a child the year before or during pregnancy decreased to 1.41 (95% CI 1.15–1.73) in normotensive women and to 7.56 (95% CI 0.94–60.61) in women with chronic hypertension (interaction p = 0.002).
When analysing the risk of the composite outcome of placental abruption and unspecified bleeding according to (i) bereavement of any relative, (ii) the time of exposure and (iii) the type of relative, the observed point estimates were generally comparable with or somewhat lower than those observed in our primary analysis (data not shown).
Discussion
Death of a child the year before or in the first trimester of pregnancy was associated with an increased risk of placental abruption, especially among women with chronic hypertension. Women who lost a child during the second or third trimester or women who lost other relatives were not at increased risk of placental abruption.
Comparison with earlier investigations
To our knowledge, this is the first prospective study to analyse the association between psychological stress during pregnancy and placental abruption. Our results corroborate those of the only case–control study on this topic, reporting a two-fold higher risk of placental abruption in women with frequent stress symptoms during pregnancy compared with their less exposed counterparts (de Paz et al. Reference de Paz, Sanchez, Huaman, Chang, Pacora, Garcia, Ananth, Qiu and Williams2011). Our findings are also consistent with an animal model showing that retroplacental hematomas were substantially more frequent among pregnant rodents exposed to cold stress to the soles than among non-exposed (Khatun et al. Reference Khatun, Kobayashi, Belayet, Sumimoto, Iwaki and Kanayama2001). Our results complement and extend findings regarding the link between mood disorders – also related to the dysregulation of the hypothalamic–pituitary–adrenal (HPA) axis – and the risk of placental abruption (Jablensky et al. Reference Jablensky, Morgan, Zubrick, Bower and Yellachich2005; de Paz et al. Reference de Paz, Sanchez, Huaman, Chang, Pacora, Garcia, Ananth, Qiu and Williams2011).
The observation that maternal bereavement of an older child, but not of other relatives, was associated with placental abruption suggests that only high levels of stress have an effect. The axis IV of the DSM-III-R – a widely accepted rating system of sources of stress – considers the death of a child to be a catastrophic stressor and rates it 6 on its six-step scale of severity; deaths of other relatives are generally considered extreme stressors and are rated 5 (APA, 1987; László et al. Reference László, Liu, Svensson, Wikström, Li, Olsen, Obel, Vestergaard and Cnattingius2013a , Reference László, Svensson, Li, Obel, Vestergaard, Olsen and Cnattingius b ). Our findings are also in line with several earlier investigations suggesting that women exposed shortly before or during pregnancy to negative life events affecting children are at a higher risk of preterm delivery (Khashan et al. Reference Khashan, McNamee, Abel, Mortensen, Kenny, Pedersen, Webb and Baker2009), early-onset pre-eclampsia (László et al. Reference László, Liu, Svensson, Wikström, Li, Olsen, Obel, Vestergaard and Cnattingius2013a ) or having an infant with congenital malformations (Hansen et al. Reference Hansen, Lou and Olsen2000) than their counterparts experiencing similar events in other type of relatives. In the case–control study performed by de Paz et al. (Reference de Paz, Sanchez, Huaman, Chang, Pacora, Garcia, Ananth, Qiu and Williams2011), the risk of placental abruption increased with symptoms of stress, anxiety and depression. An intensity- and duration-dependent effect of cold stimulation on retroplacental hematoma rates was also observed in the animal study of Khatun et al. (Reference Khatun, Kobayashi, Belayet, Sumimoto, Iwaki and Kanayama2001).
Possible pathways linking stress to placental abruption
At least two mechanisms may contribute to the explanation of the association between antenatal stress and placental abruption (de Paz et al. Reference de Paz, Sanchez, Huaman, Chang, Pacora, Garcia, Ananth, Qiu and Williams2011). One involves biological effects of stress, including prolonged secretion of stress hormones, subsequent vasoconstriction, blood pressure elevations (Markovitz et al. Reference Markovitz, Jonas and Davidson2001), pro-inflammatory (Coussons-Read et al. Reference Coussons-Read, Okun and Nettles2007; Steptoe et al. Reference Steptoe, Hamer and Chida2007) and pro-thrombotic (von Känel et al. Reference von Känel, Mills, Fainman and Dimsdale2001) alterations and uteroplacental ischemia (Khatun et al. Reference Khatun, Kobayashi, Belayet, Sumimoto, Iwaki and Kanayama2001; László et al. Reference László, Liu, Svensson, Wikström, Li, Olsen, Obel, Vestergaard and Cnattingius2013a , Reference László, Svensson, Li, Obel, Vestergaard, Olsen and Cnattingius b ). In line with this hypothesis, a study by Rydhström et al. (Reference Rydhström, Walles and Owman1989) found substantially higher uterine norepinephrine levels in women undergoing emergency caesarean delivery due to placental abruption/hemorrhage than in a group of women whose labor started normally, but ended with an emergency caesarean due to umbilical cord prolapse or transverse or breech fetal position; this may suggest that an increased sympathetic activity may be implicated in the etiology of placental abruption (Rydhström et al. Reference Rydhström, Walles and Owman1989). A potential synergy between sympathetic overactivity specific to both hypertension and to severe stress might explain the high risk of placental abruption observed in chronic hypertensive women who lost a child; in addition, severe stress may increase blood pressure in hypertensive women to harmful levels. The second mechanism involves a bereavement-induced change in life-style. Smoking (Oyelese & Ananth, Reference Oyelese and Ananth2006), alcohol consumption (Burd et al. Reference Burd, Roberts, Olson and Odendaal2007), inflammation (Nath et al. Reference Nath, Ananth, Smulian, Shen-Schwarz and Kaminsky2007), hypertension and pre-eclampsia (Oyelese & Ananth, Reference Oyelese and Ananth2006) are associated with increased abruption risk. Furthermore, thrombotic lesions are frequent in spiral arteries of women with this disorder (Ananth et al. Reference Ananth, Oyelese, Prasad, Getahun and Smulian2006; Kinzler et al. Reference Kinzler, Prasad and Ananth2009; Elsasser et al. Reference Elsasser, Ananth, Prasad and Vintzileos2010; Brosens et al. Reference Brosens, Pijnenborg, Vercruysse and Romero2011). Despite the biologic plausibility, we found no evidence that smoking in early pregnancy or pre-eclampsia may link prenatal stress to placental abruption. Whether other factors, such as depression, anxiety, use of antidepressant medications, inflammation or coagulation, may contribute to the explanation of the investigated association needs further examination.
Our finding that only losses occurring during the year before pregnancy or in the first trimester were associated with placental abruption suggests that severe stress may affect the priming of the uterus, implantation and placental development. An alternative explanation may be that women's perception of stress (Glynn et al. Reference Glynn, Wadhwa, Dunkel-Schetter, Chicz-Demet and Sandman2001, Reference Glynn, Schetter, Wadhwa and Sandman2004) and their physiological reactivity to stress (Entringer et al. Reference Entringer, Buss, Shirtcliff, Cammack, Yim, Chicz-DeMet, Sandman and Wadhwa2010) may be more pronounced in earlier than in later periods of pregnancy, which would be an evolutionary advantage. Accordingly, a number of studies found the highest risk of some other placental dysfunction disorders – preterm delivery (Glynn et al. Reference Glynn, Wadhwa, Dunkel-Schetter, Chicz-Demet and Sandman2001; Khashan et al. Reference Khashan, McNamee, Abel, Mortensen, Kenny, Pedersen, Webb and Baker2009), fetal growth restriction in very preterm deliveries (Precht et al. Reference Precht, Andersen and Olsen2007) and pre-eclampsia (László et al. Reference László, Liu, Svensson, Wikström, Li, Olsen, Obel, Vestergaard and Cnattingius2013a ) – for stress exposure shortly before or during the time of placentation. However, other investigations did not find substantial differences in the risk of fetal growth restriction (Khashan et al. Reference Khashan, McNamee, Abel, Mortensen, Kenny, Pedersen, Webb and Baker2009; Class et al. Reference Class, Lichtenstein, Långström and D'Onofrio2011) or stillbirth (László et al. Reference László, Svensson, Li, Obel, Vestergaard, Olsen and Cnattingius2013b ) according to the time of exposure to stress, whereas two studies reported the highest preterm birth risk among women exposed to stress in mid-gestation (Hedegaard et al. Reference Hedegaard, Henriksen, Secher, Hatch and Sabroe1996; Class et al. Reference Class, Lichtenstein, Långström and D'Onofrio2011).
Limitations
Our study has a few limitations. First, we cannot exclude the possibility that in some cases placental abruption might have been diagnosed as unspecified late pregnancy bleeding; nevertheless, this possible misclassification is likely to be non-differential and thus attenuate the association measures toward the null. Second, although several sociodemographic, health and pregnancy-related factors were considered in our multivariable analysis, the possibility of residual confounding from genetic, familial life-style and other health-related factors is possible; potential candidates include nutritional factors and infections related to stress exposure. Third, since prior to 1995 the Danish National Hospital Register contains only in-patient diagnoses, maternal hypertension is likely to be underascertained in Denmark in earlier periods, i.e. only the most severe cases are likely to have been identified. Fourth, despite our overall large sample size, the power for some of the subanalyses is low, particularly in the group with chronic hypertension. Though some of the investigated associations in this group were statistically significant, precision was generally low when exposure was classified according to the time of death and the mother's relationship to the deceased; caution is thus needed when interpreting these results.
Conclusions
Death of a child the year before or in the first trimester of pregnancy is associated with an increased risk of placental abruption; this relationship is particularly strong among women with chronic hypertension. These findings support the hypothesis that a state of increased sympathetic or HPA axis activity may be related to placental abruption. Studies are needed to investigate the effect of less severe, but more frequent, sources of stress on placental abruption risk.
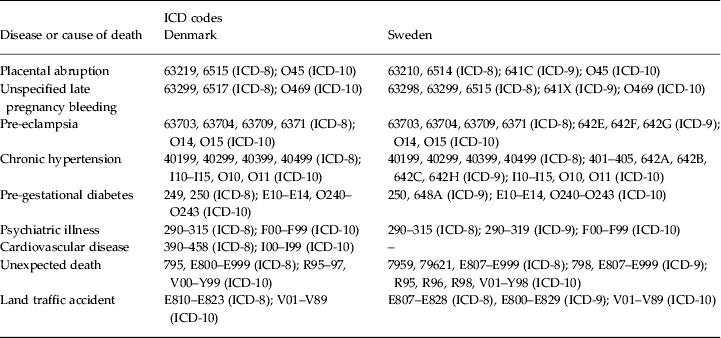
Appendix. ICD codes used to identify medical conditions and causes of death
Acknowledgements
The study was supported by the Swedish Council for Working Life and Social Research (grant no. 2010-0092) and by the European Research Council (ERC-2010-StG no. 260242 to J.L.). M.V. is supported by the Lundbeck Foundation. C.O. is supported by the Tryg Foundation.
Declaration of Interest
None.






