Introduction
Major depressive disorder (MDD) represents a leading cause of disability burden globally (Ferrari et al., Reference Ferrari, Charlson, Norman, Patten, Freedman, Murray, Vos and Whiteford2013; GBD 2015 Mortality and Causes of Death Collaborators, 2016), with high economic costs for society (Katon, Reference Katon2009; Sobocki et al., Reference Sobocki, Jönsson, Angst and Rehnberg2006; Gelenberg, Reference Gelenberg2010). MDD is associated with substantial disability across many domains of life (Coryell et al., Reference Coryell, Scheftner, Keller, Endicott, Maser and Klerman1993; Ormel et al., Reference Ormel, von Korff, van den Brink, Katon, Brilman and Oldehinkel1993; Judd et al., Reference Judd, Akiskal, Zeller, Paulus, Leon, Maser, Endicott, Coryell, Kunovac, Mueller, Rice and Keller2000; Greer et al., Reference Greer, Kurian and Trivedi2010; Kamenov et al., Reference Kamenov, Caballero, Miret, Leonardi, Sainio, Tobiasz-Adamczyk, Haro, Chatterji, Ayuso-Mateos and Cabello2016), affecting patients' ability to fulfil family roles, to work and to participate in the society at levels similar to or worse than those reported in chronic somatic diseases (Hays et al., Reference Hays, Wells, Sherbourne, Rogers and Spritzer1995; Buist-Bouwman et al., Reference Buist-Bouwman, De Graaf, Vollebergh, Alonso, Bruffaerts and Ormel2006).
Numerous studies have found that a more severe depression is associated with more disability (Ormel et al., Reference Ormel, von Korff, van den Brink, Katon, Brilman and Oldehinkel1993; Judd et al., Reference Judd, Akiskal, Zeller, Paulus, Leon, Maser, Endicott, Coryell, Kunovac, Mueller, Rice and Keller2000; Kruijshaar et al., Reference Kruijshaar, Hoeymans, Bijl, Spijker and Essink-Bot2003; Spijker et al., Reference Spijker, De Graaf, Bijl, Beekman, Ormel and Nolen2004a) and that a decrease in MDD severity is accompanied by a decrease in disability (Coryell et al., Reference Coryell, Scheftner, Keller, Endicott, Maser and Klerman1993; Ormel et al., Reference Ormel, von Korff, van den Brink, Katon, Brilman and Oldehinkel1993; Judd et al., Reference Judd, Akiskal, Zeller, Paulus, Leon, Maser, Endicott, Coryell, Kunovac, Mueller, Rice and Keller2000; Hirschfeld et al., Reference Hirschfeld, Dunner, Keitner, Klein, Koran, Kornstein, Markowitz, Miller, Nemeroff, Ninan, Rush, Schatzberg, Thase, Trivedi, Borian, Crits-Christoph and Keller2002; Buist-Bouwman et al., Reference Buist-Bouwman, Ormel, De Graaf and Vollebergh2004). However, the longitudinal relationship between disability and symptomatic course of MDD is not fully understood. For example, two studies that followed primary care patients for 1 (Judd et al., Reference Judd, Akiskal, Zeller, Paulus, Leon, Maser, Endicott, Coryell, Kunovac, Mueller, Rice and Keller2000) and 3.5 years (Ormel et al., Reference Ormel, von Korff, van den Brink, Katon, Brilman and Oldehinkel1993), as well as one 10-year study conducted in tertiary care centres (Von Korff et al., Reference Von Korff, Ormel, Katon and Lin1992) reported that disability disappears when patients become asymptomatic. However, later community-based studies with a 3-year follow-up (Buist-Bouwman et al., Reference Buist-Bouwman, Ormel, De Graaf and Vollebergh2004; Rhebergen et al., Reference Rhebergen, Beekman, de Graaf, Nolen, Spijker, Hoogendijk and Penninx2010) and an outpatient study of 5 years (Coryell et al., Reference Coryell, Scheftner, Keller, Endicott, Maser and Klerman1993) found residual disability after symptom remission. Similarly, the assumption that a chronic or recurrent course of depression leads to chronic disability was supported by some (Ormel et al., Reference Ormel, von Korff, van den Brink, Katon, Brilman and Oldehinkel1993; Judd et al., Reference Judd, Akiskal, Zeller, Paulus, Leon, Maser, Endicott, Coryell, Kunovac, Mueller, Rice and Keller2000) but not all (Kruijshaar et al., Reference Kruijshaar, Hoeymans, Bijl, Spijker and Essink-Bot2003; Spijker et al., Reference Spijker, Graaf, Bijl, Beekman, Ormel and Nolen2004b) studies.
Comparison across these studies might be limited by differences in methodologies. As Buist-Bouwman et al. (Reference Buist-Bouwman, Ormel, De Graaf and Vollebergh2004) pointed out, samples were recruited from different settings (community, primary and specialized mental care) and thus probably reflect different severities of MDD symptoms. Similarly, studies used different criteria to define MDD remission. For example, Von Korf et al. (Reference Von Korff, Ormel, Katon and Lin1992) defined MDD remission as a drop in MDD severity to the mean population level, whereas Buist-Bouwman et al. (Reference Buist-Bouwman, Ormel, De Graaf and Vollebergh2004) used dichotomous criteria for MDD diagnosis. Comparison across studies might further be complicated by the different ways in which disability was conceptualized. Most studies assessed work and social disability, which are known to be high in MDD (McKnight and Kashdan, Reference McKnight and Kashdan2009). However, various instruments were used, ranging from well-established to research-specific questionnaires, making the direct comparison of results difficult (McKnight and Kashdan, Reference McKnight and Kashdan2009). Furthermore, although most questionnaires also assessed other aspects of living with MDD, such as physical impairment, pain, impaired vitality or sexual activity, this was not done uniformly across the studies. Finally, since all but two studies have a duration of <3.5 years, insights into the long-term disability remain limited.
To overcome these limitations, the present study examines the 6-year disability in participants recruited from different settings, using a multiple-domain disability assessment scale consistent with the International Classification of Functioning, Disability and Health (ICF). We first examine whether MDD course influences disability by comparing the 6-year disability in participants with chronic, recurrent and remitting MDD and in healthy controls. Since sum-scores might obscure potential differences in disability across various domains (Kamenov et al., Reference Kamenov, Caballero, Miret, Leonardi, Sainio, Tobiasz-Adamczyk, Haro, Chatterji, Ayuso-Mateos and Cabello2016), we examine both total and domain-specific disability. Second, we examine whether disability persists after recovery from MDD, by comparing levels of disability in people with remitted MDD and healthy controls. The current practice assumes that patients who reach remission can also resume normal functioning. The study of residual disability and its predictors could help the timely identification of patients with increased risk for long-term disability and thus help alleviate the burden on individuals and society. According to the ICF, disability stems not only from illness, but also from its interaction with contextual factors (WHO, 2001). Therefore, our third aim is to identify socio-demographic, clinical and personality variables that predict residual disability in persons with remitting MDD. To our knowledge, such studies are still lacking.
Methods
Design
Data were drawn from the Netherlands Study of Depression and Anxiety (NESDA), a longitudinal cohort study examining the long-term course and consequences of depressive and anxiety disorders. Between September 2004 and February 2007, 2981 adults (18–65 years) with a current or remitted depressive and/or anxiety disorder (78%) and healthy controls (22%) were recruited from community (19%), primary (54%) and specialized (27%) mental health care. Exclusion criteria were: (1) a primary diagnosis of psychotic, obsessive-compulsive, bipolar or severe addiction disorder and (2) not being fluent in Dutch (Penninx et al., Reference Penninx, Beekman, Smit, Zitman, Nolen, Spinhoven, Cuijpers, De Jong, Van Marwijk, Assendelft, van der Meer, Verhaak, Wensing, de Graaf, Hoogendijk, Ormel and van Dyck2008). Baseline assessments (T0) were conducted at three study sites (Amsterdam, Groningen, Leiden), by trained research staff. The face-to-face interviews explored a wide range of domains, including socio-demographics, psychopathology, risk factors and consequences of mental disorders. Follow-up assessments took place at 2 years (T1; N = 2596, 87.1%), at 4 years (T2; N = 2402, 80.6%) and at 6 years (T3, until April 2013; N = 2256, 75.7%). The Ethical Review Boards of all participating centres approved the study. All participants provided written informed consent after the procedures were fully explained.
Study sample
Since we were interested in the course of disability in MDD, we selected respondents with a 1-month diagnosis of MDD with or without dysthymia at baseline who participated in all subsequent assessments and who fulfilled diagnostic criteria for chronic, recurrent or remitting MDD (see below). To examine whether residual disability persists following remission, we additionally selected healthy controls, i.e. participants without (a history of) depressive or anxiety disorders at all assessments. Incident MDDs (i.e. healthy controls at baseline with an MDD diagnosis at T1, T2 or T3) were not included. The final sample consisted of 914 participants: 57 (6.3%) with chronic MDD, 210 (23.0%) with recurrent MDD, 217 (23.7%) with remitting MDD and 430 (47.0%) healthy controls. Factors associated with attrition were identified among baseline socio-demographic and clinical variables, using logistic regression. Attrition was significantly associated with education (β = −0.3, p ⩽ 0.001) and severity of anxiety (β = 0.3, p = 0.007) but not with age (β = −0.1, p = 0.378), gender (β = 0.1, p = 0.396), severity of MDD (β = 0.2, p = 0.117), severity of avoidance (β = 0.1, p = 229), alcohol consumption (β = −0.1, p = 0.968) or chronic somatic diseases (β = −0.1, p = 0.834).
Diagnosis
MDD was diagnosed with the Composite International Diagnostic Interview (CIDI), version 2.1, Dutch version (Smitten et al., Reference Smitten, Smeets and Brink1998), a structured interview with high reliability (Wittchen et al., Reference Wittchen, Robins, Cottler, Sartorius, Burke and Regier1991; Wacker et al., Reference Wacker, Battegay, Mullejans, Schlosser, Stefanis, Rabavilas and Soldatos2006) and high validity for depressive disorders (Wittchen, Reference Wittchen1989, Reference Wittchen1994). Chronic MDD (i.e. MDD during the entire study period) was defined as meeting 1-month DSM-IV-criteria for MDD at baseline and a diagnosis of MDD or dysthymia at all subsequent assessments. Recurrent MDD was defined as meeting 1-month criteria for MDD at baseline, followed by at least 1 month of remission (not fulfilling criteria of MDD nor dysthymia) and a subsequent recurrence of MDD or dysthymia at any of the later assessments. In this group, 111 participants (52.9%) reported remission at T1 and recurrence at T2, 61 participants (29.0%) reported remission at T1 and recurrence at T3 and 38 participants (18.1%) reported remission at T2 and recurrence at T3. Remitting MDD was defined as meeting the 1-month criteria for MDD at baseline, followed by remission of MDD and dysthymia at T1 (early remission, n = 146, 67.3%), at T2 (intermediate remission, n = 48, 22.1%) or at T3 (late remission, n = 23, 10.6%).
Disability
Disability was assessed at each wave using the World Health Organization Disability Assessment Schedule II (WHO DAS II) (Chwastiak and Von Korff, Reference Chwastiak and Von Korff2003). This self-report questionnaire assesses on a Likert scale how much difficulty participants experienced over the past 30 days in performing activities such as, for example, doing the most important work/school tasks well; maintaining friendships or joining community activities in the same way as everyone else. Seven domains were assessed: household activities, work/school activities, interpersonal functioning, participation in society, cognition, mobility and self-care. Domain-specific scores were added to calculate total disability. Higher scores indicate more disability. The WHO DAS II is highly sensitive to symptom change in MDD (Perini et al., Reference Perini, Slade and Andrews2006) and has sufficient test–retest reliability (Chopra et al., Reference Chopra, Chopra, Couper and Herrman2004). Since a large proportion of participants (n = 266, 29.1%) did not work nor go to school, we used the 32-item version of the questionnaire (excluding work disability). The internal consistency was high (Cronbach's α = 0.90).
Putative predictors of disability in remitting MDD
We considered baseline socio-demographic, clinical and personality variables. Socio-demographic variables included: age, gender, education, presence of a partner and employment status. Clinical variables. Age of onset of depression was determined using the CIDI (Smitten et al., Reference Smitten, Smeets and Brink1998). Severity of MDD was assessed with the 30-item Inventory of Depressive Symptomatology (IDS) (Rush et al., Reference Rush, Giles, Schlesser, Fulton, Weissenburger and Burns1986). The presence of comorbid 1-month dysthymia and anxiety disorders (panic disorder with/without agoraphobia, social phobia, agoraphobia or generalized anxiety disorder) was assessed with the CIDI (Smitten et al., Reference Smitten, Smeets and Brink1998). Severity of anxiety and of avoidance symptoms was assessed using the 21-item Beck Anxiety Inventory (BAI) (Beck et al., Reference Beck, Epstein, Brown and Steer1988) and the 15-item Fear Questionnaire (FQ) (Marks and Mathews, Reference Marks and Mathews1979), respectively. Alcohol consumption (number of drinks containing alcohol on a typical day) was assessed using the Alcohol Use Disorders Identification Test (AUDIT) (Saunders et al., Reference Saunders, Aasland, Babor, de la Fuente and Grant1993). Consumption of 1–2 drinks was labelled as mild, 3–4 drinks as moderate; higher consumption was considered excessive drinking. The presence of somatic disease was operationalized as the number of chronic somatic diseases under medical treatment and was assessed using a comprehensive, self-reported inventory of 20 chronic conditions (Penninx et al., Reference Penninx, Beekman, Smit, Zitman, Nolen, Spinhoven, Cuijpers, De Jong, Van Marwijk, Assendelft, van der Meer, Verhaak, Wensing, de Graaf, Hoogendijk, Ormel and van Dyck2008). Psychiatric treatment referred to use of antidepressant medication or psychological treatment. Current medication use was considered when taken at least 50% of the time and referred to use of antidepressants [serotonin reuptake inhibitors, Anatomical Therapeutic Chemical (ATC) code N06AB; tricyclic antidepressants, ATC N06AA; and other antidepressants, ATC N06AF/N06AX]. Psychological treatment included formal psychotherapy, counselling or skills training. Care setting referred to whether participants were recruited from specialized mental health outpatient clinics, from primary care or from the community. Personality variables, assessed with the 60-item Neuroticism–Extraversion–Openness (NEO) personality self-report questionnaire (Costa and McCrae, Reference Costa and McCrae1995), included neuroticism, extraversion, agreeableness, conscientiousness and openness to experience.
Data analysis
Baseline characteristics across groups were compared using two-tailed χ2 tests for categorical variables, one-way analysis of variance statistics (ANOVA) for continuous variables, and non-parametric Kruskal–Wallis test for skewed variables. The course of total and domain-specific disability over time was examined using Linear Mixed Models (LMM). To correct for correlation in data due to the repeated measure design, ‘participant’ was introduced as a random factor. Group, time and group × time interaction were entered as fixed factors. The group × time interaction term compared changes in disability over time across groups. LMM analyses were adjusted for all socio-demographics, entered as fixed factors.
In remitting MDD (n = 217), residual disability was analysed using descriptive statistics (mean, s.d., minimum and maximum values). Predictors of residual disability in the remitting group were identified through regression analyses. Strength of association between putative predictors and disability was assessed through bivariate analysis (p value set at 0.1). Multicollinearity was suspected if Pearson's correlation coefficients were larger than 0.80 and if the variance inflation factor (VIF) was larger than 10 (Field, Reference Field2009). Independent predictors of disability were identified through four multivariate models (significance value set at 0.05). Models 1, 2 and 3 included socio-demographic, clinical and personality variables, respectively, that were significant in bivariate analyses. Significant factors obtained from Models 1, 2 and 3 were introduced in Model 4. We finally conducted a post-hoc analysis using the Spearman correlation coefficient and the coefficient of determination to examine the relationship between residual disability and residual depressive symptoms and to estimate their shared variance. Data were analysed using SPSS version 24.0 (IBM SPSS Statistics for Windows, 2016).
Results
Study sample
Baseline characteristics of the study sample (n = 914) are summarized in Table 1. Mean age was 41.8 years (s.d. 13.4). The majority of participants were female (62.0%), had followed 12.4 years of education (s.d. 3.2) and had a partner (69.3%). Compared to controls, MDD groups were significantly less educated, were less likely to have a partner or to be employed, were more likely to report abstinence from alcohol use, had more somatic diseases and reported higher neuroticism and lower extraversion, agreeableness and conscientiousness. Depressive symptoms were most severe in chronic MDD, followed by recurrent MDD, remitting MDD and healthy controls. Comorbid dysthymia was significantly less common in remitting MDD, compared to chronic and recurrent MDD.
Table 1. Baseline characteristics of participants with chronic, recurrent or remitting depressive disorder and of healthy controls (n = 914)
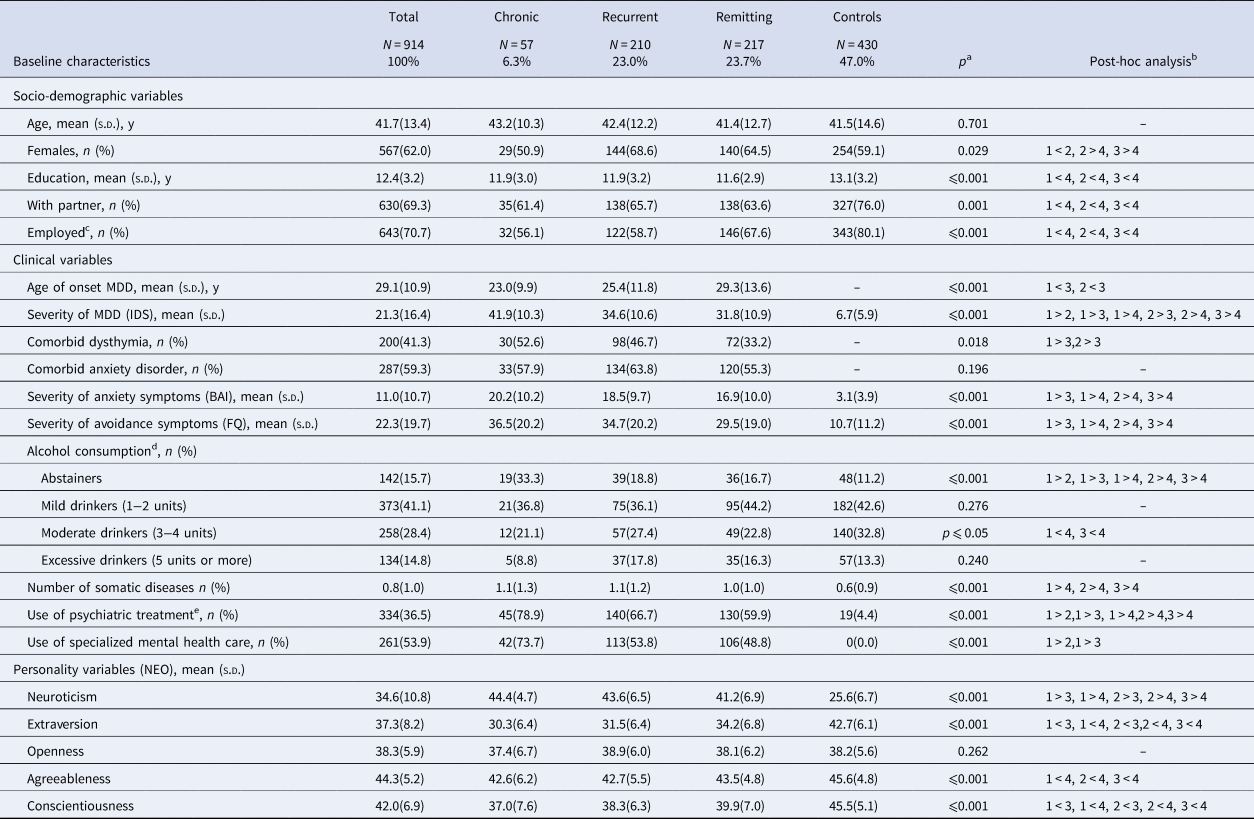
BAI, Beck Anxiety Inventory; FQ, Fear Questionnaire; IDS, Inventory of Depressive Symptomatology; MDD, Major Depressive Disorder; NEO, Neuroticism–Extraversion–Openness personality self-report questionnaire.
a Overall group differences, based on χ2 statistics for categorical variables, ANOVA for continuous variables and Kruskal–Wallis for skewed variables.
b Groups in post-hoc analysis noted as: 1 = chronic depressive disorder; 2 = recurrent depressive disorder; 3 = remitting depressive disorder; 4 = healthy controls. p ⩽ 0.05 is used as a level of significance.
c Analysis based on 909 cases (data on employment was missing for five out of 914 cases, 0.5%).
d Analysis based on 907 cases (data on alcohol consumption was missing for seven out of 914 cases, 0.8%).
e Antidepressant medication or psychological treatment.
Total disability
Total disability is presented Fig. 1 and in online Supplementary Table S1. At baseline, all diagnostic groups reported significantly more disability compared to healthy controls. Most disability was found in chronic MDD [β (s.e.) = 39.0 (1.8), p ⩽ 0.001], followed by recurrent MDD [β (s.e.) = 31.5 (1.1), p ⩽ 0.001] and remitting MDD [β (s.e.) = 27.2 (1.1), p ⩽ 0.001] (reference: healthy controls). Furthermore, we found a significant gradient in disability across diagnostic groups: chronic MDD functioned significantly worse than recurrent MDD [β (s.e.) = 6.0 (2.5), p ⩽ 0.05], which functioned significantly worse than remitting MDD [β (s.e.) = 3.8 (1.6), p ⩽ 0.05]. These differences remained statistically significant at T2 and T3.
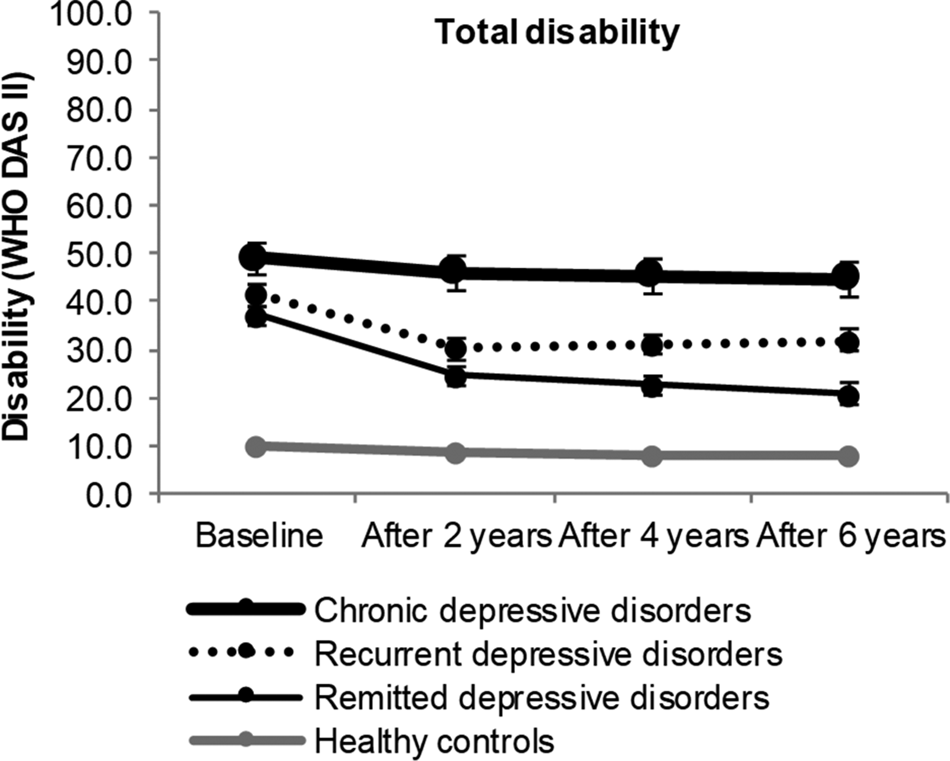
Fig. 1. Six-year course of total disability in participants with chronic depressive disorder, recurrent depressive disorder, remitting depressive disorder and healthy controls. Analyses are adjusted for age, gender, education and partner status at baseline. Differences between depression groups and healthy controls are significant at all measurements (p ⩽ 0.001).
Interaction with time between baseline and T3 was not significant for chronic MDD (see also Fig. 1), suggesting stable levels of disability during follow-up. By contrast, we found a significant negative interaction with time between baseline and T3 for recurrent MDD [β (s.e.) = −7.5 (1.1), p ⩽ 0.001] and for remitting MDD [β (s.e.) = −14.5 (1.0), p ⩽ 0.001] (reference: healthy controls), thus disability decreased over time in these groups. The significant decrease in disability occurred exclusively during the first 2 years of follow-up, when the majority of participants (81.9% for the recurrent group and 67.3% for the remitting group) achieved remission.
At T3, all MDD groups were significantly more disabled than healthy controls. Thus, significant residual disability persisted despite remission of MDD [β (s.e.) = 12.7 (1.1), p ⩽ 0.001]. Notably, residual disability showed significant heterogeneity in this group, with scores ranging between 0.0 and 81.1 (mean 19.2, s.d. 15.8). Furthermore, 18.0% of participants reported disability scores lower than the mean disability score of healthy controls, whereas 7.8% reported disability scores larger than the mean disability scores of chronic MDD.
Domain-specific disability
Domain-specific disability is presented in Fig. 2 and in online Supplementary Table S2. At baseline, all diagnostic groups reported significant disability in all domains. Regardless of the diagnostic group, most disability was found in household activities, followed by interpersonal functioning, participation in society and cognition, and least disability in mobility and self-care. Chronic MDD reported significantly more disability in household activities, interpersonal functioning, participation in society and cognition, but not in mobility and self-care, compared to recurrent and remitting MDD. In turn, recurrent MDD was significantly more disabled in interpersonal functioning, cognition and self-care, but not in household activities, participation in society and mobility, compared to remitting MDD.
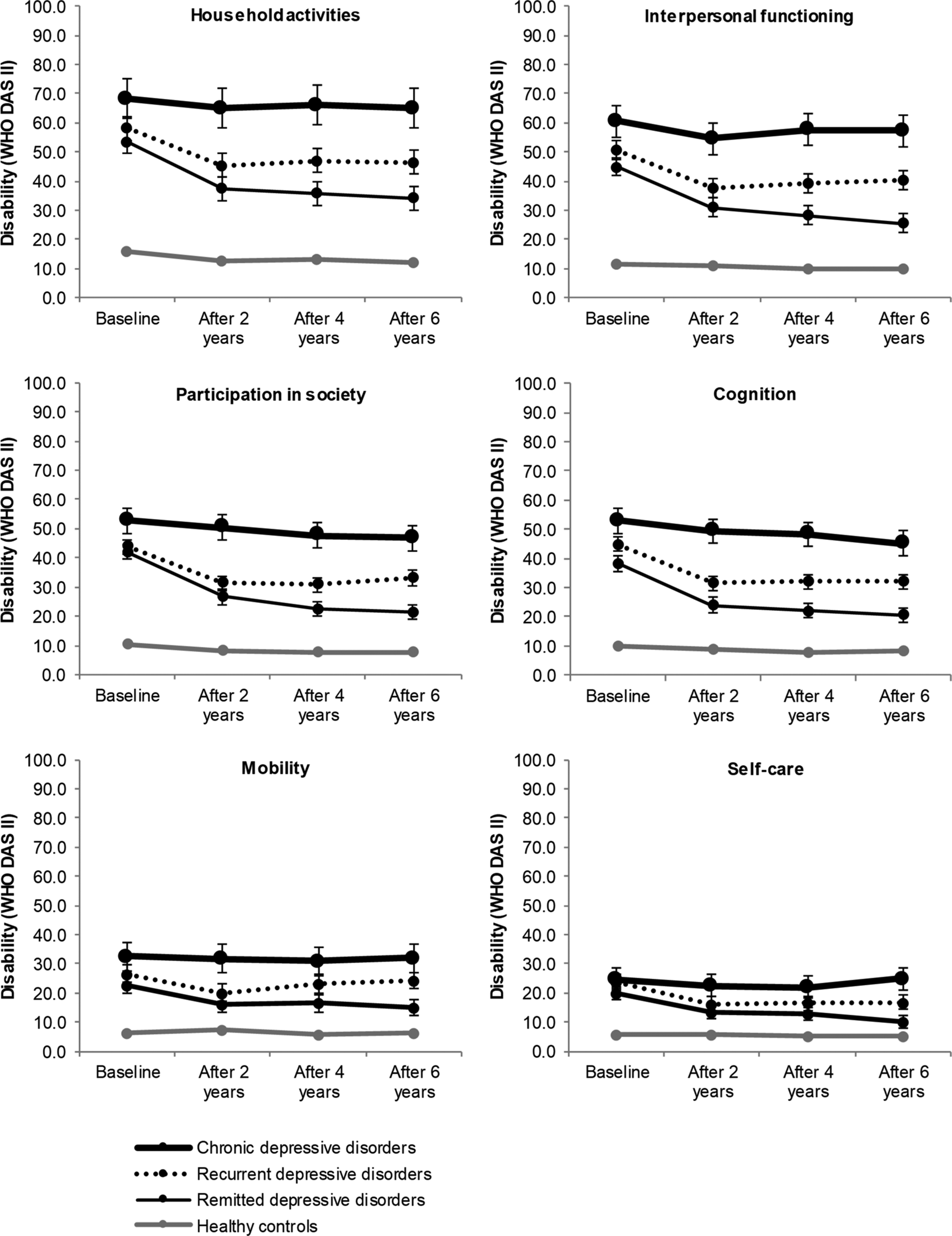
Fig. 2. Six-year course across domains of disability in participants with chronic depressive disorder, recurrent depressive disorder, remitting depressive disorder and healthy controls. Analyses are adjusted for age, gender, education and partner status at baseline. Differences between depression groups and healthy controls are significant at all measurements (p ⩽ 0.001).
Similar to total disability, domain-specific disability in recurrent and remitted MDD decreased significantly over time across all domains, due to a significant decrease during the first 2 years. Notably, in remitted MDD, three domains (participation in society, interpersonal functioning and self-care) continued to improve even after the first 2 years of follow-up, suggesting that these domains need more time to improve. In chronic MDD, for which total disability did not change over time, disability was stable in all domains, except for cognition. Cognition improved significantly from baseline to T3 [β (s.e.) = −6.3 (2.2), p ⩽ 0.05, reference: healthy controls]. At T3, all domain-specific disability differed significantly across the groups, with worse disability reported by chronic MDD, followed by recurrent and remitting MDD and by healthy controls.
Predictors of residual disability
Predictors of disability in remitting MDD are shown in Table 2. In bivariate analysis, disability was associated with several predictors. Multicollinearity was not a problem (the largest Pearson's correlation coefficient was 0.5, the largest VIF was 1.6). Among socio-demographic variables (Model 1), age, education and employment status were predictive of residual disability. Among clinical variables (Model 2), residual disability was predicted by time-point of remission of MDD, severity of avoidance, number of somatic diseases and baseline disability. Among personality variables (Model 3), only neuroticism and extraversion remained significant. Our final model (Model 4) showed residual disability in remitting MDD was significantly predicted by older age, late remission of MDD (at T3), more severe avoidance symptoms and higher baseline disability. The total variance explained by the model was 43.9% (F = 15.2, df = 10, p ⩽ 0.001).
Table 2. Bivariate and multivariate predictors of disability at 6-year follow-up in participants with remitting depressive disorder (n = 217)
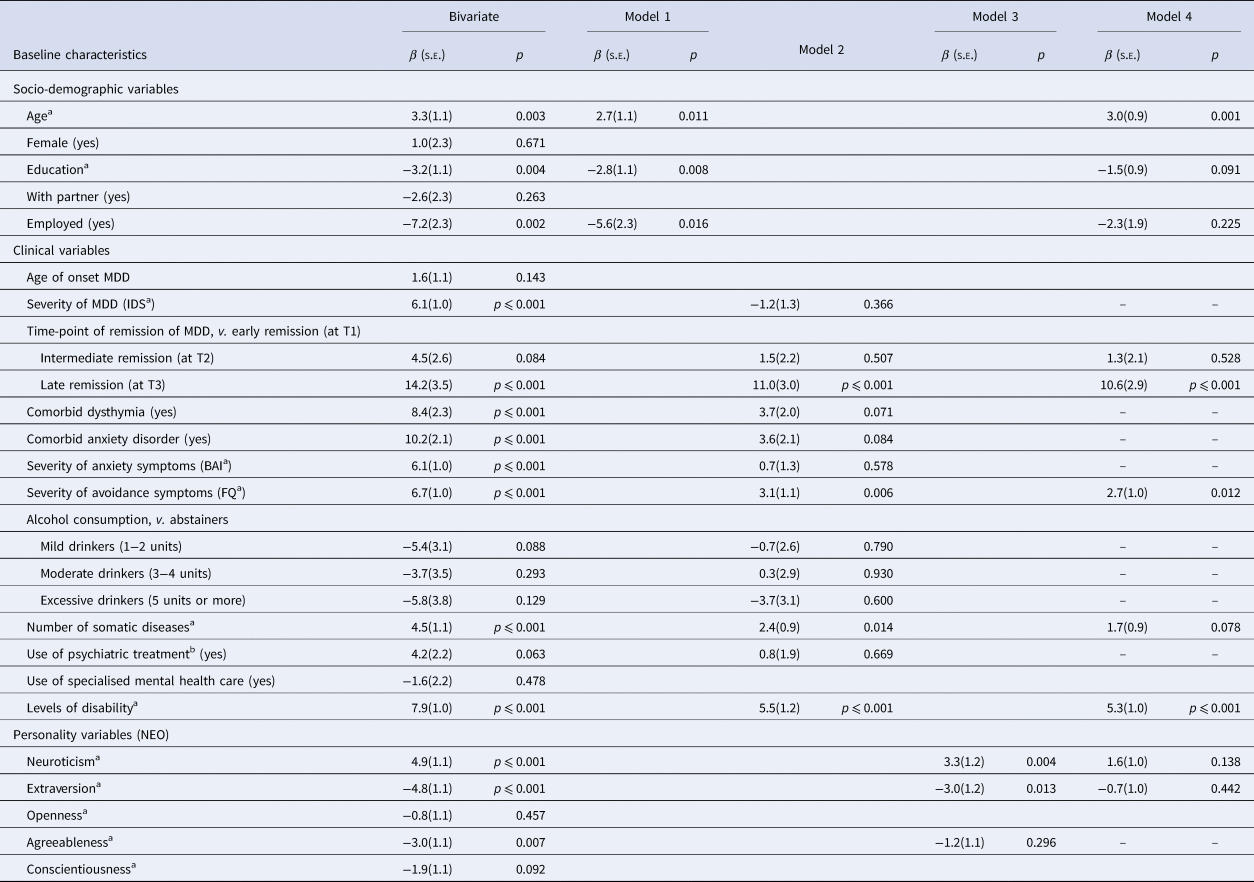
BAI, Beck Anxiety Inventory; FQ, Fear Questionnaire; IDS, Inventory of Depressive Symptomatology; MDD, Major Depressive Disorder; NEO, Neuroticism–Extraversion–Openness personality self-report questionnaire.
Model 1: socio-demographic variables; Model 2: clinical variables; Model 3: personality variables; Model 4: final model.
a Per s.d. increase.
b Antidepressant medication or psychological treatment.
Discussion
Our primary aim was to examine the relationship between the course of depression and disability over a 6-year period. Results showed that MDD was associated with significantly more disability than no diagnosis and that most disability was reported by chronic MDD, followed by recurrent and remitting MDD. A chronic course of depression was associated with a chronic, stable course of disability. By contrast, remission of depressive symptoms was associated with a significant decrease in disability, which however did not reach the level of healthy controls. This synchrony of change between the symptomatic course of depression and the course of disability is in line with previous findings (Ormel, Reference Ormel2000).
MDD was associated with significant disability across all domains. Most disability was found in household activities, interpersonal functioning, participation in society and cognition, and least disability was found in mobility and self-care. Similar findings were reported in a recent study, in which domestic life and interpersonal activities, and to a lesser extent mobility and cognition (but not self-care) had a high impact on the quality of life in depressive disorder in nine countries (Kamenov et al., Reference Kamenov, Caballero, Miret, Leonardi, Sainio, Tobiasz-Adamczyk, Haro, Chatterji, Ayuso-Mateos and Cabello2016). This suggests that depression has a lower impact on mobility, compared to other domains of functioning. In line with Konecky et al. (Reference Konecky, Meyer, Marx, Kimbrel and Sandra2014), we conclude that the assessment of mobility might be less indicative of disability in MDD.
Across all disability domains, we found a synchrony of change, with significant changes within the first 2 years of follow-up. Chronic depression was associated with chronic domain-specific disability, except for cognition. This suggests that symptomatic remission might be a prerequisite for improvements in disability. In remitting MDD, interpersonal functioning and participation in society continued to improve even after the first 2 years of follow-up. This suggests that different domains improve at a different speed. It is possible that depressive symptoms remit at differential speeds, which in turn impacts on the domains of disability. This relationship was shown in a recent study, which found that early insomnia had strong effects on work disability, while self-blame impacted on close relationships, interest loss on social activities and fatigue on home management (Fried and Nesse, Reference Fried and Nesse2014).
Total and domain-specific disability persisted after symptomatic remission. This is remarkable, considering that at T3 the majority of participants with remitting MDD had been in remission for at least 2 years. Four scenarios are possible. First, remission of disability might lag behind symptomatic remission (Rhebergen et al., Reference Rhebergen, Beekman, de Graaf, Nolen, Spijker, Hoogendijk and Penninx2010). A late remission (at T3) appeared to be the most important predictor of high levels of residual disability. It is thus possible that those who remitted at the end of the follow-up simply lack time for improvements in disability. Second, residual disability might be due to subthreshold symptoms (state effect) (Karsten et al., Reference Karsten, Hartman, Ormel, Nolen and Penninx2010). In our study, residual disability showed substantial heterogeneity. Post-hoc analyses in remitting MDD revealed a strong, positive correlation between the severity of residual disability and the severity of residual depressive symptoms (Spearman's r = 0.7, p ⩽ 0.001). Furthermore, we found that the severity of residual depressive symptoms explained nearly half of the variance in residual disability in this group (coefficient of determination = 48.6). This suggests that residual disability might be largely due to subthreshold depressive symptoms. Third, residual disability could be a continuation of pre-morbid disability (trait effect) (Ormel et al., Reference Ormel, Oldehinkel, Nolen and Vollebergh2004; Bos et al., Reference Bos, ten Have, van Dorsselaer, Jeronimus, de Graaf and de Jonge2018). In the absence of pre-morbid assessments, we cannot verify trait effects, but trait effects have been documented previously (Ormel et al., Reference Ormel, Oldehinkel, Nolen and Vollebergh2004). Fourth, residual disability might represent persistent disability developed during MDD (scar effects) (Ormel et al., Reference Ormel, Oldehinkel, Nolen and Vollebergh2004; Bos et al., Reference Bos, ten Have, van Dorsselaer, Jeronimus, de Graaf and de Jonge2018). According to Ormel et al. (Reference Ormel, Oldehinkel, Nolen and Vollebergh2004), scarring may occur in severe recurrent episodes. In our study, baseline severity of MDD did not predict residual disability.
Higher residual disability was predicted by late symptom remission, as well as by older age, more severe avoidance symptoms and higher disability at baseline. Severity of avoidance symptoms predicts disability in anxiety disorders (Iancu et al., Reference Iancu, Batelaan, Zweekhorst, Bunders, Veltman, Penninx and Van Balkom2014; Hendriks et al., Reference Hendriks, Spijker, Licht, Hardeveld, de Graaf, Batelaan, Penninx and Beekman2016), but to our knowledge, no studies examined this predictor in relation to disability in MDD. Use of psychiatric treatment did not reach statistical significance in predicting residual disability (p = 0.063). This may seem surprising, since both antidepressant medication and psychotherapy can reduce disability even in patients with severe MDD (Hirschfeld et al., Reference Hirschfeld, Dunner, Keitner, Klein, Koran, Kornstein, Markowitz, Miller, Nemeroff, Ninan, Rush, Schatzberg, Thase, Trivedi, Borian, Crits-Christoph and Keller2002). However, one should bear in mind that this study has a naturalistic design. Hence, this finding is likely due to the fact that treatment is used most often by the most severe patients, who also happen to have the worst outcomes (Cabello et al., Reference Cabello, Caballero, Chatterji, Cieza and Ayuso-Mateos2014).
Strengths and limitations
Strengths of this study include the large sample size, recruitment from different settings, use of structured diagnostic procedures, follow-up of 6 years and assessment of several domains of disability, in line with the ICF. One limitation resides in the assessment of disability. We rely on self-reported measures, which are important to assess the patient's perspectives but might be impacted by information-processing biases. Depressive symptoms are known to alter the interpretation of one's own functioning (Kempen et al., Reference Kempen, van Heuvelen, van den Brink, Kooijman, Klein, Houx and Ormel1996) and of socially relevant information (Weightman et al., Reference Weightman, Air and Baune2014). Since the NESDA study did not collect any information from other sources (such as family members, friends or healthcare providers), it is not possible to assess whether the disability scores of participants with MDD were erroneously increased. Further limitations are the lack of insights regarding the level of premorbid functioning and the fact that work disability could not be assessed because a large proportion of included participants did not work. Also, MDD course neglected symptom fluctuations between assessments. This study did not include data on income and therefore could not provide a complete assessment of the role played by the socio-economic status in predicting disability in people with remitted MDD. Finally, we do not know whether the predictors of residual disability are causal, and hence, we cannot be sure that targeting these predictors will improve functioning after symptomatic remission.
Clinical implications
Chronic MDD was associated with a chronic course of disability, whereas symptomatic remission was associated with a significant decrease in disability, suggesting that symptomatic remission is a prerequisite for improvements in disability. However, symptom remission does not imply full remission of disability. Therefore, treatment of MDD should explicitly focus on disability, especially on the more complex domains. Older age, high levels of disability and severe avoidance symptoms represent risk factors for residual disability. This underlines the importance of current treatment strategies that promote behavioural activation (i.e. diminishing avoidance). Furthermore, severity of residual disability correlated with the severity of residual depressive symptoms. Therefore, treating subthreshold depressive symptoms in patients with remitted MDD might prevent recurrence of MDD and thus reduce the burden of this disease.
Author ORCIDs
Sorana C. Iancu, 0000-0001-6269-8123
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291719001612.






