Introduction
Mild traumatic brain injury (mTBI) is a frequent medical condition with an incidence of about 100 to 300 per 100 000. mTBI is often used interchangeably with related terms such as concussion, cerebral concussion or mild head injury (Anderson et al. Reference Anderson, Heitger and Macleod2006). Various definitions of mTBI or concussion exist, mainly including an induction by biomechanical forces, a rapid onset of neurological functional impairments, and grossly normal neuroimaging studies (mTBI Committee, 1993; Aubry et al. Reference Aubry, Cantu, Dvorak, Graf-Baumann, Johnston, Kelly, Lovell, McCrory, Meeuwisse and Schamasch2002; Carroll et al. Reference Carroll, Cassidy, Holm, Kraus and Coronado2004; Cantu et al. Reference Cantu, Aubry, Dvorak, Graf-Baumann, Johnston, Kelly, Lovell, McCrory, Meeuwisse, Schamasch, Kevin, Bruce, Ferrara, Kelly, McCrea, Putukian and McLeod2006; Ruff, Reference Ruff2009). mTBI may be caused by relatively minor traumata, such as sport injuries, household accidents, whiplash injuries or falls (Rickels et al. Reference Rickels, Wild, Wenzlaff and Bock2006). Possible acute symptoms of mTBI comprise short-time unconsciousness, headache, dizziness, irritability, anxiety and impaired neuropsychological functions such as reduced attention, concentration or memory problems (Evans, Reference Evans1992; Hall et al. Reference Hall, Hall and Chapman2005). Remission frequently occurs within hours up to a 1 or 2 months (Jacobson, Reference Jacobson1995; Binder et al. Reference Binder, Rohling and Larrabee1997; Schretlen & Shapiro, Reference Schretlen and Shapiro2003; Frencham et al. Reference Frencham, Fox and Maybery2005), but may take up to 1 year (Dikmen et al. Reference Dikmen, McLean and Temkin1986, Reference Dikmen, Ross, Machamer and Temkin1995; Alexander, Reference Alexander1995). However, some patients suffer from physical, cognitive or psychological impairments even years after the trauma, which may hamper their reintegration into social, familial and professional life (Barth et al. Reference Barth, Diamond and Errico1996; Gasquoine, Reference Gasquoine1997; McAllister & Arciniegas, Reference McAllister and Arciniegas2002; Ponsford & Schönberger, Reference Ponsford and Schönberger2010).
Long-lasting symptoms are often referred to as ‘post-concussion syndrome’ and include somatic symptoms such as chronic headaches, chronic pain syndromes, dizziness and visual disturbances. Frequently encountered cognitive symptoms include attentional deficits, slowed information processing, reduced verbal and working memory, and impaired executive functions. Psychiatric symptoms such as depressed mood, anxiety, irritability, agitation, poor motivation, social withdrawal and interpersonal difficulties are also reported frequently (Binder, Reference Binder1986; Ashman et al. Reference Ashman, Spielman, Hibbard, Silver, Chandna and Gordon2004). The reported prevalence rates for long-lasting post-concussion symptoms that occur after mTBI vary from 7–8% (Binder, Reference Binder1986) to 10–20% (Alexander, Reference Alexander1995), up to 33% (Rimel et al. Reference Rimel, Giordani, Barth, Boll and Jane1981). Patients with post-concussion symptoms repeatedly consult health-care services, constituting a therapeutic challenge for neurologists, psychiatrists and psychologists. As these patients often present with symptoms several years after mTBI, in the absence of visible brain damage according to standard clinical neuroimaging methods, they often arouse the suspicion of malingering and stir up a debate about the necessity of treatment and the fair forensic evaluation of patients who sustained mTBI earlier in life (Green et al. Reference Green, Iverson and Allen1999; Paniak et al. Reference Paniak, Reynolds, Toller-Lobe, Melnyk, Nagy and Schmidt2002; Flaro et al. Reference Flaro, Green and Robertson2007; Ruff et al. Reference Ruff, Iverson, Barth, Bush and Broshek2009).
Despite their social and scientific relevance, convincing studies on long-term sequelae of mTBI are rare, and as a recent review pointed out, there is insufficient evidence to determine whether mTBI is associated with cognitive deficits 6 months or longer post-injury (Dikmen et al. Reference Dikmen, Corrigan, Levin, Machamer, Stiers and Weisskopf2009). The few studies covering a time period of several years differ with respect to methods and reveal heterogeneous and sometimes conflicting results (Table 1). Segalowitz et al. (Reference Segalowitz, Bernstein and Lawson2001) investigated students who, on average, suffered from mTBI 6.4 years ago and did not report any subjective impairment at the time of investigation. Nevertheless, a comprehensive neuropsychological assessment revealed attention and information processing deficits. Similarly, Vanderploeg et al. (Reference Vanderploeg, Curtiss and Belanger2005, Reference Vanderploeg, Curtiss, Luis and Salazar2007) found impairments in partial aspects of complex attention and working memory, on average 8 years post-injury. By contrast, several studies did not find evidence of cognitive or psychological deficits several years after the trauma. For example, Ettenhofer & Abeles (Reference Ettenhofer and Abeles2009) reported that mTBI does not result in cognitive impairment or psychiatric dysfunction; however, follow-up testing was performed 2.9 years after injury.
Table 1. Studies investigating long-term cognitive and emotional consequences after a minimum of 6 years after mild traumatic brain injury (mTBI)

a Self-reported TBI.
b TBI defined by diagnostic criteria.
The first five studies included patients with mild TBI only. The inclusion was based on self-report in four cases or defined diagnostic criteria in one case, but not on magnetic resonance imaging (MRI)-based exclusion of brain lesions at the time of testing. The next six studies included patients with mild to moderate or very severe TBI; thus their results may be influenced by the higher degree of severity.
Diverging methodological approaches, such as differences in study design or in the assessment of confounding variables, constitute a crucial issue (Gasquoine, Reference Gasquoine1997; Mathias & Coats, Reference Mathias and Coats1999). The lack of a consistent definition of mTBI is one of several methodological problems. Many authors refer exclusively to the initial Glasgow Coma Scale (Teasdale & Jennett, Reference Teasdale and Jennett1974) to assess the degree of trauma, whereas others base their diagnosis on additional criteria, such as those suggested by the Mild Traumatic Brain Injury Committee of the American Congress of Rehabilitation Medicine (ACRM) (mTBI Committee, 1993; Carroll et al. Reference Carroll, Cassidy, Holm, Kraus and Coronado2004; Lezak et al. Reference Lezak, Howieson and Loring2004). Most investigations rely on retrospective self-reports, that is diagnosis and classification are based on patients' reports years after the trauma. The selection of the study sample constitutes another difficulty in mTBI research. Many studies were unable to exclude false-positive findings influenced by secondary reinforcers such as compensation claims (Paniak et al. Reference Paniak, Reynolds, Toller-Lobe, Melnyk, Nagy and Schmidt2002; Flaro et al. Reference Flaro, Green and Robertson2007), an issue of paramount importance in evaluating mTBI (Arciniegas et al. Reference Arciniegas, Anderson, Topkoff and McAllister2005; Merten et al. Reference Merten, Green, Henry, Blaskewitz and Brockhaus2005). Furthermore, there is the possibility that investigators overestimate the consequences and subjective meaning of mTBI in the life of the patients by selecting their study sample from a clinical population still receiving medical care at the time of assessment. This issue was confirmed by the meta-analysis of Belanger et al. (Reference Belanger, Curtiss, Demery, Lebowitz and Vanderploeg2005), who reported that cognitive impairments after mTBI in clinical populations yielded effect sizes of d=0.74, compared to unselected prospective samples with effect sizes of d=0.04. This underlines the importance of the recruitment procedure for this kind of study. It is essential to include subjects based on objective criteria (e.g. time of injury) rather than selecting them according to subjective criteria such as the presence of self-reported complaints.
Given that the long-term consequences of mTBI remain inconclusive, we conducted an investigation of patients who had sustained mTBI on average 6 years ago, but with a methodological improvement. Patients who fulfilled the criteria of the Mild Traumatic Brain Injury Committee of the ACRM were selected from the initial clinical charts and included irrespective of current symptoms. State-of-the-art magnetic resonance imaging (MRI) was conducted to exclude any structural brain damage at time of testing. We investigated a broad range of cognitive domains, while controlling for psychiatric conditions and malingering, using established psychometric methods. We hypothesized that mTBI leads to cognitive impairments that are still detectable 6 years after trauma and relevant for daily living. We further hypothesized that emotional disturbances are also more frequent after having sustained mTBI.
Method
Ethical guidelines
All procedures were approved by the Institutional Ethical Review Board. The ethical standards of the Declaration of Helsinki were met and all participants provided written informed consent.
Subjects
Patients with the clinical diagnosis of mTBI treated at the University of Muenster in the years 2001 to 2003 were identified by systematic screening of patients' charts. Inclusion criteria were documentation of the clinical diagnosis, age at time of testing between 18 and 65 years, and conformance to the research diagnostic criteria of the ACRM (mTBI Committee, 1993). These criteria include any period of loss of consciousness for a maximum of 30 min and/or post-traumatic amnesia for a maximum of 24 h and/or any alteration in mental state at the time of the accident and/or focal neurological deficit(s) that may or may not be transient. Furthermore, an initial Glasgow Coma Scale score (if available) of 13–15 measured 30 min post-injury was required. As language-based neuropsychological tests were used, only native German-speaking patients were included. An initial telephone screening was performed to exclude individuals with head injuries additional to the index mTBI, psychopharmacological medication, neurological diseases, or MRI contra-indications. According to the charts, 233 patients suffered from mTBI. Of these, 136 could not be contacted because of missing or inaccurate contact information. Of the remaining 97 patients who could be contacted, 53 did not give written informed consent. In the remaining 44, two patients suffered from claustrophobia and terminated the MRI scan prematurely and three others did not attend for neuropsychological testing. Three more patients completed the neuropsychological testing but denied participation in the MRI. In two out of 36 patients (5.6%) and one out of 36 controls (2.8%), the MRI scan showed structural lesions. These patients and the associated control subjects were excluded from further analysis, thus 33 matched pairs entered the final analysis.
Healthy volunteers were recruited by advertisement and matched individually to the patients according to gender (same gender), age (±2 years) and education level (defined as highest graduation level). As in patients, an initial telephone screening was performed to exclude prior head injury, any medical or neurological diseases, or MRI contra-indications.
Inclusion into final analysis using additional neuroimaging criteria
Thirty-six patients and 36 matched healthy controls met the inclusion criteria. As a unique step for quality assurance distinguishing this study from other neuropsychological investigations and as part of a larger investigation, a structural MRI was performed at the time of testing to screen for previously undetected brain lesions. MRI data were acquired on a 3-T whole-body scanner (Gyroscan Intera T30, Philips Medical Systems, The Netherlands). The imaging protocol comprised the following sequences:
(1) Axial T2-weighted turbo-spin-echo [echo time (TE)/repetition time (TR) 80/3000 ms; flip angle 90°; 36 contiguous slices; slice thickness 3.6 mm; field of view (FOV) 240 mm; matrix 400×512; scan duration 5:15 min].
(2) Axial T2*-weighted fast-field-echo sequence (TE/TR 16 ms/shortest; flip angle 18°; 36 contiguous slices; slice thickness 3.6 mm; FOV 230 mm; matrix 256×512; scan duration 4:09 min).
(3) Sagittally acquired 3D T1-weighted turbo-field-echo (TE/TR 3.4/7.4 ms; flip angle 9°; 320 slices; FOV 256 mm×205 mm×160 mm; matrix 512×410×320 resulting in isotropic voxels with an edge length of 0.5 mm; scan duration 11:09 min).
All scans were evaluated by a radiologist experienced in neurosurgery (H.S.) who was blinded to subjects status (patient or control). For two out of 36 patients (5.6%) and one out of 36 controls (2.8%), the MRI scan showed structural lesions. These patients and the associated control subjects were excluded from further analysis, thus 33 matched pairs entered the final analysis.
Characteristics
The group demographic characteristics of the remaining 66 participants are displayed in Table 2. None of the characteristics differed significantly between groups. In the final cohort, 18 patients had sustained mTBI due to a traffic accident, six due to a sports injury, and nine due to a fall or a collision. The mean time elapsed between injury and testing was 6.02 years (range 4.75–7.25 years).
Table 2. Characteristics of the study population
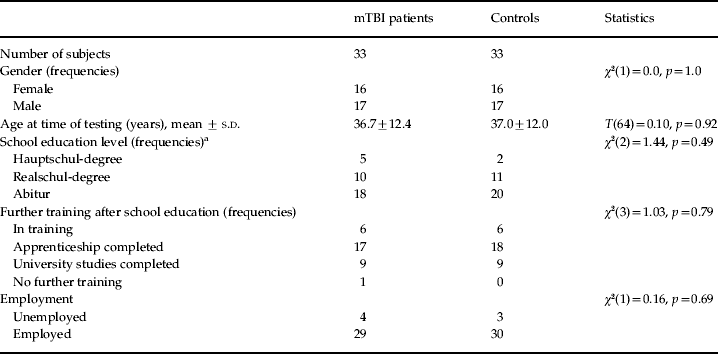
mTBI, Mild traumatic brain injury; s.d., standard deviation.
a German terms for school education are not translated into English, as the education systems are fundamentally different. The ‘Hauptschul-degree’ requires 9 years of education at a basic school, the ‘Realschul-degree’ requires 10 years at a higher level of education, and the ‘Abitur’ 12–13 years at the highest level of school education.
Materials and procedures
A neuropsychological and psychiatric test battery containing the following standardized instruments was performed:
(1) Auditory Verbal Learning Test (AVLT), German version: Verbaler Lern- und Merkfähigkeitstest (Rey, Reference Rey1964; Helmstaedter et al. Reference Helmstaedter, Lendt and Lux1996). Subjects learn, reproduce and recognize a list of 15 common nouns presented over five trials.
(2) Tests for Attentional Performance (TAP), German version: Testbatterie zur Aufmerksamkeitsprüfung, versions 1.02c and 1.7, computer-based (Zimmermann & Fimm, Reference Zimmermann and Fimm1992). Attentional performance was tested using the working-memory, divided attention and go/nogo subtests of the TAP. In the working-memory test, subjects perform a two-back task based on digits, in the divided attention test subjects have to attend to and react to visual and auditory stimuli presented simultaneously, and in the go/nogo subtest subjects differentiate between critical and non-critical visual stimuli and prevent inadequate reactions.
(3) Trail Making Test Parts A and B (TMT-A and TMT-B; Reitan, Reference Reitan1958; Spreen & Strauss, Reference Spreen and Strauss1998). Subjects connect numbered only circles, or both numbered and lettered circles.
(4) Word fluency tasks (Regensburger Wortflüssigkeits-Test, RWT). Subjects name as many words as possible for the categories ‘initial letter S’, ‘animals’ and ‘alteration sports/fruit’ (Aschenbrenner et al. Reference Aschenbrenner, Tucha and Lange2000).
(5) Digit span of the Wechsler Memory Scale, Revised, German adaptation (WMS-R). Subjects repeat progressively longer strings of digits in the same or in reverse order respectively (Härting et al. Reference Härting, Markowitsch, Neufeld, Calabrese, Deisinger and Kessler2000).
(6) Beck Depression Inventory (BDI, German adaptation) to assess subjective symptoms of depressed mood (Beck et al. Reference Beck, Ward, Mendelson, Mock and Erbaugh1961; Hautzinger et al. Reference Hautzinger, Bailer, Worall and Keller1995).
(7) To measure patients' impairment in daily life, we constructed a 25-item questionnaire (Q-25), comparable with the Rivermead Post Concussion Symptoms Questionnaire (RPQ). Subjects rated subjectively perceived performance changes that occurred since their injury, taking into account different domains of daily living and cognitive functioning (King et al. Reference King, Crawford, Wenden, Moss and Wade1995).
(8) A standardized Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) was conducted with all participants (Wittchen et al. Reference Wittchen, Zaudig and Fydrich1997).
(9) A German adaptation of the Word Memory Test (WMT) was performed. This computer-based verbal learning test provides information on possible negative response bias during test taking, and measures immediate recognition (IR), delayed recognition (DR) and consistency (CNS) below 82.5% (Green, Reference Green2005).
The complete testing and clinical interview session was conducted individually for each participant and lasted about 2–3 h. Test scores were categorized into five cognitive domains: (1) episodic memory: acquisition and consolidation, (2) episodic memory: retention and recognition, (3) working memory, (4) attention, and (5) executive functions. To control for mood effects, we also assessed impairment in daily life and depression (see Table 3) .
Table 3. List of (neuropsychological) test scores categorized into five cognitive domains, one mood domain and one suboptimal effort domain

AVLT, Auditory Verbal Learning Test; WMT, Word Memory Test; TAP, Tests for Attentional Performance; TMT, Trail Making Test; BDI, Beck Depression Inventory; Q-25, 25-item questionnaire; SCID-I, Structured Clinical Interview for DSM-IV Axis I Disorders; IR, immediate recognition; DR, delayed recognition; CNS, consistency of immediate and delayed recognition responses.
For descriptive analysis, means and standard deviations (s.d.) were calculated. For statistical analysis, for each domain the effect of group (patient or control) on test scores was assessed using multivariate analysis of variance (MANOVA). Calculations were performed using SPSS version 15.0. The results of the SCID were analyzed descriptively.
Results
Group comparisons of neuropsychological and behavioral data
All seven MANOVAs revealed significant differences in cognitive performance between mTBI patients and control subjects. Table 4 shows means, standard deviations and univariate effects for the 21 neuropsychological, two mood and three suboptimal effort measures across both groups. Fig. 1 displays deviations in cognitive performance of mTBI patients.

Fig. 1. Mean deviation from average of the controls (z scores). AVLT, Auditory Verbal Learning Test; WMT, Word Memory Test; MC, Multiple Choice; PA, Paired Associations; FR, Free Recall; LDFR, Long Delay Free Recall; TAP, Tests for Attentional Performance; WM, Working Memory; DS_F, Digit Span Forwards; DS_B, Digit Span Backwards; DA, Divided Attention; TMT, Trail Making Test, WF_S, Word Fluency – S; AN, Animals; S, Sports/Fruits.
Table 4. Results

mTBI, Mild traumatic brain injury; AVLT, Auditory Verbal Learning Test; WMT, Word Memory Test; TAP, Tests for Attentional Performance; TMT, Trail Making Test; BDI, Beck Depression Inventory; Q-25, 25-item questionnaire; IR, immediate recognition; DR, delayed recognition; CNS, consistency of immediate and delayed recognition responses; df, degrees of freedom; s.d., standard deviation.
Significant group difference at: * p<0.05, ** p<0.01, *** p<0.001.
(1) Episodic memory: acquisition and consolidation. Group differences were highly significant [Wilks' λ=0.742, F(3.62)=7.18, p<0.001, partial ε2=0.26]. Between-group effects were significant for the parameters WMT-Paired-Associations and AVLT-Sum Trial 1 to 5. (2) Episodic memory: retention and recognition. The overall effect was highly significant [Wilks' λ=0.753, F(6.59)=3.23, p<0.01, partial ε2=0.25]. Between-group effects were significant for all measures except for AVLT-Trial 5 minus 7. (3) Working memory. Group differences were highly significant in multivariate analysis [Wilks' λ=0.654, F(4.61)=8.07, p<0.001, partial ε2=0.35]. Univariate statistics were significant for three out of four outcomes. (4) Attention. The main effect was highly significant for this analysis [Wilks' λ=0.785, F(4.61)=4.17, p<0.01, partial ε2=0.22]. Group differences were significant in the Trail Making Subtests. (5) Executive functions (word fluency). For this cognitive function, multivariate analysis was highly significant [Wilks' λ=0.700, F(3.61)=8.73, p<0.001, partial ε2=0.30]. Group differences were significant for semantic and lexical fluency. (6) Impairment in daily life and Depression. This analysis showed a highly significant overall analysis [Wilks' λ=0.718, F(2.63)=12.39, p<0.001, partial ε2=0.29]. Both the BDI and the Q-25 resulted in significant group differences. Three patients, but no controls, had a BDI sum score ⩾18. (7) Response bias. No subject fulfilled criteria for underachievement, defined as a score in the effort measures of the WMT <82.5%. There were no significant group differences in the effort measures of the WMT [Wilks' λ=0.931, F(3.62)=1.532, p=0.215, partial ε2=0.07]. None of the patients were involved in compensation claims due to cognitive and emotional consequences of mTBI.
To determine whether the above-reported group differences between mTBI patients and control subjects were modulated by self-reported impairment in daily life or by actual depressive symptoms, we performed an additional MANCOVA for each of the five cognitive domains; including BDI and Q-25 as covariates affected the reported results only minimally, and all main group differences remained significant.
Estimation of the prevalence of manifest neuropsychological impairment in mTBI patients was based on deteriorated performance with at least 1.5 s.d. below the mean of the controls in two or more cognitive domains. This resulted in 42.4% of the patients (14 out of 33) presenting with manifest neuropsychological impairment.
Results of the SCID diagnostic assessment
Healthy controls did not reveal any Axis I psychiatric disorders as assessed by SCID. Three patients had a mild episode of major depression at time of testing, two of these as a symptom of recurrent depression. One of these had already experienced two depressive episodes before mTBI. Another patient reported one mild episode of major depression in the time between mTBI and testing, but was remitted at the time of current assessment.
Discussion
In this study we investigated a broad range of cognitive domains in patients who sustained mTBI on average 6 years prior to the study, while controlling for psychiatric conditions and malingering. We could confirm our initial hypothesis that considerable cognitive deficits are present in a broad range of cognitive domains even 6 years after mTBI. Differences between mTBI patients and controls amounted to medium to large effect sizes in learning and long-term memory, working memory, attention, and executive functions.
Previous research on long-lasting consequences of mTBI yielded ambiguous results. Some investigators reported no or only subtle differences between control groups and patients several years after mTBI (Dikmen et al. Reference Dikmen, McLean and Temkin1986, Reference Dikmen, Ross, Machamer and Temkin1995; Frencham et al. Reference Frencham, Fox and Maybery2005; Vanderploeg et al. Reference Vanderploeg, Curtiss and Belanger2005). In a comparison of mild to moderate TBI within 2 months of trauma, Goldstein et al. (Reference Goldstein, Levin, Goldman, Clark and Altonen2001) identified no cognitive deficits in the mTBI group. By contrast, other researchers found significant cognitive impairments even many years after mTBI (Leininger et al. Reference Leininger, Gramling, Farrell, Kreutzer and Peck1990; Bernstein, Reference Bernstein2002; Sterr et al. Reference Sterr, Herron, Hayward and Montaldi2006). Thus, the debate about long-term deficits of mTBI remains unresolved. A recent review concluded that, to date, there is insufficient evidence to determine whether mTBI is associated with cognitive deficits 6 months or longer post-injury (Dikmen et al. Reference Dikmen, Corrigan, Levin, Machamer, Stiers and Weisskopf2009).
It is possible that factors such as depression or negative response bias influence test performance; we therefore assessed these variables in the current study (Bessell et al. Reference Bessell, Watkins and Williams2008). Furthermore, methodological differences between studies are large and complicate comparisons. Some of these investigations suffer from low sample size, diagnosis relying on self-reports instead of standardized diagnostic assessment at time of injury, inclusion of various degrees of severity, variable duration between injury and testing, and finally a large variety of neuropsychological assessment methods. In addition, the results of previous investigations may be influenced by the presence of undetected brain lesions. For our investigation, patients were selected from the initial clinical charts, the presence of established diagnostic criteria for mTBI in the initial clinical charts was assured, and patients were contacted irrespective of current treatment status. Absence of structural brain damage at the time of investigation was secured by performing a structural MRI scan specifically sensitive to TBI. In two out of the initial 36 patients and in one of the initial 36 controls, structural damage was detected and led to exclusion. This finding is in line with other investigations; for example, Bruns & Jagoda (Reference Bruns and Jagoda2009) reported that about 15% of patients with clinically diagnosed mTBI present with acute intracranial lesions detected by cerebral computed tomography. Using 3-T MRI, as we did in this investigation, the rate of parenchymal lesions was reported to be even higher (Lee et al. Reference Lee, Wintermark, Gean, Ghajar, Manley and Mukherjee2008). The highest incidence rate was reported by a group who found structural lesions in 11 out of 20 mTBI patients (Datta et al. Reference Datta, Pillai, Rao, Kovoor and Chandramouli2009). Our finding underlines the significance of initial brain imaging in TBI patients (Stein et al. Reference Stein, Burnett and Glick2006).
In our investigation, we identified significant deficits in working memory and attention about 6 years after mTBI. Short-term memory and attention are known to be affected by mTBI shortly after injury (Malojcic et al. Reference Malojcic, Mubrin, Coric, Susnic and Spilich2008), but long-term consequences are less evident. Vanderploeg et al. (Reference Vanderploeg, Curtiss and Belanger2005) investigated patients with mTBI about 8 years after trauma. Using non-traditional analysis methods, focusing on the continuation rate of the Paced Auditory Serial Addition Test and pro-active interference in the California Verbal Learning Test, they found subtle deficits in working memory and attention. Investigating 38 patients 6.8 years after mTBI, Sterr et al. (Reference Sterr, Herron, Hayward and Montaldi2006) also found deficits of working memory and attention. As mainly error rates, but not reaction times, were affected, they concluded that the threshold for inefficient simultaneous management of response accuracy and speed may be lowered even years after mTBI. In contrast to our investigation, the digit span forward and backward tests did not indicate working memory deficits in the sample of mTBI subjects; however, the validity of this observation is limited by the small sample size of 10 subjects (Segalowitz et al. Reference Segalowitz, Bernstein and Lawson2001). Nevertheless, the authors corroborated deficits of attention by electrophysiological correlates in the P300 component of an oddball task (Segalowitz et al. Reference Segalowitz, Bernstein and Lawson2001). Our findings on working memory are in line with a recent electroencephalographic (EEG) study in mTBI. mTBI patients showed impaired verbal and visuospatial attention span, and EEG coherence measures indicated impaired functional connectivity 2.13 months after mTBI (Kumar et al. 2009 b). Thus, previous work on working memory and attention supports our observations of deficits even 6 years after mTBI.
In our investigation episodic memory as assessed by the AVLT and the memory measures of the WMT was significantly worse in patients compared to controls. This finding is of interest because there is still considerable debate about whether mTBI might cause lasting deficits in long-term memory, for example in encoding or retrieval. Most previous reports of memory deficits in mTBI are contaminated by the inclusion of more severe forms of TBI. Klein et al. (Reference Klein, Houx and Jolles1996) identified impaired memory performance both for consolidating material into memory and for passive retrieval in 45 patients 3 years after mild or moderate TBI, even though subjects had no subjective complaints. Draper et al. (Reference Draper, Ponsford and Schönberger2007) and Ponsford et al. (Reference Ponsford, Draper and Schönberger2008) reported that memory performance correlated with functional outcome about 10 years after mild to severe TBI. Compromised memory functions were also found in one quarter of TBI patients with relatively preserved intellectual capacities (Levin et al. Reference Levin, Goldstein, High and Eisenberg1988). Gupta & Ghai (Reference Gupta and Ghai1991) reported poorer immediate and delayed free recall after TBI, whereas Hall & Bornstein (Reference Hall and Bornstein1991) concluded that poorer recall is a lasting feature of memory function after brain injury. By contrast, Himanen et al. (Reference Himanen, Portin, Isoniemi, Helenius, Kurki and Tenovuo2006) reported good recovery of semantic memory during a 30-year follow-up in patients with mild to severe TBI. For isolated mTBI, evidence is sparse. A recent investigation in high-school athletes showed that memory deficits persisted for about 7 days, but resolved by day 10 (Sim et al. Reference Sim, Terryberry-Spohr and Wilson2008). However, Nolin (Reference Nolin2006) reported poor performance in free recall as assessed by the California Verbal Memory Test in patients after mTBI, whereas cued recall remained unimpaired, pointing towards a selective dysfunction of registration and retrieval processes rather than a general storage problem. Divergent results may be explained by other influencing factors, such as attentional load. Recall performances of individuals with mTBI was similar to controls when words were encoded under full attention, whereas they performed worse when encoding in a divided attention condition (Blanchet et al. Reference Blanchet, Paradis-Giroux, Pépin and McKerral2009). Our findings indicate that memory encoding and recall deficits are not confined to higher degrees of TBI severity, but may be detected several years after mTBI if a set of sophisticated neuropsychological tests is used.
In the domain of executive functions, word fluency tasks tap complex cognitive processes, involving the perception of a target word, online-maintenance in working memory, retrieval of its meaning, activation of related concepts and instruction-dependent active search for concepts with equivalent meaning (Konrad et al. Reference Konrad, Engelien, Schöning, Zwitserlood, Jansen, Pletziger, Beizai, Kersting, Ohrmann, Luders, Greb, Heindel, Arolt and Kugel2008). Word fluency tasks have proven to be very sensitive for the characterization of patients after head trauma, and impaired performance in word fluency was observed in patients after TBI (Aschenbrenner et al. Reference Aschenbrenner, Tucha and Lange2000). Word fluency tasks also have proven sensitivity in long-term follow-up of patients after TBI (Mathias & Coats Reference Mathias and Coats1999; Belanger et al. Reference Belanger, Curtiss, Demery, Lebowitz and Vanderploeg2005; McHugh et al. Reference McHugh, Laforce, Gallagher, Quinn, Diggle and Buchanan2006). We also found that word fluency was impaired in patients after mTBI. This is in line with the impairments of working and semantic memory reported above, and with the findings of Draper et al. (Reference Draper, Ponsford and Schönberger2007) and Ponsford et al. (Reference Ponsford, Draper and Schönberger2008), who reported that performance in the Controlled Oral Word Association Test (COWAT) correlated with functional outcome after mild to severe TBI.
Impairments in daily life were also assessed in our investigation and differed significantly between groups. Thus, subtle cognitive impairments indeed have consequences for daily living. Our finding is in line with Draper et al. (Reference Draper, Ponsford and Schönberger2007), who found impairments in self-assessed psychosocial outcome after mild to severe TBI, that is occupational activity, interpersonal relationships and independent living skills. By contrast, Ettenhofer & Abeles (Reference Ettenhofer and Abeles2009) reported that single-incident mTBI is of little clinical significance to long-term cognitive and symptom outcome. Temkin et al. (Reference Temkin, Corrigan, Dikmen and Machamer2009) reported a dose–response relationship between severity of injury and social outcomes, but insufficient evidence for mTBI. Comparisons between studies are complicated by divergent definitions and assessment methods of impairments in daily life functioning. Furthermore, the interval between injury and testing differs between studies. Notably, self-assessed psychosocial impairments do not explain the cognitive impairments measured in this study, as the effect sizes remained stable after correction for Q-25.
The assessment of emotional consequences 6 years after mTBI represents another major point of our study. In daily clinical practice, complaints of depressive mood after mTBI are a frequent clinical and forensic problem. In studies investigating the occurrence of major depression in mTBI, in general about 10–20% of the patients meet diagnostic criteria for major depression (Deb et al. Reference Deb, Lyons, Koutzoukis, Ali and McCarthy1999; Rapoport et al. Reference Rapoport, McCullagh, Streiner and Feinstein2003, Reference Rapoport, Herrmann, Shammi, Kiss, Phillips and Feinstein2006). Long-term assessments are often hampered because patients with different degrees of TBI severity are included. For example, Draper et al. (Reference Draper, Ponsford and Schönberger2007) reported that 46% of patients with mild or moderate TBI met criteria for clinical depression, whereas Koponen et al. (Reference Koponen, Taiminen, Portin, Himanen, Isoniemi, Heinonen, Hinkka and Tenovuo2002) found that 26.7% experienced major depression in the time interval between mild to very severe TBI and the assessment but only 10% showed depression at the time of the interview about 30 years after TBI. Depression rates decreased from 31% after 1 month to 17% after 3–5 years in a sample including patients with mild to severe TBI (Dikmen et al. Reference Dikmen, Bombardier, Machamer, Fann and Temkin2004). A decreasing depression rate with time after injury was also supported by Ashman et al. (Reference Ashman, Spielman, Hibbard, Silver, Chandna and Gordon2004) . However, Whelan-Goodinson et al. (Reference Whelan-Goodinson, Ponsford, Johnston and Grant2009), who retrospectively established pre- and post-traumatic frequencies of psychiatric disorders (DSM-IV, Axis I), reported a dramatic increase of major depressive disorder from 17% before to 45% after head trauma, and anxiety from 13% before to 38% after head trauma. So far, investigations do not directly support a link between severity of TBI and occurrence of depression; that is, more severe trauma is not related to more frequent or severe depression (Hibbard et al. Reference Hibbard, Uysal, Kepler, Bogdany and Silver1998). This is supported by our observation indicating an increased rate of depression even after mTBI.
In our investigation, three out of 33 (9%) patients fulfilled diagnostic criteria (SCID) of a mild episode of major depression about 6 years after mTBI. [Even if the patient who had recurrent episodes before the trauma is excluded, two out of 33 (6%) still meet diagnostic criteria.] At first sight, this rate seems low. However, bearing in mind that we rigorously excluded patients after moderate or severe TBI and that the interval between injury and assessment was 6 years on average, our observation is in line with the rates reported in the literature. Depressive symptoms were further assessed using BDI self-rating, as depressive symptoms are often remarked by the patients themselves even when third-party raters evaluate normal depression ratings (Schöning et al. Reference Schöning, Zwitserlood, Engelien, Behnken, Kugel, Schiffbauer, Lipina, Pachur, Kersting, Dannlowski, Baune, Zwanzger, Reker, Heindel, Arolt and Konrad2009). In line with the SCID diagnosis, patients scored significantly worse than healthy patients [mean=6.52 (s.d.=6.05) compared to mean=3.85 (s.d.=4.00), p<0.05]. BDI scores did not interact with cognitive performance measures, as demonstrated in a MANCOVA model, thus neuropsychological impairments cannot be explained by depression.
Malingering is another frequent concern in the assessment of neurocognitive function in patients after TBI (Bordini et al. Reference Bordini, Chaknis, Ekman-Turner and Perna2002). The rate of negative response bias in adults after mTBI is much higher than in second-grade or disabled children (Green et al. Reference Green, Flaro and Courtney2009). This is particularly evident when monetary compensation claims come into play, but may also be triggered by other conscious or unconscious motives (Boone & Lu, Reference Boone and Lu2003). Although in our investigation no patients were involved in compensation claims, we tested putative symptoms fabrication using the WMT, a state-of-the-art measure for non-credible cognitive performance (Iverson et al. Reference Iverson, Green and Gervais1999; Green, Reference Green2005). We were thus able to rule out any negative response bias in our mTBI group and could ascertain the validity of our findings.
In summary, our investigation demonstrates significant cognitive and emotional impairments on average 6 years after mTBI. In this study we do not examine the causes of these impairments, thus we can only raise some hypotheses. Most frequently, deficits after TBI are explained by disruption of neuronal connections by the shearing and stretching forces of the incident (Graham et al. Reference Graham, McIntosh, Maxwell and Nicoll2000; Smith et al. Reference Smith, Meaney and Shull2003). TBI might cause disruption of neurofunctional circuits, decreasing cerebral processing speed and interacting with cerebral functions. External forces seem to be transmitted mainly to certain predeliction sites that are more affected than other regions, such as the corpus callosum. Using diffusion tensor imaging (DTI), reduced fractional anisotropy (FA) as a marker of reduced fiber integrity has been observed in the corpus callosum of patients with moderate TBI, and this finding was associated with poor neuropsychological outcome (Kumar et al. 2009 a). mTBI patients also showed reduced FA in some brain regions (Kraus et al. Reference Kraus, Susmaras, Caughlin, Walker, Sweeney and Little2007), although findings in the corpus callosum seem to depend on the time after trauma. Patients with mild TBI investigated <3 months post-trauma had reduced FA in the genu, whereas patients with mild TBI investigated ⩾3 months post-trauma showed no significant differences (Rutgers et al. Reference Rutgers, Fillard, Paradot, Tadié, Lasjaunias and Ducreux2008). Altered EEG coherence demonstrates that these pathophysiological processes might indeed occur after mTBI (Kumar et al. 2009 b). The emotional disturbances observed after mTBI may represent an indirect psychological reaction to the trauma, itself representing a major life event that is frequently associated with changes in psychosocial conditions. Emotional changes may also be a direct consequence of damage to the emotion regulation system (Draper et al. Reference Draper, Ponsford and Schönberger2007); for example, left dorsofrontal brain lesions may increase the probability of developing depression after TBI (Fedoroff et al. Reference Fedoroff, Starkstein, Forrester, Geisler, Jorge, Arndt and Robinson1992). A possible explanation for our observations on impaired cognitive and emotional function in mTBI may thus be explained by disruption of important neurofunctional circuits. Although not assessed by the T1-, T2- and T2*-weighted imaging sequences used routinely in the clinical context and applied in this study, such disruptions might still be present in the investigated population. DTI might be a useful imaging modality for assessing more subtle disruptions of structural brain connectivity.
Some limitations of our study need to be acknowledged. Although subjects were matched carefully for gender, age and education, we cannot fully exclude systematic differences between groups in other aspects. For example, it is possible that a subset of patients were more interested in participating than others, although the systematic screening of patients' charts and the systematic contact of all available patients independent from actual symptoms was performed to reduce selection bias. The broad age range (19–64 years) and the balanced gender distribution (16 females, 17 males) in each group do not favor this hypothesis; however, the high proportion of individuals with higher school education in both groups might indicate a stronger interest in scientific research. As education levels were matched across groups, this should not influence our results. Furthermore, our study cannot answer the question of cause and consequence. It is theoretically possible that subjects in the mTBI group sustained TBI because their pre-morbid cognitive or emotional function was already impaired before mTBI. However, this explanation seems rather far-fetched, and the fact that no differences existed between groups concerning age, gender, school and further education level makes it unlikely that systematic differences existed before the brain injury occurred. As a strength of this study, pre-injury and lifetime psychiatric diagnosis were excluded by the SCID. Using healthy subjects as the control sample, the effects we describe may not be specific to mTBI, but may instead reflect general changes due to traumatic events. As specificity was not the aim of our study, further research differentiating various traumatic events is needed.
In conclusion, our investigation contradicts claims that mTBI does not result in long-term cognitive or emotional impairments. By contrast, our investigation demonstrates that patients, even 6 years after mTBI, still show major impairments in a broad range of cognitive domains that can be revealed by thorough neuropsychological testing. In contrast to many other studies, we can exclude a significant influence of major confounding factors, such as depression, malingering or undetected structural brain damage. Effect sizes of cognitive deficits were medium to large in a variety of cognitive domains (partial ε2=0.22–0.35, d=0.2–1.3). According to our findings, the notion of complete recovery after mTBI is not supported. We suggest that long-term cognitive and emotional deficits in mTBI need to be taken seriously when evaluating or treating patients after mTBI.
Acknowledgments
This study was supported by an IMF grant from the Medical Faculty of the Westfälische Wilhelms-University Münster, Germany (KO 210710).
Declaration of Interest
None.







