Introduction
Initially, research in behavioural and psychiatric genetics focused on disentangling the genetic and environmental influences on traits or disorders. The findings of these studies were highly relevant in showing the influence of genetic factors in the aetiology of almost all traits and disorders. Most of these studies assumed that the effects of genes and environment act independently, meaning that the effect of an environmental risk factor does not depend on the genotype. In a seminal paper, Kendler & Eaves (Reference Kendler and Eaves1986) presented two alternative models that represent how genes and environment jointly influence variation in a trait or disorder: genotype–environment correlation (r GE) and genotype–environment interaction (G×E). r GE occurs when genes that influence a trait also influence the exposure to an environmental risk factor (Plomin et al. Reference Plomin, Defries and Loehlin1977; Kendler & Eaves, Reference Kendler and Eaves1986). G×E occurs when the effect of exposure to environmental factors depends on a person's genotype. In the presence of G×E, individuals with a ‘sensitive’ genotype will be at greater risk if the predisposing environment is present than individuals with an ‘insensitive’ genotype (Boomsma & Martin, Reference Boomsma, Martin, D'Haenen, den Boer and Willner2002; Rutter, Reference Rutter2007).
Borderline personality disorder (BPD) is characterized by emotional lability, impulsivity, interpersonal difficulties, identity disturbance, and stress-related cognitive distortion (APA, 2000). A combination of factors from various domains influences the risk to develop BPD. Many studies in clinical samples have demonstrated that traumatic life events such as sexual or physical abuse and parental divorce, loss or illness are generally more common in patients with BPD than in non-patients or patients with other personality disorders (Paris, Reference Paris1997; Zanarini et al. Reference Zanarini, Williams, Lewis, Reich, Vera, Marino, Levin, Yong and Frankenburg1997; Helgeland & Torgersen, Reference Helgeland and Torgersen2004; Bandelow et al. Reference Bandelow, Krause, Wedekind, Broocks, Hajak and Ruther2005). The total number of negative life events experienced is also higher for BPD patients than for control subjects (Jovev & Jackson, Reference Jovev and Jackson2006; Horesh et al. Reference Horesh, Ratner, Laor and Toren2008).
Recently, several twin and twin family studies provided evidence that genetic factors explain familial clustering of BPD, with heritability estimates ranging from 35% to 45% (Distel et al. Reference Distel, Trull, Derom, Thiery, Grimmer, Martin, Willemsen and Boomsma2008 a; Kendler et al. Reference Kendler, Aggen, Czajkowski, Roysamb, Tambs, Torgersen, Neale and Reichborn-Kjennerud2008; Torgersen et al. Reference Torgersen, Czajkowski, Jacobson, Reichborn-Kjennerud, Roysamb, Neale and Kendler2008). Although many researchers and psychiatrists acknowledge the importance of both traumatic life events and biological vulnerabilities (Livesley, Reference Livesley2008; Distel et al. Reference Distel, Trull, Boomsma, Jackson and Westbrook2009 a), the joint influence of life events and genetic vulnerability on the development of BPD has not yet been investigated.
The present study aimed to explore the influence of G×E on individual differences in BPD. As the presence of r GE can lead to spurious findings of G×E, we applied two methods to test for r GE. To assess the extent to which individuals are at risk for developing BPD, participants completed the Personality Assessment Inventory – Borderline features scale (PAI-BOR; Morey, Reference Morey1991), a self-report questionnaire designed to quantify features clinically associated with BPD. The use of a quantitative measure allows for the assessment of a large number of individuals needed to reliably explore the presence of G×E and r GE. The exposure to life events was assessed by self-report.
Method
Participants
Data were collected as part of an ongoing project on health, lifestyle and personality in twin families registered voluntarily with the Netherlands Twin Registry (Boomsma et al. Reference Boomsma, de Geus, Vink, Stubbe, Distel, Hottenga, Posthuma, van Beijsterveld, Hudziak, Bartels and Willemsen2006) and the East Flanders Prospective Twin Survey (Derom et al. Reference Derom, Vlietinck, Thiery, Leroy, Fryns and Derom2006). We focus on data on BPD traits and the exposure to life events collected in 2004–2005. In The Netherlands, a total of 12 785 twins and 3323 siblings were approached, of whom some individuals had participated before (n=10 099) and some had never participated (n=6009). In total, 5281 (33%) twins and siblings returned the survey. To examine the reasons for not participating, a non-response study was conducted. Addresses proved incorrect in 23.8% of the individuals who participated before and in 42.0% who had never participated. Thus, a substantial group of targeted participants never received the questionnaire. After subtracting the estimated number of incorrect addresses from the number of questionnaires sent, the estimated ‘true’ response rates were 52% and 15% for twins who respectively did or did not participate before and 52% and 27% for siblings who respectively did or did not participate before (Distel et al. Reference Distel, Ligthart, Willemsen, Nyholt, Trull and Boomsma2007). In Belgium, a total of 3979 twins were approached, of whom 932 (23%) twins returned the survey. The total sample for analysis consisted of 5083 twins, 477 brothers and 808 sisters from 3688 families. The mean age of the twins and the siblings was 34.1 (s.d.=10.9, range 18–86) and 38.6 (s.d.=12.2, range 18–90) years respectively.
The zygosity of same-sex twins was determined by placental examination, blood groups, DNA typing or on self-report answers to a validated survey containing questions on physical twin resemblance. There were 764 monozygotic (MZ) male twins, 386 dizygotic (DZ) male twins, 1932 MZ female twins, 944 DZ female twins, 421 male DZ opposite sex twins and 636 female DZ opposite sex twins. Further details can be found elsewhere (Derom & Derom, Reference Derom, Derom, Blickstein and Keith2005; Distel et al. Reference Distel, Ligthart, Willemsen, Nyholt, Trull and Boomsma2007).
Measures
The risk of developing BPD was assessed by the PAI-BOR (Morey, Reference Morey1991). The 24 items of this scale concern, for example, stability of mood and affects, anger control, self-image, feelings of emptiness, intense and unstable relationships and self-harm and are rated on a four-point scale (0–3; false, slightly true, mainly true, very true). Multigroup confirmatory factor analysis showed that the PAI-BOR is measurement invariant across sex and age (De Moor et al. Reference De Moor, Distel, Trull and Boomsma2009). Receiver operating character analysis with a group of BPD patients and a group of non-BPD depressed patients showed an area under the curve of 0.78, indicating that the PAI-BOR discriminates between BPD patients and non-BPD depressed psychiatric patients reasonably well. At the best cut-off point of 42, the sensitivity was 71% and the specificity 69%. The positive predictive value and negative predictive value were 76% and 64% respectively (Distel et al. Reference Distel, Hottenga, Trull and Boomsma2008 b). The 6-month test–retest reliability and internal consistency (Cronbach's α) of the Dutch version of the PAI-BOR are 0.78 and 0.84 respectively (Distel et al. Reference Distel, Trull, Derom, Thiery, Grimmer, Martin, Willemsen and Boomsma2008 a). The exposure to life events was assessed by the self-report Dutch life events scale (the Schokverwerkings Inventarisatie Lijst; Van der Velden et al. Reference Van der Velden, Van der Burg, Steinmetz and Van den Bout1992). Question were asked about the experience of a divorce/break-up of an intimate relationship, traffic accident, violent and sexual assault, robbery and job loss. The response categories were: ‘never experienced’, ‘0–6 months ago’, ‘6–12 months ago’, ‘1–5 years ago’ and ‘more than 5 years ago’.
Statistical analysis
Gene–environment interaction
Structural equation modelling (SEM) was used to test whether genetic and environmental effects on the variance in BPD features interact with self-reported exposure to life events. Variance in a trait can be caused by genetic (G) or environmental (E) factors. If the contributions of genes to the variance in BPD are independent of each other, the genetic effects are additive (A). However, if alleles interact within a particular locus (D; dominance) or across different loci (epistasis), the genetic effects are non-additive. The relative influence of, for example, A is calculated by the additive genetic variance divided by the total variance. In an interaction model, the variance of a trait might depend on a moderator, such as the experience of a life event. The moderator might affect one of the variance components, thus A, D or E, but also two of the components or all of them. In every case, the total variance will differ depending on the moderator. This means that a moderator with a significant influence on E will also affect the relative influence of A even when the additive genetic variance is unchanged.
A series of interaction models was fitted for each life event. Fig. 1 shows this model for a pair of relatives. MZ twins reared together share (nearly) all genes (r=1) and DZ twins and sibling pairs share on average 50% of their segregating genes (r=0.5). Because offspring receive only one allele from each parent and not a combination of two alleles, the chance that two siblings receive the same allele is 0.5×0.5, resulting in a correlation of 0.25 between the latent D factor for DZ twins and sibling pairs (Posthuma et al. Reference Posthuma, Beem, de Geus, van Baal, von Hjelmborg, Lachine and Boomsma2003). The exposure to a life event was included as a moderator in the path from latent factors A, D and E. In Fig. 1 this is represented as a+βaModT1 for the path from A to the phenotype. Here a represents the effect of A independent of the moderator, and ModT1 represents the exposure to a life event (0 for non-exposed individuals and 1 for exposed individuals). If βa is significantly different from zero, an interaction between the latent additive genetic factor and the life event is present. In the same way, interaction effects are tested by constraining βd and βe to equal zero (Purcell, Reference Purcell2002).

Fig. 1. Gene–environment interaction model for borderline personality with the exposure to a life event included as a moderator (Mod) in a pair of relatives [monozygotic twins (MZ), dizygotic twins (DZ) or non-twin siblings (sib)]. BPD, borderline personality disorder; T1, twin one; T2, twin two; m, mean Personality Assessment Inventory – Borderline features scale (PAI-BOR) score; A, additive genetic variance; a, factor loading of A; D, dominant genetic variance; d, factor loading of D; E, unique environmental variance; e, factor loading of E.
Earlier analyses showed that the heritability of BPD features is equal for men and women, that there is no shared environmental effect and that the same genes influence BPD traits in men and women (r MZ males=r MZ females=0.43 and r DZ males=r DZ females=r DZ opposite sex=0.18) (Distel et al. Reference Distel, Trull, Boomsma, Jackson and Westbrook2008 a). Therefore, in the present analyses, sex differences in variance components were not included in the model. Sex, age and country of origin were included in the analyses as fixed effects (regression on the mean PAI-BOR score). Based on the correlation structure (r DZ<½ r MZ), we included the effect of dominance in the model instead of the effect of shared environmental factors.
G×E analyses were performed in the software package Mx (Neale et al. Reference Neale, Boker, Xie and Maes2006). The fit of the different models was evaluated by using a hierarchical log-likelihood ratio test to select the simplest model that would best explain the data among a set of possible models. The difference between the negative log likelihood of the two models has a χ2 distribution and the number of degrees of freedom for this test is equal to the difference in the number of estimated parameters in the two models. A non-significant p value (p>0.05) means that the constrained model is not significantly worse than the less constrained model, and is kept as the most parsimonious and best-fitting model. The PAI-BOR data showed a somewhat skewed distribution so a square root transformation was applied.
Gene–environment correlation
The presence of r GE can lead to spurious findings in G×E if it is not accounted for in the model (Purcell, Reference Purcell2002). The presence of r GE can be tested for by fitting a bivariate genetic model in which a genetic correlation is estimated between the genetic factor that influences borderline personality traits and the genetic factor that influences the exposure to a certain life event. As it is sometimes argued that the assumption of an underlying normal distribution is violated in the case of a dichotomous variable, such as being exposed or unexposed to a life event, we first applied the co-twin control method (Cederlof et al. Reference Cederlof, Friberg and Lundman1977; Kendler et al. Reference Kendler, Neale, Maclean, Heath, Eaves and Kessler1993; Middeldorp et al. Reference Middeldorp, Cath, Beem, Willemsen and Boomsma2008) to test for r GE.
The co-twin control design makes use of three groups of subjects: (1) a group of MZ twin pairs discordant for the life event, (2) a group of DZ twin pairs discordant for the life event, and (3) a group of pairs of genetically unrelated individuals discordant for the life event. The first two groups are automatically matched for age and sex (only same-sex twins were included) whereas subjects in the third group were matched for age and sex by creating pairs of men and women of the same age. The group of unrelated discordant individuals was selected from the families without an MZ or a DZ discordant twin pair. Given the different degrees of genetic relationships between the three groups (100, 50 and 0%), a distinct pattern for the difference scores between exposed and non-exposed individuals is expected in each group in the absence and presence of r GE. In the absence of r GE, the difference in PAI-BOR scores between the exposed and non-exposed subjects will be similar in the three groups. In other words, if the genes influencing BPD features and the genes influencing exposure to a life event are not correlated, the difference between the PAI-BOR score of the exposed and non-exposed subjects does not depend on the degree of the genetic relationship. In the presence of r GE, it is expected that non-exposed subjects from the unrelated group will score lower than the DZ non-exposed subjects who will score lower than the MZ non-exposed subjects. In other words, if the association between BPD features and the exposure to a life event is caused by common genetic effects, non-exposed and exposed subjects who share all genetic make-up will have more similar PAI-BOR scores than non-exposed subjects and exposed subjects who share half of their genetic make-up, who in turn will have more similar scores than genetically unrelated subjects. Differences in scores of the non-exposed subjects across the three groups were tested by regression analyses, with the PAI-BOR score in the non-exposed subjects as the dependent variable and group membership (MZ, DZ and unrelated, coded as 0, −1 and −2 respectively) as the independent variable. The regression equation for discordant pairs can be written as: Y=α+βX, where Y is the PAI-BOR score in the non-exposed subjects, α is the intercept, β the regression coefficient and X the group membership (MZ, DZ and unrelated, coded as 0, −1 and −2 respectively). In the presence of r GE, group membership will significantly predict the PAI-BOR scores in the non-exposed subjects.
Second, r GE was investigated by conducting bivariate genetic analyses. The bivariate genetic model tests whether the phenotypic association between BPD and the exposure to a life event can be explained by an overlap in genetic factors. In a univariate genetic model, the variance in a trait is decomposed in part due to genetic factors and in part due to environmental factors, by comparing MZ and DZ cross-twin correlations. In the bivariate case, cross-twin cross-trait MZ and DZ correlations are used to decompose the covariance between the traits into a genetic and an environmental part. The bivariate genetic analyses were performed in the software program Mplus (Muthén & Muthén, Reference Muthén and Muthén2005) because the exposure to life events was modelled as a dichotomous variable (coded as 0 for the non-exposed individuals and 1 for the exposed individuals) and BPD features as a continuous variable.
Results
Main effects
Table 1 shows the prevalence of exposure to each life event and the mean PAI-BOR scores of the exposed and unexposed subjects. Prevalences ranged from 29% (robbery) to 7% (violent and sexual assault). For all life events, except for robbery (F 1=3.834, p=0.05), the exposed subjects had significantly higher mean PAI-BOR scores than the non-exposed subjects (all p<0.001). To investigate whether the strength of the effect of exposure to a life event on BPD features depends on the time interval between completing the PAI-BOR and the occurrence of life events and on the number of experienced life events, we separately analysed the effect of life events experienced in the past 5 years and life events experienced more than 5 years ago. Both the number of life events experienced in the past 5 years and more than 5 years ago were associated with higher PAI-BOR scores, but the effect was strongest when the life events occurred more recently (r=0.229, p<0.001 v. r=0.095, p<0.001).
Table 1. Prevalence of having experienced a divorce/break-up, traffic accident, violent or sexual assault, robbery and job loss and the mean PAI-BOR score of the exposed and unexposed subjects for each life event

PAI-BOR, Personality Assessment Inventory – Borderline features Scale; BPD, borderline personality disorder; s.d., standard deviation.
Gene–environment interaction
SEM was used to explore which life events moderate the genetic architecture of BPD features. As more recent life events have a stronger effect on the PAI-BOR score, we gave life events from the past 5 years a higher weight (1.5) than life events from more than 5 years ago. As r GE may be present for some life events (see results section on r GE), the mean PAI-BOR score was corrected for the effect of the moderator by including it as a fixed effect in the mean model (Purcell, Reference Purcell2002).
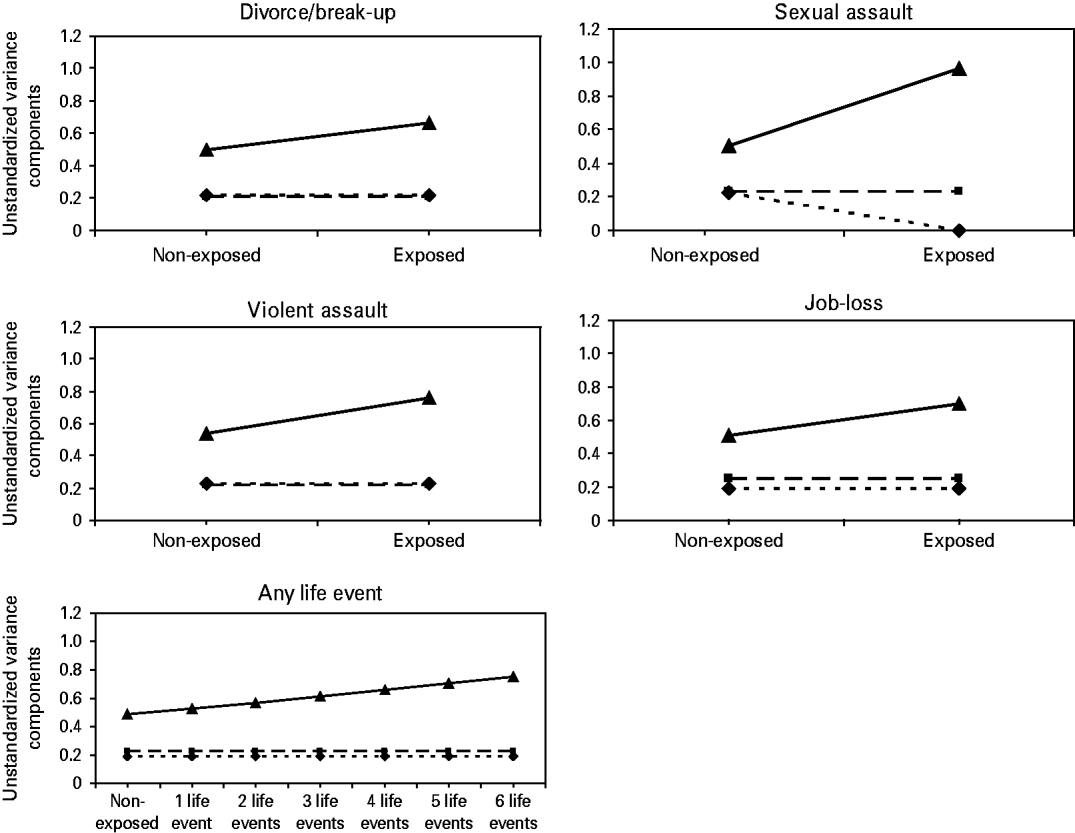
The results of the genetic model fitting are shown in Table 2. For each life event, the full model (model 1) contains all moderation effects on the paths from A, D and E to the phenotype. Models 2 and 3 subsequently constrained the effect of moderation on A and D and the moderation on E at zero, and the model fit was then compared to the most parsimonious model at that point. In model 4 the significance of D was tested. For traffic accident and robbery all moderation effects could be dropped from the models without a significant deterioration in fit. Broad-sense heritability (A+D) of BPD features was estimated at 47%. There were no significant moderator effects of a divorce/break-up, violent assault or job loss on D and A. However, the positive moderation effect of these life events on E could not be dropped from the model, indicating that the variance in BPD features due to unique environment increases in individuals who have experienced a divorce/break-up, violent assault or job loss. This results in lower relative contributions of A and D in individuals exposed to these life events. The broad-sense heritability estimate of BPD features is about 45% in non-exposed and about 40% in individuals exposed to a divorce/break-up, violent assault or job loss.
Table 2. Model fitting results for an interaction model of borderline personality with the life events as moderators. Standardized parameter estimates are given for the best fitting model (95% confidence intervals)

A, Additive genetic variance; D, dominant genetic variance; E, unique environmental variance; v., versus; −2LL, −2 log likelihood; df, degrees of freedom.
For sexual assault a strong negative moderation effect on A was found, resulting in no significant contribution of A in individuals who have experienced sexual assault. A small positive moderation effect was found for D and E. The exposure to sexual assault thus leads to a lower heritability estimate for BPD features. The broad-sense heritability is estimated at 47% in non-exposed and 24% in exposed individuals. As a result of the strong negative moderation on A, A is estimated at zero in exposed individuals. Confidence intervals for A and D, however, include zero, indicating that the influence of A and D cannot be reliably distinguished.
The total number of life events to which an individual has been exposed does not interact with genetic effects on BPD features but E is more important in individuals who have experienced more life events. The heritability estimate thus decreases as a function of the number of experienced life events, from 46% in non-exposed subjects to 36% in those who experienced six life events. Fig. 2 shows the absolute contribution of A, D and E to variation in BPD features for individuals non-exposed and exposed to a divorce/break-up, violent assault, sexual assault, job loss or any life event.

Fig. 2. The absolute contribution of genetic (A, D) and environmental (E) factors to variation in borderline personality for non-exposed and exposed individuals. A (–♦–), D (–▪–), E (–▴–).
Gene–environment correlation
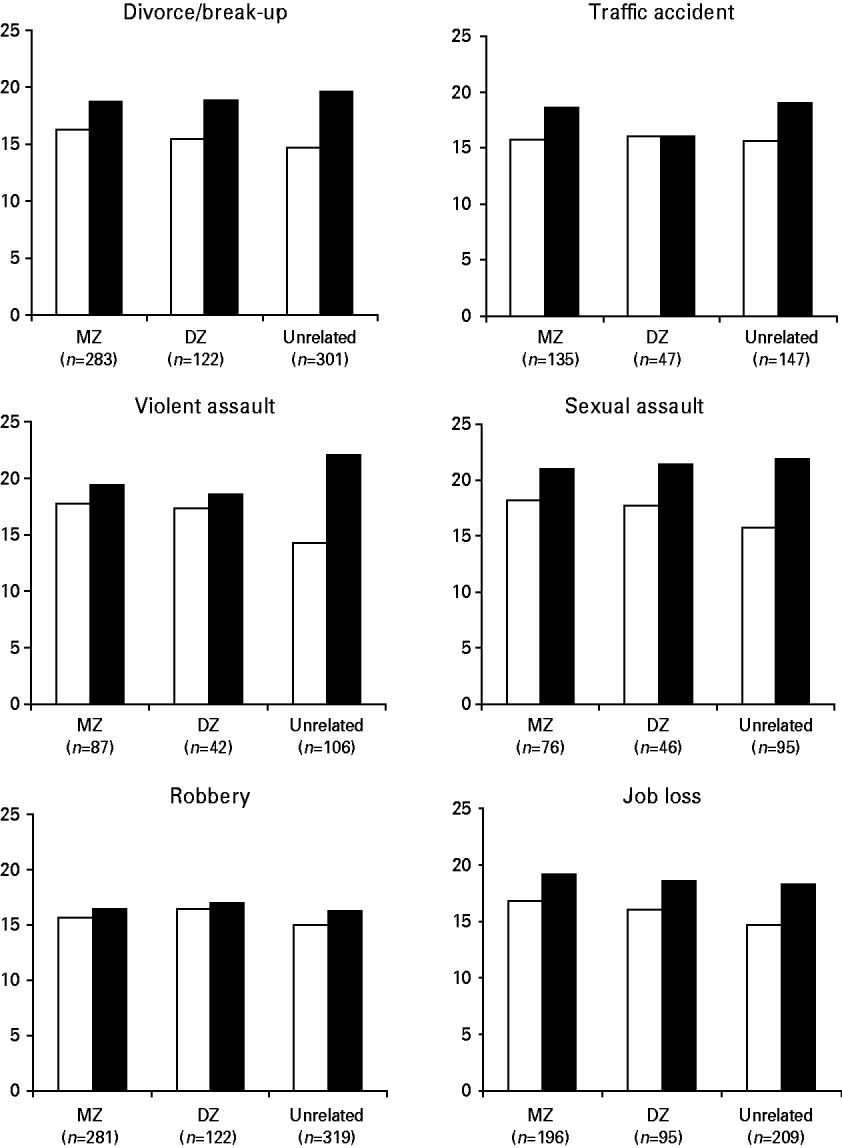
Fig. 3 gives a graphical representation of the pattern of PAI-BOR scores for subjects in the MZ discordant, DZ discordant and unrelated discordant subjects for each life event. The difference between the exposed and non-exposed subjects was larger in the unrelated group for all life events. In addition, the non-exposed subjects scored highest in the MZ discordant group and lowest in the unrelated discordant group. Regression analyses showed that genetic relatedness to subjects who had been exposed to a divorce/break-up (F 1=7.361, p=0.007), violent assault (F 1=8.265, p=0.004) and job loss (F 1=8.122, p=0.005) significantly predicted the PAI-BOR score of the unexposed subjects. This was not true for traffic accident (F 1=0.009, p=0.926), sexual assault (F 1=3.070, p=0.081) and robbery (F 1=1.222, p=0.269). These results strongly suggest r GE between some life events and BPD features, although the association cannot entirely be explained by r GE because the scores of the exposed and non-exposed MZ twins also differ.

Fig. 3. Graphical representation of the pattern of Personality Assessment Inventory – Borderline features Scale (PAI-BOR) scores of subjects in the monozygotic (MZ) discordant, dizygotic (DZ) discordant and unrelated discordant subjects for each life event. □, Non-exposed; ▪, exposed.
To strengthen our results on the presence of r GE we also conducted bivariate genetic analyses. Table 3 shows the results of these analyses for five out of six life events. The bivariate genetic analyses could not be carried out for robbery because the effect of robbery on the PAI-BOR score was too small (see Table 1). In line with the results of the co-twin control design, significant genetic correlations with BPD were found for divorce, violent assault and job loss. Whereas in the co-twin control design no r GE was found for sexual assault, the more powerful bivariate genetic analysis showed a genetic correlation of 0.388. For divorce, sexual assault and job loss, significant environmental correlations with BPD existed in addition to the genetic correlation.
Table 3. Phenotypic, genetic and environmental correlation between BPD and divorce, traffic accident, violent assault, sexual assault and job loss (95% confidence intervals)

BPD, Borderline personality disorder.
Discussion
This study corroborates previous findings in clinical and non-clinical studies that showed a strong relationship between having experienced (one or more) traumatic life events and (the severity of) BPD symptoms (Silk et al. Reference Silk, Lee, Hill and Lohr1995; Johnson et al. Reference Johnson, Cohen, Brown, Smailes and Bernstein1999; Jovev & Jackson, Reference Jovev and Jackson2006; Horesh et al. Reference Horesh, Ratner, Laor and Toren2008). We explored how genes and environment jointly affect BPD features.
We aimed to identify specific environmental influences that moderate the genetic and environmental influences on BPD features (i.e. G×E interaction). Additive genetic influences on BPD features were only found to interact negatively with the exposure to sexual assault. This suggests that sexual assault has such a large effect that, even in less genetically vulnerable individuals, it is associated with more BPD features. Environmental influences on BPD features were found to interact positively with the experience of sexual assault and also with divorce/break-up, violent assault or job loss and the total number of life events. This may point to large individual differences in the event and the way individuals experiences such a life event.
Because the presence of r GE can lead to spurious findings of G×E interaction if it is not accounted for in the model, we also investigated the presence of r GE. The r GE was found for a divorce/break-up, violent assault, sexual assault and job loss. Genes influencing BPD features thus increase the likelihood of being exposed to these life events. Kendler et al. (Reference Kendler, Gardner and Prescott2003) also found evidence for r GE for neuroticism, a personality trait strongly associated with BPD (McCrae et al. Reference McCrae, Yang, Costa, Dai, Yao, Cai and Gao2001; Distel et al. Reference Distel, Trull, Willemsen, Vink, Derom, Lynskey, Martin and Boomsma2009 b), and marital problems, job loss and problems getting along with people. For traffic accidents and robbery no evidence for r GE was found. Our results also indicate that common genes are not the only explanation for the association. The significant genetic and environmental correlations found in our bivariate genetic analyses support the hypothesis that causality plays a role in the association between divorce, sexual assault and job loss and BPD features. Whether this causality is unidirectional or reciprocal cannot be concluded from these analyses. It is likely that the association of violent assault with increased PAI-BOR scores is explained by common genetic factors because the environmental correlation was not significant.
G×E interaction has been tested for a range of behavioural phenotypes (e.g. Caspi et al. Reference Caspi, McClay, Moffitt, Mill, Martin, Craig, Taylor and Poulton2002), personality traits (e.g. Terracciano et al. Reference Terracciano, Tanaka, Sutin, Deiana, Balaci, Sanna, Olla, Maschio, Uda, Ferrucci, Schlessinger and Costa2010) and psychiatric disorders (e.g. Risch et al. Reference Risch, Herrell, Lehner, Liang, Eaves, Hoh, Griem, Kovacs, Ott and Merikangas2009). For BPD features, this is the first study that investigated whether the exposure to serious life events moderates the latent genetic and environmental influences. Two previous studies reported on an interaction between measured genes, serious or stressful life events and the risk for BPD. Wagner et al. (Reference Wagner, Baskaya, Lieb, Dahmen and Tadic2009) investigated whether the serotonin-transporter-linked promoter region (5-HTTLPR) S/L polymorphism modulates the effects of serious life events on impulsivity in patients with BPD. Their study showed that, in individuals with SS/SL genotypes, physical maltreatment, childhood sexual abuse and the cumulative number of serious life events had an effect on the impulsivity score. In individuals carrying the LL variant, no serious life events measure significantly explained the variance in the impulsivity score. In a second study into G×E interaction in patients with BPD, Wagner et al. (Reference Wagner, Baskaya, Dahmen, Lieb and Tadic2010) investigated the inter-relationships between serious life events, impulsive aggression and the brain-derived neurotrophic factor (BDNF) Val66Met polymorphism. The study showed that, in BPD patients carrying the BDNF Val/Val polymorphism, childhood sexual abuse had a decreasing effect on impulsive aggression. The authors speculate how this counter-intuitive result may be explained but conclude by stating that: ‘the interrelations between serious life events, impulsive aggression and the BDNF Val66Met polymorphism as well as their implication for BPD are far from understood and require further investigations’.
Several limitations of this study should be noted. First, some selection bias may have been present in the sample. In general, the Dutch sample, which constituted 85% of the sample in the present study, was shown to be representative of the general Dutch population with regard to several variables such as socio-economic status, smoking behaviour, and religion (Boomsma et al. Reference Boomsma, Vink, van Beijsterveldt, de Geus, Beem, Mulder, Derks, Riese, Willemsen, Bartels, van den Berg, Kupper, Polderman, Posthuma, Rietveld, Stubbe, Knol, Stroet and van Baal2002). However, individuals from families in which only some individuals participate show slightly more BPD features than individuals from families in which most individuals participate (Distel et al. Reference Distel, Ligthart, Willemsen, Nyholt, Trull and Boomsma2007). Second, although the large sample size (n>6300) offered the possibility of testing for different models of genetic and cultural inheritance, a limitation of the present study is that the large sample size precluded a clinical diagnosis of the phenotype. The third limitation concerns the measurement of life events. Life events are assessed retrospectively, which is sometimes argued to cause reported life events to be in part due to biased memory. If biased memory plays a role, this would influence the reliability of the measured exposure to life events if biased memory is not randomly distributed in the population. If individuals with a certain mood are more likely to recall certain life events than others, the association between the life event and a certain mood may be inflated. Middeldorp et al. (Reference Middeldorp, Cath, Beem, Willemsen and Boomsma2008) investigated whether mood congruence bias was present for depression scores. Depression and the exposure to life events were measured on two occasions, which made it possible to investigate whether inconsistent life event reports were associated with depression scores. Their analysis did not show evidence for mood congruence bias. In our dataset only data on life events were also collected at a prior occasion. It was therefore only possible to test whether individuals reported on life events consistently. A total of 75% of the subjects who reported a life event on the first occasion also reported that life event on the second occasion. Fourth, as we only used cross-sectional data it was not possible to investigate whether reciprocal or unidirectional causality influences the association between the stressful life events and BPD, in addition to genetic factors. Longitudinal data are currently being collected, providing the opportunity to address this issue in the future.
We showed that the association between life events and BPD features can be explained by shared genetic influences, causal effects and an interaction between genes and environment depending on the type of life event. These findings hold several important implications for clinical settings and research. The fact that individuals with BPD features have a higher risk of experiencing a divorce/break-up, violent assault, sexual assault and job loss based on their genotype, and that the exposure to a divorce, sexual assault and job loss increases the number of BPD features, indicates how important it is during treatment to pay attention to problems in relationships and at work. Furthermore, although it is already well known that sexual assault is highly associated with psychopathology, the finding that sexual assault can increase BPD features in genetically less vulnerable subjects emphasizes the impact of this kind of life event. In future studies that aim to find genes that influence BPD features, individuals exposed to sexual assault and possibly other severe life events could be excluded from the analyses because the importance of genes in the development of BPD is much lower in individuals who experienced such life events compared to individuals who did not.
Acknowledgments
This study was supported by the Borderline Personality Disorder Research Foundation, Spinozapremie (NWO/SPI 56-464-14192), the Centre for Neurogenomics and Cognitive Research, the Centre for Medical Systems Biology (NWO Genomics), and the Twin-family database for behaviour genetics and genomics studies (NWO 480-04-004). C.M. was supported by NWO-ZonMw (91676125).
Declaration of Interest
None.