Introduction
Several lines of evidence implicate disrupted functional connectivity as a key feature of schizophrenia (Friston & Frith, Reference Friston and Frith1995; Weinberger, Reference Weinberger1995; Andreasen et al. Reference Andreasen, Nopoulos, O'Leary, Miller, Wassink and Flaum1999). Using functional imaging techniques, altered functional interactions in cortical and subcortical systems of patients with schizophrenia were described on the basis of correlational (Jennings et al. Reference Jennings, McIntosh, Kapur, Zipursky and Houle1998; Stephan et al. Reference Stephan, Magnotta, White, Arndt, Flaum, O'Leary and Andreasen2001; Lawrie et al. Reference Lawrie, Buechel, Whalley, Frith, Friston and Johnstone2002; Kim et al. Reference Kim, Ho Seok, Park, Soo Lee, Chul Lee and Soo Kwon2005; Boksman et al. Reference Boksman, Theberge, Williamson, Drost, Malla, Densmore, Takhar, Pavlosky, Menon and Neufeld2005; Whalley et al. Reference Whalley, Simonotto, Marshall, Owens, Goddard, Johnstone and Lawrie2005; Wolf et al. Reference Wolf, Gur, Valdez, Loughead, Elliott, Gur and Ragland2007) or principal component analysis (Meyer-Lindenberg et al. Reference Meyer-Lindenberg, Poline, Kohn, Holt, Egan, Weinberger and Berman2001) as well as structural equation modelling (Schlösser et al. Reference Schlösser, Gesierich, Kaufmann, Vucurevic, Hunsche, Gawehn and Stoeter2003a) and dynamic causal modelling (Mechelli et al. Reference Mechelli, Allen, Amaro, Fu, Williams, Brammer, Johns and McGuire2007).
These findings give reason to assume that the frequently reported working memory impairments in schizophrenia which have been shown to go along with both hypo- (Barch et al. Reference Barch, Carter, Braver, Sabb, MacDonald, Noll and Cohen2001; Perlstein et al. Reference Perlstein, Carter, Noll and Cohen2001; Mendrek et al. Reference Mendrek, Kiehl, Smith, Irwin, Forster and Liddle2005) and hyperactivation (Callicott et al. Reference Callicott, Bertolino, Mattay, Langheim, Duyn, Coppola, Goldberg and Weinberger2000; Manoach et al. Reference Manoach, Gollub, Benson, Searl, Goff, Halpern, Saper and Rauch2000; Schneider et al. Reference Schneider, Habel, Reske, Kellermann, Stocker, Shah, Zilles, Braus, Schmitt, Schlösser, Wagner, Frommann, Kircher, Rapp, Meisenzahl, Ufer, Ruhrmann, Thienel, Sauer, Henn and Gaebel2007; Schlösser et al. Reference Schlösser, Koch, Wagner, Nenadic, Roebel, Schachtzabel, Axer, Schultz, Reichenbach and Sauer2008) in various brain areas might be based on a disruption in the interplay or functional connectivity between these areas. Meyer-Lindenberg et al. (Reference Meyer-Lindenberg, Poline, Kohn, Holt, Egan, Weinberger and Berman2001) applied an n-back task to investigate functional connectivity during working memory processing in schizophrenia patients using positron-emission tomography (PET) and a canonical variates analysis. High intra-temporal and low intra-frontal functional correlations were found in patients, with the reverse finding in controls. Comparable findings could be detected in a preceding study of our group (Schlösser et al. Reference Schlösser, Gesierich, Kaufmann, Vucurevic, Hunsche, Gawehn and Stoeter2003a). Using an n-back working memory task and structural equation modelling we were able to detect decreased intra-frontal connectivity in patients in terms of a significantly weaker interhemispheric interaction between right and left dorsolateral prefrontal cortex (DLPFC) as well as right and left ventrolateral prefrontal cortex (VLPFC). In a later n-back study Meyer-Lindenberg et al. (Reference Meyer-Lindenberg, Olsen, Kohn, Brown, Egan, Weinberger and Berman2005) used voxelwise analyses of covariance to examine potential alterations in functional connectivity in patients with schizophrenia. An increased fronto-temporal connectivity with stronger voxelwise correlations between right DLPFC and left hippocampus was detected in patients. Furthermore, there is evidence that working memory processing might be associated with altered connectivity in schizophrenia patients in terms of an increased functional interaction between ventrolateral prefrontal and superior parietal areas in association with a decreased connectivity between dorsolateral prefrontal and inferior parietal areas (Tan et al. Reference Tan, Sust, Buckholtz, Mattay, Meyer-Lindenberg, Egan, Weinberger and Callicott2006). Hence, findings regarding alterations in the functional interplay between task-relevant regions in patients during working memory processing are still rather inconsistent. In sum, they, however, indicate an altered functional connectivity in predominantly frontal areas. In order to examine whether this alteration in connectivity is a stable psychopathological symptom of the disorder or might be dependent on task difficulty we applied a short-term memory task which demands the increasing overlearning of verbal stimulus material. Previous studies of our group demonstrated that the overlearning process with the transition from controlled to automatic processing goes along with a continuous decrease of prefrontal cortical activation, suggesting decreasing demand on cognitive control processes (Koch et al. Reference Koch, Wagner, von Consbruch, Nenadic, Schultz, Ehle, Reichenbach, Sauer and Schlösser2006, Reference Koch, Wagner, Nenadic, Schachtzabel, Roebel, Schultz, Axer, Reichenbach, Sauer and Schlösser2007). We could furthermore confirm that in particular the DLPFC showed marked activation changes over time during asymptotic learning. Patients, as compared with healthy controls, exhibited significantly stronger activation decreases in association with distinct and extended activation abnormalities at the beginning of the learning process.
Both the DLPFC and the VLPFC have been shown to be critically involved in this working memory task. While the DLPFC can be assumed to constitute the neural substrate of executive functioning during retrieval of working memory information, the VLPFC is known to be strongly involved in the maintenance of working memory stimuli (D'Esposito et al. Reference D'Esposito, Postle, Ballard and Lease1999; Postle et al. Reference Postle, Berger and D'Esposito1999). Both processes play an essential role in the present task and have been demonstrated to demand fewer cortical resources with increasing working memory practice across time (Koch et al. Reference Koch, Wagner, von Consbruch, Nenadic, Schultz, Ehle, Reichenbach, Sauer and Schlösser2006). Abnormally increased activation in these regions has been found in patients predominantly during the initial stages of the working memory learning process. Based on the connectivity studies which indicate altered functional interaction between frontal areas in patients with schizophrenia (Meyer-Lindenberg et al. Reference Meyer-Lindenberg, Poline, Kohn, Holt, Egan, Weinberger and Berman2001; Schlösser et al. Reference Schlösser, Gesierich, Kaufmann, Vucurevic, Hunsche, Gawehn and Stoeter2003a) and based on our previous findings as described above we hypothesized that the normalization of activation in schizophrenia patients during the course from initial to late learning stages would be associated with increased functional interactions between dorsolateral and ventrolateral prefrontal and other task-specific areas.
In a study in normal control subjects Büchel et al. (Reference Büchel, Coull and Friston1999) reported that in parallel to decreasing activation in specialized brain areas during learning, increases of effective connectivity occur between distinct cortical systems. However, previous studies have demonstrated enhanced connectivity in short-term memory tasks in schizophrenia relative to healthy controls (Honey et al. Reference Honey, Fu, Kim, Brammer, Croudace, Suckling, Pich, Williams and Bullmore2002; Schlösser et al. Reference Schlösser, Gesierich, Kaufmann, Vucurevic, Hunsche, Gawehn and Stoeter2003a). This was partially attributed to the compensatory recruitment of brain areas and resource allocation in order to maintain task performance.
Therefore, based on the previous studies, we hypothesized that (1) the functional connectivity between task-relevant areas in the prefrontal cortex and other cortical and subcortical regions of the network during the course of overlearning is significantly modulated by the task in terms of the exponential learning process and (2) this task-related modulation of functional connectivity is significantly stronger in schizophrenia patients thereby reflecting a compensatory recruitment of brain areas in order to perform the learning task.
To investigate these hypotheses we applied the analysis of psychophysiological interactions (PPI) in order to reveal task-dependent modulation of functional interactions between the relevant frontal areas (DLPFC, VLPFC) and other areas within the working memory network during the course of an asymptotic overlearning task. The PPI analysis as performed in the current study intends to examine the task-dependent modulation of functional connectivity between different nodes of the network. The PPI variable itself is created by multiplying the source area functional magnetic resonance imaging (fMRI) time series with the task component as included in the statistical parametric mapping (SPM) design matrix. The present fMRI paradigm design was deliberately chosen to allow enhanced practice and overlearning taking place. This was established by repeatedly using identical stimulus material leading eventually to an asymptotic performance level. The study was performed in a new sample of patients suffering from schizophrenia and healthy controls expanding on the previously reported findings with regard to the overlearning task (Koch et al. Reference Koch, Wagner, von Consbruch, Nenadic, Schultz, Ehle, Reichenbach, Sauer and Schlösser2006, Reference Koch, Wagner, Nenadic, Schachtzabel, Roebel, Schultz, Axer, Reichenbach, Sauer and Schlösser2007).
Method
Participants
Participants were 13 (nine male, four female) right-handed patients with the DSM-IV diagnosis of schizophrenia and 13 right-handed healthy subjects who were matched to the patients according to gender, age and years of education (means±2). Patients were recruited from the Department of Psychiatry and Psychotherapy at the University of Jena (Jena, Germany). They were all in-patients. Diagnosis was established using a symptom checklist on the basis of DSM-IV criteria [abbreviated Structured Clinical Interview for DSM-IV (SCID) interview] and confirmed by two clinical psychiatrists (R.S. and C.S.). Patients with co-morbid axis I disorder were not included in the study. All of the patients were clinically stabilized for at least 14 days with atypical antipsychotic medication.
The patients were aged 26.4 [standard deviation (s.d.)=7.6] years and had an education of 11.7 (s.d.=1.8) years. In the healthy controls mean age was 25.6 (s.d.=8.5) years and mean education 12.8 (s.d.=0.8) years. There were no significant differences between both groups with respect to age [t(24)=−0.2, n.s.] and education (Z=−1.9, n.s.).
Volunteer subjects were screened by comprehensive assessment procedures for medical, neurological and psychiatric history. Exclusion criteria were current and potentially interfering medical conditions, any current or previous neurological or psychiatric disorder, and first-degree relatives with neurological or psychiatric disorders. Psychopathological status of the patients was assessed by the Positive and Negative Syndrome Scale (Kay et al. Reference Kay, Fiszbein and Opler1987). Ratings were 17.4 (s.d.=7.0) on the Positive Symptom Scale and 16.1 (s.d.=5.8) on the Negative Symptom Scale.
The protocol was approved by the local ethical committee and all participants gave written informed consent prior to the study.
Task design
Participants were presented a modified delayed match-to-sample task as described earlier (Koch et al. Reference Koch, Wagner, von Consbruch, Nenadic, Schultz, Ehle, Reichenbach, Sauer and Schlösser2006, Reference Koch, Wagner, Nenadic, Schachtzabel, Roebel, Schultz, Axer, Reichenbach, Sauer and Schlösser2007). The task was implemented by means of the presentation software package (Neurobehavioral Systems Inc., Albany, CA, USA).
Four pairs of consonants (target sets) were simultaneously presented on the screen for 5340 ms (encoding phase). This was immediately followed by a retrieval phase which started with the presentation of an asterisk in the centre of the screen. After 500 ms the asterisk was replaced by a pair of two consonants simultaneously presented for 1670 ms. Subjects were instructed to memorize the target set and to subsequently decide as quickly as possible for each presented pair of consonants whether it belonged to the target set or not. Ten consonant pairs were displayed within these blocks, each preceded by an asterisk for 500 ms yielding a total retrieval block length of 21 700 ms. This duration guaranteed optimal sensitivity and maximal orthogonality between the single regressors (i.e. encoding and retrieval).
We formally assessed the collinearity of our encoding and maintenance-retrieval covariates by dividing encoding and maintenance-retrieval into four sections each and analysing the inner product x Ty=|x||y| cos(θ) where θ is the angle between the respective encoding vector x and retrieval vector y (i.e. the columns for encoding and maintenance-retrieval in the design matrix). The two vectors are orthogonal if cos(θ)=0 (i.e. the inner product of both vectors is zero). The cos(θ) values were ranging from −0.005 to 0.044 with a modality of cos(θ)mod=0 indicating a negligible collinearity of the two covariates.
Each block was followed by a 10 000 ms break during which participants were instructed not to memorize the target set. This was controlled by a short debriefing after the measurement. The whole design consisted of three different target set sessions comprising 15 blocks each across which the target sets stayed constant. Thus, all examined learning effects relate to the change in behavioural performance or blood oxygenation level-dependent (BOLD) signal across sessions, i.e. the signal change across the 15 retrieval blocks (each consisting of 10 consonant pairs) averaged across the three sessions.
Data acquisition
Imaging was performed on a 1.5 T Magnetom Vision whole-body system (Siemens Medical Solutions, Erlangen, Germany) equipped with a fast gradient system for echo-planar imaging. A custom head-holder was used to restrict movement. T2*-weighted images sensitive to BOLD contrast were obtained using a gradient-echo echo-planar planar sequence [repetition time (TR)=2700 ms, echo time (TE)=60 ms, flip angle=90°, gap=0.5 mm] with 24 contiguous transverse slices of 5 mm thickness. The matrix size was 64×64 pixels with an in-plane resolution of 3.75×3.75 mm corresponding to a field of view of 220 mm. Additionally high-resolution anatomical T1-weighted volume scans with isotropic voxel resolution were obtained in sagittal orientation (TR=15 ms, TE=5 ms, flip angle=30° and field of view of 256 mm).
Data analysis
Behavioural performance
Performance was assessed by the percentage of correct responses and the mean response times for all correct responses during retrieval. A repeated-measures analysis of variance (ANOVA) was performed with group (patients, controls) as the between-subject factor and block (block 1 to 15) as the within-subject factor.
fMRI data
fMRI data analysis was performed with SPM2 (Wellcome Trust Centre for Neuroimaging, London, UK; http://www.fil.ion.ucl.ac.uk/spm). For the first three functional scans data were discarded in order to allow for signal saturation. Scans were corrected for motion effects and for differences in slice time acquisition by sinc interpolation. The anatomical high-resolution images were linearly and nonlinearly transformed to the reference brain of the Montreal Neurological Institute, corresponding to the Talairach & Tournoux (Reference Talairach and Tournoux1988) coordinate system. An 8 mm full-width-at-half-maximum Gaussian smoothing kernel was applied to the data to optimize the signal:noise ratio and compensate for inter-subject anatomical variation. Analysis was based on the autoregressive AR(1) model.
Univariate data analysis
A fixed-effects model was used for first-level analysis. Phases of encoding, retrieval and resting state were assigned to the respective scans. The trials for each condition and participant were modelled using a boxcar model convolved with a canonical haemodynamic response function to form covariates in a general linear model.
PPI
In order to prepare time series extraction for the a priori-defined functional nodes of the model, all suprathreshold activations for the overall versus baseline contrasts were identified. Subsequently, activations were parcellated into areas of DLPFC [Brodmann area (BA) 46 and BA 9] and VLPFC (BA 44, 45, 47) bilaterally according to the automated Talairach atlas (Lancaster et al. Reference Lancaster, Woldorff, Parsons, Liotti, Freitas, Rainey, Kochunov, Nickerson, Mikiten and Fox2000).
For each individual subject, local maxima (with coordinates as x, y, z) were identified for the left DLPFC (x=−52, y=6, z=42), right DLPFC (x=48, y=10, z=28), left VLPFC (x=−34, y=18, z=−2) and right VLPFC (x=32, y=28, z=−20). Spherical regions of interest (ROIs) with 8 mm radius were defined around these locations. A single time series representative of this region was obtained based on the first eigenvariate. This denoising technique is equivalent to using the first principal component time series of the ROIs (Sadasivan & Dutt, Reference Sadasivan and Dutt1996). All time series were adjusted for confounds (e.g. global mean, low-frequency components) after applying the general linear model with condition-specific predictors within the framework of SPM2. Details of this approach have been described earlier (Büchel & Friston, Reference Büchel and Friston1997, Fletcher et al. Reference Fletcher, McKenna, Friston, Frith and Dolan1999).
In order to examine the functional interaction between selected prefrontal brain areas and functionally connected regions, we performed a PPI analysis. The PPI analysis is based on using bilinear interaction terms (Friston et al. Reference Friston, Buechel, Fink, Morris, Rolls and Dolan1997). Analogous to the use of modulatory bilinear terms in systems engineering the interaction term can be regarded as an expression of the modulatory input of an external factor or signal on the interaction between a target and a source region. This use of PPI does not include directionality. Thus, instead of effective connectivity, the use of the terms functional connectivity, functional integration or functional interaction (Friston et al. Reference Friston, Buechel, Fink, Morris, Rolls and Dolan1997) has been proposed. In the present implementation of the PPI analysis, we followed the approach used by Egner & Hirsch (Reference Egner and Hirsch2005). First, the regional time series were deconvolved with regard to the haemodynamic response function, thus revealing the underlying neuronal signal for further processing.
We constructed one regressor representing the activation time course for the overall versus baseline contrast in the given volumes of interest (i.e. left/right DLPFC, left/right VLPFC), one regressor representing the time-dependent change as the psychological variable of interest (from the design matrix) and a third regressor representing the element×element product of the previous two (the PPI term).
As the psychological variable, the modelled exponential signal decrease during the overlearning paradigm was used. This strategy was in accordance with the time-dependent monoexponential BOLD signal decay characteristics of an extended cortical–subcortical–cerebellar network in schizophrenia patients and controls as described previously in detail (Koch et al. Reference Koch, Wagner, von Consbruch, Nenadic, Schultz, Ehle, Reichenbach, Sauer and Schlösser2006, Reference Koch, Wagner, Nenadic, Schachtzabel, Roebel, Schultz, Axer, Reichenbach, Sauer and Schlösser2007).
In a subsequent univariate SPM analysis we used the interaction term as a covariate of interest and the activation time course for the overall versus baseline contrast as a covariate of no interest.
With this implementation of the PPI analysis, significant SPM activations of a particular area would reflect changes in functional connectivity between the source area and the activated regions associated with learning-related BOLD signal decreases. Analyses were based on a false discovery rate (FDR)-corrected threshold of p<0.05. From each significant activation cluster of the PPI analysis we then extracted its time series at the maximally activated voxel and correlated it with the PPI whole-brain result. The number of expected voxels per cluster (k E) was taken as a spatial extent threshold. The interaction between task-dependent functional integration for the chosen seed regions and group (patients versus controls/controls versus patients) was inclusively masked with the main effect of group to restrict analyses to the PPI group-specific results and based on a threshold of p<0.005 uncorrected with a cluster threshold of five activated voxels. Montreal Neurological Institute (MNI) coordinates were converted to Talairach coordinates using the mni2tal algorithm (Brett et al. Reference Brett, Johnsrude and Owen2002).
In addition, the learning half time, based on the learning rate parameter p3 was determined by an exponential model fit (for details, see Koch et al. Reference Koch, Wagner, Nenadic, Schachtzabel, Roebel, Schultz, Axer, Reichenbach, Sauer and Schlösser2007). This was used to more precisely characterize the learning-associated signal changes and functional connectivity in both groups by investigating the PPI effects during the first half time of the learning process.
Results
Behavioural results
The percentage of correct responses ranged between 90.2 and 99.0 in healthy controls and between 83.6 and 96.7 in patients. In the first practice section the percentage of correct responses was 92.6 (s.d.=1.9) in controls and 86.8 (s.d.=2.4) in patients. In the last practice section controls showed 97.5 (s.d.=1.1) % of correct responses, patients showed 89.7.0 (s.d.=1.3) %. Response times ranged between 757.4 ms and 644.7 ms in healthy controls and between 919.6 ms and 777.9 ms in patients. In the first practice section mean response times were 718.5 (s.d.=32.9) ms in controls and 889.2.1 (s.d.=35.6) ms in patients. In the last practice section mean response times reached 655.5 (s.d.=14.5) ms in controls and 797.7 (s.d.=29.4) ms in patients. As illustrated in Fig. 1 reaction time decreased exponentially across the retrieval period in both groups.
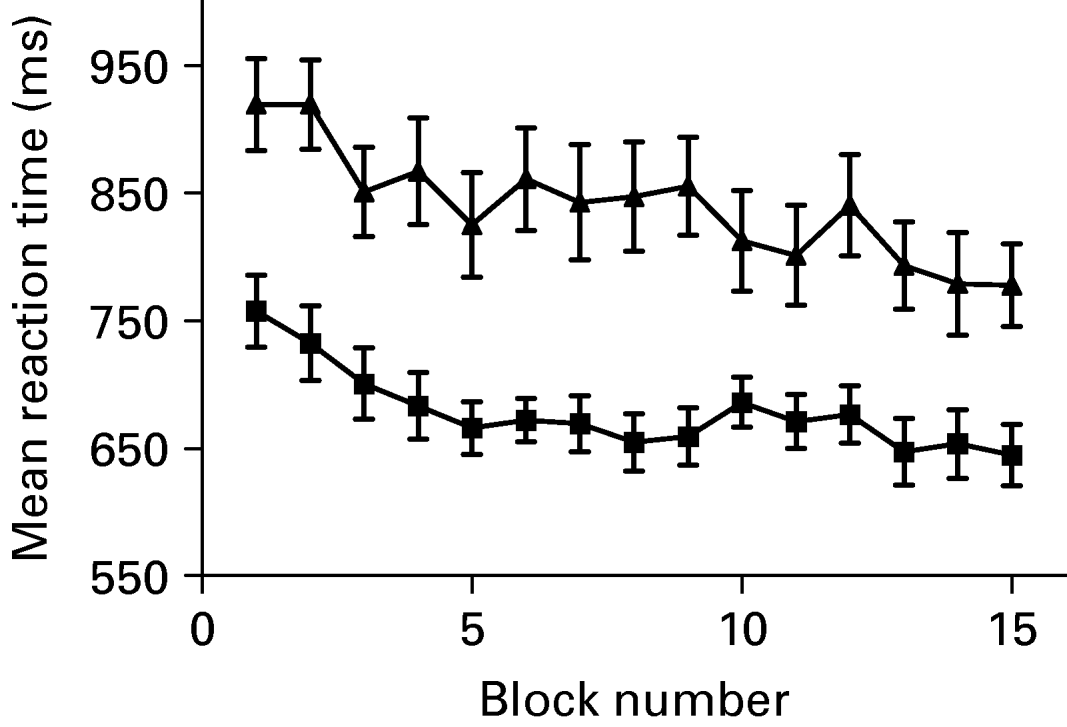
Fig. 1. Time course of the reaction time (ms) for patients (–▴–) and controls (–▪–) during the overlearning task. Values are means, with standard errors represented by vertical bars.
The repeated-measures ANOVA yielded a significant main effect of block [F(14, 336)=52.2, p<0.001] and a significant main effect of group [F(1, 24)=1346.6, p<0.001], indicating significant learning-associated performance improvements in both groups. However, in this ANOVA design no significant group×block interaction emerged. So far this repeated-measures ANOVA does not provide evidence for both the time curves showing group-specific characteristics depending on the time point examined.
Imaging results
fMRI analysis
In the univariate statistical analysis practice-related exponential signal decreases were distinguishable in patients and controls. Patients showed abnormally increased activation during early stages of learning in superior frontal, parietal, middle temporal and occipital regions as well as the anterior cingulate that normalized with increasing practice. Patients exhibited stronger signal decreases relative to controls predominantly in prefrontal, parietal and superior temporal brain areas. Details have been described elsewhere (Koch et al. Reference Koch, Wagner, Nenadic, Schachtzabel, Roebel, Schultz, Axer, Reichenbach, Sauer and Schlösser2007).
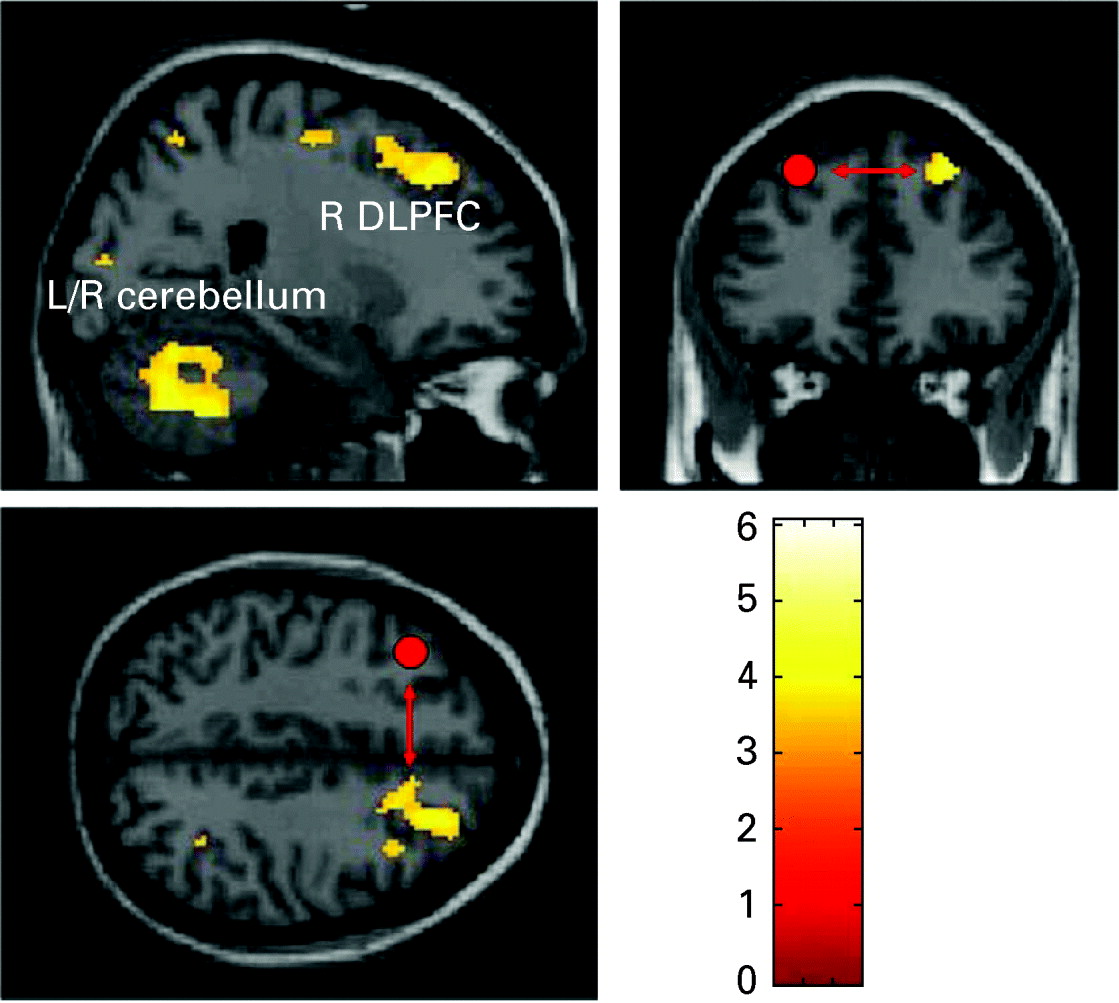
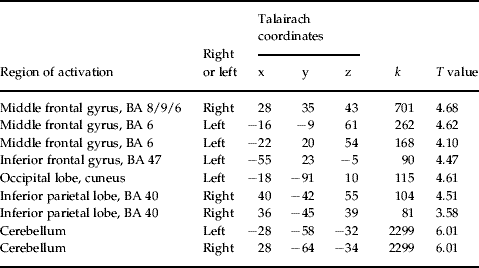
fMRI-PPI
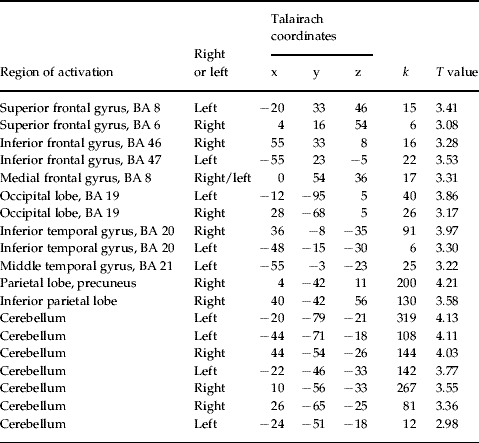
In order to examine the primary hypotheses the learning-related change of functional interactions between prefrontal areas bilaterally was evaluated by creating PPI variables. Only in the patient group were there task-dependent interaction patterns between the left DLPFC and other brain areas including the right DLPFC, left VLPFC, premotor cortex, right inferior parietal cortex, left and right cerebellum and left occipital lobe (see Table 1, Figs 2, 3). The direct group comparison with the DLPFC as seed region revealed significantly stronger task-dependent interaction patterns between the left DLPFC and mainly cerebellar, but also inferior and middle temporal, superior and inferior frontal regions, as well as the occipital and parietal lobe in patients compared with healthy controls (see Table 2). The opposite contrast investigating significantly stronger task-dependent interaction patterns in controls compared with patients yielded activation in the right cerebellum (x=16, y=−24, z=−22, k=23, T=3.32).

Fig. 2. Regions showing significant task-dependent interaction with the left dorsolateral prefrontal cortex in patients (p<0.05, false discovery rate corrected). BA, Brodmann area.
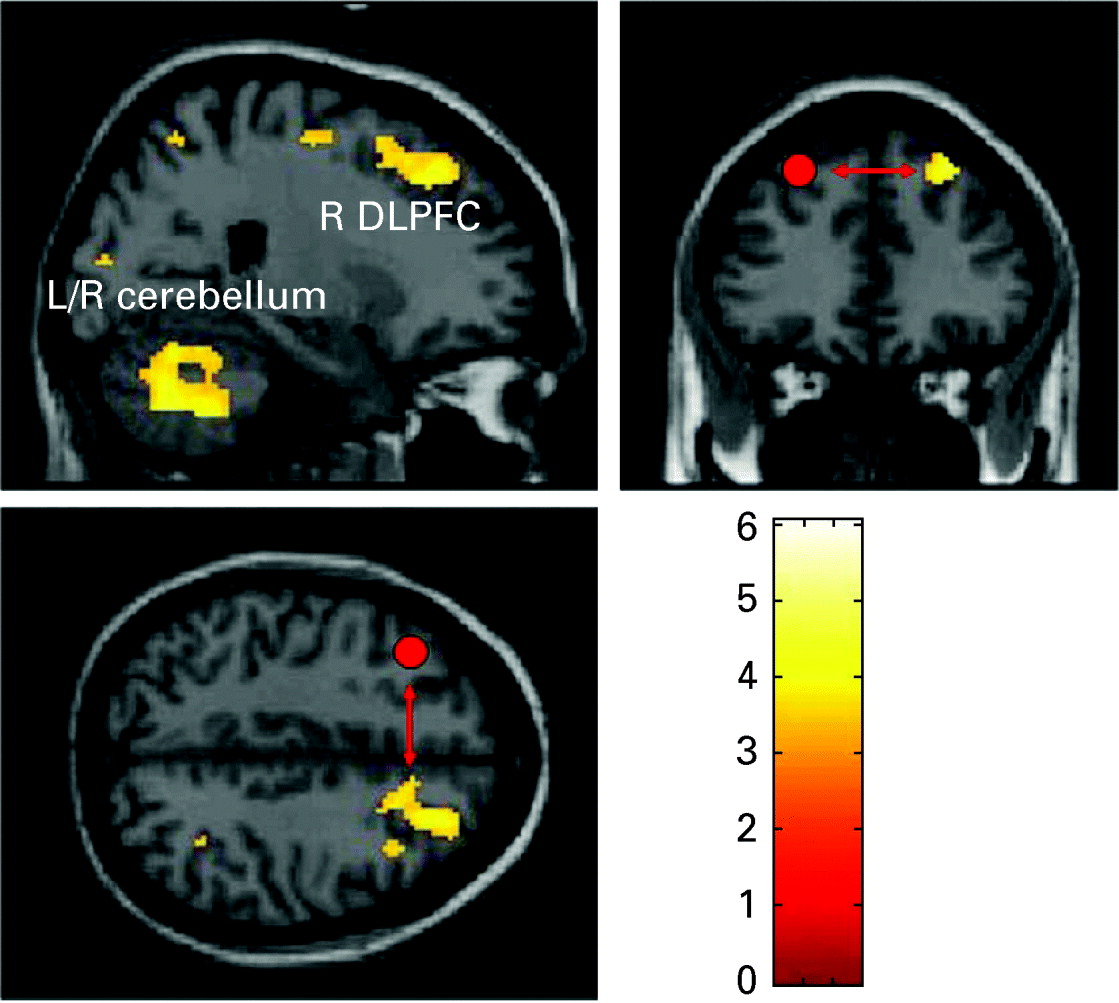
Fig. 3. Right dorsolateral prefrontal and cerebellar regions that showed a task-dependent interaction with the left dorsolateral prefrontal cortex (DLPFC) seed region (illustrated in red) in patients (p<0.05, false discovery rate corrected). L, Left; R, right.
Table 1. Talairach coordinates of activation maxima [SPM(T) value] for the regions showing significant task-dependent interaction with the left DLPFC in patients (p<0.05, FDR corrected)
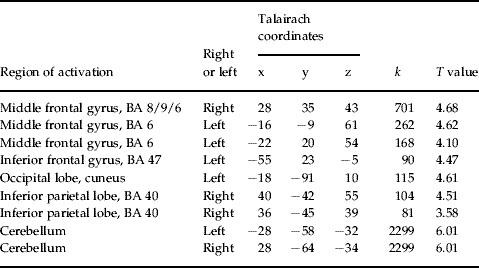
SPM, Statistical parametric mapping; DLPFC, dorsolateral prefrontal cortex; FDR, false discovery rate; BA, Brodmann area.
Table 2. Talairach coordinates of activation maxima [SPM(T) value] for the regions showing significantly stronger task-dependent interaction with the left DLPFC in patients compared with controls (p<0.005, uncorrected)
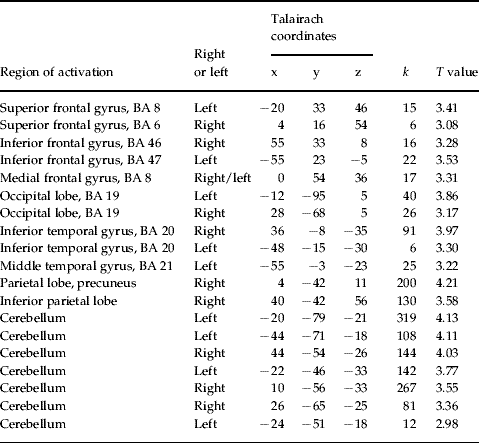
SPM, Statistical parametric mapping; DLPFC, dorsolateral prefrontal cortex; BA, Brodmann area.
The PPI covariance analyses for the right DLPFC as well as the right and left VLPFC yielded no significant activation patterns (p<0.05, FDR corrected), neither in controls nor in patients.
In order to investigate the temporal characteristics of the BOLD signal in more detail, we performed a repeated-measures ANOVA for the extracted BOLD time series of the left and right DLPFC. These areas were chosen as they constituted the seed region and major interaction areas of the PPI results.
For the left DLPFC a significant main effect of block [F(14, 336)=7.799, p<0.001] and no significant effect of group or group×block interaction were observed. For the right DLPFC a significant main effect of block [F(14, 336)=3.938, p<0.001] but no overall significant main effect of group could be detected. However, a marginally significant block×group interaction could be found [F(14, 336)=1.712, p=0.05]. Inspection of the estimated marginal means for the BOLD activation data or time demonstrated that this interaction was associated with higher BOLD data in patients relative to controls in the first six blocks and lower BOLD data in patients compared with controls throughout the last nine blocks. Planned comparisons of all 15 blocks by one-way ANOVAs performed subsequently revealed that for block 1 and block 4 these differences were significant (both p<0.03).
This finding provides evidence that the difference between patients and controls might be partially attributable to the higher activation during the initial stages of learning in patients. This activation is subsequently decreasing to a level comparable with the normal controls and no statistical differences between groups are observable.
Aside from the ANOVA, in order to take into account the nonlinear nature of the different time curves, we directly compared the fitted learning rates for both groups. We used the exponential model as outlined in detail in Koch et al. (Reference Koch, Wagner, von Consbruch, Nenadic, Schultz, Ehle, Reichenbach, Sauer and Schlösser2006, Reference Koch, Wagner, Nenadic, Schachtzabel, Roebel, Schultz, Axer, Reichenbach, Sauer and Schlösser2007). For the healthy subject group the analysis yielded a p3 of 2.22 for the control group and p3 of 15.99 for the patient group. The learning rate multiplied with the natural logarithm of 2 equals the time where half of the maximal learning occurred. Given that the natural logarithm of 2 is approximately equal to 0.693, the learning half time for controls is equal to 1.53 blocks and 11.08 blocks for the patients. Thus, normal controls already reached half of their maximum learning performance within the second learning block whereas the patients needed about 11 blocks to reach the same state.
In order to examine whether differences in the learning characteristics might contribute to different PPI findings we separately analysed the data for the healthy controls and patients with schizophrenia for the time interval in terms of learning blocks during which the subjects reached the learning half time. According to the calculations above we chose for the healthy controls two blocks corresponding to 30 fMRI scans and for the patients 11 blocks corresponding to 166 fMRI scans. This analysis was performed for the connection between the left DLPFC and right DLPFC in accordance with the major finding from the SPM analysis reported previously. For the time period examined in the group of the healthy subjects a non-significant correlation of 0.15 (p=0.4) could be detected. For the scans investigated in patients the observed correlation of 0.46 was highly significant (p<0.001).
Discussion
The present study sought to investigate whether learning-related changes in BOLD activation in schizophrenia patients and healthy controls are associated with differential task-related modulation of functional connectivity in both groups.
As hypothesized we found that intensive practice of the cognitive task was associated with an enhanced functional connectivity between the left frontal cortex and further relevant regions within the working memory network in schizophrenia. Task-related modulation of functional connectivity is significantly stronger in schizophrenia patients, thereby reflecting the observed capacity of patients to normalize the compensatory enhanced activation during the course from the initial to the late learning stages.
Thus, the increasing overlearning of verbal stimulus material was based on a significant functional interaction between the left DLPFC and an extended fronto-parieto-cerebellar network in schizophrenia patients. Since the learning task interaction variable has been modelled by a nonlinear function in terms of an exponential decrease, these interactions represent a network which holds close functional interactions that gain significance following a pattern of asymptotic decrease of reaction times.
The present data indicate that the left DLPFC which has already been shown to play a major role in verbal working memory and cognitive control processes (Wager & Smith, Reference Wager and Smith2003; Altamura et al. Reference Altamura, Elvevag, Blasi, Bertolino, Callicott, Weinberger, Mattay and Goldberg2007; Schlösser et al. Reference Schlösser, Koch, Wagner, Nenadic, Roebel, Schachtzabel, Axer, Schultz, Reichenbach and Sauer2008) appears to be in close cooperation with several other task-relevant areas of the brain during overlearning in patients. The findings suggest that the left DLPFC constitutes one of the central nodes within the fronto-parietal network which has been demonstrated to be modulated in activation during short-term learning processes. Here, the left-sided lateralization is in keeping with the concept of left-hemisphere dominance in verbal processing and potential cognitive control exerted by the left DLPFC.
Our preceding findings demonstrated that repeated presentation of stimulus material (i.e. increasing practice or decreasing task demands) was associated with decreasing activation strength in a fronto-parieto-cerebellar network in patients and controls. Patients, however, exhibited abnormally increased activation in a similar network during initial learning stages (i.e. increased task demands) with an activation normalization as practice increased.
The present findings suggest that in patients this activation normalization is associated with a high level of functional connectivity between the DLPFC and other task-relevant areas during practice. On the contrary, there were no significant PPI results in controls at the chosen threshold.
Relatively enhanced connectivity in schizophrenia in the presence of performance deficits in short-term memory tasks has been mentioned in other studies examining functional connectivity, e.g. using structural equation modelling (Honey et al. Reference Honey, Fu, Kim, Brammer, Croudace, Suckling, Pich, Williams and Bullmore2002; Schlösser et al. Reference Schlösser, Gesierich, Kaufmann, Vucurevic, Hunsche, Gawehn and Stoeter2003a). In particular, enhanced prefronto-prefrontal connectivity was observed as a correlate of increased memory load. This has been interpreted as a reflection of greater demand for executive processes. In the same vein, the present findings of higher connectivity in patients with schizophrenia might be an effective physiological mechanism which enables successful short-term learning in patients. This higher connectivity is present despite disorder-related cognitive impairments and thus might be regarded as an indirect correlate of an underlying cortical inefficiency and the attempt to maintain a task performance level comparable with healthy controls (Honey et al. Reference Honey, Fu, Kim, Brammer, Croudace, Suckling, Pich, Williams and Bullmore2002). Within this model, healthy subjects do not require this enhanced level of connectivity due to their more efficient processing. Based on the current findings we, therefore, postulated that enhanced compensatory connectivity in the context of cortical inefficiency may be a specific neurophysiological signature underlying the pathophysiology of schizophrenia.
This interpretation is also supported by previous results from univariate data analyses suggesting cortical inefficiency may be related to higher task demands (Potkin et al. Reference Potkin, Turner, Brown, McCarthy, Greve, Glover, Manoach, Belger, Diaz, Wible, Ford, Mathalon, Gollub, Lauriello, O'Leary, van Erp, Toga, Preda and Lim2009). Likewise, the current learning task involves intra-scan differences with higher and lower task demands during the time of the learning trial. As evident from the time series analysis described above as well as indicated by our preceding univariate analyses (Koch et al. Reference Koch, Wagner, Nenadic, Schachtzabel, Roebel, Schultz, Axer, Reichenbach, Sauer and Schlösser2007) hyperactivation is present particularly in the first quarter of the learning trial. To establish this transition from inefficient hyperactivation status to a normalized activation level a higher degree of cortical integration might be necessary. Or, in other words, a more effective interaction between task-relevant regions might allow single areas of the network to reduce their activation.
Both groups might demonstrate differences in the dynamics of their learning curve. To rule out any influence of the difference with regard to different time points at which subjects are receiving their asymptotic performance level, we performed separate analyses for the time-frame in which both groups were within a half time of the maximum learning capacity. Presumably, cognitive demand was on a high level for both groups in this learning phase which was still in the declining limb of the exponential learning curve. The results from this additional analysis demonstrated that even if the correlation is restricted to the steeper decline section of the exponential learning curve the previously reported findings remained essentially valid. Thus, even if it is assumed that healthy subjects have a shorter learning half rate than schizophrenia patients the observation of higher connectivity in patients still remains valid.
This higher integration as evident by higher PPI effects on cortico-cortical connectivity could also serve different functional purposes. One could be the compensatory recruitment of additional brain areas, for example the contralateral right DLPFC in conjunction with the left DLPFC. This recruitment might enable the temporary resource allocation until learning traces are more established. Although not observed in the present task, other learning tasks have demonstrated a shift from prefrontal to temporal brain systems as learning performance and task uncertainty decrease (Schlösser et al. Reference Schlösser, Nenadic, Wagner, Zysset, Koch and Sauer2009) and that learning on the basis of predictable stimulus–outcome contingencies enables the brain to reduce resources (Koch et al. Reference Koch, Schachtzabel, Wagner, Reichenbach, Sauer and Schlösser2008a).
The intact and strong functional interplay within the distributed network enables the group of schizophrenia patients to establish a sufficient level of functional efficiency. This finding adds an important complement to the previously reported univariate findings demonstrating exponential signal decreases in a frontal-parietal network.
The enhanced functional connectivity with the cerebellar cortices during overlearning in the schizophrenia patient group concurs with earlier works that show connections from the DLPFC to the cerebellar cortex (Ito, Reference Ito1984). Fronto-cerebellar effective connectivity is assumed to be of major importance in the context of working memory studies and has been found to be unimpaired in drug-free patients with schizophrenia (Schlösser et al. Reference Schlösser, Gesierich, Kaufmann, Vucurevic and Stoeter2003b).
The present findings of substantial learning processes and associated BOLD changes which occur within the scan time during short-term memory tasks might have implications for the analysis and interpretation of other fMRI studies. However, in other working memory paradigms used in schizophrenia research in combination with fMRI the high repetition of stimulus material as in the present study is usually not taking place. For example, during the Sternberg item recognition task, which involves solving a delayed match-to-sample task, the stimulus material is usually varied across the scanning session and repetition of stimulus material or stimuli series is sought to be avoided (Schlösser et al. Reference Schlösser, Nenadic, Wagner, Gullmar, von Consbruch, Kohler, Schultz, Koch, Fitzek, Matthews, Reichenbach and Sauer2007a, Reference Schlösser, Koch, Wagner, Nenadic, Roebel, Schachtzabel, Axer, Schultz, Reichenbach and Sauer2008; Koch et al. Reference Koch, Wagner, Nenadic, Schachtzabel, Schultz, Roebel, Reichenbach, Sauer and Schlösser2008b). Thus, limited or no learning might occur during these types of working memory paradigms.
It can only be speculated whether the observed functional alterations in patients are related to underlying morphological changes in the brain. Studies with diffusion tensor imaging reported reduced rather than enhanced integrity of white matter fibre tracts linking different cortical and subcortical brain areas (Kanaan et al. Reference Kanaan, Kim, Kaufmann, Pearlson, Barker and McGuire2005; Federspiel et al. Reference Federspiel, Begre, Kiefer, Schroth, Strik and Dierks2006). A potential solution to this controversy with the current functional findings could be that the PPI analysis does not imply information about directed information flow within the brain which might be transmitted by the long fibre tracts. Rather, it comprises information about the correlation of different regional temporal activation vectors.
The PPI analysis as performed in the current study intends to examine the task-dependent modulation of functional connectivity between different nodes of the network. The PPI variable itself is created by multiplying the source area fMRI time series with the task component as included in the SPM design matrix. We multiplied the left DLPFC BOLD time series with the time course of the exponential decay (i.e. the exponential decrease in reaction time). This resulted in the PPI interaction term used as a covariate of interest in the ensuing SPM analysis. Since the exponential decay as a whole contributed to the psychological interaction variable it will be methodologically not feasible to just compare different parts of the time series without preserving the exponentially decaying overall characteristics of the interaction term. Although the psychological variable in terms of exponential decrease reflects a process of learning, the estimated regression coefficient of the general linear model is based on a stationary relationship. This provides some limitation for the model since it does not allow the detection of time-varying connectivity parameters (Schlösser et al. Reference Schlösser, Wagner and Sauer2006, Reference Schlösser, Koch and Wagner2007b).
Taken together, the present study provides evidence for a learning-related modulation of functional interaction between the left DLPFC and other task-related cortical and subcortical brain areas. This task-related connectivity modulation is relatively enhanced in schizophrenia patients consistent with the ability of this group to normalize initially increased task activation during practice and overlearning as reported earlier. Moreover, it suggests preserved functional capacities and the ability to cope with new memory material in patients. The present findings, therefore, provide an optimistic outlook for pharmacological as well as non-pharmacological therapeutic interventions which might be able to capitalize on preserved functional plasticity by pharmacological enhancement and/or cognitive training.
Acknowledgements
This work was supported by the German Federal Ministry of Education and Research (BMBF, grant FKZ01ZZ0405), the Interdisciplinary Centre for Clinical Research of the University of Jena (IZKF) and Thuringian Ministry of Science, Research, and Art (TMWFK, grant B307-04004).
Declaration of Interest
None.