Introduction
Obsessive–compulsive disorder (OCD) is characterized by intrusive distressing ideas, repeated behaviors and neutralizing thoughts, which aim to control internal and external events (Aouizerate et al. Reference Aouizerate, Guehl, Cuny, Rougier, Bioulac, Tignol and Burbaud2004). In this context, OCD patients typically develop overactive self-performance monitoring (Endrass & Ullsperger, Reference Endrass and Ullsperger2014). Such an alteration is accompanied by event-related potential (ERP) abnormalities in components indexing monitoring processes, such as the error-related negativity (Gehring et al. Reference Gehring, Himle and Nisenson2000; Riesel et al. Reference Riesel, Kathmann and Endrass2014), the correct-response negativity (CRN) (Riesel et al. Reference Riesel, Endrass, Kaufmann and Kathmann2011; Carrasco et al. Reference Carrasco, Harbin, Nienhuis, Fitzgerald, Gehring and Hanna2013), and the motor potential (MP) (Johannes et al. Reference Johannes, Wieringa, Mantey, Nager, Rada, Muller-Vahl, Emrich, Dengler, Munte and Dietrich2001). Note that the latter potential, which is elicited by voluntary finger or foot movements (Brunia & Van den Bosch, Reference Brunia and Van den Bosch1984; Deecke et al. Reference Deecke, Bashore, Brunia, Grunewald-Zuberbier, Grunewald and Kristeva1984), exhibits larger amplitudes for correct than incorrect responses, thus yielding information about monitoring processes (Vaughan et al. Reference Vaughan, Costa and Ritter1968; Deecke et al. Reference Deecke, Bashore, Brunia, Grunewald-Zuberbier, Grunewald and Kristeva1984; Simons, Reference Simons2010). While overactive monitoring is well established in OCD (Gehring et al. Reference Gehring, Himle and Nisenson2000; Endrass & Ullsperger, Reference Endrass and Ullsperger2014) and may even represent an endophenotype of the condition (Riesel et al. Reference Riesel, Endrass, Kaufmann and Kathmann2011, Reference Riesel, Endrass, Auerbach and Kathmann2015), little is known about the specificity and scope of this pattern. Only a few studies have explored it by comparing OCD and other psychiatric conditions (e.g. Xiao et al. Reference Xiao, Wang, Zhang, Li, Tang, Wang, Fan and Fromson2011), and none has examined it in relation to inwards attention. Here we address both issues combining behavioral and neurophysiological measures.
Available findings stem from responses to external stimuli. However, given the role of peripheral physiological processes in homeostasis and emotional regulation (Cameron, Reference Cameron2001; Craig, Reference Craig2002; Weiss et al. Reference Weiss, Sack, Henningsen and Pollatos2014), OCD may also involve overactive monitoring of internal signals, such as those indexing bodily states. A crucial function in this respect is interoception, the perceptual and physiological sensing of visceral signals (Craig, Reference Craig2003). This multidimensional function (Craig, Reference Craig2002; Garfinkel et al. Reference Garfinkel, Seth, Barrett, Suzuki and Critchley2015; García-Cordero et al. Reference García-Cordero, Sedeño, de la Fuente, Slachevsky, Forno, Klein, Lillo, Ferrari, Rodriguez, Bustin, Torralva, Baez, Yoris, Esteves, Melloni, Salamone, Huepe, Manes, García and Ibañez2016) differentially engages the insula, the anterior cingulate cortex and the prefrontal cortex (Critchley et al. Reference Critchley, Wiens, Rotshtein, Ohman and Dolan2004; Zaki et al. Reference Zaki, Davis and Ochsner2012). Notably, the former two areas evidence increased activity and volume in OCD patients (Song et al. Reference Song, Jung, Jang, Kim, Shim, Park, Choi and Kwon2011). Thus, interoceptive alterations may be reasonably expected in this population.
Critical evidence on bodily signal monitoring is afforded by heartbeat detection (HBD) tasks, in which participants follow their own heartbeats through mental (Schandry et al. Reference Schandry, Bestler and Montoya1993) or motor (Couto et al. Reference Couto, Salles, Sedeno, Peradejordi, Barttfeld, Canales-Johnson, Dos Santos, Huepe, Bekinschtein, Sigman, Favaloro, Manes and Ibanez2014) tracking. These tasks tap interoceptive accuracy (IAcc) – the participants’ objective precision in detecting visceral sensations (Garfinkel et al. Reference Garfinkel, Seth, Barrett, Suzuki and Critchley2015) –, and they also allow assessment of two related dimensions: interoceptive sensibility (ISen) – the subject's confidence in his/her own performance – and interoceptive awareness (IAw) – a metacognitive index of interoceptive performance (Zaki et al. Reference Zaki, Davis and Ochsner2012; Garfinkel et al. Reference Garfinkel, Seth, Barrett, Suzuki and Critchley2015). Together with the evidence above, reports of metacognitive impairments in OCD (Cucchi et al. Reference Cucchi, Bottelli, Cavadini, Ricci, Conca, Ronchi and Smeraldi2012; Coles et al. Reference Coles, Schofield and Nota2015) suggest that such three interoceptive dimensions may be altered in this group.
Finally, electrophysiological correlates of heartbeat sensing are indexed by the heart evoked potential (HEP). This ERP deflection reflects attention allocation to cardiac modulations irrespective of response accuracy (Schandry et al. Reference Schandry, Sparrer and Weitkunat1986; Pollatos & Schandry, Reference Pollatos and Schandry2004). If overactive monitoring in OCD extends to the interoceptive domain, then distinct HEP modulations should also be observed during the HBD task.
Our study examined whether overactive monitoring in OCD is also present in the interoceptive domain, and whether this potential pattern is different in other clinical conditions, with a specific focus on panic disorder. To this end, we compared the performance of three samples (OCD patients, panic disorder patients and healthy controls) on a motor-tracking HBD task. We measured IAcc, ISen and IAw, together with associated neurophysiological processes. Since the HBD task does not include high-conflict trials but requires precise manual responses to cardiac signals, our analysis focused on the MP and the HEP rather than the ERN. These two components offer complementary measures of attention allocation to inner-body modulations. Specifically, we hypothesized that, relative to both other samples, OCD patients would exhibit: (i) enhanced IAcc and impaired IAw; (ii) increased MP amplitude for correct relative to incorrect trials; and (iii) enlarged HEP modulations, indexing overactive monitoring of internal signals. Our study thus offers an unprecedented cross-dimensional view into inner monitoring in OCD. Insights derived therefrom could contribute to: (a) a better characterization of the patients’ cognitive profile and the relation between inner monitoring and obsessive–compulsive mechanisms and symptoms; (b) the formalization of potentially sensitive and specific biomarkers of the disease; and (c) the establishment of new avenues to assess disease progression and response to clinical treatment.
Method
Participants
The study comprised 15 OCD patients, 15 panic disorder patients and 25 healthy volunteers. The patients’ diagnoses were established by two OCD experts (R.K. and A.Y.) using the Structured Clinical Interview for DSM-IV Axis I SCID-I (First et al. Reference First, Spitzer, Gibbon and Williams2002) and following Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV) criteria (American Psychiatric Association, 1994). OCD patients scored well above the 21-point cut-off (mean = 30.27) on the Obsessive–Compulsive Inventory (OCI-R) (Foa et al. Reference Foa, Huppert, Leiberg, Langner, Kichic, Hajcak and Salkovskis2002), and they presented high scores (mean = 80.93) in the Obsessive Belief Questionnaire (OBQ-TRIP) (Moulding et al. Reference Moulding, Anglim, Nedeljkovic, Doron, Kyrios and Ayalon2011) (see online Supplementary Table S1.1). OCD and panic patients reported psychiatric medication intake during recruitment (see online Supplementary Table S1.2). Healthy controls had no history of drug abuse or neuropsychiatric disease. The groups were matched for age, gender, education, handedness and body mass index (Table 1). Participants provided informed consent in accordance with the Declaration of Helsinki. The study was approved by the institutional ethics committee.
Table 1. Demographic and mood/anxiety results

Data are given as mean (standard deviation) unless otherwise indicated.
OCD, Obsessive–compulsive disorder; CTRL, control; PANIC, panic; HADS-A, Hospital Anxiety and Depression Scale, anxiety subscale; HADS-D, Hospital Anxiety and Depression Scale, depression subscale; STAI-S, State Trait Anxiety Inventory, state version.
* Significant difference.
Mood and anxiety measures
Anxiety and depression symptoms were evaluated using the Hospital Anxiety and Depression Scale (HADS). The HADS has two subscales, addressing recent depressive symptoms (HADS-D) and trait anxiety (HADS-A) (Zigmond & Snaith, Reference Zigmond and Snaith1983). Anxiety levels during the HBD task were assessed via the state version of the State Trait Anxiety Inventory (STAI-S; Spielberger et al. Reference Spielberger, Gorsuch and Lushene1970).
HBD task
Cardiac interoception was assessed through a modified version of a validated HBD task (Sedeno et al. Reference Sedeno, Couto, Melloni, Canales-Johnson, Yoris, Baez, Esteves, Velasquez, Barttfeld, Sigman, Kichic, Chialvo, Manes, Bekinschtein and Ibanez2014; Couto et al. Reference Couto, Adolfi, Velasquez, Mesow, Feinstein, Canales-Johnson, Mikulan, Martinez-Pernia, Bekinschtein, Sigman, Manes and Ibanez2015). Two conditions were considered, both involving dominant-hand responses. In the exteroceptive condition, participants tapped a keyboard to follow an audio-recording of a typical heartbeat; this indexed monitoring of external stimuli. In the interoceptive condition, participants followed their own heartbeats without any external cues, which provided a measure of IAcc (Garfinkel et al. Reference Garfinkel, Seth, Barrett, Suzuki and Critchley2015) (see online Supplementary material section 2 for further details). In addition, we assessed ISen and IAw (Garfinkel et al. Reference Garfinkel, Seth, Barrett, Suzuki and Critchley2015), as defined in the Introduction. Finally, during this task, neurophysiological and cardiac signals were recorded via electroencephalography (EEG) and electrocardiography (ECG).
IAcc
Behavioral interoceptive performance was indexed by tapping accuracy (TA) (Canales-Johnson et al. Reference Canales-Johnson, Silva, Huepe, Rivera-Rei, Noreika, Garcia, Silva, Ciraolo, Vaucheret, Sedeno, Couto, Kargieman, Baglivo, Sigman, Chennu, Ibanez, Rodriguez and Bekinschtein2015). First, correct answers were selected based on responses that were temporally locked to the R-peak of the heartbeats. We used this R-wave deflection given that the time window between 200 to 300 ms after it is a critical proxy of heartbeat perception (Brener & Kluvitse, Reference Brener and Kluvitse1988; Wiens & Palmer, Reference Wiens and Palmer2001; Critchley et al. Reference Critchley, Wiens, Rotshtein, Ohman and Dolan2004; Kleckner et al. Reference Kleckner, Wormwood, Simmons, Barrett and Quigley2015).
TA was defined as the average of the absolute value of the time difference between the R-peak and the motor tap in correct answers (Canales-Johnson et al. Reference Canales-Johnson, Silva, Huepe, Rivera-Rei, Noreika, Garcia, Silva, Ciraolo, Vaucheret, Sedeno, Couto, Kargieman, Baglivo, Sigman, Chennu, Ibanez, Rodriguez and Bekinschtein2015), namely: TA = ∑(motor tap time − R-peak time)/n. Accordingly, given that TA is a reaction-time measure, smaller values indicate better behavioral performance (IAcc) during the HBD task.
ISen
ISen represents a subject's self-confidence relative to his/her objective performance in an interoceptive task (Garfinkel et al. Reference Garfinkel, Seth, Barrett, Suzuki and Critchley2015). To measure this variable, at the end of the interoceptive condition of the HBD task, we asked participants to rate their confidence in their performance from 1 (not confident at all) to 9 (fully confident).
IAw
IAw represents the degree to which objective performance during the HBD task (IAcc) is associated with subjective confidence in such performance (ISen) (Garfinkel et al. Reference Garfinkel, Seth, Barrett, Suzuki and Critchley2015). This index was calculated for each group (given that we only had one measure for IAcc and ISen per participant) based on an indirect correlation between IAcc (based on TA) and ISen. A high correspondence between these two measures, represented by a high correlation value, indicates the ability of participants to recognize that their HBD performance was either good or bad (Garfinkel et al. Reference Garfinkel, Seth, Barrett, Suzuki and Critchley2015).
ERP measures
EEG recordings and preprocessing steps
EEG signals were recorded with a Biosemi Active-two 128-channel system at 1024 Hz, resampled offline at 256 Hz. The reference was set by default to link mastoids, and re-referenced off-line to the average electrodes. Segments with eye movement contamination were removed from further analysis using independent component analysis (ICA) and a visual inspection procedure. To control for potential cardiac artifacts, we also removed cardiac components via ICA. Two external electrodes were used to collect the ECG signal. R-wave-ECG detection was achieved by a peak finder function implemented on MATLAB (MathWorks, Inc., USA), which quickly identifies local peaks or valleys (local extrema) in a noisy vector using the alternating nature of the derivatives and a user-defined magnitude threshold to determine whether each peak is significantly larger (or smaller) than the data around it (Kruczyk et al. Reference Kruczyk, Umer, Enroth and Komorowski2013). See online Supplementary material section 3 for EEG preprocessing details.
HEP
The HEP is a modulation emerging 200–500 ms after the R-wave peak (Montoya et al. Reference Montoya, Schandry and Muller1993; Pollatos & Schandry, Reference Pollatos and Schandry2004; Fukushima et al. Reference Fukushima, Terasawa and Umeda2011; Canales-Johnson et al. Reference Canales-Johnson, Silva, Huepe, Rivera-Rei, Noreika, Garcia, Silva, Ciraolo, Vaucheret, Sedeno, Couto, Kargieman, Baglivo, Sigman, Chennu, Ibanez, Rodriguez and Bekinschtein2015) that indexes attention to cardiac modulations (Schandry & Weitkunat, Reference Schandry and Weitkunat1990; Montoya et al. Reference Montoya, Schandry and Muller1993), and also other processes related to body–brain communication, such as emotional processes (Canales-Johnson et al. Reference Canales-Johnson, Silva, Huepe, Rivera-Rei, Noreika, Garcia, Silva, Ciraolo, Vaucheret, Sedeno, Couto, Kargieman, Baglivo, Sigman, Chennu, Ibanez, Rodriguez and Bekinschtein2015), motivation (Schandry & Weitkunat, Reference Schandry and Weitkunat1990), stress (Gray et al. Reference Gray, Taggart, Sutton, Groves, Holdright, Bradbury, Brull and Critchley2007) and pain perception (Shao et al. Reference Shao, Shen, Wilder-Smith and Li2011). In all of these, HEP amplitude seems to be triggered by attention to body sensations.
Regarding cardiac perception, one main pathway of heartbeat information relies on signals from baroreceptors in the aortic arch and carotid bodies (Critchley et al. Reference Critchley, Wiens, Rotshtein, Ohman and Dolan2004; Critchley & Harrison, Reference Critchley and Harrison2013) which, primarily via the vagus nerve and its connections to the nucleus of the solitary tracks (Craig, Reference Craig2002, Janig, Reference Janig1996), provide projections to centers such as the hypothalamus, thalamus and cerebral cortex (Craig, Reference Craig2002). Here, the insular cortex and the ACC play a major role as a viscerosensory (Craig, Reference Craig2002, Reference Craig2003) and visceromotor centers, respectively (Craig, Reference Craig2002, Reference Craig2004; Dum et al. Reference Dum, Levinthal and Strick2009). Primary and secondary somatosensory cortices constitute another pathway of cardiac information, given that sensory information from the skin of the chest is also essential for interoceptive perception (Khalsa et al. Reference Khalsa, Rudrauf, Feinstein and Tranel2009; Couto et al. Reference Couto, Salles, Sedeno, Peradejordi, Barttfeld, Canales-Johnson, Dos Santos, Huepe, Bekinschtein, Sigman, Favaloro, Manes and Ibanez2014). The key role of these cortical areas (insula, ACC and somatosensory cortices) has been supported by lesion models (Khalsa et al. Reference Khalsa, Rudrauf, Feinstein and Tranel2009; Couto et al. Reference Couto, Salles, Sedeno, Peradejordi, Barttfeld, Canales-Johnson, Dos Santos, Huepe, Bekinschtein, Sigman, Favaloro, Manes and Ibanez2014; Ronchi et al. Reference Ronchi, Bello-Ruiz, Lukowska, Herbelin, Cabrilo, Schaller and Blanke2015; Terasawa et al. Reference Terasawa, Kurosaki, Ibata, Moriguchi and Umeda2015; García-Cordero et al. Reference García-Cordero, Sedeño, de la Fuente, Slachevsky, Forno, Klein, Lillo, Ferrari, Rodriguez, Bustin, Torralva, Baez, Yoris, Esteves, Melloni, Salamone, Huepe, Manes, García and Ibañez2016), fMRI studies and meta-analyses (Critchley et al. Reference Critchley, Wiens, Rotshtein, Ohman and Dolan2004; Pollatos et al. Reference Pollatos, Matthias and Schandry2007; Schulz et al. Reference Schulz, Matthey, Vogele, Schaan, Schachinger, Adler, Beutel and Michal2016), and brain stimulation studies (Pollatos et al. Reference Pollatos, Herbert, Mai and Kammer2016). Critically, HEP source analysis has also revealed the involvement of these areas in cardiac perception (Pollatos et al. Reference Pollatos, Kirsch and Schandry2005), which supports the relationship between this ERP and interoceptive processing.
Based on previous studies (Pollatos & Schandry, Reference Pollatos and Schandry2004; Gray et al. Reference Gray, Taggart, Sutton, Groves, Holdright, Bradbury, Brull and Critchley2007), we measured this potential in a region of interest (ROI) comprising four right-prefrontal electrodes (C14, C15, C16, C10). A baseline was defined at 200 ms before the R-peak and the epochs were selected between −0.2 and 0.5 s. We predicted greater amplitude of the HEP for internally than externally triggered cardiac stimuli, and for OCD patients in general.
MP
The MP waveform begins 150 to 100 ms prior to the onset of a self-paced movement, although it can peak 50 ms after the movement (Deecke et al. Reference Deecke, Eisinger and Kornhuber1980). Following standard recommendations for MP research (Johannes et al. Reference Johannes, Wieringa, Mantey, Nager, Rada, Muller-Vahl, Emrich, Dengler, Munte and Dietrich2001) and previous protocols (Caldara et al. Reference Caldara, Deiber, Andrey, Michel, Thut and Hauert2004), we selected six motor-related electrodes to construct a left-hemisphere ROI, considering that all subjects were right-handed (D11, D12, D13, D18, D19, D20). The MP can be a promising ERP for interoceptive performance, given that interoceptive processes are linked to insular-motor input of internal and external stimuli (Nagai et al. Reference Nagai, Kishi and Kato2007). Recent findings have shown cortical alterations in the insula and related structures in OCD (Song et al. Reference Song, Jung, Jang, Kim, Shim, Park, Choi and Kwon2011). Furthermore, ERP analysis comparing OCD and panic patients have shown similar inhibitory deficits in early potentials associated to motor response during a Go/NoGo task (Bannon et al. Reference Bannon, Gonsalvez, Croft and Boyce2002). Building on previous research (Caldara et al. Reference Caldara, Deiber, Andrey, Michel, Thut and Hauert2004), we analysed intra-group variance comparing right and wrong answers. We assumed that correct responses should be affected by higher effort or monitoring, while incorrect responses should not show such modulations. Specifically, we expected MP amplitude to increase for correct relative to incorrect responses, thus framing it as an indicator of precision and quickness of monitoring functions.
Data analysis
Demographic and clinical data were assessed through analyses of variance. Gender was analysed with the Pearson χ2 test. Considering the possible influence of depression and anxiety symptoms on interoception (Dunn et al. Reference Dunn, Dalgleish, Ogilvie and Lawrence2007; Domschke et al. Reference Domschke, Stevens, Pfleiderer and Gerlach2010) and the significant mood differences between groups (Table 1), we performed an analysis of covariance using HADS scores as covariates for the HBD measures (only significant results surviving covariance are reported). Effect sizes were calculated with partial ηp 2. Post-hoc analyses were performed with Tukey's honestly significant difference test. We also used multiple regression models (p < 0.05) to assess the relationship between attention to heartbeats (IAcc) and body signals (HEP) from the HBD task, on the one hand, and the dysfunctional beliefs of OCD patients (perfectionism, inflated responsibility, intolerance of uncertainly, need to control, and overestimation of threat), as measured by the OBQ-TRIP, on the other (see online Supplementary material section 4).
Point-by-point comparisons along the ERP were made via the Monte Carlo permutation test (Manly, Reference Manly2007), as done in previous HEP (Couto et al. Reference Couto, Adolfi, Velasquez, Mesow, Feinstein, Canales-Johnson, Mikulan, Martinez-Pernia, Bekinschtein, Sigman, Manes and Ibanez2015) and MP (Ibanez et al. Reference Ibanez, Cardona, Dos Santos, Blenkmann, Aravena, Roca, Hurtado, Nerguizian, Amoruso, Gomez-Arevalo, Chade, Dubrovsky, Gershanik, Kochen, Glenberg, Manes and Bekinschtein2013) studies. If the ensuing p values are smaller than the critical α-level of 0.05, then the data can be concluded to reveal significant differences. This method circumvents the multiple comparisons problem and does not depend on multiple comparison corrections or Gaussian distribution assumptions (Nichols & Holmes, Reference Nichols and Holmes2002). In addition, non-a priori windows were selected to analyse the ERPs. Importantly, by adopting a data-driven approach in our analyses, we avoided the bias of a priori window selection.
Results
Demographic results
No group differences were found in gender, age, formal education, handedness or body mass index (Table 1).
Mood and anxiety results
Data gleaned through the HADS-A revealed more anxiety symptoms in OCD and panic patients than in controls. No differences were found between the patient groups. Results from the HADS-D subscale showed that depression scores were highest in OCD patients, followed by panic patients, and then by controls. No between-group differences were found in the STAI-S (Table 1).
HBD task
IAcc
All groups performed similarly in the exteroceptive condition. However, significant differences emerged in the IAcc measure (Fig. 1 a and online Supplementary Table S5.1). Compared with controls and panic patients, OCD patients presented significantly lower values of TA, indicating higher IAcc (i.e. better synchronization of motor responses to heartbeats).
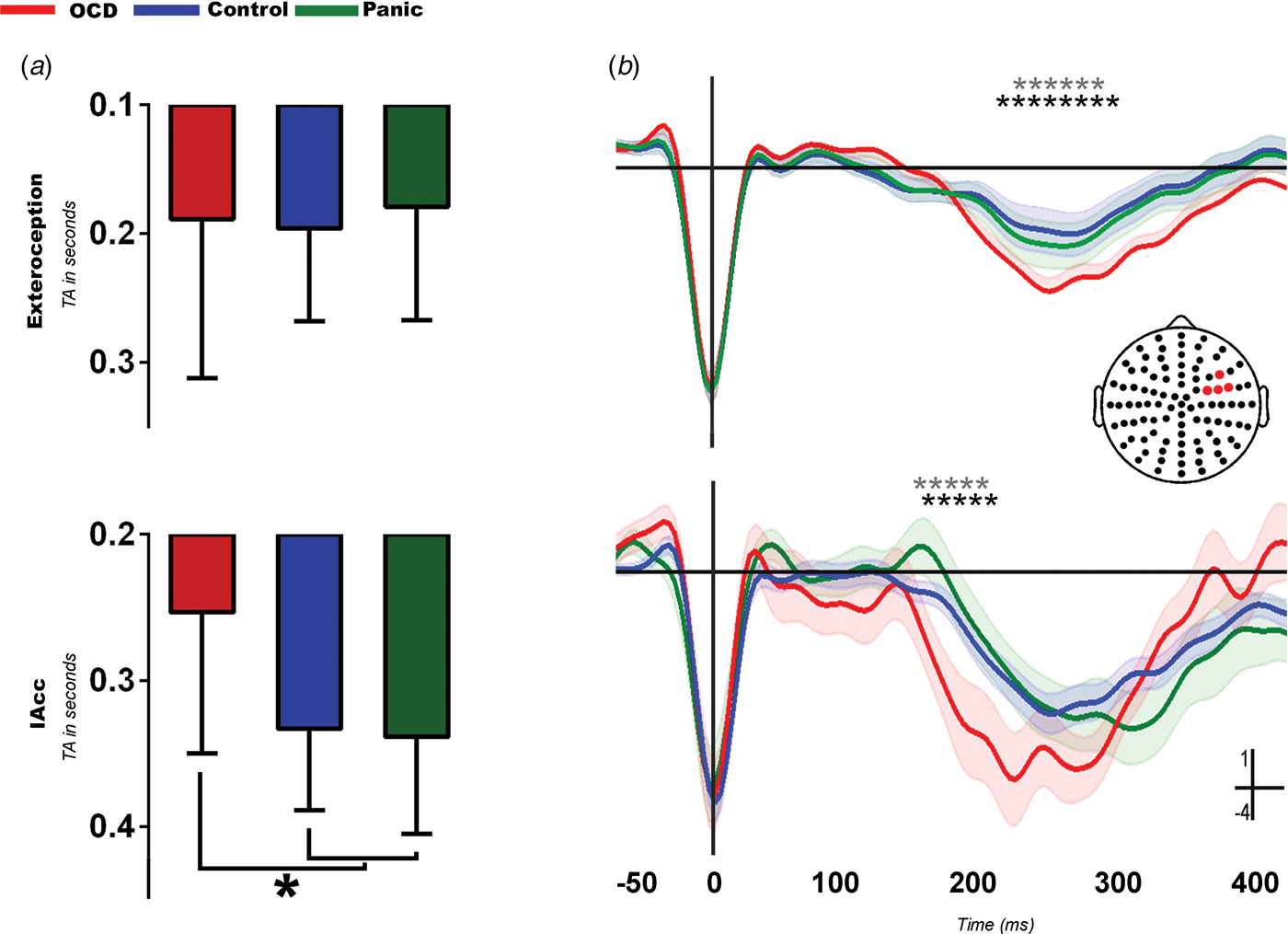
Fig. 1. Heartbeat detection task and associated heart evoked potential (HEP) modulations. (a) Behavioral results. Obsessive–compulsive disorder (OCD) obtained lower tapping accuracy (TA) scores, which indicates higher interoceptive accuracy (IAcc) than controls (p < 0.01) and panic patients (p < 0.01) (see online Supplementary Table S5.1 for details). The y axis indicates TA (in s). Values are means, with standard deviations represented by vertical bars. * Significant differences (p<0.05). (b) HEP results. More negative HEP modulations for OCD in the exteroceptive (top) and interoceptive (bottom) conditions. In the exteroceptive condition, OCD patients showed a significantly more negative HEP than controls [latency = 303 ms, t = 36.49, degrees of freedom (df) = 12.46, p < 0.01] and panic patients (latency = 262 ms, t = 29.77, df = 91.62, p < 0.01). The same was true of the interoceptive condition, where OCD patients also showed a larger HEP than controls (latency = 190 ms, t = 27.04, df = 87.63, p < 0.01) and panic patients (latency = 182 ms, t = 28.01, df = 11.4, p < 0.01). Shadowed bars around potentials indicate standard errors of the mean. * Significant difference between OCD and controls (p < 0.05). * Significant difference between OCD and panic (p < 0.05). No differences were found in either condition between controls and panic patients.
ISen
Analysis of ISen scores revealed significantly lower self-confidence in OCD patients relative to controls (p = 0.03) and panic patients (p = 0.04), who did not differ from each other (p = 0.1) (see online Supplementary Table S5.1).
IAw
Spearman correlations revealed a significant association between IAcc and ISen in controls (R = −0.46, p = 0.02) and panic patients (R = −0.55, p = 0.03). Given that IAcc is a reaction time score (TA), the negative direction of the relationship indicates a correspondence between high subjective confidence and high objective performance, and vice versa. No correspondence between objective performance and subjective confidence was found in OCD (R = 0.11, p = 0.67) (online Supplementary Table S5.1 and online Supplementary Fig. S5.2).
ERP
Our data-driven windows for the HEP (150–320 ms) and the MP (−50 to 50 ms) resemble the canonical ones for both components (Deecke et al. Reference Deecke, Bashore, Brunia, Grunewald-Zuberbier, Grunewald and Kristeva1984; Montoya et al. Reference Montoya, Schandry and Muller1993; Johannes et al. Reference Johannes, Wieringa, Mantey, Nager, Rada, Muller-Vahl, Emrich, Dengler, Munte and Dietrich2001; Leopold & Schandry, Reference Leopold and Schandry2001; Caldara et al. Reference Caldara, Deiber, Andrey, Michel, Thut and Hauert2004; Pollatos & Schandry, Reference Pollatos and Schandry2004; Aravena et al. Reference Aravena, Hurtado, Riveros, Cardona, Manes and Ibanez2010).
HEP
First, to test the influence of cardiac attention on the HEP (Montoya et al. Reference Montoya, Schandry and Muller1993; Pollatos et al. Reference Pollatos, Kirsch and Schandry2005), we compared its modulation during the interoceptive condition against an exteroceptive one and a 7-min resting-state condition across groups. Controls showed more negative HEP amplitude in the interoceptive relative to both the exteroceptive and the resting-state conditions. The same modulation was observed in OCD, but no differences emerged in the panic sample (see online Supplementary material section 6.1 and Supplementary Fig. S6.1). While the latter is an interesting finding, it falls outside the main focus of our study; thus, we address it in online Supplementary material section 7.
A between-group comparison revealed significantly larger HEP amplitudes in OCD patients than in both other samples across conditions (Fig. 1 b). As shown through a point-by-point comparison along the ERP via the permutation test, these differences emerged in the following expected windows (Leopold & Schandry, Reference Leopold and Schandry2001; Fukushima et al. Reference Fukushima, Terasawa and Umeda2011; Shao et al. Reference Shao, Shen, Wilder-Smith and Li2011): 200–300 ms for the exteroceptive condition, and 160–230 ms for the interoceptive condition. No differences were found between controls and panic patients. Based on other studies that reported HEP modulations after the 500-ms mark, we replicated our analysis including epochs from −50 to 800 ms, and found the same pattern of increased amplitude in OCD patients in later windows (see online Supplementary Fig. S6.2).
MP
In the exteroceptive condition, the MP was more negative for correct than incorrect trials across groups (Fig. 2 a). However, in line with previous research (Johannes et al. Reference Johannes, Wieringa, Mantey, Nager, Rada, Muller-Vahl, Emrich, Dengler, Munte and Dietrich2001; Ibanez et al. Reference Ibanez, Cardona, Dos Santos, Blenkmann, Aravena, Roca, Hurtado, Nerguizian, Amoruso, Gomez-Arevalo, Chade, Dubrovsky, Gershanik, Kochen, Glenberg, Manes and Bekinschtein2013), this modulation was more negative and wider (from −70 to 50 ms) in OCD patients. In panic patients, significant differences among categories emerged in a smaller window (from 40 to 70 ms). Between-group differences in these MP modulations are shown upon subtracting incorrect from correct trials (Fig. 2 b): more negative amplitudes were found in OCD patients relative to the other two groups, which showed similar MP amplitudes.

Fig. 2. Motor potential (MP) modulations. (a) Intra-group comparisons: MP modulations differed between correct and incorrect responses in both conditions for obsessive–compulsive disorder (OCD) patients [exteroceptive condition: latency = 0.05 ms, t = 55.45, degrees of freedom (df) = 19.90, p < 0.01; interoceptive condition: latency = −0.04 ms, t = 33.51, df = 10.63, p < 0.01]. Differences in the exteroceptive condition were also observed in controls (latency = 0.03 ms, t = 20.31, df = 33.41, p < 0.01) and panic patients (latency = 0.08 ms, t = 20.36, df = 17.14, p < 0.01). The interoceptive condition yielded no significant results in panic patients and significant differences in only one point of the event-related potential for controls (latency = 0.8 ms; t = 25.28, df = 32.68, p < 0.01). (b) Inter-group comparisons. The subtraction between correct and incorrect responses showed a more negative amplitude of the MP in both conditions for OCD patients compared with controls and panic patients (exteroceptive condition: OCD > controls: latency = 0.03 ms, t = 46.69, df = 19.12, p < 0.01; OCD > panic: latency = 0.01 ms, 50.28, df = 18.21, p < 0.01; interoceptive condition: OCD > controls: latency = 0.05 ms, t = 42.04, df = 20.41, p < 0.01; OCD > panic: latency = 0.05 ms, t = 46.41, df = 13.69, p < 0.01). Shadowed bars around potentials indicate standard errors of the mean. * Significant difference between OCD and controls (p < 0.05). * Significant difference between OCD and panic (p < 0.05). No differences were found between controls and panic patients.
Additional differences were observed in the interoceptive condition (Fig. 2 a). A larger negative amplitude for correct than incorrect trials was observed only in OCD patients. This pattern emerged in a window between −120 and −50 ms. The healthy control group showed a small difference (only one point of the ERP) in the same direction. No differences were found in the panic control sample. To gain further insights into these differential modulations, we compared the correct–incorrect difference wave between groups (Fig 2 b). The OCD sample exhibited a significantly larger modulation than the other two groups in the window from −80 to 20 ms. In sum, the difference in MP modulations between correct and incorrect responses was significantly higher in the OCD group than in the other two samples.
Interoception and dysfunctional beliefs in OCD
Using multiple regression models, we found that only the perfectionism subscale from the OBQ-TRIP was associated with the predictors mentioned in the Data analysis section (R 2 = 0.48, p < 0.01). More particularly, this association was significant relative to HEP modulations in the 300–400 ms window (β = −0.81, p < 0.01), but it was not significant relative to IAcc scores (β = 0.21, p = 0.40). No other significant associations were found (see online Supplementary Fig. S4 and Supplementary Table S4).
Discussion
By combining behavioral and electrophysiological measures, we evaluated the scope and distinctiveness of overactive monitoring in OCD. Patients with this condition showed increased IAcc and deficits in metacognitive abilities relative to controls and panic patients. Moreover, as compared with these two samples, they exhibited enhanced negativity of interoceptively relevant ERPs. This suggests the existence of condition-distinctive neural markers of altered inner monitoring in OCD. Below we discuss our key findings separately.
The scope of self-monitoring alterations in OCD
Previous behavioral studies failed to find increased monitoring in pediatric OCD samples (Hajcak et al. Reference Hajcak, Franklin, Foa and Simons2008; Hanna et al. Reference Hanna, Carrasco, Harbin, Nienhuis, LaRosa, Chen, Fitzgerald and Gehring2012) and healthy but obsessive adults (Kaczkurkin, Reference Kaczkurkin2013). However, the behavioral performance of OCD patients in our study was superior to that of controls, specifically during the interoceptive condition of the HBD task. Together with evidence of fewer errors in flanker tasks (Riesel et al. Reference Riesel, Endrass, Kaufmann and Kathmann2011, Reference Riesel, Kathmann and Endrass2014), our results add to the empirical corpus of enhanced monitoring accuracy in this population. Moreover, they highlight the sensitivity of cardiac monitoring tasks to address the issue.
Yet, as was the case in previous research (Endrass et al. Reference Endrass, Klawohn, Schuster and Kathmann2008; Riesel et al. Reference Riesel, Kathmann and Endrass2014), the most robust evidence of altered monitoring was obtained through electrophysiological measures. OCD patients consistently exhibited enhanced HEP and MP negativity during both external and internal monitoring. Although there are some discrepancies relative to the positive or negative deflection of the HEP, studies with parameters similar to our own have also reported negative deflections of this ERP (for a more detailed treatment of this, see online Supplementary material section 8). Thus, not only have we replicated this population's enhanced monitoring of environmental stimuli (Gehring et al. Reference Gehring, Himle and Nisenson2000; Riesel et al. Reference Riesel, Endrass, Kaufmann and Kathmann2011), but we have also shown that this pattern extends to inner monitoring skills.
In fact, interoceptive monitoring, as indexed by the MP, seems to be even more enhanced than its exteroceptive counterpart in OCD. Although MP modulations between correct and incorrect responses were observed in all groups, only OCD patients showed such an effect in the interoceptive condition. Moreover, their behavioral advantages were observed only when cardiac signals were triggered internally.
This pattern of inner overmonitoring may reflect biofunctional mechanisms associated with obsessive–compulsive cognition and symptoms. Indeed, inner monitoring (as indexed by HEP modulations) correlated with perfectionism ratings in OCD patients. Perfectionism is a core cognitive trait in OCD (Salkovskis, Reference Salkovskis1985; Coles et al. Reference Coles, Frost, Heimberg and Rheaume2003; Belloch et al. Reference Belloch, Morillo, Luciano, Garcia-Soriano, Cabedo and Carrio2010; Schrijvers et al. Reference Schrijvers, De Bruijn, Destoop, Hulstijn and Sabbe2010) and it has been linked to the ‘just right or not just right’ (NJR) sensation (Coles et al. Reference Coles, Frost, Heimberg and Rheaume2003; Starcevic et al. Reference Starcevic, Berle, Brakoulias, Sammut, Moses, Milicevic and Hannan2011). The latter has been described as a sensory or inner-drive feeling that disrupts homeostasis and prompts compulsive behaviors until things are ‘right or perfect’ (Coles et al. Reference Coles, Frost, Heimberg and Rheaume2003) or until they reach a sense of completeness (Salkovskis et al. Reference Salkovskis, Wroe, Gledhill, Morrison, Forrester, Richards, Reynolds and Thorpe2000). It has been suggested that this difficulty to stop can also explain the prolonged periods of mental rituals that patients with OCD perform to control their unwanted thoughts. Cognitive theories of obsessions state that patients with OCD struggle to decide whether they were successful at removing intrusive distressing thoughts (Salkovskis, Reference Salkovskis1985). This persistent doubt triggers either mental or behavioral rituals aimed at ensuring certainty that they do not hold any threatening thought (e.g. a patient who prays repeatedly to dispel blasphemous ideas). Thus, OCD patients with high levels of perfectionism might present an attentional bias to bodily signals, which might sustain or even increase this NJR sensation. Such a conjecture aligns with evidence of an association between this cognitive domain and body checking in OCD (Vartanian & Germeroth, Reference Vartanian and Germeroth2011). Hence, the alteration in the monitoring of bodily signals in OCD may represent an important mechanism affecting cognitive traits like perfectionism and associated symptoms such as checking behaviors, symmetry and ordering.
Although preliminary (due to the patients’ sample size and the lack of a division according to OCD subtypes), our results open a novel avenue to assess the relationship between inner monitoring and cognitive/behavioral hallmarks of OCD. For example, patients who show fear reduction and become less hypervigilant about their obsessions after response prevention (EX/RP) treatment could also show a profile of reduced inner monitoring that could be tapped via HEP analysis. Future studies might include HEP modulations as a dependent measure in clinical trials to further our understanding of brain changes after EX/RP treatment.
Metacognition of inner over-monitoring in OCD
The OCD patients were less confident about their actual performance than the other samples. This was true despite their objectively superior performance. Such a dissociation between objective performance and subjective confidence was further supported by IAw results. Indeed, the OCD patients were the only ones showing no correspondence between objective performance and subjective estimation of their outcome.
This finding aligns with previous reports showing metacognitive deficits in OCD (Cucchi et al. Reference Cucchi, Bottelli, Cavadini, Ricci, Conca, Ronchi and Smeraldi2012; Coles et al. Reference Coles, Schofield and Nota2015), which are acknowledged as core symptoms in psychopathological models of the disorder (Hermans et al. Reference Hermans, Engelen, Grouwels, Joos, Lemmens and Pieters2008). Compatibly, our results suggest that classical metacognitive alterations relative to external monitoring (Endrass et al. Reference Endrass, Klawohn, Schuster and Kathmann2008, Reference Endrass, Schuermann, Kaufmann, Spielberg, Kniesche and Kathmann2010) also extend to the monitoring of inner bodily signals. Unlike previous metacognition results showing no differences between OCD and other clinical samples (Janeck et al. Reference Janeck, Calamari, Riemann and Heffelfinger2003; Cucchi et al. Reference Cucchi, Bottelli, Cavadini, Ricci, Conca, Ronchi and Smeraldi2012; Brevers et al. Reference Brevers, Cleeremans, Bechara, Greisen, Kornreich, Verbanck and Noel2013; Odlaug et al. Reference Odlaug, Chamberlain, Derbyshire, Leppink and Grant2014), our findings suggest that OCD could be characterized by alterations in a specific metacognitive subskill: the ability to estimate one's own internal attentional abilities.
The ‘specificity’ of self-monitoring alterations in OCD
While the external overmonitoring pattern found in OCD has also been observed in patients with general anxiety (Stern et al. Reference Stern, Liu, Gehring, Lister, Yin, Zhang, Fitzgerald, Himle, Abelson and Taylor2010; Xiao et al. Reference Xiao, Wang, Zhang, Li, Tang, Wang, Fan and Fromson2011) and post-traumatic stress disorders (Rabinak et al. Reference Rabinak, Holman, Angstadt, Kennedy, Hajcak and Phan2013), research on the specificity of self-monitoring alterations in OCD has so far yielded inconclusive results (Gehring et al. Reference Gehring, Himle and Nisenson2000; Endrass et al. Reference Endrass, Klawohn, Schuster and Kathmann2008; Weinberg et al. Reference Weinberg, Olvet and Hajcak2010; Riesel et al. Reference Riesel, Endrass, Kaufmann and Kathmann2011, Reference Riesel, Kathmann and Endrass2014; Xiao et al. Reference Xiao, Wang, Zhang, Li, Tang, Wang, Fan and Fromson2011; Carrasco et al. Reference Carrasco, Harbin, Nienhuis, Fitzgerald, Gehring and Hanna2013; Moser et al. Reference Moser, Moran, Schroder, Donnellan and Yeung2013; Lin et al. Reference Lin, Moran, Schroder and Moser2015). The same is true of electrophysiological studies on processing external stimulus monitoring: whereas some studies have shown higher ERN and CRN amplitude in OCD patients compared with general anxiety disorder patients (Carrasco et al. Reference Carrasco, Harbin, Nienhuis, Fitzgerald, Gehring and Hanna2013), others yielded the opposite pattern (Xiao et al. Reference Xiao, Wang, Zhang, Li, Tang, Wang, Fan and Fromson2011).
However, no study has assessed inner monitoring in OCD via interoceptive tasks. Previous findings in other anxiety disorders are inconclusive, with patients performing either better than (Ehlers & Breuer, Reference Ehlers and Breuer1992), worse than (Willem Van der Does et al. Reference Willem Van der Does, Antony, Ehlers and Barsky2000) or similar to (Yoris et al. Reference Yoris, Esteves, Couto, Melloni, Kichic, Cetkovich, Favaloro, Moser, Manes, Ibanez and Sedeno2015) controls. Despite this controversy, these reports highlight that patients with anxiety disorders might not present an overmonitoring of bodily signals as we found in our OCD sample. Also, no study seems to have assessed HEP modulations in OCD solely, or in comparison with other pathological conditions. However, there are a few studies that have found reduced HEP modulation in other psychiatric conditions, such as depression (Terhaar et al. Reference Terhaar, Viola, Bar and Debener2012), borderline personality disorder (Muller et al. Reference Muller, Schulz, Andermann, Gabel, Gescher, Spohn, Herpertz and Bertsch2015) and depersonalization disorder (Schulz et al. Reference Schulz, Koster, Beutel, Schachinger, Vogele, Rost, Rauh and Michal2015). These findings suggest that inner over-monitoring alterations, as indexed by IAcc scores and HEP modulations, might represent a distinctive hallmark of OCD patients.
In sum, focusing on interoceptive monitoring, we found significant differences between OCD and panic disorder patients across behavioral and electrophysiological dimensions. Specifically, OCD patients exhibited: (i) more accurate performance in the interoceptive than the exteroceptive condition; (ii) more negative HEP and MP amplitude in both conditions; (iii) earlier modulations of these components in the interoceptive condition; and (iv) lower ISen and IAw scores. Thus, cross-dimensional aspects of internal monitoring seem distinctively altered in OCD.
Given that OCD shares co-morbid symptoms with other psychiatric disorders (e.g. 75.6% with any other anxiety disorder, 40.7% with major mood disorder, and 20% with panic; Ruscio et al. Reference Ruscio, Stein, Chiu and Kessler2010), the apparent specificity of interoceptive alterations across the anxiety spectrum may offer a new, valuable contribution for accurate diagnosis and longitudinal tracking of this condition. Thus, in line with evidence of differential neural markers of symptom groups in OCD (Mataix-Cols et al. Reference Mataix-Cols, Wooderson, Lawrence, Brammer, Speckens and Phillips2004), our results suggest that psychiatric assessment may be fruitfully complemented with behavioral and neurocognitive assessment of target domains. Moreover, by evincing a differential link between cognitive and bodily functions, they contribute to forging post-dualistic approaches to the study of psychiatric conditions (Zinbarg & Barlow, Reference Zinbarg and Barlow1996; Barlow & Campbell, Reference Barlow and Campbell2000).
Limitations and avenues for further research
Our sample size was modest. However, previous studies with similar numbers of participants also reported robust results (Gehring et al. Reference Gehring, Himle and Nisenson2000; Johannes et al. Reference Johannes, Wieringa, Mantey, Nager, Rada, Muller-Vahl, Emrich, Dengler, Munte and Dietrich2001; Endrass et al. Reference Endrass, Klawohn, Schuster and Kathmann2008; Stern et al. Reference Stern, Liu, Gehring, Lister, Yin, Zhang, Fitzgerald, Himle, Abelson and Taylor2010). Besides, we were unable to consider different subtypes of OCD. Yet, all our patients featured similar symptoms and each of them had OCI-R scores two standard deviations above those of the panic group, which speaks to the sample's broad homogeneity. That being said, since this disorder features high symptomatic variability (Foa et al. Reference Foa, Huppert, Leiberg, Langner, Kichic, Hajcak and Salkovskis2002), future research should favor a dimensional approach comparing self-monitoring patterns across subtypes of OCD. In addition, although the inclusion of panic patients represents an initial effort to evaluate the specificity of inner overmonitoring alterations in OCD, future studies should include samples featuring other anxiety disorders to more rigorously test this possibility.
Finally, different interoceptive accuracy tasks have been used in the literature (e.g. the discrimination task and the mental tracking task), each with its own advantages and disadvantages (Domschke et al. Reference Domschke, Stevens, Pfleiderer and Gerlach2010; Yoris et al. Reference Yoris, Esteves, Couto, Melloni, Kichic, Cetkovich, Favaloro, Moser, Manes, Ibanez and Sedeno2015). We selected an interoceptive task that requires motor tapping because external over-monitoring in OCD is generally tested with motor tasks (Endrass & Ullsperger, Reference Endrass and Ullsperger2014; Riesel et al. Reference Riesel, Kathmann and Endrass2014, Reference Riesel, Endrass, Auerbach and Kathmann2015). Importantly, even if one assumes that a motor confound exists in the results of the OCD group, behavioral differences between groups should have emerged across conditions rather than only in the interoceptive task. Also, in the latter, OCD patients exhibited greater IAcc than the other groups, which was coherent with HEP differences. Finally, previous studies have also shown that OCD performance in motor tasks varies widely, from good to bad (Gehring et al. Reference Gehring, Himle and Nisenson2000). Still, a replication of our study with non-motor interoceptive tasks could offer useful insights on the issue.
Conclusion
Our work offers unprecedented cross-dimensional evidence of altered interoceptive over-monitoring in OCD. Convergent behavioral and electrophysiological data showed that overactive monitoring in OCD extends to the sensing of internal bodily signals. Notably, this pattern discriminated OCD from controls and panic patients, suggesting a condition-distinctive alteration. These results highlight the potential of exploring interoceptive processes in the OCD spectrum to: (i) better characterize the population's cognitive profile; (ii) evaluate their sensitivity and specificity as a potential endophenotype (Riesel et al. Reference Riesel, Endrass, Auerbach and Kathmann2015); (iii) assess their relation with characteristic obsessive–compulsive cognitive profiles and symptoms; and (iv) evaluate their potential relevance to track disease progression, therapeutic impact and other forms of clinical response. Finally, our findings pave the way for new insights into the links between overmonitoring, cognition and body sensing. This is the first study to show that one of the most replicated hallmarks of OCD seems to extend to the body's inner world, opening new fields of research and assessment.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291717000368
Acknowledgements
This work was partially supported by grants from CONICET, CONICYT/FONDECYT Regular (1130920 and 1140114), FONCyT-PICT 2012-0412, FONCyT-PICT 2012-1309, FONDAP 15150012, and the INECO Foundation.
Declaration of Interest
None.





