Introduction
The ability to detect signs of impending danger is crucial for the survival of an organism. Most animals, including humans, have been afforded a hard-wired fear system involving phylogenetically old neural structures such as the amygdala, which is important for detecting environmental threat and rapidly encoding new stimulus–threat relationships. Indeed, a large body of work using fear conditioning, a widely used model of learning about danger, has shown striking similarities in the threat-related behavioural responses and their neural substrates across species (LeDoux, Reference LeDoux1996). Functional neuroimaging studies of fear conditioning or conditioning-related anticipatory anxiety paradigms in healthy human volunteers have largely confirmed the significant role of the amygdala in the learning, expression and extinction of fear and anxiety (Furmark et al. Reference Furmark, Fischer, Wik, Larsson and Fredrikson1997; Buchel et al. Reference Buchel, Dolan, Armony and Friston1999; Phelps et al. Reference Phelps, O'Connor, Gatenby, Gore, Grillon and Davis2001; Cheng et al. Reference Cheng, Knight, Smith, Stein and Helmstetter2003; Phelps et al. Reference Phelps, Delgado, Nearing and LeDoux2004; Knight et al. Reference Knight, Nguyen and Bandettini2005; Nitschke et al. Reference Nitschke, Sarinopoulos, Mackiewicz, Schaefer and Davidson2006).
In addition to detecting dangerous signals, however, an efficient defence system should be able to distinguish these stimuli from those indicating relative safety, and to quickly and efficiently adapt to changes in the reinforcement value of a particular stimulus or to the presence of conflicting information. For instance, such a system should allow for the, implicit or explicit, learning that something that was once feared is no longer a threat. A key structure thought to be involved in this process is the prefrontal cortex, specifically the infra-limbic cortex in rodents (Morgan et al. Reference Morgan, Romanski and LeDoux1993; Milad & Quirk, Reference Milad and Quirk2002; Quirk, Reference Quirk2002) and its probable homologue in humans, the anterior cingulate cortex (ACC), particularly its subgenual aspect (Milad et al. Reference Milad, Rauch, Pitman and Quirk2006). Based on numerous studies in experimental animals, it has been suggested that top-down inhibition of the amygdala by the prefrontal region is necessary for the extinction of the fear response, that is in acquiring and/or implementing the new learning that a defence response is no longer appropriate (Milad & Quirk, Reference Milad and Quirk2002; Quirk & Gehlert, Reference Quirk and Gehlert2003; Sotres-Bayon et al. Reference Sotres-Bayon, Bush and LeDoux2004). In general, the involvement of the ACC in the extinction of conditioned fear has received some support from functional (Gottfried & Dolan, Reference Gottfried and Dolan2004; Phelps et al. Reference Phelps, Delgado, Nearing and LeDoux2004) and structural (Milad et al. Reference Milad, Quinn, Pitman, Orr, Fischl and Rauch2005) neuroimaging studies in humans, although the precise nature of the relationship between the amygdala and ACC in the fear response is yet to be fully understood.
Fear conditioning is thought to capture the biologically adaptive response to the anticipation of threat. However, this response can become maladaptive, for instance when fear responses are exaggerated and continue to be elicited by stimuli that no longer signal threat, such as in the case of some anxiety disorders. Thus, fear conditioning has been proposed as a useful model to explore the physiological and neural correlates of the dysfunctional or hypersensitive fear responses observed in anxious individuals (Armony & LeDoux, Reference Armony and LeDoux1997; Brewin, Reference Brewin2001; Rauch et al. Reference Rauch, Shin and Phelps2006; Etkin & Wager, Reference Etkin and Wager2007). Specifically, in the context of conditioning, high anxiety has been proposed to be associated with a number of (potentially interacting) mechanisms including (i) impaired inhibitory conditioning to safety signals, (ii) greater excitatory conditioning to danger cues and (iii) enhanced conditionability (Mineka & Zinbarg, Reference Mineka and Zinbarg1996; Hermann et al. Reference Hermann, Ziegler, Birbaumer and Flor2002; Lissek et al. Reference Lissek, Powers, McClure, Phelps, Woldehawariat, Grillon and Pine2005). A hyper-responsive amygdala and/or a hypoactive ACC have been typically proposed as the neural underpinnings of these hypothesized behavioural mechanisms (Armony & LeDoux, Reference Armony and LeDoux1997; Quirk & Gehlert, Reference Quirk and Gehlert2003; Rauch et al. Reference Rauch, Shin and Phelps2006; Etkin & Wager, Reference Etkin and Wager2007).
Enhanced amygdala responses to fearful faces in anxious individuals, in particular under conditions of limited attention or awareness, have been observed in functional neuroimaging studies (Bishop et al. Reference Bishop, Duncan and Lawrence2004; Etkin et al. Reference Etkin, Klemenhagen, Dudman, Rogan, Hen, Kandel and Hirsch2004; Dickie & Armony, Reference Dickie and Armony2008; reviewed in Bishop, Reference Bishop2007), lending support to the hypothesized role of this structure in the aetiology of anxiety disorders. However, despite the importance of the fear conditioning paradigm as a model for studying anxiety-related behaviour, little is known about the potential influence of individual differences in trait anxiety (TA) on amygdala and prefrontal activity during the acquisition and extinction of conditioned fear (Bishop, Reference Bishop2007).
We therefore conducted a functional magnetic resonance imaging (fMRI) study to examine the influence of anxiety vulnerability, indexed by Spielberger's TA scores (Spielberger, Reference Spielberger1983), on amygdala and subgenual ACC activity during the acquisition and extinction of contextual fear conditioning. We chose a contextual conditioning paradigm, in which the conditioned stimulus (CS) is longer lasting and less predictive of the unconditioned stimulus (US) than in the traditional cue-conditioning design because it has been suggested that the sustained anxious apprehension often observed during context conditioning with unpredictable US presentation is a more accurate model of anxiety states than explicitly cued fear conditioning (Grillon, Reference Grillon2002). Importantly, our paradigm was previously shown to be able to be sensitive to individual differences in behavioural conditioned responses as a function of TA (Barrett & Armony, Reference Barrett and Armony2006).
Method
Participants
Participants were recruited through advertisements posted at McGill University, directing interested individuals to a website, where they completed the State-Trait Anxiety Inventory – Trait Version (STAI-T; Spielberger, Reference Spielberger1983) and provided demographic information. Twenty-four healthy volunteers (11 male and 13 female) participated in the study. All participants were right-handed according to 12 representative items from the Edinburgh Handedness Inventory (Oldfield, Reference Oldfield1971). Subjects reported no past or current neurological or psychiatric disorder (including substance abuse) and were not taking any psychotropic medications. All subjects had normal hearing and normal or corrected-to-normal vision and none were colour-blind. Informed written consent was obtained from all participants according to the institutional guidelines established by the Ethics Committee of the Montreal Neurological Hospital and Institute. Participants received Can$50 as compensation for their time and inconvenience.
Data from six participants (three female) were excluded from the analyses because of problems with the auditory system (two), significant image artefacts (one), poor performance on the decision-making task (two) or self-report of being in a ‘trance-like’ state during the fMRI session (one). Thus, the total number of participants included in the final analysis was 18 (10 female, mean age 23.1±4.9 years).
Questionnaires
Visual analogue scales measuring valence and arousal (scale 0–100) were administered immediately before and after the fMRI session; participants were instructed to rate how they felt during the Acquisition and Extinction sessions. Following the fMRI procedure participants also completed the STAI-T, the Penn State Worry Questionnaire (PSWQ; Stober & Bittencourt, Reference Stober and Bittencourt1998) and the Intolerance of Uncertainty Scale (IUS; Buhr & Dugas, Reference Buhr and Dugas2002). In addition, participants completed a debriefing questionnaire that asked questions regarding task difficulty, task pace, background colours and noise, as well as general impressions.
Experimental paradigm
The task used, shown in Fig. 1, was similar to one conducted previously in a behavioural study (Barrett & Armony, Reference Barrett and Armony2006). Participants were told that they would complete a computerized decision-making task during which trials would be grouped into two alternating background screen colour blocks (i.e. the background colour of computer screen alternated between green and purple). One of the colour blocks would be occasionally accompanied by a loud noise-burst presented through headphones; participants were told of the colour–noise relationship at the start of the study (half of the subjects had green paired with noise and the other half purple). For the decision-making task, participants had to decide which of two letters were of larger size by pressing either the left or right button of an MR-compatible mouse.
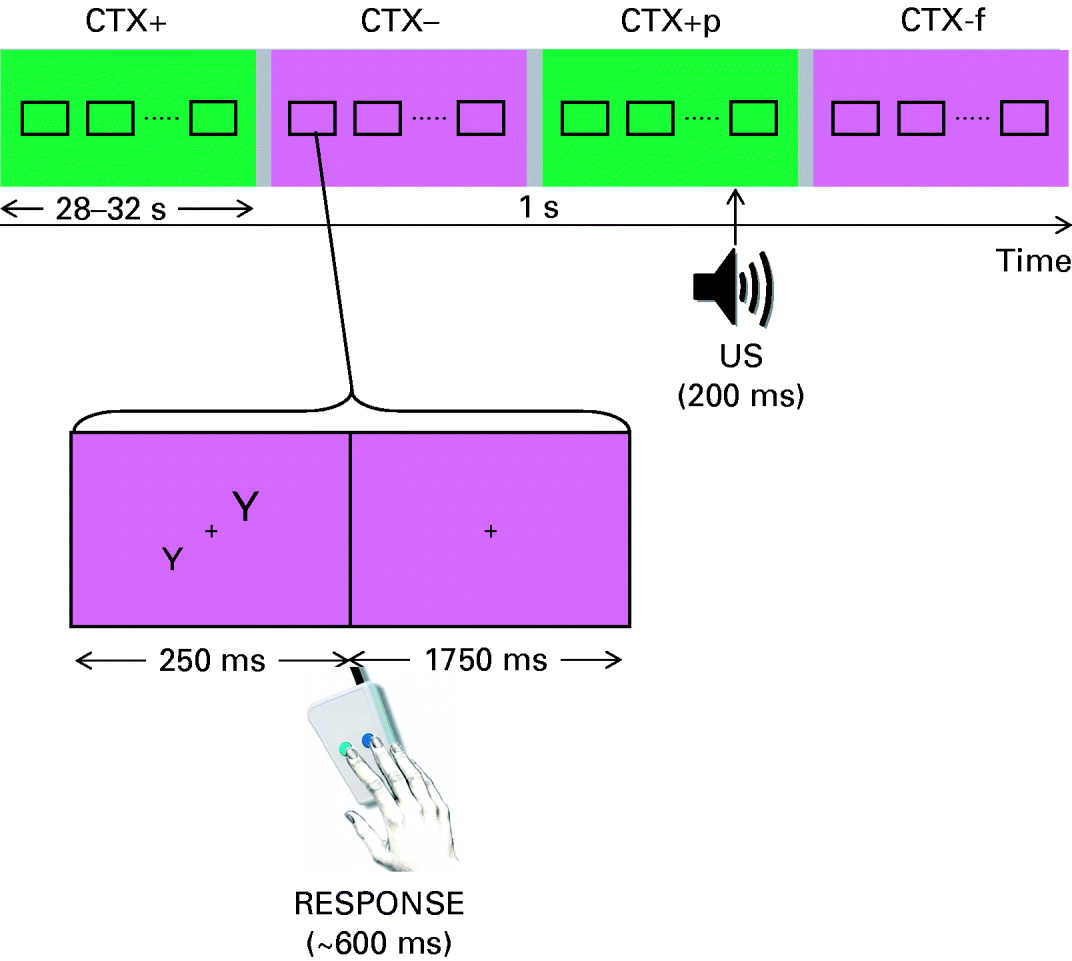
Fig. 1. Schematic of the experimental design. Participants were asked to decide which of the two letters, presented simultaneously for 250 ms, was larger by pressing a button. The background colour (irrelevant to the decision-making task) alternated between two colour blocks (average duration 27 s) corresponding to the CTX+ condition [associated with the probability of delivery of an unconditioned stimulus (US)] and the CTX− condition (no US). The actual colours used were counterbalanced across participants. The time of US within each CTX+ block varied from trial to trial.
Participants completed two sessions: Acquisition and Extinction. In the Acquisition session, there were two different colour-block, or context, conditions: noise expected (CTX+, 15 colour blocks) and noise not expected (CTX−, 15 colour blocks). During five CTX+ colour blocks, a single noise (different in each case) was presented. In addition, a CTX− colour block always followed a CTX+ colour block containing noise. As we were not interested in the immediate or lasting effect of the actual noise, data collected during the CTX+ blocks paired with noise as well as the CTX− block that immediately followed were excluded from the behavioural and fMRI analyses of interest. For CTX+ and CTX− conditions, letter-pair trials were combined into 15 blocks. Block length was jittered between 14 and 16 trials (i.e. 28–32 s) to reduce the predictability of block changes. Blocks for all condition types were pseudo-randomly presented across subjects to ensure that (i) 3/5 CTX+ paired blocks were presented in the first half of the experiment and (ii) no more than two CTX+ or CTX− colour blocks could be presented in succession. A 1-s grey screen and white fixation cross marked the change between blocks (i.e. inter-stimulus interval or ISI).
The task procedure of the Extinction session was identical to the Acquisition session except that no noise was ever presented and only 10 CTX+ colour blocks and 10 CTX− colour blocks were presented. For the analysis of behavioural responses in each session, median response time and mean accuracy (percentage correct) values were determined for all trials in the CTX+ and CTX− conditions.
Unconditioned stimulus (US)
A loud, unpleasant noise was used as the US (Buchel et al. Reference Buchel, Dolan, Armony and Friston1999; Armony & Dolan, Reference Armony and Dolan2001; Barrett & Armony, Reference Barrett and Armony2006). In the Acquisition session, a different noise was presented during each CTX+ colour block paired with noise, 1000 ms after presentation of one letter-pair and at variable points throughout the blocks. Five different 200-ms noises were created using CoolEdit Pro (Syntrillium Software Corp., Phoenix, AZ, USA) and comprised of high-frequency (⩾0.5 kHz) frequency-modulated tones. The mean sound pressure level of the noises was 96 dBA (s.d.=2) and the mean maximum sound pressure level was 102.8 dBA (s.d.=0.8). Participants listened to the noise with the highest mean and maximum sound pressure level prior to the start of the experiment; no one described the noise as painful or unbearable.
fMRI procedure
Brain imaging was conducted at the Montreal Neurological Institute (MNI) with a 1.5-T Siemens Sonata whole-body scanner equipped with a standard head coil and using gradient EPI sequences. Auditory stimuli were presented through pneumatic tubes and customized MR-compatible headphones connected to a laptop computer through an amplifier. A vacuum-cushion placed around the headphones was used to stabilize the subject's head. Visual stimuli were generated using E-Prime (Psychology Software Tools, Pittsburgh, PA, USA) and projected using an LCD projector and mirror system. A two-button response pad connected to the computer recorded subject responses. Functional T2*-weighted images were acquired with blood oxygenation level-dependent (BOLD) contrast (Acquisition: 380 volumes, Extinction: 260 volumes, TR=2540 ms, TE=50 ms, Flip angle=90°, FOV=256 mm, Matrix=64×64), covering the entire brain (30 interleaved slices parallel to the anterior–posterior commissural plane, in plane resolution 4×4 mm2, 4 mm thickness). Between the Acquisition and Extinction sessions, a high-resolution T1-weighted anatomical volume was acquired using a gradient echo pulse sequence (TR=22 ms, TE=9.2 ms, Flip angle=30°, voxel size 1×1×1 mm3), lasting approximately 15 min.
fMRI data analysis
Functional MRI data were analysed using Statistical Parametric Mapping software (SPM2, Wellcome Department of Cognitive Neurology, London, UK). Images were pre-processed according to standard procedures (Ashburner & Friston, Reference Ashburner and Friston1997). In brief, images were time-corrected to account for differences in sampling times for different slices, realigned to the first volume to correct for inter-scan movement, spatially normalized (final voxel size 2×2×2 mm3) to the standard space of Talairach and Tournoux (Talairach & Tournoux, Reference Talairach and Tournoux1988) using the MNI template (Collins et al. Reference Collins, Neelin, Peters and Evans1994) and smoothed with an isotropic 8-mm full width at half maximum (FWHM) Gaussian kernel. Low-frequency temporal drifts were removed by applying a high-pass filter (cut-off 128 s). Data were analysed using the general linear model, in which individual events were modelled by a finite impulse response function (nine 3-s bins). In the Acquisition session, five event types were defined: (i) CTX+, (ii) CTX−, (iii) CTX+ containing noise, (iv) CTX− following noise, and (v) Noise. In the Extinction session, two event types were defined: (i) CTX+ and (ii) CTX−. The six covariates corresponding to the movement parameters obtained from the realignment procedure were also included in the model. Regions of interest (ROIs) for the amygdala and subgenual ACC (sgACC) (Brodmann area 25) were defined using masks created by WFU PickAtlas (Maldjian et al. Reference Maldjian, Laurienti, Kraft and Burdette2003). Skin conductance results from our previous study using the same paradigm showed a differential effect of conditioning on the first and second halves of the CTX blocks (Barrett & Armony, Reference Barrett and Armony2006). We therefore focused our current fMRI analysis on the early (i.e. the first four 3-s bins) and late (last four bins) parts of each colour block. We constructed two contrasts for each session, (CTX+ – CTX−)EARLY and (CTX+ – CTX−)LATE, and parameters estimates for these contrasts, for each subject, were taken to second-level, random-effects analyses. Within each ROI, voxel-specific results were corrected for multiple comparisons [family-wise error (FWE), p<0.05] (Worsley et al. Reference Worsley, Marrett, Neelin, Vandal, Friston and Evans1996). To examine the effect of anxiety vulnerability on the conditioned responses in the amygdala and ACC, parameter estimates from significant voxels were correlated with STAI-T scores. Importantly, TA scores were entered in the analyses as a parametric variable rather than a categorical one based on median split as there is a growing body of literature suggesting that the former approach is preferable because of increased power and fewer potential statistical artefacts (Bissonnette et al. Reference Bissonnette, Ickes, Bernstein and Knowles1990; Maxwell & Delaney, Reference Maxwell and Delaney1993; MacCallum et al. Reference MacCallum, Zhang, Preacher and Rucker2002).
Results
Behavioural results
Anxiety-related questionnaires
The mean TA score (post-test) was 38.2 (s.d.=9.5, range 21–54), similar to the values reported previously for this age group (Kendall & Sheldrick, Reference Kendall and Sheldrick2000). The participants reported a mean PSWQ score of 45.7 (s.d.=15.5, range 15–69) and an average value of 56.2 (s.d.=15.6, range 28–91) in the IUS, consistent with previous studies with this population (Meyer et al. Reference Meyer, Miller, Metzger and Borkovec1990; Buhr & Dugas, Reference Buhr and Dugas2002). As expected (Meyer et al. Reference Meyer, Miller, Metzger and Borkovec1990; Buhr & Dugas, Reference Buhr and Dugas2002), the three measures were significantly correlated (0.62<r<0.67, all p<0.01). Female participants reported higher average values than men on the three scales but the differences did not reach statistical significance, although there was a trend (p=0.09) for the PSWQ.
Visual analogue scales
A repeated-measures ANOVA with session (pre-fMRI, Acquisition, Extinction) as a within-subject factor yielded a main effect of session for both arousal [F(2, 30)=5.1, p=0.02] and pleasantness [F(2, 30)=13.1, p=0.001]. Post-hoc tests revealed that pleasantness ratings significantly decreased between pre-scanning (mean=74, s.d.=18) and the Acquisition phase (mean=51, s.d.=21) and remained at a similar level throughout the Extinction session (mean=52, s.d.=26). Arousal ratings increased significantly after Acquisition (before: mean=40, s.d.=15; after: mean=52, s.d.=19) but returned to the pre-scanning level during Extinction (mean=39, s.d.=20). High-anxious individuals reported a larger increase in arousal as a result of conditioning than low-anxiety participants, as evidenced by a marginally significant [F(2, 28)=3.2, p=0.06] interaction between session and STAI-T.
Debriefing questionnaire
Means for ratings of task difficulty (1=extremely easy, 7=extremely difficult), task pace (1=extremely slow, 7=extremely fast) and pleasantness of the noise (1=extremely pleasant, 7=extremely unpleasant) were 4.28 (s.d.=1.49), 5.22 (s.d.=0.88) and 5 (s.d.=0.67) respectively. There were no sex differences or interactions with anxiety scores.
For the CTX+ condition, mean noise expectancy (never, 50%, 75%, 100%) was 47.5% (s.d.=23.4) for Acquisition and 42.5% (s.d.=27.8) for Extinction. For the CTX− condition, mean noise expectancy was 1.1 (s.d.=4.7) for Acquisition and 2.8 (s.d.=11.8) for Extinction. An ANOVA with Condition (CTX+, CTX−) and Session (Acquisition, Extinction) as within-subject factors revealed a main effect of condition only [CTX+: mean=45, s.d.=25; CTX−: mean=1.9, s.d.=8.9; F(1, 16)=52.15, p<0.05], with subjects expecting noise significantly more often in the CTX+ condition compared to the CTX− condition. Of note, for both Acquisition and Extinction, only one subject reported that they expected to hear noise more than ‘never’ in the CTX− condition. Finally, mean subject ratings of how much they liked the background screen colours were 3.6 (s.d.=1.2) for the CTX+ condition and 3.3 (s.d.=1.2) for the CTX− condition.
Decision-making task
A repeated-measures ANOVA for response times in the letter discrimination task revealed a main effect of Session [Acquisition: mean=640.0, s.d.=105.7; Extinction: mean=580.2, s.d.=71.1; F(1, 14)=20.9, p<0.001], with subjects displaying faster response times during Extinction compared to Acquisition, and a Session by Condition interaction [Acquisition, CTX+: mean=634.5, s.d.=112.0, CTX−: mean=645.6, s.d.=102.0; Extinction, CTX+: mean=581.6, s.d.=71.4, CTX−: mean=578.9, s.d.=73.0; F(1, 16)=5.37, p<0.05]. To examine the interaction further, separate ANOVAs were conducted for each Session with Condition (CTX+, CTX−) as a within-subject factor. The results revealed that, during Acquisition, participants responded significantly faster in the CTX+ condition compared to the CTX− condition [F(1, 17)=4.37, p=0.05]. No significant differences were found for response times during Extinction (all F<1).
No interactions between response times and STAI, PSWQ or IUS scores were observed during acquisition. By contrast, a significant interaction between condition and IUS was present during the Extinction session [F(1, 14)=5.7, p<0.005), indicating a positive correlation between the difference in response time between CTX− and CTX+ and IUS scores (r=0.51).
There were no significant main effects or interactions (all F<1) for response accuracy during either Acquisition or Extinction.
Functional MRI results
Acquisition session
A significant increase in BOLD signal during the late phase (last 12 s) of the CTX+ block compared to CTX− was observed in the left amygdala [(−30, −2, −18), p<0.05 corrected; z=2.8; see Fig. 2]. This activity did not correlate with STAI-T scores (r=−0.12, p=0.3). No other amygdala cluster exhibited increased responses to the CTX+ in either the first or last part of the block, even at a lower significance threshold (p<0.05 uncorrected). No significant activations in the contrast CTX+ minus CTX− or in the regression with STAI-T scores were observed within the ROI corresponding to the sgACC.

Fig. 2. (a) Three-dimensional renderings of the left (green) and right (red) amygdala activation associated with the contrast CTX+ minus CTX− during the Acquisition and Extinction phases respectively. The anatomically defined region of interest (ROI) is depicted in grey. The map is thresholded at p<0.05 for visualization. (b) Time-course of the blood oxygenation level-dependent (BOLD) signal during the presentation of CTX+ (relative to CTX−) showing the significant late (15–27 s) activation during Acquisition in the left amygdala and the early (0–12 s) response during Extinction for the right amygdala. (c) Correlations between BOLD signal and individuals' trait anxiety (TA) scores on the State-Trait Anxiety Inventory – Trait Version (STAI-T).
Extinction session
The contrast CTX+ minus CTX− revealed significant right amygdala activation in the early period [(18, 4, −16), p<0.05 corrected, z=3.36], as shown in Fig. 2. A trend was also observed for an amygdala activation in the last 12 s of the CTX+ block [(30, 2, −18), p=0.09 corrected, z=2.62]. A regression analysis revealed that only the early conditioned response in the amygdala correlated significantly with STAI-T scores (r=0.45, p=0.03, see Fig. 2), such that higher TA was associated with greater conditioned responses in the amygdala. No significant correlations were found for the late conditioned response.
Significant activations within the sgACC [(12, 32, −18); (8, 18, −10), p<0.05 FWE, z=3.22, z=3.02 respectively; see Fig. 3] were associated with the early component of the CTX+ block, compared to the safe context. These responses were not significantly correlated with STAI-T scores (r=−0.01, p=0.48 and r=0.06, p=0.41 respectively).

Fig. 3. (a) Three-dimensional reconstruction of the activation within the subgenual anterior cingulate cortex (sgACC) during Extinction for the contrast CTX+ minus CTX−. (b) Time-course of the blood oxygenation level-dependent (BOLD) signal during the presentation of CTX+ (relative to CTX−) for the two peak voxels, showing the significant early (0–12 s) response.
Extinction versus Acquisition sessions
To isolate the specific contribution of the ACC to the extinction process, we conducted an interaction analysis with Condition (CTX+ minus CTX−) and Session (Acquisition minus Extinction) as within-subject factors. This analysis revealed a significant bilateral activation with the sgACC [(4, 28, −20), z=3.63; (−2, 28, −20), z=3.14, both p<0.05 FWE]. Of interest, the difference in BOLD signal between sessions in these voxels correlated significantly with individuals' TA scores, as shown in Fig. 4 (right: r=0.60, left: r=0.52).

Fig. 4. (a) Statistical parametric map showing the significant left and right subgenual anterior cingulate cortex (sgACC) activation for the interaction between condition (CTX+ minus CTX−) and session (Extinction minus Acquisition). (b) Mean effect sizes for the early (0–12 s) and late (15–27 s) periods during the Acquisition (![]() ) and Extinction (□) sessions. (c) Correlations between blood oxygenation level-dependent (BOLD) signal and individuals' trait anxiety (TA) scores on the State-Trait Anxiety Inventory – Trait Version (STAI-T).
) and Extinction (□) sessions. (c) Correlations between blood oxygenation level-dependent (BOLD) signal and individuals' trait anxiety (TA) scores on the State-Trait Anxiety Inventory – Trait Version (STAI-T).
Discussion
In this study we examined how individual differences in the expression of anxiety-like traits influence the neural responses associated with the acquisition and extinction of anticipatory anxiety elicited through a context conditioning paradigm, with particular focus on the amygdala and the sgACC. During the Acquisition session, 30% of the CTX+ blocks were paired with an aversive noise, whereas during the Extinction session, unbeknown to the participants, no US was presented. Post-experiment debriefing indicated that, regardless of the level of TA, all participants found the US (noise) unpleasant and expected it to be presented in almost half of the CTX+ blocks in both sessions.
Amygdala
As expected, significant amygdala activation was observed during the presentation of the CTX+ (associated with the probability of a US delivery), compared to CTX− (safe context), consistent with previous functional neuroimaging studies (e.g. LaBar et al. Reference LaBar, Gatenby, Gore, LeDoux and Phelps1998; Buchel et al. Reference Buchel, Dolan, Armony and Friston1999; Armony & Dolan, Reference Armony and Dolan2001; Phelps et al. Reference Phelps, Delgado, Nearing and LeDoux2004; Knight et al. Reference Knight, Nguyen and Bandettini2005). Some of the characteristics of this amygdala activation are noteworthy. First, the activation was significant during the second half of the context block only (i.e. the last 12 s). This type of response is different from the conditioned response typically observed after successful conditioning, where the presence of a CS immediately elicits the conditioned response (Lavond & Steinmetz, Reference Lavond and Steinmetz2003). Instead, this late response could represent the learning of the CTX−US contingency taking place during the acquisition phase. Indeed, given that the exact time of the presentation of the US during the CTX+ was unpredictable, and that there was in fact no US presentation during the CTX+ blocks included in the analysis, the expected probability of US delivery, and the corresponding anticipatory response, should have increased with time, becoming stronger (and hence statistically significant) in the second half of the context block. Second, there was no correlation between the response in the amygdala to the aversive context and TA. This finding suggests that anxiety vulnerability does not directly influence amygdala activity associated with the acquisition of conditioned fear responses.
During the Extinction session, presentation of the CTX+ was also associated with increases in BOLD signal in the amygdala, consistent with previous neuroimaging studies (LaBar et al. Reference LaBar, Gatenby, Gore, LeDoux and Phelps1998; Gottfried & Dolan, Reference Gottfried and Dolan2004; Phelps et al. Reference Phelps, Delgado, Nearing and LeDoux2004; Kalisch et al. Reference Kalisch, Korenfeld, Stephan, Weiskopf, Seymour and Dolan2006; Milad et al. Reference Milad, Wright, Orr, Pitman, Quirk and Rauch2007). Of note, these signal increases were observed during the early phase of the CTX+ (the first 12 s of the block). These early responses are consistent with single-unit recordings in experimental animals (Quirk et al. Reference Quirk, Armony and LeDoux1997) and support the hypothesis that, following successful conditioning, the CTX+ itself acquires aversive properties and thus it becomes capable of triggering the neural circuit associated with the fear system, especially when the precise time of expected US presentation, during the CTX+, cannot be predicted.
In contrast to what we observed during the acquisition phase, the magnitude of the conditioned amygdala response during extinction was significantly modulated by individual differences in anxiety vulnerability. Specifically, higher levels of TA were associated with a greater amygdala response in blocks in which an aversive stimulus was anticipated, compared to safe trials. Thus, although individual differences in TA may not influence the learning of anticipatory anxiety (i.e. acquisition), they do seem to play a role in the magnitude of these responses in the absence of reinforcement during extinction.
Subgenual anterior cingulate cortex (sgACC)
The observed greater involvement of the sgACC during the Extinction than the Acquisition session, confirmed both by simple main effects for each phase and by an interaction analysis, is consistent with previous neuroimaging (Gottfried & Dolan, Reference Gottfried and Dolan2004; Phelps et al. Reference Phelps, Delgado, Nearing and LeDoux2004; Kalisch et al. Reference Kalisch, Korenfeld, Stephan, Weiskopf, Seymour and Dolan2006; Milad et al. Reference Milad, Wright, Orr, Pitman, Quirk and Rauch2007) and experimental animal (Morgan et al. Reference Morgan, Romanski and LeDoux1993; Milad & Quirk, Reference Milad and Quirk2002; Quirk, Reference Quirk2002) studies and supports the hypothesis that this region is preferentially engaged during the extinction and/or inhibition of previously acquired aversive responses.
It is noteworthy that the magnitude of the differential involvement of the sgACC during the extinction session was modulated by individual differences in anxiety vulnerability, such that higher levels of TA were associated with a greater ACC conditioned response during the Extinction session (compared to the Acquisition session). This result is somewhat counterintuitive, as it is typically reported that anxiety disorders, especially post-traumatic stress disorder (PTSD), are associated with a decreased response in the ACC (Etkin & Wager, Reference Etkin and Wager2007), resulting in inadequate inhibition of the amygdala, which in turn leads to exaggerated fear responses (Quirk & Gehlert, Reference Quirk and Gehlert2003). Nonetheless, a number of studies have also reported concurrent increased activity within the ACC and amygdala in PTSD (Rauch et al. Reference Rauch, van der Kolk, Fisler, Alpert, Orr, Savage, Fischman, Jenike and Pitman1996; Liberzon et al. Reference Liberzon, Taylor, Amdur, Jung, Chamberlain, Minoshima, Koeppe and Fig1999; Bryant et al. Reference Bryant, Felmingham, Kemp, Das, Hughes, Peduto and Williams2008). Furthermore, connectivity analyses have shown that these two regions appear to be more positively correlated in individuals with PTSD than in trauma-exposed controls (Gilboa et al. Reference Gilboa, Shalev, Laor, Lester, Louzoun, Chisin and Bonne2004). In line with these results, one possible interpretation of our sgACC finding is that it reflects a compensatory mechanism whereby, in high-anxious individuals, this structure is more actively engaged to inhibit the hyper-responsive amygdala.
Implications for anxiety disorders
Several behavioural and physiological studies have shown that individuals suffering from anxiety disorders, including PTSD (Grillon & Morgan, Reference Grillon and Morgan1999; Orr et al. Reference Orr, Metzger, Lasko, Macklin, Peri and Pitman2000; Peri et al. Reference Peri, Ben-Shakhar, Orr and Shalev2000; Blechert et al. Reference Blechert, Michael, Vriends, Margraf and Wilhelm2007; Wessa & Flor, Reference Wessa and Flor2007), social phobia (Hermann et al. Reference Hermann, Ziegler, Birbaumer and Flor2002), anxiety neurosis (Pitman & Orr, Reference Pitman and Orr1986) and panic disorder (Michael et al. Reference Michael, Blechert, Vriends, Margraf and Wilhelm2007), exhibit a reduced extinction, compared to control subjects, to previously conditioned aversive stimuli. In most cases this resistance to extinction in the anxious group occurred in the absence of significant group differences during acquisition, although conflicting findings have been reported (for a review, see Lissek et al. Reference Lissek, Powers, McClure, Phelps, Woldehawariat, Grillon and Pine2005). Thus, our results could provide a neural interpretation of these findings, namely that high anxiety is associated with a hyper-responsive amygdala during the extinction processes, possibly resulting in longer-lasting, resilient anxiety responses to stimuli that no longer signal threat. This resistance-to-extinction hypothesis is consistent with the hypothesis that PTSD can be thought of as a failure to recover from the normative effects of traumatic stress. Indeed, epidemiological studies of trauma-exposed individuals have demonstrated that the majority of these individuals experience PTSD symptoms soon after the traumatic event (Rothbaum et al. Reference Rothbaum, Foa, Riggs, Murdock and Walsh1992; Brewin et al. Reference Brewin, Andrews and Rose2000), but that, in most cases, these symptoms disappear with time (Kessler et al. Reference Kessler, Sonnega, Bromet, Hughes and Nelson1995; Breslau et al. Reference Breslau, Kessler, Chilcoat, Schultz, Davis and Andreski1998). In some individuals, however, symptoms including exaggerated fear responses to trauma-reminders persist for months or years, suggesting an impaired ability to learn that these stimuli no longer signal the presence of threat. It has been suggested that this impaired learning is mediated by a functional deficit in the amygdala–prefrontal circuit (Armony & LeDoux, Reference Armony and LeDoux1997; Brewin, Reference Brewin2001; Rauch et al. Reference Rauch, Shin and Phelps2006).
Limitations
Our study has a number of limitations that need to be considered when interpreting the findings presented here. The relatively few subjects tested may limit the generalizability of our results, as other individual differences besides TA may have existed in our sample. In particular, we were not able to examine possible interactions between sex and TA on brain activity (Dickie & Armony, Reference Dickie and Armony2008). Furthermore, we did not have a concurrent physiological measure (e.g. skin conductance) during scanning to obtain an independent index of acquisition and extinction of conditioned responses. Without such a measure, we were unable to determine the time-course of conditioning and extinction for inclusion in the fMRI analysis, or to unequivocally determine whether the observed sgACC activation reflected recall of extinction or late acquisition responses to the CTX+.
Conclusion
Overall, our results support the idea that individuals with high levels of anxiety-relevant traits and vulnerable to developing an anxiety disorder display a more resilient anxiety response during extinction that is characterized by hyper-responsivity in the amygdala. Thus, anxiety-related personality characteristics may account for some of the inter-subject variability observed in conditioning experiments. Further studies focusing on the extinction of conditioned responses in individuals suffering from anxiety disorders as well as those vulnerable to developing them should help to elucidate the nature of the interaction of the ACC and the amygdala in the development and aetiology of anxiety disorders.
Acknowledgements
We thank the staff at the Brain Imaging Centre at the Montreal Neurological Institute for their help and Karine Sergerie for her comments on this manuscript. This work was supported by grants from the Canadian Institutes for Health Research and the Natural Sciences and Engineering Research Council of Canada. J. L. A. was supported by the Canada Research Chairs Program.
Declaration of Interest
None.






