Introduction
Borderline personality disorder (BPD) is a mental disorder that affects about 6% of the population (Grant et al. Reference Grant, Chou, Goldstein, Huang, Stinson, Saha, Smith, Dawson, Pulay, Pickering and Ruan2008) and is characterized by severe problems in emotion regulation. Emotion dysregulation in BPD patients involves high emotional reactivity (Rosenthal et al. Reference Rosenthal, Gratz, Kosson, Cheavens, Lejuez and Lynch2008), high emotional arousal (Kuo & Linehan, Reference Kuo and Linehan2009), and impaired inhibition of emotional processing, which suggests an increased emotional distractibility (Domes et al. Reference Domes, Winter, Schnell, Vohs, Fast and Herpertz2006; Fertuck et al. Reference Fertuck, Lenzenweger, Clarkin, Hoermann and Stanley2006; Silbersweig et al. Reference Silbersweig, Clarkin, Goldstein, Kernberg, Tuescher, Levy, Brendel, Pan, Beutel, Pavony, Epstein, Lenzenweger, Thomas, Posner and Stern2007). A growing body of emotional challenge studies has revealed a dysfunctional network of brain regions, including a hyper-reactive amygdala and hypoactivation in frontal regions implicated in attention and arousal modulation [e.g. the anterior cingulate cortex (ACC), orbitofrontal cortex (OFC) and dorsolateral prefrontal cortex (DLPFC)] in BPD patients compared to healthy participants (see Lis et al. Reference Lis, Greenfield, Henry, Guilé and Dougherty2007).
Emotional arousal has been proposed to impair cognitive functioning in BPD, for example causing serious difficulties in memory and goal-directed behavior (Skodol et al. Reference Skodol, Gunderson, Pfohl, Widiger, Livesley and Siever2002; Fertuck et al. Reference Fertuck, Lenzenweger, Clarkin, Hoermann and Stanley2006). Hurlemann et al. (Reference Hurlemann, Hawellek, Maier and Dolan2007) found enhanced retrograde and anterograde amnesia for neutral stimuli in response to negatively arousing stimuli in individuals with BPD. Mensebach et al. (Reference Mensebach, Wingenfeld, Driessen, Rullkoetter, Schlosser, Steil, Schaffrath, Bulla-Hellwig, Markowitsch, Woermann and Beblo2009) revealed impaired verbal memory performances after negatively valenced interference, but not during a neutral control condition in BPD patients. However, neuroimaging studies investigating the influence of emotional hyper-reactivity on cognitive functions, such as working memory (WM), in BPD are still lacking.
As another core feature, BPD patients frequently report states of dissociation, that is disruptions of usually integrated cognitive and perceptual functions (e.g. consciousness, memory, identity, body awareness and perception), especially during emotional arousal (APA, 2000; Stiglmayr et al. Reference Stiglmayr, Shapiro, Stieglitz, Limberger and Bohus2001, Reference Stiglmayr, Ebner-Priemer, Bretz, Behm, Mohse, Lammers, Anghelescu, Schmahl, Schlotz, Kleindienst and Bohus2008). Trait dissociation has been associated with altered WM performance in BPD patients (Haaland & Landrø, Reference Haaland and Landrø2009) and in patients with dissociative disorders (Elzinga et al. Reference Elzinga, Ardon, Heijnis, De Ruiter, van Dyck and Veltman2007). Moreover, it has been proposed that dissociation dampens emotional arousal (Sierra & Berrios, Reference Sierra and Berrios1998; Lanius et al. Reference Lanius, Vermetten, Loewenstein, Brand, Schmahl, Bremner and Spiegel2010). Despite the high clinical relevance of stress-related dissociation in BPD, however, little is known about its neurobiological basis and its influence on emotional cognitive processing.
General models of emotion–cognitive interactions suggest that increased emotional distractibility is associated with altered activation in a ventral brain system involved in emotional processing and a dorsal brain system involved in executive functioning (Drevets & Raichle, Reference Drevets and Raichle1998; Davis & Whalen, Reference Davis and Whalen2001). For example, impaired inhibition of emotional distractors has been associated with stronger activation in the amygdala and insula and weaker activation in the DLPFC (involved in the suppression of task-irrelevant information in WM) in healthy participants (Dolcos & McCarthy, Reference Dolcos and McCarthy2006) and also in traumatized participants (Morey et al. Reference Morey, Dolcos, Petty, Cooper, Hayes, LaBar and McCarthy2009). Furthermore, we recently found that activation in ventral brain regions was even more increased and associated with impaired WM performance during emotional distraction after social stress induction (Oei et al. Reference Oei, Veer, Wolf, Spinhoven, Rombouts and Elzinga2011).
Adapting a well-established emotional working memory paradigm (Oei et al. Reference Oei, Tollenaar, Spinhoven and Elzinga2009, Reference Oei, Tollenaar, Elzinga and Spinhoven2010, Reference Oei, Veer, Wolf, Spinhoven, Rombouts and Elzinga2011), the present study aimed to investigate the influence of emotional distraction on WM in BPD. During functional magnetic resonance imaging (fMRI), participants performed a modified Sternberg item recognition task, while being distracted by task-irrelevant neutral versus negatively arousing pictures from the International Affective Picture System (IAPS; Lang et al. Reference Lang, Bradley and Cuthbert2005). We assumed that emotional distraction would be associated with stronger amygdala activation and weaker DLPFC activation in both groups, replicating previous findings in healthy subjects (Dolcos & McCarthy, Reference Dolcos and McCarthy2006). Moreover, we assumed that this pattern of brain activation would be even more pronounced in BPD patients compared to healthy participants during emotional distraction. Another aim of the present study was to investigate the role of self-reported dissociation, assessed immediately before scanning, on WM performance and neural activation during emotional distraction in BPD patients.
Method
Sample
The sample comprised 44 females aged between 18 and 45 years. All participants underwent diagnostic assessments, including the Structured Clinical Interview for DSM-V Axis I Disorders – Clinical Version (SCID-CV; First et al. Reference First, Spitzer, Gibbon and Williams1997) and the International Personality Disorder Examination (IPDE; Loranger, Reference Loranger1999), by trained diagnosticians (inter-rater reliability: κ=0.77). Intelligence and basic WM were estimated using subscales of the Wechsler Adult Intelligence Scale III (WAIS-III; Wechsler, Reference Wechsler1997; subscales Information, Similarities, Block Design) and the Wechsler Memory Scale – Revised (WMS-R; Wechsler, Reference Wechsler1987; subscales Digit Span forward/backward). Clinical assessment included questionnaires on BPD symptom severity [Borderline Symptom List 95 (BSL-95); Bohus et al. Reference Bohus, Limberger, Frank, Sender, Gratwohl and Stieglitz2001, Reference Bohus, Limberger, Frank, Chapman, Kuehler and Stieglitz2007], trauma history [Posttraumatic Stress Diagnostic Scale (PDS); Foa, Reference Foa1995; Childhood Trauma Questionnaire (CTQ); Bernstein et al. Reference Bernstein, Stein, Newcomb, Walker, Pogge, Ahluvalia, Stokes, Handelsman, Medrano, Desmond and Zule2003], trait dissociation [Dissociative Experience Scale (DES); Bernstein & Putnam, Reference Bernstein and Putnam1986], impulsivity [Barratt Impulsiveness Scale 10 (BIS-10); Patton et al. Reference Patton, Stanford and Barratt1995], attention deficit hyperactivity disorder (ADHD) symptoms [ADHD-Checklist (ADHD-CL); Rösler et al. Reference Rösler, Retz, Thome, Schneider, Stieglitz and Falkai2006], emotion regulation [Difficulties in Emotion Regulation Scale (DERS); Gratz & Roemer, Reference Gratz and Roemer2004], dysphoric mood [Beck Depression Inventory (BDI); Beck et al. Reference Beck, Ward, Mendelson, Mock and Erbaugh1961] and state anxiety [State Anxiety Inventory (STAI-X1); Spielberger et al. Reference Spielberger, Gorsuch and Lushene1970]. Arousal and dissociative states were assessed by the Dissociation Stress Scale 4 (DSS-4; Stiglmayr et al. Reference Stiglmayr, Schmahl, Bremner, Bohus and Ebner-Priemer2009), a self-rating scale consisting of four items on current dissociative experience (e.g. derealization, depersonalization) and an arousal rating (all between ‘0=not at all’ and ‘9=extremely’). Self-rated dissociation (DSS-4) immediately before scanning was positively correlated with DSS-4 scores after scanning (ρ=0.809, p<0.0001) and DES scores (ρ=0.662, p<0.0001).
General exclusion criteria related to MRI were metal implants, pregnancy and left-handedness. All BPD patients were free of severe somatic illness and had not suffered from alcohol or other substance abuse within the past 6 months. Further exclusion criteria were medication within the past 14 days (in the case of fluoxetine 28 days), current major depression, lifetime psychotic disorder, bipolar affective disorder, mental retardation, developmental disorder, and a life-threatening suicidal crisis.
The patient sample consisted of 22 females meeting criteria for BPD according to DSM-IV (APA, 2000). All patients fulfilled DSM-IV criterion 6 for emotional instability and reported a history of psychological, physical and/or sexual trauma as assessed by the PDS and CTQ. Clinical characteristics (DSS-4, DES, BSL-95, BIS-10, ADHD-CL, STAI, and BDI) and co-occurring mental conditions in the present patient sample are reported in Table 1. Nine out of 22 BPD patients currently met diagnosis for post-traumatic stress disorder (PTSD).
Table 1. Demographic and clinical variables in healthy controls (HC) and patients with borderline personality disorder (BPD) and results of the t tests
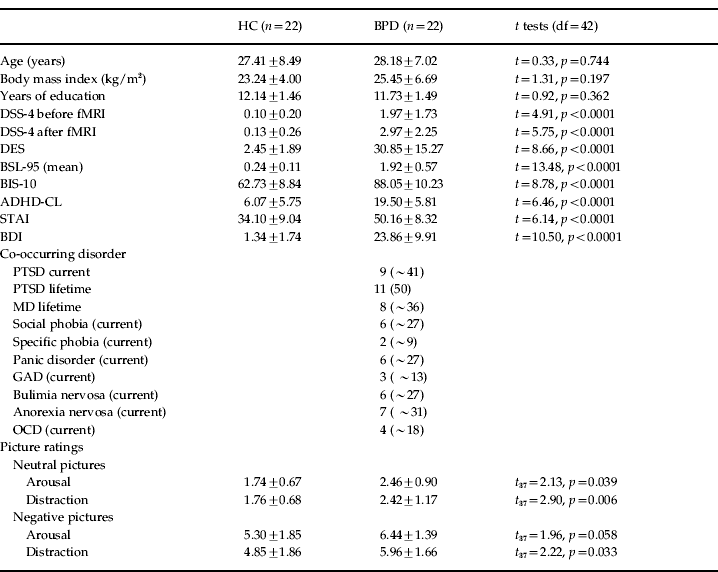
df, Degrees of freedom; DSS-4, Dissociation Stress Scale 4; fMRI, functional magnetic resonance imaging; DES, Dissociation Experience Scale; BSL-95, Borderline Symptom List 95; BIS-10, Barratt Impulsiveness Scale 10; ADHD-CL, Attention Deficit Hyperactivity Disorder Checklist; STAI, State Anxiety Inventory; BDI, Beck Depression Inventory; PTSD, post-traumatic stress disorder; MD, major depression; GAD, generalized anxiety disorder; OCD, obsessive–compulsive disorder.
Data are presented as mean ± standard deviation or n (%).
The control group consisted of 22 unmedicated healthy controls (HC) without a history of psychiatric disorders and trauma. The two groups did not differ regarding age, education level, and body mass index (BMI) (see Table 1) or on estimators of WM and intelligence, hours of sleep, and average amount of drinking (all p>0.05; data not shown).
Emotional Working Memory Task (EWMT)
The EWMT is an adapted Sternberg item recognition task (Sternberg, Reference Sternberg1966), modified and validated by Oei et al. (Reference Oei, Tollenaar, Spinhoven and Elzinga2009, Reference Oei, Tollenaar, Elzinga and Spinhoven2010, Reference Oei, Veer, Wolf, Spinhoven, Rombouts and Elzinga2011). The present version consisted of 48 trials, each starting with the presentation of three letters (memoranda, 1000 ms). After a delay interval (1500 ms), three letters were again displayed (probe, 2000 ms). In half of the trials, one of the three memoranda was present in the probe. Participants had to press the ‘yes’ or ‘no’ button indicating whether they had recognized a target or not. During the delay interval, either neutral or negatively arousing pictures from the IAPS (Lang et al. Reference Lang, Bradley and Cuthbert2005) were presented as distractors. The presentation of pictures was pseudo-randomized to control for expectancy effects. Pictures were selected based on the results of a pilot study in an independent sample of 15 BPD patients and 15 HC. Pictures in the ‘emotional’ condition included negatively arousing interpersonal scenes on physical and sexual violence, emotional neglect, or mutilation. To control for confounding differences in visual information processing, neutral and emotional pictures were matched regarding number of persons and complexity of the sceneFootnote 1 Footnote †. In the control condition within the EWMT, a fixation cross was presented during the delay interval. The resting phase between the trials was jittered to prevent temporal correlation. Participants were instructed to focus on the middle of the screen, concentrating only on the task and ignoring the pictures. Presentation® (neurobehavioral systems: www.neurobs.com/) was used to present stimuli and record behavioral data. After scanning, participants rated the pictures together with 30 similar looking IAPS pictures regarding arousal and distraction (difficulty of shifting attention away from the picture) as perceived during the task (between ‘0=not at all’ and ‘9=extremely’) and post-hoc recognition of the pictures was tested.
Procedure
The experiment was approved by the local ethics committee (University of Heidelberg, in accordance with the World Medical Association's Declaration of Helsinki) and conducted at the Central Institute of Mental Health in Mannheim, Germany. All participants received information about the experiment and scanning procedure. After giving signed informed consent, participants underwent diagnostic (SCID-CV, IPDE) and basic clinical assessments (WAIS-III, WMS-R, BSL-95, CTQ, PDS, DES, BIS-10, ADHD-CL, DERS, BDI, STAI).
Participants practised 15 trials of the EWMT outside the scanner to ensure that they understood the instruction correctly (feedback was given by the experimenter). Immediately before and after scanning, participants completed the DSS-4. Inside the scanner, participants first performed 15 trials of the working memory task without distraction, only viewing a fixation cross during the delay interval (‘Basic WMT without distraction’). Then they performed the EWMT. At the end, participants were debriefed, thanked, and paid for their participation.
Scan protocol
Scanning was conducted by using a Siemens TRIO-3T MRI (Siemens Medical Solutions, Germany). Using three-dimensional (3D) magnetization-prepared rapid acquisition gradient echo (MPRAGE; T1-weighted contrast, voxel size 1×1×1 mm3), a high-resolution anatomical scan was acquired for each participant as an individual template for the functional data. For fMRI scans, T2-weighted gradient echo planar imaging (EPI) was used for measurement of the blood oxygen level-dependent (BOLD) signal [field of view (FOV)=210×210 mm, voxel size=3×3×3 mm, echo time (TE)=30 ms, repetition time (TR)=250 0 ms], with 40 contiguous 3-mm sagittal slices in a 64×64 matrix (Ogawa et al. Reference Ogawa, Lee, Kay and Tank1990). The first five scans were discarded to minimize T1 effects. Head movement artifacts and scanning noise were restricted using head cushions and headphones within the scanner coil.
Statistical analysis
Behavioral data
Several ratings and recognition tests of four BPD patients and one HC were missing data and were excluded from the analyses. Picture ratings were evaluated using t tests for independent samples. Post-hoc recognition was evaluated by subtracting the number of falsely recognized pictures from the number of correctly recognized pictures. Subsequently, group differences regarding the number of correctly recognized neutral and emotional pictures were evaluated with t tests.
Reaction times (RTs) were checked for outliers, which were replaced by the mean+2 standard deviations. Mean RTs and accuracy (percentage of correct trials) were evaluated using repeated-measures analyses of variance (rm-ANOVAs) with group (HC, BPD) as the between-subjects factor and condition (control condition, neutral pictures, negative pictures) as the within-subjects factor (p<0.05). In the case of significant effects for the dependent variables, post-hoc t tests and effect sizes [partial eta-squared (ηp 2) and Cohen's d respectively; Cohen, Reference Cohen1988] were computed. Custom statistical software (SPSS version 15.0.1; SPSS Inc., USA) was used for analyses.
fMRI data
Functional imaging data were analyzed using standard procedures implemented in Statistical Parametric Mapping (SPM) version 8 (SPM8; Wellcome Department of Cognitive Neurology, London, UK; www.fil.ion.ucl.ac.uk/spm/). The EPI time series were preprocessed according to usual practiceFootnote 2 . This was followed by statistical parametric mapping on a voxel-by-voxel basis using the general linear model and random effects procedures in SPM8 (Woolrich et al. Reference Woolrich, Behrens, Beckmann and Smith2004). First-level analyses were modeled using the respective conditions as regressors. Data analysis at group level involved both a whole-brain voxel-wise analysis and region-of-interest (ROI) analyses (i.e. rm-ANOVAs, t tests) to evaluate group differences during different conditions (control condition, neutral pictures, negative pictures). A full factorial design (two groups×three conditions) was used including the F contrasts ‘main effect of condition’ (control condition versus neutral pictures versus negative pictures; across groups), ‘main effect of group’ (HC versus BPD; across all conditions), and ‘interaction effect group×condition’. An intensity threshold of p [false discovery rate (FDR) corrected] <0.05 and an extent threshold of p>10 contiguous voxels were defined. The ROIs were identified based on a twofold decision rule: according to theoretical models of emotion–cognitive interaction, the amygdala, insula, hippocampus, ACC, and DLFPC were defined as ROIs (Drevets & Raichle, Reference Drevets and Raichle1998; Davis & Whalen, Reference Davis and Whalen2001). ROI analyses in these a priori-defined brain regions were conducted, when significant activation was revealed by the whole-brain F contrast ‘main effect of condition’. In this case, functional ROIs were defined by a sphere of radius 9 mm on the local maximum of this whole-brain contrast. With respect to the amygdala and hippocampus, which are anatomically well-defined regions, ROIs were computed using anatomical masks defined by the Automated Anatomical Labeling software (AAL; Tzourio-Mazoyer et al. Reference Tzourio-Mazoyer, Landeau, Papathanassiou, Crivello, Etard, Delcroix, Mazoyer and Joliot2002), smoothed with a cube of voxels of size (FWHM) 9 mm. For this purpose, values for percentage signal change during each condition (relative to baseline activation) were extracted for each participant using the rfxplot toolbox (Gläscher, Reference Gläscher2009) and followed up statistically with post-hoc t tests (p<0.05, two-tailed) using SPSS. In the case of significant effects, effect sizes (ηp 2 and Cohen's d) were computed.
Covariate analyses, correlation analyses and subgroup analyses
Analyses of covariance (ANCOVAs) with symptom severity (BSL-95), depressive symptoms (BDI) and anxiety (STAI) were computed to control for a confounding influence of these covariates on behavioral data and the percentage signal change in each brain region. As none of these covariates turned out to be a significant predictor for any dependent variable, they were not included in the final rm-ANOVA.
Spearman's rank correlations between brain activation and behavioral parameters were computed for both groups. In addition, Spearman's rank correlations between DSS-4 scores immediately before scanning and behavioral parameters and also brain activation were computed in the BPD group (two-tailed, p<0.05). To account for the influence of symptom severity (BSL), depressive symptoms (BDI), state anxiety (STAI), and DSS-4 scores after scanning, partial correlations were computed.
As nine of the 22 BPD patients in the present study met the diagnosis for PTSD and previous research has revealed amygdala hyper-reactivity and increased emotional distractibility in PTSD patients (Morey et al. Reference Morey, Dolcos, Petty, Cooper, Hayes, LaBar and McCarthy2009; Wingenfeld et al. Reference Wingenfeld, Mensebach, Rullkoetter, Schlosser, Schaffrath, Woermann, Driessen and Beblo2009), we computed additional t tests including only BPD patients without co-occurring PTSD (n=13) and HC (n=22).
Results
Behavioral results
Subjective picture ratings
Means and standard deviations of pictures ratings are shown in Table 1. Negative pictures were rated as more arousing (HC: t 20=10.10, p<0.0001; BPD: t 17=14.28, p<0.0001) and distracting (HC: t 20=8.16, p<0.0001; BPD: t 17=9.20, p<0.0001) than neutral pictures. Arousal ratings for negative and neutral pictures were significantly higher in BPD patients compared to HC. Moreover, BPD patients rated neutral pictures as significantly more distracting than HC.
Post-hoc recognition test
There were no significant group differences in the post-hoc recognition test (all p's >0.05, data not shown).
Emotional WM performance
There were no significant group differences regarding RT and accuracy in the first 15 trials of the WM task (Basic WMT without distraction) (all p's >0.05, data not shown). Concerning RTs during the EWMT, the rm-ANOVA revealed a significant main effect for condition (F 2,41=6.11, p=0.005, ηp 2=0.23), a significant group effect (F 1,41=4.90, p=0.032, ηp 2=0.11) and a significant interaction effect group×condition (F 2,41=3.35, p=0.048, ηp 2=0.14), with BPD patients showing higher RTs during emotional distraction compared to HC (t 42=2.95, p=0.005, d=0.89) (Fig. 1). Post-hoc t tests revealed significantly increased RTs during emotional distraction compared to the control condition in BPD patients (t 21=4.57, p<0.001, d=0.41) but not in HC (t 21=0.79, p=0.440). The rm-ANOVA for accuracy revealed no significant effects (all p's >0.05, data not shown).
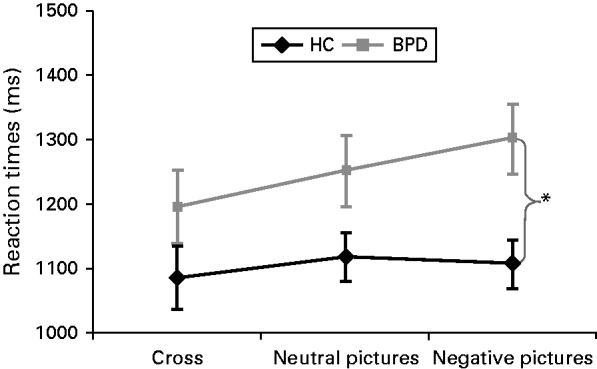
Fig. 1. Means ± standard error of the mean (s.e.m.) of reaction times (RTs) in healthy participants (HC) and patients with borderline personality disorder (BPD).
Neuroimaging results
Whole-brain analyses
The results of the F contrast for condition (whole-brain analysis) are presented in Table 2. Contrasting brain activation between the two groups, differences in the amygdala (e.g. x, y, z=21, −6, −18), insula (42, 0, −15), DLPFC (42, 27, 33; −45, 30, 33) and ACC (−21, 39, 0) were found, among others.
Table 2. Whole-brain results for the F contrast ‘main effect of condition’ (within the 2×3 full factorial design)
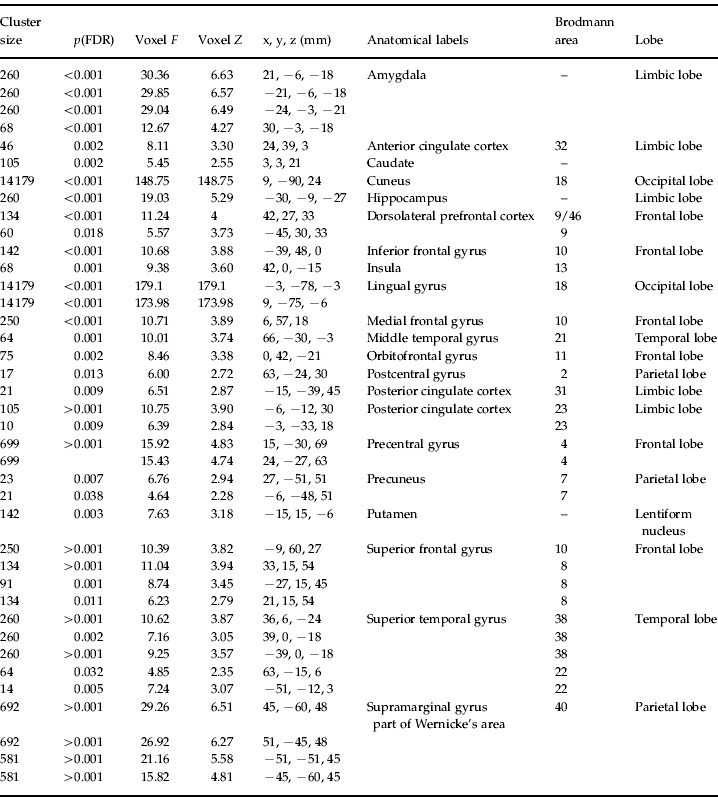
FDR, False discovery rate.
ROI analyses
Amygdala
Means and standard error of the mean (s.e.m.) of percentage signal change in the amygdala are displayed in Fig. 2 a. The rm-ANOVAs revealed a significant interaction effect group×condition (F 2,4=3.98, p=0.026, ηp 2=0.16) next to a significant main effect for condition (F 2,41=30.76, p<0.0001, ηp 2=0.60). Compared to the HC group, BPD patients showed significantly higher amygdala activation during the presentation of neutral pictures (t 42=2.13, p=0.041, d=0.67) and negatively arousing pictures (t 42=2.32, p=0.025, d=0.64). Post-hoc t tests in both groups revealed significantly higher amygdala activation during the presentation of negative pictures compared to the control condition (HC: t 21=4.04, p=0.001, d=0.59; BPD: t 21=6.16, p<0.0001, d=1.02) and the presentation of neutral pictures (HC: t 21=3.73, p=0.0001, d=0.58; BPD: t 21=2.36, p=0.028, d=0.34).

Fig. 2. Results of the whole-brain analysis (F contrast ‘main effect of condition’) and region of interest (ROI) analyses (percentage signal change during the control condition, neutral pictures and negative pictures) for (a) the amygdala, (b) hippocampus, insula, (c) insula, (d) dorsolateral prefrontal cortex (DLPFC) and (e) anterior cingulate cortex (ACC) in healthy participants (HC) and patients with borderline personality disorder (BPD).
Hippocampus
Means and s.e.m. of percentage signal change in the hippocampus are displayed in Fig. 2 b. The rm-ANOVAs revealed a significant interaction effect (F 2,41=3.79, p=0.031, ηp 2=0.16) next to the significant main effect for condition (F 2,41=31.68, p<0.0001, ηp 2=0.61). There was no significant group effect. Post-hoc t tests revealed higher activation during the presentation of negative pictures compared to the control condition (HC: t 21=4.94, p<0.0001, d=0.89; BPD: t 21=6.09, p<0.0001, d=1.50) and the presentation of neutral pictures (HC: t 21=1.97, p=0.062; BPD: t 21=2.46, p=0.023, d=0.38) in both groups. Activation was significantly higher in BPD patients compared to HC during the presentation of negative pictures (t 42=2.19, p=0.034, d=0.66).
Insula
Means and s.e.m. of percentage signal change in the insula ROI (peak voxel: 42, 0, −15) are displayed in Fig. 2 c. The rm-ANOVAs revealed a significant interaction effect condition×group (F 2,42=3.79, p=0.031, ηp 2=0.16) next to the significant main effect for condition (F 2,41=30.30, p<0.0001, ηp 2=0.60), but no significant group effect. Post-hoc t tests revealed significantly higher activation during the presentation of negative pictures compared to the control condition in both groups (HC: t 21=4.08, p=0.001, d=0.51; BPD: t 21=6.65, p<0.0001, d=1.21).
DLPFC
Means and s.e.m. for percentage signal change in the right DLPFC ROI (peak voxel: 42, 27, 33) are depicted in Fig. 2 d. The rm-ANOVAs revealed a significant main effect for condition (F 2,41=3.45, p=0.041, ηp 2=0.14) with reduced activation during emotional distraction.
ACC
Means and s.e.m. for percentage signal change in the ACC ROI (peak voxel: 24, 39, 3) are depicted in Fig. 2 e. The rm-ANOVAs revealed only a significant main effect for condition (F 2,42=13.01, p<0.0001, ηp 2=0.39) and no other effects. Post-hoc t tests revealed significantly lower activation during the presentation of negative pictures compared to the control condition (HC: t 21=2.63, p=0.016, d=0.48; BPD: t 21=4.05, p=0.001, d=0.88) and compared to the presentation of neutral pictures (HC: t 21=3.08, p=0.006, d=0.53; BPD: t 21=2.72, p=0.013, d=0.61).
Correlation and subgroup analyses
In BPD patients, there was a significant positive correlation between amygdala activation and RT during emotional distraction (ρ=0.393, p=0.035); in HC, this was only a trend (ρ=0.298, p=0.095). In both groups, DLPFC activation was negatively correlated with RT during emotional distraction (BPD: ρ=−0.462, p=0.015; HC: ρ=−0.452, p=0.017).
In the BPD group, there were significant negative correlations between DSS-4 scores before the experiment and activation in the amygdala (ρ=−0.540, p=0.010; Fig. 3), hippocampus (ρ=−0.513, p=0.015), insula (ρ=−0.462, p=0.030) and ACC (ρ=−0.510, p=0.015) (all two-tailed), which remained significant when accounting for symptom severity, depressive symptoms, state anxiety, and DSS-4 scores (after the experiment) in partial correlation analysis.
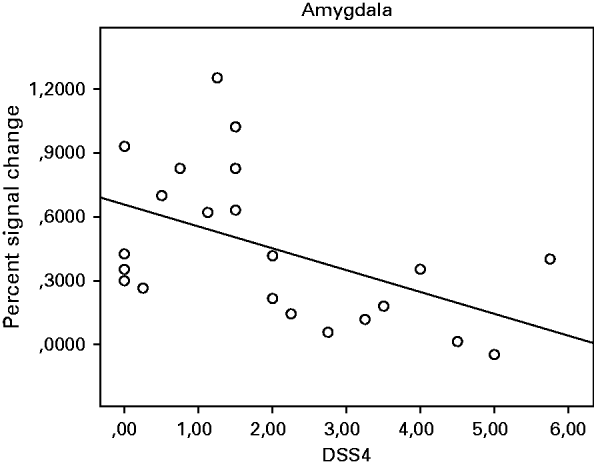
Fig. 3. Spearman's rank correlations between activation in the amygdala and dissociative states (DSS-4) during the presentation of negative pictures in the borderline personality disorder (BPD) group.
Post-hoc t tests including only BPD patients without co-occurring PTSD (n=13) and HC (n=22) revealed significantly prolonged RT during emotional distraction in the patients compared to HC (t 33=2.53, p=0.016, d=0.85). Moreover, significantly higher activation in the amygdala (neutral pictures: t 33=2.18, p=0.045, d=0.82; negative pictures: t 33=2.88, p=0.004, d=1.04), hippocampus (neutral pictures: t 33=3.69, p=0.001, d=1.89; negative pictures: t 33=3.60, p=0.001, d=1.24) and DLPFC (neutral pictures: t 33=3.73, p=0.001, d=1.31; negative pictures: t 33=2.34, p=0.026, d=0.79) was found in this patient group compared to the HC group. The trend towards higher insula activation in the patient group compared to the HC group became significant after excluding BPD patients with co-occurring PTSD (t 33=2.77, p=0.009, d=1.01).
Discussion
This fMRI study aimed to investigate the influence of emotional distraction on WM in BPD and HC. Twenty-two unmedicated BPD patients and 22 HC performed an adapted Sternberg item recognition task (EWMT; Oei et al. Reference Oei, Tollenaar, Spinhoven and Elzinga2009, Reference Oei, Tollenaar, Elzinga and Spinhoven2010, Reference Oei, Veer, Wolf, Spinhoven, Rombouts and Elzinga2011), while being distracted by neutral versus negatively arousing pictures from the IAPS (Lang et al. Reference Lang, Bradley and Cuthbert2005). The main findings of the present study are:
-
(1) Patients with BPD showed significantly longer RTs and higher activation in limbic(-related) brain regions (amygdala, hippocampus, insula) compared to HC during the presentation of negatively arousing pictures.
-
(2) In the BPD group, negative correlations between self-reported dissociation and activation in the amygdala, insula, hippocampus, and ACC were found, suggesting a dampening effect of dissociation on brain regions involved in emotional processing.
In line with previous studies using the EWM paradigm (Dolcos & McCarthy, Reference Dolcos and McCarthy2006; Morey et al. Reference Morey, Dolcos, Petty, Cooper, Hayes, LaBar and McCarthy2009), emotional distraction was associated with increased amygdala activation and decreased DLPFC activation. Activation in these brain areas was correlated with RT during emotional distraction. Of note, decreased WM performance during emotional distraction in HC was mirrored only on a neurobiological level, but not on the behavioral level in the present study. In contrast to HC, BPD patients showed significantly increased RT during emotional distraction compared to the control condition. At the same time, RT during the control condition and accuracy did not differ between the groups in the EWMT. Thus, enhanced RTs during emotional distraction may mirror greater difficulties in shifting attention away from emotional pictures rather than general WM impairments in BPD patients. This extends previous findings on impaired inhibition of emotional processing in BPD patients (Domes et al. Reference Domes, Winter, Schnell, Vohs, Fast and Herpertz2006; Fertuck et al. Reference Fertuck, Lenzenweger, Clarkin, Hoermann and Stanley2006; Hurlemann et al. Reference Hurlemann, Hawellek, Maier and Dolan2007; Silbersweig et al. Reference Silbersweig, Clarkin, Goldstein, Kernberg, Tuescher, Levy, Brendel, Pan, Beutel, Pavony, Epstein, Lenzenweger, Thomas, Posner and Stern2007; Mensebach et al. Reference Mensebach, Wingenfeld, Driessen, Rullkoetter, Schlosser, Steil, Schaffrath, Bulla-Hellwig, Markowitsch, Woermann and Beblo2009) and might reflect the clinically relevant difficulties of BPD patients maintaining cognitive functioning (e.g. goal-directed behavior, task-related attention) during emotional stress.
At the neurobiological level, ROI analyses revealed that limbic activation was even more increased in BPD patients compared to HC during the presentation of negatively arousing pictures whereas no significant group differences were found during the control condition. Extending previous findings on amygdala hyper-reactivity in BPD (see Lis et al. Reference Lis, Greenfield, Henry, Guilé and Dougherty2007), amygdala activation in BPD patients compared to HC was significantly higher during the presentation of negative and neutral pictures. Affective hyper-reactivity to neutral interpersonal pictures has been found in previous studies in BPD patients (Donegan et al. Reference Donegan, Sanislow, Blumenberg, Fulbright, Lacadie, Skudlarski, Gore, Olson, McGlashan and Wexler2003; Fertuck et al. Reference Fertuck, Jekal, Song, Wyman, Morris, Wilson, Brodsky and Stanley2009) and might be related to the ambiguity and greater threat potential of neutral interpersonal stimuli.
Notably, we found negative correlations between dissociation and activation in the amygdala, hippocampus, right insula, and ACC during the presentation of negative pictures in BPD patients. These correlations remained significant after accounting for symptom severity, dysphoric mood, state anxiety, and dissociation at the end of the experiment. Although correlations do not imply causal conclusions, the relationship between dissociation and dampened limbic brain activation might be an important step in a better understanding of the neurobiology underlying dissociation in BPD. Our findings are in line with theoretical models by Sierra & Berrios (Reference Sierra and Berrios1998) and Lanius et al. (Reference Lanius, Vermetten, Loewenstein, Brand, Schmahl, Bremner and Spiegel2010), which propose a prefrontal inhibition of limbic regions (including the amygdala and right anterior insula) during dissociative states. Dissociation is considered to be a regulatory strategy to cope with the overwhelming emotional arousal during confrontation with stressful stimuli in the sense of ‘shutting down’ the affective system (Sierra & Berrios, Reference Sierra and Berrios1998; Lanius et al. Reference Lanius, Vermetten, Loewenstein, Brand, Schmahl, Bremner and Spiegel2010). The cost of this subjective detachment from emotional experience might be an avoidance of necessary cognitive and affective coping. Dissociative states might prevent habituation to negatively arousing situations and new learning (Lanius et al. Reference Lanius, Vermetten, Loewenstein, Brand, Schmahl, Bremner and Spiegel2010). Previous studies have demonstrated reduced (amygdala-moderated) startle response (Ebner-Priemer et al. Reference Ebner-Priemer, Badeck, Beckmann, Wagner, Feige, Weiss, Lieb and Bohus2005) and diminished acquisition of emotional conditioning (Ebner-Priemer et al. Reference Ebner-Priemer, Mauchnik, Kleindienst, Schmahl, Peper, Rosenthal, Flor and Bohus2009) in BPD patients with high peri-experimental dissociation compared to those without dissociation. Furthermore, a close relationship between high levels of dissociation and poor response to dialectical behavior therapy and brief psychodynamic psychotherapy has been found (Spitzer et al. Reference Spitzer, Barnow, Freyberger and Grabe2007; Kleindienst et al. Reference Kleindienst, Limberger, Ebner-Priemer, Mauchnik, Dyer, Berger, Schmahl and Bohus2011). However, to our knowledge, the present study is the first to show a direct relationship between self-reported dissociation and limbic brain activation during emotional challenge in BPD. In future studies, the influence of experimentally induced dissociation on emotional cognitive processing in BPD should be investigated.
Further strengths of the present study are the relatively large sample size of unmedicated, well-characterized BPD patients and the use of an established paradigm. One limitation is that we did not include a clinical control group. Therefore, conclusions regarding the specificity of our findings for BPD are limited. For example, our findings might be related to the fact that we only included patients with a history of interpersonal traumatization. Nine out of 22 BPD patients additionally met PTSD diagnosis, which has also been associated with amygdala hyper-reactivity and increased emotional distractibility (Morey et al. Reference Morey, Dolcos, Petty, Cooper, Hayes, LaBar and McCarthy2009). However, group effects remained significant or became even clearer, when excluding BPD patients with co-occurring PTSD, suggesting that the current findings are not driven by the BPD patients with PTSD. Nevertheless, future research should compare BPD patients to patients with other diagnoses, who also report a history of interpersonal trauma.
In general, our findings are in line with the well-known clinical characteristics of BPD, particularly affective instability, difficulties in emotion regulation, and dissociation. These findings suggest not only that patients may be sensitive to experiences of abandonment but also that the negative affect that this may cause may have consequences on a cognitive level, for example by impairing performance at work. Similarly, it may also hinder cognitive reflections on interpersonal problems. This behavioral pattern of results applied to dissociative and non-dissociative patients, even though in the limbic structures we could also demonstrate that state dissociation had an important dampening effect on these affective processes in BPD.
In conclusion, this fMRI study revealed increased emotional distractibility in BPD patients in terms of prolonged RT and increased limbic activation during emotional distraction. Moreover, our findings suggest dampened limbic brain activation during self-reported dissociative states in BPD patients. These results emphasize the importance of emotional hyper-reactivity in the context of cognitive functioning and may help to better understand the role of dissociative states in emotional cognitive processing in BPD.
Acknowledgements
A. Krause-Utz was funded by a Ph.D. stipend (SFB636) from the German Research Foundation. B. M. Elzinga was funded by a VIDI grant from the Netherlands Organization for Scientific Research (grant no. 016·085·353). We thank all the participants in this study for their collaboration and J. Mauchnik, P. Ludäscher, C. Stief, B. Sarun, I. Veer and S. Rombouts for their contributions to this study.
Declaration of Interest
None.







