Introduction
It is becoming increasingly clear that no country can afford to provide universal healthcare coverage for all illnesses to all citizens. Triage rules are needed to allocate available healthcare resources to deal with the inevitable shortfall between resources and need. Among the several kinds of information used to help develop these rules, comparative illness burden estimates have been especially valuable as a reference standard for government health policy planners (Murray & Lopez, Reference Murray and Lopez1996; Murray et al. Reference Murray, Lopez, Mathers and Stein2001; Lopez & Mathers, Reference Lopez, Mathers and Matlin2007). A central component of these estimates is the condition-specific severity weight, a statistic obtained by having expert raters evaluate the relative burdens of different conditions using the person trade-off method (Murray & Lopez, Reference Murray and Lopez1996; Murray et al. Reference Murray, Lopez, Mathers and Stein2001; WHO, 2004). An important limitation of this approach is that the vignettes represent single conditions rather than more realistic cases where an individual suffers from a number of different conditions (Fortin et al. Reference Fortin, Soubhi, Hudon, Bayliss and van den Akker2007). This is an important limitation because methodological research has shown that condition-specific severity weights vary as a function of the presence of co-morbidity (Moussavi et al. Reference Moussavi, Chatterji, Verdes, Tandon, Patel and Ustun2007).
Previous attempts to take co-morbidity into consideration in estimating condition-specific illness burden have been limited by the fact that simplistic models were used to estimate effects (Verbrugge et al. Reference Verbrugge, Lepkowski and Imanaka1989; Maddigan et al. Reference Maddigan, Feeny and Johnson2005). The current report presents the results of an analysis aimed at generating condition-specific estimates of disease burden in a more realistic way. The method is illustrated in an analysis of data collected in general population surveys on the joint associations of health conditions reported by respondents and overall respondent ratings of perceived health, although the same logic could be applied to the analysis of complex vignettes describing co-morbid condition profiles.
Method
The sample
Data come from surveys carried out in 15 countries by the World Health Organization (WHO) World Mental Health (WMH) Survey Initiative (Kessler & Üstün, Reference Kessler and Üstün2008). Of the countries, six are classified by the World Bank as developing (Colombia, Lebanon, Nigeria, Mexico, People's Republic of China, Ukraine) and nine as developed (Belgium, France, Germany, Italy, Israel, Japan, The Netherlands, Spain, and United States of America) (Table 1). Country-specific response rates ranged from 45.9% (France) to 87.7% (Colombia), with a weighted (by sample size) average response rate across surveys of 69.6%. All surveys were based on probability samples of the adult household populations in the participating countries or regions within the countries. Respondents were aged 18+ years other than in Israel, where the minimum age was 21 years. The upper end of the age range was unbounded in all countries other than Colombia, Mexico and the People's Republic of China, where the upper bound was 65 years. More details about WMH sampling and eligibility are reported elsewhere (Heeringa et al. Reference Heeringa, Wells, Hubbard, Mneimneh, Chiu, Sampson, Berglund, Kessler and Üstün2008).
Table 1. Sample characteristics of the WMH Surveys
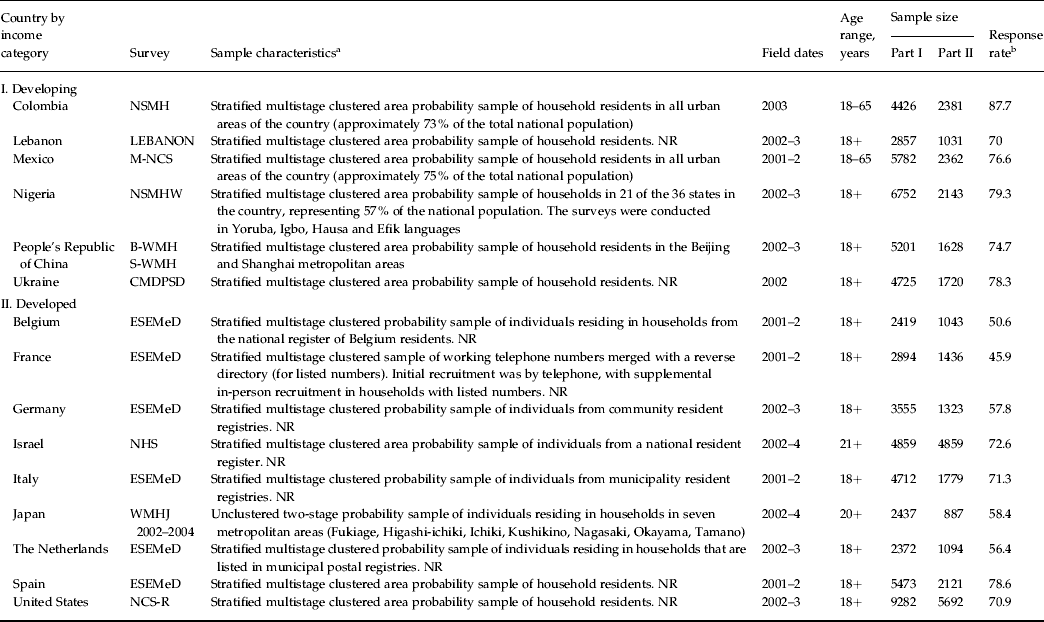
WMH, World Mental Health; NSMH, The Colombian National Study of Mental Health; LEBANON, Lebanese Evaluation of the Burden of Ailments and Needs of the Nation; NR, nationally representative; M-NCS, The Mexico National Comorbidity Survey; NSMHW, The Nigerian Survey of Mental Health and Wellbeing; B-WMH, The Beijing World Mental Health Survey; S-WMH, The Shanghai World Mental Health Survey; CMDPSD, Comorbid Mental Disorders during Periods of Social Disruption; ESEMeD, The European Study of the Epidemiology of Mental Disorders; NHS, Israel National Health Survey; WMHJ 2002–2004, World Mental Health Japan Survey; NCS-R, The US National Comorbidity Survey Replication.
a Most WMH Surveys are based on stratified multistage clustered area probability household samples in which samples of areas equivalent to counties or municipalities in the USA were selected in the first stage followed by one or more subsequent stages of geographic sampling (e.g. towns within counties, blocks within towns, households within blocks) to arrive at a sample of households, in each of which a listing of household members was created and one or two people were selected from this listing to be interviewed. No substitution was allowed when the originally sampled household resident could not be interviewed. These household samples were selected from Census area data in all countries other than France (where telephone directories were used to select households) and The Netherlands (where postal registries were used to select households). Several WMH surveys (Belgium, Germany, Italy) used municipal resident registries to select respondents without listing households. The Japanese sample is the only totally unclustered sample, with households randomly selected in each of the four sample areas and one random respondent selected in each sample household. Of the 15 surveys, 10 are based on nationally representative household samples, while two others are based on nationally representative household samples in urbanized areas (Colombia, Mexico).
b The response rate is calculated as the ratio of the number of households in which an interview was completed to the number of households originally sampled, excluding from the denominator households those known not to be eligible either because of being vacant at the time of initial contact or because the residents were unable to speak the designated languages of the survey. The weighted average response rate is 73%.
All WMH interviews were conducted face-to-face by trained lay interviewers. Standardized interviewer training and quality-control procedures were used (Pennell et al. Reference Pennell, Mneimneh, Bowers, Chardoul, Wells, Viana, Dinkelmann, Gebler, Florescu, He, Huang, Tomov, Vilagut, Kessler and Üstün2008). Informed consent was obtained before beginning interviews. Each interview had two parts. All respondents completed part I, which contained assessments of core mental disorders. The part II interview, which assessed physical disorders and correlates, was administered to 100% of respondents who met lifetime criteria for any of part I mental disorder plus a probability subsample of other part I respondents. A part II weight equal to the inverse of the respondent's probability of selection into part II was used to adjust for differential selection into part II.
Measures
Chronic physical conditions
Physical conditions were assessed with a chronic conditions checklist based on the US National Health Interview Survey list (Schoenborn et al. Reference Schoenborn, Adams and Schiller2003; Center for Disease Control and Prevention, 2004). Respondents were asked to report whether they ever had a series of symptom-based conditions (e.g. chronic headaches) and whether a health professional ever told them they had a series of silent conditions (e.g. cancer). Information was obtained whether episodic conditions were still present in the previous 12 months. Checklists like this yield more accurate reports than estimates derived from responses to open-ended questions (Baker et al. Reference Baker, Stabile and Deri2001; Knight et al. Reference Knight, Stewart-Brown and Fletcher2001). These reports were grouped into ten categories to maximize comparability with previous studies (Murray et al. Reference Murray, Lopez, Mathers and Stein2001). The categories include arthritis, cancer, cardiovascular disorders (heart attack, heart disease, hypertension, stroke), chronic pain conditions (chronic back or neck pain, other chronic pain conditions), diabetes, frequent or severe headaches or migraines, chronic insomnia, neurological disorders (multiple sclerosis, Parkinson's, epilepsy, seizure disorders), digestive disorders (stomach or intestinal ulcer, irritable bowel disorder) and respiratory disorders (seasonal allergies, asthma, chronic obstructive pulmonary disease, emphysema).
Mental disorders
Mental disorders were assessed with the WHO Composite International Diagnostic Interview, version 3.0 (CIDI), a fully structured lay-administered interview designed to generate diagnoses of common mental disorders according to the definitions and criteria of both the International Classification of Diseases, 10th revision (ICD-10) and Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) systems (Kessler & Üstün, Reference Kessler and Üstün2004, Reference Kessler and Üstün2008). DSM-IV criteria are used here. The nine mental disorders include major depressive episode, bipolar disorder I–II, panic–agoraphobia (panic disorder or agoraphobia without a history of panic disorder), specific phobia, social phobia, generalized anxiety disorder, post-traumatic stress disorder, alcohol abuse with or without dependence, and drug abuse with or without dependence. WMH clinical reappraisal studies have shown that the diagnoses of these disorders based on the CIDI have generally good concordance with diagnoses based on blinded clinician-administered reappraisal interviews (Haro et al. Reference Haro, Arbabzadeh-Bouchez, Brugha, de Girolamo, Guyer, Jin, Lepine, Mazzi, Reneses, Vilagut, Sampson and Kessler2006). As with physical conditions, we focus on mental conditions present at some time in the 12 months before interview.
Health valuation
Respondents were asked to make a health valuation after all physical and mental conditions had been assessed. We used a 0–100 visual analog scale (VAS) where 0 represents ‘the worst possible health a person can have’ and 100 represents ‘perfect health’ to describe their own overall physical and mental health during the previous 30 days taking into consideration all the physical and mental conditions reviewed in the survey. The recall period for the VAS (30 days) is different from that for the conditions (12 months) because we wanted to include effects not only of active conditions but also of recent conditions that, although not active, might still have an important effect on health valuations (e.g. a heart attack that occurred several months before the interview). The decision to anchor the low end of the scale as defining ‘the worst possible health’ rather than ‘death’ is consistent with the approach taken in the widely-used EQ-5DTM self-report questionnaire (http://www.euroqol.org) and was taken in the WMH surveys based on the finding in previous research that some health states are valued lower than death (Macran & Kind, Reference Macran and Kind2001). While the decision regarding which of these alternative lower-bound anchors to use probably had little effect on the estimates of relative disease burden reported here, it is noteworthy that an explicit valuation of death would be needed if we wanted to use the data to calculate years of life lived in less than perfect health.
Analysis methods
A series of multiple regression models was used to estimate joint predictive associations of conditions with VAS scores controlling age, sex and country. As the sample size was too small to allow each of the 524 288 (219) logically possible multivariate condition profiles to be a separate predictor, the models necessarily made simplifying assumptions about effects of co-morbidity. The first multivariate model (M1) assumed additivity; that is, a separate predictor for each condition without interactions. M2 included a series of predictors for number of conditions (e.g. one predictor for having exactly one condition, another for exactly two, etc) without information about type of condition. M3 included 19 predictors for type and number of conditions. The number-of-conditions dummies in this model represent aggregate patterns of co-morbidity assumed independent of types. M4 allowed for the effects of type to be a linear function of number of other conditions. More complex models allowed for interactions of type with number using weighted counts based on type coefficients, but these results are not reported because the models did not fit the data as well as the simpler models.
The skewed distribution of the VAS scores made ordinary least-squares (OLS) regression analysis both biased and inefficient. This problem was addressed in two ways. First, a two-part modeling approach (Duan et al. Reference Duan, Manning, Morris and Newhouse1984) was used where a part I logistic regression equation (Hosmer & Lemeshow, Reference Hosmer and Lemeshow2001) predicted having a VAS score of 100 v. <100 in the total sample and a part II linear regression equation predicted scores in the 0–99 range. Individual-level predicted scores were estimated by multiplying predicted values based on the two equations. A problem with this approach is that non-random variance in prediction errors can lead to bias even when sophisticated transformation methods are used (Manning, Reference Manning1998). A second approach, generalized linear models (GLM), was used to address that problem by pre-specifying non-linear associations and non-random error structures in one-part models. Such models can sometimes fit highly skewed data better than two-part models (McCullagh & Nelder, Reference McCullagh and Nelder1989; Mullahy, Reference Mullahy1998; Manning & Mullahy, Reference Manning and Mullahy2001). We used a number of different two-part model specifications and a number of standard GLM specifications and then selected the best specification using standard empirical model comparison procedures (Buntin & Zaslavsky, Reference Buntin and Zaslavsky2004). All models were estimated separately in developed and developing countries in an effort to obtain a rough indication of variation in results by development, but no attempt was made to estimate country-specific models.
M4, which allowed the effects of co-morbidity to vary by type of condition as a linear function of number of other conditions, was the best-fitting model. This is a model of intermediate complexity in that it allows interactions to vary across conditions but not across particular pairs or higher numbers of disorders. Although this is unlikely to be the optimal interaction model, the fact that it provides the best fit across the range of models considered suggests that it is a useful first approximation. But a complication, as in any interaction model, is that the coefficients have no intuitive interpretation. We addressed this problem by using individual-level simulation to transform coefficients to a scale of average decrement in VAS scores associated with each condition. This was done by generating two estimates of predicted VAS scores for each respondent from each simulation. The first estimate was based on the model parameters in M4, while the second estimate was based on a revision of this model that assumed none of the respondents had one particular focal condition. The first estimate was then subtracted from the second and the sum across respondents was divided by the number of respondents with the focal condition to estimate the average individual-level decrease in VAS scores associated with that condition taking co-morbidity into consideration. This estimate was then projected to the societal level (i.e. the effect on the mean VAS score) by multiplying it by condition prevalence.
It is noteworthy that the simulation approach, by virtue of the fact that it works with mean VAS scores, treats the VAS as an interval scale. This assumption has been called into question in some previous studies (Krabbe et al. Reference Krabbe, Stalmeier, Lamers and Busschbach2006; Parkin & Devlin, Reference Parkin and Devlin2006) and non-linear monotonic transformations have been proposed to approximate interval scale properties (Krabbe, Reference Krabbe2008). However, strong linear associations have been found between health state values based on VAS scores and ordinal (Craig et al. Reference Craig, Busschbach and Salomon2009) or partially metric (Krabbe et al. Reference Krabbe, Salomon and Murray2007) scaling methods. As a result, and given that we explored a number of different non-linear transformations of the VAS in the GLM models, we treated the VAS as an interval scale in the current analysis.
Because the WMH sample design featured weighting and clustering, all multiple regression analyses used the Taylor series linearization method (Wolter, Reference Wolter1985) implemented in the SUDAAN software system (2002; Research Triangle Institute, USA). Standard errors of simulation estimates were obtained using the method of Jackknife repeated replications (Wolter, Reference Wolter1985) implemented with a SAS macro (SAS/STAT® software, version 9.1 for Unix, SAS Institute, Inc., USA). Statistical significance was consistently evaluated using two-sided 0.05 level tests.
Results
Condition prevalence estimates
More than half of all respondents reported having one or more conditions in the 12 months before interview (Table 2). Of those with any conditions, 54.6% had more than one and 51% of those with more than one had more than two conditions. The majority of conditions were reported to be more prevalent in developed than developing countries.
Table 2. Twelve-month prevalence estimates of chronic physical conditions and mental conditions separately in WMH Surveys in developing and developed countries

WMH, World Mental Health.
Values are given as percentage (standard error).
a Bipolar disorder was not assessed in Belgium, France, Germany, Israel, Italy, The Netherlands, Nigeria, Spain and Ukraine.
b Drug abuse was not assessed in Belgium, France, Germany, Italy, The Netherlands and Spain.
c Social phobia was not assessed in Israel.
d Specific phobia was not assessed in Israel and Ukraine.
Distribution of VAS scores
VAS scores are distributed quite similarly in developing and developed countries. Fewer than 10% of respondents in either set of countries have scores below 50, while 20.8% have scores of 100 and an additional 7.4% have scores in the range 91–100. The median among respondents with scores less than 100 is 80 [interquartile range (IQR)=70–90] in both developing and developed countries.
Selecting a functional form and error structure for the models
We estimated seven one-part GLM models and seven two-part models. We evaluated comparative model fit by plotting associations between predicted mean VAS scores and observed mean scores for each decile of predicted VAS scores and using a number of other model-fitting tests that have been proposed in the econometrics literature (Buntin & Zaslavsky, Reference Buntin and Zaslavsky2004) (detailed results are available on request). The GLM model with a square root functional form and independent error structure and the one-part OLS model were found to be the best-fitting models in terms of all the tests we considered. Based on this result and the simpler interpretation of the OLS model than the GLM model, we chose the OLS model.
The individual-level predictive associations of conditions with VAS scores
The coefficients in M1 are significant as a set and show each condition to have a negative predictive association with VAS scores (Table 3). (Only a single illustrative fit statistic is shown in Table 3. More detailed results for each model are available on request.) The coefficients in M2 are also significant as a set and show that VAS scores decrease monotonically with number of conditions. The M3 results show that the individual conditions continue to have generally negative coefficients when controlling for number of conditions and that the coefficients vary significantly across conditions. The coefficients associated with number of conditions in M3 are significantly negative. This indicates sub-additive interactions: that the joint adverse associations of co-morbid condition clusters with VAS scores are less than the sum of the associations of the individual pure conditions in the clusters taken one at a time. M4 shows that these non-additive associations vary significantly across conditions.
Table 3. Model comparisons for the multivariate associations of conditions on VAS scores separately in WMH Surveys in developing and developed countries

VAS, Visual analog scale; WMH, World Mental Health; AIC, Akaike's Information Criterion.
a Only one illustrative test statistic, AIC, is reported in this table, but model comparison was based on a number of different tests. For a description, see the text.
b A separate dummy variable predictor for each of the 19 conditions.
c A separate dummy variable predictor for having exactly one of the 19 disorders, exactly two of the 19 disorders, etc.
d The predictors in M1 and M2 with the exception that the dummy predictor for having exactly one disorder is omitted.
e The predictors in M3 plus interactions between each of the dummy predictors for type of disorders and a continuous variable for number of disorders.
f Best-fitting model.
Simulated individual-level estimates
Transformation of the M4 coefficients using simulation shows that the condition-specific individual-level estimates are consistently negative (Table 4). Coefficients for only two conditions (digestive disorders and specific phobia) differ significantly between developing and developed countries (both higher in developed). Magnitude of estimates is also quite similar in developing versus developed countries, with median values on the 0–100 VAS of 5.4 (IQR=3.2–5.8) in developing and 4.9 (IQR=3.1–7.1) in developed countries. Differences in coefficients across conditions are statistically significant in the total sample and fairly consistent in developing versus developed countries. The Spearman rank-order correlation among condition estimates between developed and developing countries is 0.54. The most notable exception is drug abuse, ranked 1st in developing countries and 14th in developed countries.
Table 4. Simulated individual-level condition-specific severity estimates based on the best-fitting regression model separately in WMH Surveys in developing and developed countries

WMH, World Mental Health; s.e., standard error.
* p<0.05(two-sided test).
† Significant difference between developing and developed countries (p<0.05; two-sided test).
Coefficients based on the bivariate model (i.e. considering only one condition at a time in predicting VAS) are consistently higher than those in the multivariate model, with the condition-specific ratio of the latter to former in the range 0.24–0.70 and a median ratio of 0.42 (IQR=0.31–0.51) (Table 5) Very similar results are found in developing [0.53 (IQR=0.35–0.62)] and developed [0.41 (IQR=0.27–0.51)] countries. The influence of co-morbidity can be seen in the fact that the correlation across conditions between mean number of co-morbid conditions (last column, Table 5) and the ratio of the coefficient based on the bivariate model to the coefficient based on the multivariate model (penultimate column, Table 5) is a statistically significant −0.46.
Table 5. Individual-level condition-specific estimates based on bivariate and the best-fitting multivariate model in the total sample

Values are given as estimate (standard error).
a Nineteen models with one condition at a time adjusted by demographic controls.
b The ratio of the estimate based on the best-fitting model to the estimate based on the bivariate model.
c Mean co-morbidity is the mean number of other conditions reported by respondents with the condition in the row.
Simulated societal-level predictive associations of conditions with mean VAS scores
Societal-level associations are a joint function of prevalence and severity. We derived these estimates by multiplying individual-level estimates by the condition prevalence estimates to arrive at estimated associations of conditions with changes in mean VAS scores in the population (Table 6). Of the coefficients, eight differ significantly between developing and developed countries, all but one higher in developed countries. The median value of the coefficients is quite similar in developing [0.09 (IQR=0.03–0.23)] and developed [0.14 (IQR=0.07–0.40)] countries.
Table 6. Societal-level condition-specific estimates of effects on mean visual analog scale scores based on the best-fitting multivariate model for developed and developing countries

s.e., Standard error.
* p<0.05(two-sided test).
† Significant difference between developing and developed countries (p<0.05; two-sided test).
While most societal-level coefficients do not differ significantly by development, 74.8% of the 171 (19×18/2) differences between pairs of the 19 coefficients are statistically significant at the 0.05 level in the total sample. The Spearman rank-order correlation among these conditions between sets of countries is 0.80. The top five conditions are the same in developing and developed countries, although the rankings differ somewhat. These top conditions are dominated by high-prevalence conditions with intermediate magnitudes of individual-level effects (6th–13th ranks), with only chronic pain conditions major depression being in the top five in terms of magnitude of individual-level effects.
Discussion
A number of limitations must be considered in interpreting these results. First, only a restricted set of common conditions was included in the analysis and some were pooled to form larger disorder groups. A number of burdensome conditions, such as dementia and psychosis, were not included. Expansion and disaggregation is clearly needed in future research. Second, diagnoses of chronic physical conditions were based on self-reports that could have been biased. Such bias might account for the generally higher prevalence estimates of these conditions in developed than developing countries. Third, we focused on 12-month prevalence of conditions but 30-day health valuations, as these were the time-frames included in the WMH surveys. This difference in recall periods would be expected to lead to an underestimate of the severity of the active phases of episodic conditions (e.g. migraine), although it should yield an accurate estimate of the average severity of conditions in a typical month (30 days) of the year (12 months). A related limitation is that even a 12-month time-frame is relatively short compared with the time-frames used in some other health valuation studies (e.g. 10 years or lifetime).
Another limitation is that the highly skewed distribution of VAS scores and non-additive effects of co-morbid conditions might have led to instability of results. Even though we explored use of GLM rather than OLS and examined a number of different model specifications to capture effects of co-morbidity, it is possible that future research will discover better specifications either of functional form or of joint associations of co-morbid conditions with health valuations. In particular, the use of data mining techniques such as regression tree analysis (Breiman et al. Reference Breiman, Friedman, Olshen and Stone1984; Friedman, Reference Friedman1991; Breiman, Reference Breiman2001, Reference Breiman2009) might provide useful insights into better specification of interaction effects. A related limitation is that we assumed that the VAS is an interval scale. As noted above in the section on Analysis methods, this assumption has been called into question in some previous studies (Krabbe et al. Reference Krabbe, Stalmeier, Lamers and Busschbach2006; Parkin & Devlin, Reference Parkin and Devlin2006). Non-linear monotonic transformations have been proposed to approximate interval scale properties (Krabbe, Reference Krabbe2008; Craig et al. Reference Craig, Busschbach and Salomon2009). It would be very useful in future methodological research to explore the extent to which these different methods influence results.
Another limitation is that our estimates were based only on the overall adult population in developed and developing countries. The ratings of conditions might be quite different in different population segments (e.g. elderly, women, poor) or in different countries. Future research is needed to investigate these specifications. The use of anchoring vignettes has been shown to help address this problem (Salomon et al. Reference Salomon, Tandon and Murray2004). In addition, a number of statistical methods exist to improve the accuracy of comparisons across subsamples and populations that could profitably be used in future applications (Tandon et al. Reference Tandon, Murray, Salomon and King2002).
Another limitation is that our results are based on VAS scores assigned by respondents to their own health states rather than to health states based on hypothetical vignettes. While there is general agreement that perceptions of people in the general population should be taken into consideration in making health valuations (Gudex et al. Reference Gudex, Dolan, Kind and Williams1996), concerns have been raised that bias exists in the perceptual ratings of community respondents based on their own illness experiences (Stiggelbout & de Vogel-Voogt, Reference Stiggelbout and de Vogel-Voogt2008) and their familiarity with the experiences of people close to them (Krabbe et al. Reference Krabbe, Stalmeier, Lamers and Busschbach2006), resulting in a general preference for health valuations made by experts (Marquie et al. Reference Marquie, Raufaste, Lauque, Marine, Ecoiffier and Sorum2003). Furthermore, bias in self-reports in the WMH data might have been greater for mental than physical conditions because so many questions were asked in the survey about mental conditions and the VAS was administered only at the end of the survey. It would be useful to investigate this potential bias in future applications by randomizing the order of presentation of the VAS question in the survey. Methods have been developed to integrate VAS responses with responses based on other valuation methods (e.g. time trade-off, willingness to pay) that might also profitably be used in future studies to evaluate these biases (Salomon & Murray, Reference Salomon and Murray2004).
A less obvious limitation, finally, is that the simulation method evaluated marginal effects of individual conditions. This method can be faulted because it implicitly assumes that the presence versus absence of a single condition can be changed while holding constant all other conditions. This assumption would be plausible if all co-morbid conditions were either causes or risk markers (Kraemer et al. Reference Kraemer, Kazdin, Offord, Kessler, Jensen and Kupfer1997) of focal conditions. However, in cases where the co-morbid condition is a consequence of the focal condition or where two or more conditions are reciprocally related, the simulation method used here will underestimate the effect of the focal condition (assuming that co-morbidity is positive) by controlling for one or more of the intervening pathways through which that condition influences VAS scores.
This underestimation could be removed by deleting controls for all conditions that are thought to mediate the total effect of the focal condition. However, in the case where these co-morbid conditions are reciprocally related to the focal condition, exclusion of the co-morbid conditions from the prediction equation will lead to overestimation of the effect of the focal condition. The only plausible way to address that issue is to develop a methodology of partial control: that is, to control for the subset of co-morbid conditions that has causal effects on the focal conditions but not for the subset that occurs as a consequence of the focal condition. An innovative methodology known as g-estimation has been developed to do this (Young et al. Reference Young, Hernan, Picciotto and Robins2010), but this method requires access to large-scale longitudinal epidemiological data that monitor onset and course of co-morbid conditions over time. As a result of this data requirement, use of g-estimation has been minimal (Taubman et al. Reference Taubman, Robins, Mittleman and Hernan2009) and has never to our knowledge been used to study health valuation. This method is nonetheless very promising and deserves to be explored in future studies aimed at sorting out the effects of co-morbidity on health valuation.
Within the context of these limitations, our results show clearly that sensible estimates can be obtained of condition-specific effects on VAS while taking co-morbidity into consideration. As noted in the Introduction, a similar approach could be used to study informant ratings by using a series of hypothetical vignettes of people with co-morbid conditions rather than pure conditions. We find that the consideration of co-morbidity makes a substantial difference to ratings. In particular, condition-specific ratings are lower when co-morbidity is taken into consideration due to a general pattern of sub-additive interactions among co-morbid conditions in predicting VAS scores. This sub-additive pattern is consistent with the findings of the one other previous study we know that carried out a similar type of analysis (Verbrugge et al. Reference Verbrugge, Lepkowski and Imanaka1989). Furthermore, we found substantial between-condition variation in the extent to which adjustment for co-morbidity influences estimates.
Although the substantive findings regarding effects of individual conditions on VAS should be interpreted with caution given the limitations enumerated above, it is noteworthy that neurological conditions, insomnia and major depression were estimated to be the most severe conditions at the individual level. The neurological conditions we considered included epilepsy and seizure disorders, Parkinson's disease and multiple sclerosis, all of which have been shown to have high disability in previous studies (Singer et al. Reference Singer, Hopman and MacKenzie1999; Jacoby & Baker, Reference Jacoby and Baker2008). The high ranking of insomnia is surprising because previous studies, although documenting a high societal-level burden of insomnia, have generally found this to be due to high prevalence in conjunction with moderate individual-level burden rather than to high individual-level burden (Roth et al. Reference Roth, Jaeger, Jin, Kalsekar, Stang and Kessler2006). The high individual-level severity of insomnia in our study probably lies in the fact that we required a greater sleep disruption (at least 2 h of either delay in sleep onset or disruption in sleep maintenance per night most nights of the week for at least 1 month in the previous year) than previous studies of insomnia (Ohayon, Reference Ohayon2002). The high individual-level estimate we found for depression, finally, is consistent with much previous research (Donohue & Pincus, Reference Donohue and Pincus2007; Wang et al. Reference Wang, Simon and Kessler2008; Gabilondo et al. Reference Gabilondo, Rojas-Farreras, Vilagut, Haro, Fernandez, Pinto-Meza and Alonso2010).
The rank-ordering of the individual-level VAS estimates was found to be quite similar in developing and developed countries. However, several exceptions were found. These should be investigated in future studies. Digestive conditions (stomach/intestine ulcer and irritable bowel disorder) were rated considerably more severe in developed than developing countries, possibly reflecting a different mix of cases that might explain the differences in estimated severity. The individual-level estimated severity of drug abuse, in comparison, was substantially higher in developing than developed countries. Differential willingness to admit drug problems might have been involved in this result, as reported prevalence of drug abuse was much lower in developing than developed countries, possibly indicating that the cases we learned of in developing countries were more severe than those in developed countries (Schmidt & Room, Reference Schmidt and Room1999).
Comparison of our individual-level condition severity estimates with estimates in an earlier WMH analysis of condition-specific role impairment (Ormel et al. Reference Ormel, Petukhova, Chatterji, Aguilar-Gaxiola, Alonso, Angermeyer, Bromet, Burger, Demyttenaere, de Girolamo, Haro, Hwang, Karam, Kawakami, Lepine, Medina-Mora, Posada-Villa, Sampson, Scott, Ustun, Von Korff, Williams, Zhang and Kessler2008) finds that the conditions rated most severe in that earlier study were generally also rated among the most severe in the current investigation. However, a number of differences in relative ratings exist that could be attributed either to differences in the outcome (i.e. a global VAS score versus a measure of condition-specific role impairment) or to our previous analysis not adjusting for co-morbidity.
Our results regarding societal-level associations are less innovative because, consistent with previous studies, we merely multiplied the prevalence estimates of the conditions with the individual-level estimates of condition severity to arrive at societal-level estimates of burden. As in previous studies that compared individual-level and societal-level estimates (Whiteford, Reference Whiteford, Andrews and Henderson2000; Andlin-Sobocki et al. Reference Andlin-Sobocki, Jonsson, Wittchen and Olesen2005; Saarni et al. Reference Saarni, Suvisaari, Sintonen, Pirkola, Koskinen, Aromaa and Lonnqvist2007), the rank-ordering of conditions differs considerably between the two, with societal-level estimates influenced importantly by variation in prevalence and the conditions estimated to be most burdensome at the societal level dominated by high-prevalence conditions.
While our results argue clearly for the importance of considering co-morbidity when estimating disease burden, the best way to do this is not obvious. The approach we took here has the advantage of considering co-morbidities in their true distribution in the population rather than requiring hypothetical scenarios to be generated that might or might not adequately characterize the actual distribution of complex co-morbidities in the population. However, methods also exist to allow the effects of individual conditions to be estimated using expert ratings of hypothetical patient scenarios that include information about complex profiles of co-morbidity (Jasso, Reference Jasso2006; Saarni et al. Reference Saarni, Suvisaari, Sintonen, Pirkola, Koskinen, Aromaa and Lonnqvist2007). Indeed, the actual distributions of co-morbidity found in community surveys like the WMH surveys could be used to generate these vignettes so as to guarantee that they represent the distribution and range of patterns in the population. As many health policy researchers favor condition severity ratings made by experts rather than the ratings made by respondents in community surveys for a variety of other reasons (Insinga & Fryback, Reference Insinga and Fryback2003; Marquie et al. Reference Marquie, Raufaste, Lauque, Marine, Ecoiffier and Sorum2003; Ormel et al. Reference Ormel, Petukhova, Chatterji, Aguilar-Gaxiola, Alonso, Angermeyer, Bromet, Burger, Demyttenaere, de Girolamo, Haro, Hwang, Karam, Kawakami, Lepine, Medina-Mora, Posada-Villa, Sampson, Scott, Ustun, Von Korff, Williams, Zhang and Kessler2008; Schnadig et al. Reference Schnadig, Fromme, Loprinzi, Sloan, Mori, Li and Beer2008), it might be that the best approach would be to build information about co-morbidity into conventional expert rating scenarios. However, valuations of the sort presented here based on community samples also would seem to have value in representing the perceptions of actual people with real conditions in the population. It remains a challenge for the field to develop a way of integrating data of these different sorts.
Acknowledgements
The analysis for this paper was carried out in conjunction with the WHO WMH Survey Initiative. We thank the WMH staff for assistance with instrumentation, fieldwork and data analysis. These activities were supported by the United States National Institute of Mental Health (R01MH070884), the Mental Health Burden Study (contract number HHSN271200700030C), the John D. and Catherine T. MacArthur Foundation, the Pfizer Foundation, the US Public Health Service (R13-MH066849, R01-MH069864 and R01 DA016558), the Fogarty International Center (FIRCA R03-TW006481), the Pan American Health Organization, the Eli Lilly & Company Foundation, Ortho-McNeil Pharmaceutical, Inc., GlaxoSmithKline, Bristol-Myers Squibb and Shire. A complete list of WMH publications can be found at http://www.hcp.med.harvard.edu/wmh/.
The Chinese WMH Survey Initiative is supported by the Pfizer Foundation. The Colombian National Study of Mental Health (NSMH) is supported by the Ministry of Social Protection. The European Study of the Epidemiology of Mental Disorders (ESEMeD) project is funded by the European Commission (contracts QLG5-1999-01042; SANCO 2004123), the Piedmont Region (Italy), Fondo de Investigación Sanitaria, Instituto de Salud Carlos III, Spain (FIS 00/0028), Ministerio de Ciencia y Tecnología, Spain (SAF 2000-158-CE), Departament de Salut, Generalitat de Catalunya, Spain, Instituto de Salud Carlos III (CIBER CB06/02/0046, RETICS RD06/0011 REM-TAP), and other local agencies and by an unrestricted educational grant from GlaxoSmithKline. The Israel National Health Survey is funded by the Ministry of Health with support from the Israel National Institute for Health Policy and Health Services Research and the National Insurance Institute of Israel. The WMH Japan (WMHJ) Survey is supported by the Grant for Research on Psychiatric and Neurological Diseases and Mental Health (H13-SHOGAI-023, H14-TOKUBETSU-026, H16-KOKORO-013) from the Japan Ministry of Health, Labor and Welfare. The Lebanese National Mental Health Survey (Lebanese Evaluation of the Burden of Ailments and Needs of the Nation; LEBANON) is supported by the Lebanese Ministry of Public Health, the WHO (Lebanon), Fogarty International, Act for Lebanon, anonymous private donations to the Institute for Development, Research, Advocacy and Applied Care (IDRAAC), Lebanon, and unrestricted grants from Janssen Cilag, Eli Lilly, GlaxoSmithKline, Roche and Novartis. The Mexican National Co-morbidity Survey (MNCS) is supported by The National Institute of Psychiatry Ramon de la Fuente (INPRFMDIES 4280) and by the National Council on Science and Technology (CONACyT-G30544-H), with supplemental support from the PanAmerican Health Organization (PAHO). The Nigerian Survey of Mental Health and Wellbeing (NSMHW) is supported by the WHO (Geneva), the WHO (Nigeria) and the Federal Ministry of Health, Abuja, Nigeria. The Ukraine Comorbid Mental Disorders during Periods of Social Disruption (CMDPSD) study is funded by the US National Institute of Mental Health (RO1-MH61905). The US National Co-morbidity Survey Replication (NCS-R) is supported by the National Institute of Mental Health (NIMH; U01-MH60220) with supplemental support from the National Institute of Drug Abuse (NIDA), the Substance Abuse and Mental Health Services Administration (SAMHSA), the Robert Wood Johnson Foundation (RWJF; grant 044708) and the John W. Alden Trust.
Declaration of Interest
R.C.K. has been a consultant for GlaxoSmithKline Inc., Kaiser Permanente, Pfizer Inc., Sanofi-Aventis, Shire Pharmaceuticals and Wyeth-Ayerst; has served on advisory boards for Eli Lilly & Company and Wyeth-Ayerst; and has had research support for his epidemiological studies from Bristol-Myers Squibb, Eli Lilly & Company, GlaxoSmithKline, Johnson & Johnson Pharmaceuticals, Ortho-McNeil Pharmaceuticals Inc., Pfizer Inc. and Sanofi-Aventis.








