Introduction
Bipolar disorder (BD) is a complex mental disorder with multidimensional psychopathology and lifetime prevalence rates estimated at approximately 2–4% (Ketter, Reference Ketter2010; Merikangas et al., Reference Merikangas, Jin, He, Kessler, Lee, Sampson and Zarkov2011). Growing evidence has revealed that patients with BD exhibit prominent cognitive impairment, especially in the domains of executive function (Grande, Berk, Birmaher, & Vieta, Reference Grande, Berk, Birmaher and Vieta2016; Miskowiak et al., Reference Miskowiak, Burdick, Martinez-Aran, Bonnin, Bowie, Carvalho and Vieta2017). This executive dysfunction includes abnormalities in impulsivity, decision-making and risk-taking behaviours among others. Thus, high levels of impulsivity, sub-optimal decision-making and potentially dangerous risky behaviours appear to be frequent components in the course of BD in its various phases and have been proposed as promising endophenotypes (core features) of the disease (Chamorro et al., Reference Chamorro, Bernardi, Potenza, Grant, Marsh, Wang and Blanco2012; Christodoulou, Lewis, Ploubidis, & Frangou, Reference Christodoulou, Lewis, Ploubidis and Frangou2006; Najt et al., Reference Najt, Perez, Sanches, Peluso, Glahn and Soares2007).
Impulsivity is a multidimensional construct that can be defined as a predisposition to rapid, unplanned reactions to internal or external stimuli that fail to take into account the negative consequences of those reactions to the individual him-/herself or to others (Moeller, Barratt, Dougherty, Schmitz, & Swann, Reference Moeller, Barratt, Dougherty, Schmitz and Swann2001). Some authors have suggested that behavioural manifestations of this domain include three different components: (a) response inhibition: involving the ability to withhold a speeded motor response prior to its initiation or the ability to cancel a response after it has been initiated (Wright, Lipszyc, Dupuis, Thayapararajah, & Schachar, Reference Wright, Lipszyc, Dupuis, Thayapararajah and Schachar2014); (b) delay of gratification: preference for immediately-available small rewards v. large but delayed rewards; and (c) inattention: the inability to maintain one's attention in order to complete a task without being distracted (Strakowski et al., Reference Strakowski, Fleck, Delbello, Adler, Shear, Mcelroy and Arndt2009, Reference Strakowski, Fleck, Delbello, Caleb, Shear, Kotwal and Arndt2010). An extensive body of research has studied the multiple dimensions of this domain using self-report measures (e.g. the Barratt Impulsivity Scale); conversely, studies that have explored impulsivity using laboratory-based behavioural measures are limited and their results somewhat are contradictory (Christodoulou et al., Reference Christodoulou, Lewis, Ploubidis and Frangou2006; Powers et al., Reference Powers, Russo, Mahon, Brand, Braga, Malhotra and Burdick2013). To our knowledge, no meta-analysis has specifically investigated behavioural impulsivity in BD, and only two previous reviews have included studies that used both self-report and cognitive tasks measures. For example, Najt et al. (Reference Najt, Perez, Sanches, Peluso, Glahn and Soares2007) found self-reported impulsivity to be higher among BD patients compared with healthy controls, regardless of the phase of the illness. A second review explored impulsivity in the euthymic stage of BD, analysing both self-reported impulsivity and two commonly identified behavioural manifestations of impulsivity: response inhibition and the ability to delay gratification (Newman & Meyer, Reference Newman and Meyer2014). It is found that most studies using self-report measures reported significant differences between euthymic BD patients and healthy controls, whereas there was little evidence of higher impulsivity when measured by behavioural paradigms. Therefore, although there is consistent evidence that patients with BD report higher impulsivity when self-ratings are used (Najt et al., Reference Najt, Perez, Sanches, Peluso, Glahn and Soares2007; Saddichha & Schuetz, Reference Saddichha and Schuetz2014), the evidence when behavioural paradigms are applied is contradictory.
Decision-making impairment has also been a persistent finding in BD. This construct is defined as a complex set of cognitive processes which allow individuals to select the most optimal course of action following consideration of existing alternatives (Bechara, Reference Bechara2005). Recent studies have identified at least two distinct forms of reward-based decision making: (a) decision-making under risk, measured by performance in tasks with explicit outcome probabilities; and (b) decision-making under ambiguity, measured by performance in tasks with implicit outcome probabilities (Wilson & Vassileva, Reference Wilson and Vassileva2018). Overall, studies tended to report poorer outcomes on decision-making in BD patients compared with healthy controls (Powers et al., Reference Powers, Russo, Mahon, Brand, Braga, Malhotra and Burdick2013; Roiser et al., Reference Roiser, Farmer, Lam, Burke, O'Neill, Keating and McGuffin2009), but contrary results have also been found. A meta-analysis by Samamé, Martino, and Strejilevich (Reference Samamé, Martino and Strejilevich2012) found decision-making abilities to be preserved in patients with euthymic BD. In the same vein, the findings of a later meta-analysis (Edge, Johnson, Ng, & Carver, Reference Edge, Johnson, Ng and Carver2013) indicated that bipolar and control groups do not differ on the total number of risky choices they make. It is important to point out that both studies only investigated the euthymic phase and did not investigate either mania or depression states. A third meta-analysis, however, suggested the existence of various cognitive profiles of decision-making in patients diagnosed with BD (Jiménez et al., Reference Jiménez, Solé, Arias, Mitjans, Varo, Reinares and Benabarre2018). Hence, decision-making ability in BD patients remains unclear, and to date, no meta-analytic studies have reviewed the findings of empirical studies of the three phases of the disorder.
Finally, although risk-taking behaviour is often part of the clinical presentation of BD, very few studies have formally assessed risk-taking propensity in these patients. A popular laboratory procedure used for studying risk-taking involves gambling tasks, where subjects are asked to choose between safe and risky alternatives. Participants can usually choose among several options that differ in the chance for a reward or penalty. The exact probability distribution of the outcome can be evident for the participant (explicit) or not (implicit). Among the few studies that use these gambling tasks, the findings are inconsistent (Chamorro et al., Reference Chamorro, Bernardi, Potenza, Grant, Marsh, Wang and Blanco2012; Holmes et al., Reference Holmes, Bearden, Barguil, Fonseca, Monkul, Nery and Glahn2009). Likewise, no meta-analysis or review has tried to integrate the various findings. Consequently, our knowledge of risk-taking behaviour in BD patients understood as situationally determined behaviours with a high potential for harm or loss and simultaneous opportunity to obtain some form of reward (Leigh, Reference Leigh1999), is still scant.
Despite the robust body of work on cognitive aspects of BD, a clear profile of deficits in associated impulsivity, decision-making and risk-taking behaviour in BD patients has yet to be established. The aim of this study was to conduct a systematic review and meta-analysis of the degree of impairment of these domains in individuals with BD during symptomatic and remitted states, focusing on studies that used a behavioural paradigm. By considering behavioural measures only, our study provides objective results – thereby avoiding the possible biases related to the subjectivity that self-report measures entail – as well as information on certain facets that questionnaires do not detect. Understanding the extent to which impulsivity, decision-making and risk-taking behaviour are affected in individuals with BD will help us to better define the cognitive profile and core features of this disorder.
Methodology
Identification of the studies
We performed a systematic review and a meta-analysis in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) statement (Moher, Liberati, Tetzlaff, & Altman, Reference Moher, Liberati, Tetzlaff and Altman2009). The protocol was registered in the PROSPERO database (CRD42018114684). Two investigators (AR and JR) independently conducted literature searches on the PsycINFO, PubMed, ScienceDirect and Scopus databases during the period October–December 2018. Three independent searches were performed on each database, according to the following title/abstract sections: ‘impulsivity OR impulsive behaviour OR impulsiveness AND bipolar disorder’; ‘decision-making AND bipolar disorder’; ‘risk behaviour OR risk-taking AND bipolar disorder’. All papers published in either English or Spanish between January 1999 and December 2018 were considered.
The following inclusion criteria were applied: (a) type of study: cohort or case-control studies; (b) age range: adult subjects (aged 18–70 years); (c) diagnosis: any bipolar disorder (BD-I, BD-II, BD-NOS) in any of its phases (mania, depression or euthymia) diagnosed using internationally accepted assessment instruments, including clinical interviews that applied the criteria of the Diagnostic and Statistical Manual of Mental Disorders (DSM) or the International Statistical Classification of Diseases and Related Health Problems (ICD); (d) evaluation of cognitive domains performed using behavioural measures; (e) published during the period 1999–2018; (f) language: English or Spanish only. The following exclusion criteria were applied: (a) narrative or systematic reviews, qualitative or case studies; (b) children, the young and the elderly (i.e. <18 years and >70 years); (c) adult subjects with any other psychiatric disorder; (d) studies assessing impulsivity as a personality trait; (e) studies evaluating the cognitive domain using questionnaires or self-reports; (f) studies using non-standardised tests; (g) studies whose data were duplicated or not original; (h) studies offering no data for meta-analysis.
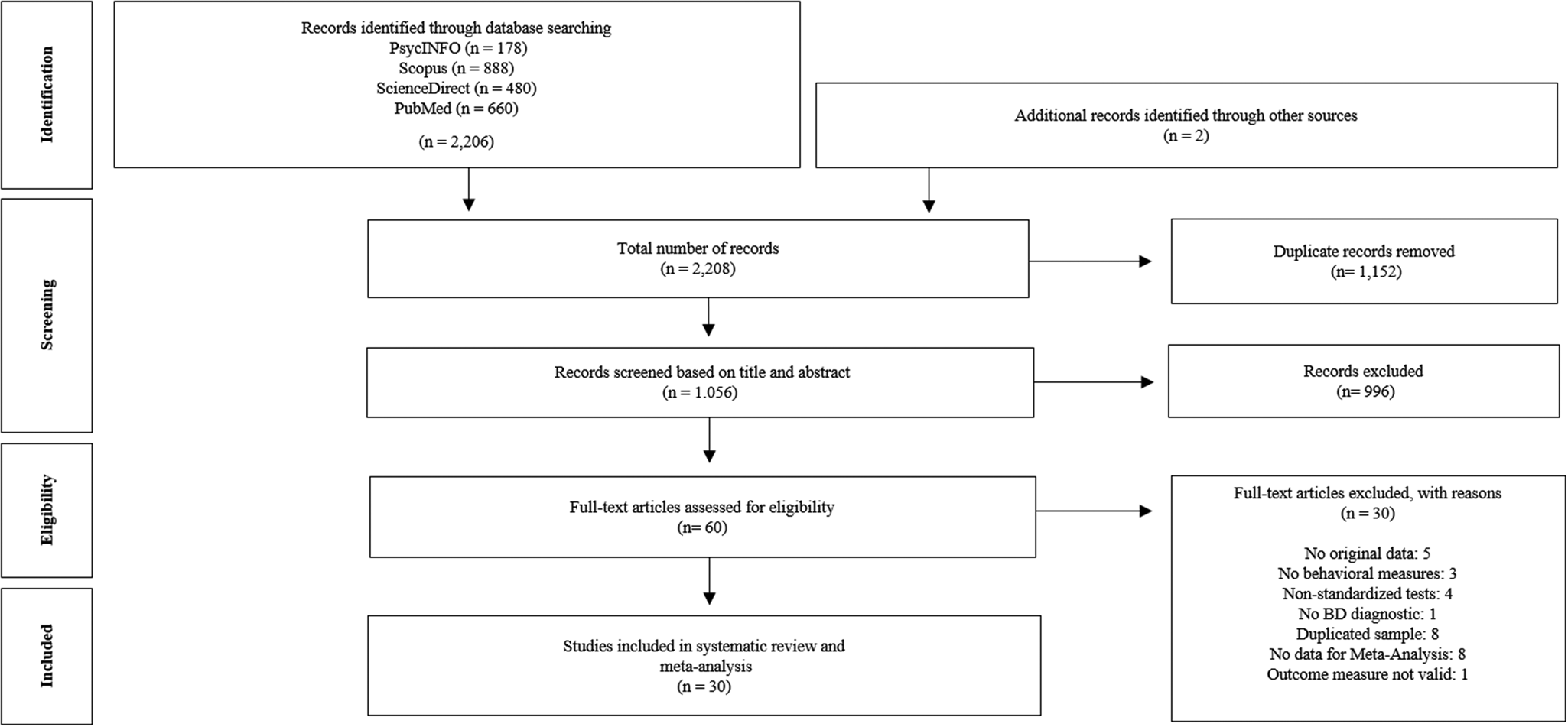
The search of the databases generated 2206 results. A further two studies were identified after searching the references of the selected articles. We excluded 1152 duplicate articles, leaving 1056 to be reviewed by title and abstract. Of these, 996 were excluded because they did not meet the inclusion criteria. Of the remaining 60 full-text articles, 30 were excluded for the following reasons: (a) no original data (n = 5); (b) no behavioural measures (n = 3); (c) use of non-standardised tests (n = 4); (d) no BD diagnosis (n = 1); (e) use of a duplicate sample (n = 8); (f) no data for meta-analysis (n = 8); and (g) outcome measure not valid (n = 1). More detailed information about the excluded studies is shown in the online Supplementary Table S1. Any differences of opinion as to which studies were to be included or excluded were resolved through discussion, with the intervention of two additional authors. In the end, a total of 30 publications were eligible for review and were included in the meta-analysis. The results of the search strategy are summarised in a flow diagram (Fig. 1).

Fig. 1. Study selection flow chart for systematic review and meta-analysis.
Data extraction and quality assessment
The two researchers independently abstracted the data from the selected articles, collecting the following information: first author, year of publication, sample, mean age of participants, phase of illness, neurocognitive test, neurocognitive outcome, results, age range, gender, study design, neuroimaging test (use of a neuroimaging test during the study), matching of controls, study quality and funding. A description of this information can be found in the supplementary material (online Supplementary Table S2). Article quality was assessed using the Newcastle–Ottawa quality assessment scale (NOS) (Wells et al., Reference Wells, Shea, Connell, Peterson, Welch, Losos and Tugwell2014). Details of the NOS can be seen in the supplementary material (online Supplementary Table S3).
Statistical analysis
For the meta-analysis, we calculated bias-corrected standardised mean differences (Hedges' g). We analysed the studies in accordance with fixed-effect and random-effects models with the inverse variance method, calculating 95% confidence intervals and p values. We analysed heterogeneity by calculating the estimated between-study variance T2, H and I 2 and the p value using Cochran's Q statistic. We performed separate meta-analyses for decision-making, risk-taking and each construct of impulsivity (response inhibition, delay of gratification and inattention). Stratified analysis was also carried out on phase of illness (euthymia, depression or mania) and diagnosis subtype (BD I or other), where there were at least three studies with these conditions. In addition, we conducted individual task (Balloon Analogue Risk Task and Cambridge Gambling Task) meta-analyses for risk-taking as they are not completely equivalent measures. For domains (decision-making, risk-taking and each construct of impulsivity) with at least ten studies, the risk of publication bias was calculated using funnel plots, Egger's test (Egger, Davey Smith, Schneider, & Minder, Reference Egger, Davey Smith, Schneider and Minder1997) and the rank correlation test (Begg & Mazumdar, Reference Begg and Mazumdar1994). All the analyses were calculated using R version 3.5.1 and the package Meta (Schwarzer, Carpenter, & Rücker, Reference Schwarzer, Carpenter and Rücker2015).
Results
A total of 30 studies (numbered to improve readability, see Table 1) were included in the meta-analysis. Table 1 summarises the main results and characteristics of the studies included. More information about the studies can be found in the supplementary material (online Supplementary Table S4).
Table 1. Main results and characteristics of the selected studies

BD, bipolar disorder; BD-I, bipolar disorder type I; BD-II, bipolar disorder type II; BD-R, bipolar disorder relatives; HC, healthy controls; RTs, reaction time; IGT, Iowa Gambling Task; DRT, delayed reward task; DSCPT, degraded stimulus version of the Continuous Performance Test; SST, Stop-Signal Task; IMT, Immediate Memory Task; DMT, Delayed Memory Task; SKIP, Single Key Impulsivity Paradigm; CGT, Cambridge Gamble Task; CPT-II, Continuous Performance Test II.
The studies were conducted on adult populations in various countries: USA [2,3,8,9,11,12,24,27,28,30], England [14,22,25], France [15,20], Germany [16,17,26], Brazil [18,21], Argentina [13,19], Italy [4], Canada [6], Egypt [23], Israel [7] and Turkey [10]. Two studies included the populations of more than one country [1,5] and one failed to specify the nationality of its population [29]. Those that specified the particular locus of their study reported that it was carried out in a hospital context; two studies did not provide this information [26,28].
Combined, the studies provided data on 1628 participants with BD, 47 BD-patient relatives and 1319 controls, with an age range of 18–67 years. Among the patients, individuals were either in a euthymic state or remission, a depressive state, a manic or a hypomanic state, or a mixed state. A total of 28 studies were exclusively cross-sectional; the remaining two [16,27] included, in addition to a cross-sectional component, a follow-up component examining a subsample of the study population in an acute illness phase and subsequent remission. Neuroimaging tests were used in 10 of the 30 studies [9,12,13,14,15,16,17,18,20,26].
In terms of the behavioural measures used in the studies: nine used the Iowa Gambling Task [1,5,8,13,14,18,19,21,30]; six used the Go/No-Go Task in its various forms [3,6,9,12,15,20]; four used the Cambridge Gambling Task [3,17,26,29]; three used the Continuous Performance Test [18,21,23]; three used the Balloon Analogue Risk Task [10,11,24]; two used the Stop Signal Task [4,27]; two used the Single Key Impulsivity Paradigm [7,28]; two used the Decision-making Task [22,25]; and four employed alternative methods, including the Delay Discounting Task [2], the Combined Stop-Signal-Go/No-Go Task [16], the Immediate Memory Task [28], the Delayed reward task and the Degraded stimulus version of the Continuous Performance Test [27]. Online Supplementary Table S5 shows which cognitive domain was measured by each neurocognitive test.
Twenty-two studies were of good methodological quality, having a score equal to or greater than 7 on the Newcastle–Ottawa Scale [1,4,5,6,7,9,10,12,13,14,15,16,17,19,20,22,23,25,26,27,29,30]. The other eight were of medium quality, having a score of 5 or 6 [2,3,8,11,18,21,24,28].
Impulsivity
It is important to note that behavioural measures of impulsivity can be subdivided into those that explore response inhibition, those that explore the ability to delay gratification and those that explore inattention. Some studies evaluated more than one modality of impulsivity at the same time.
Response inhibition
A measure of response inhibition was provided by 13 studies [3,4,6,9,12,15,16,18,20,21,23,27,28]. In six of them [3,9,18,21,23,28], BD patients performed significantly worse than healthy controls did in response inhibition tests, whereas in five studies no significant difference between the groups was found [4,6,12,15,20]. The remaining two studies, however, showed disparate results: in one, the results indicated that BD patients differed significantly from controls when the former was depressed but not when they were in remission [16]; in the other, bipolar manic/mixed subjects differed significantly from healthy subjects on the response inhibition task, but bipolar depressed and euthymic patients did not [27]. There is a moderate risk of bias, as no study had the maximum score of 9 in the quality assessment and four studies (30.8%) were not considered of high quality (score under 7).
In the meta-analysis there was no significant heterogeneity between studies [τ 2 = 0.0146; H = 1.16 (1.00–1.62); I 2 = 26.1% (0.0–61.7%); Q = 16.24, p = 0.180] and the pooled effect size was 0.49 [fixed-effect model; CI (0.38–0.60); z = 8.92; p < 0.0001]. There was no evidence of publication bias in the funnel plot (online Supplementary Fig. S1) and Egger's test was not significant (p = 0.6058). The effect size in the subgroups were very similar and significant in the separate meta-analyses of euthymic [fixed-effect model; SMD = 0.59; CI (0.43–0.75); z = 7.23; p < 0.0001], depressed [fixed-effect model; SMD = 0.48; CI (0.16–0.81); z = 2.92; p = 0.0035] and manic [fixed-effect model; SMD = 0.47; CI (0.21–0.74); z = 3.49; p = 0.0005] samples, but not significant for the subgroup of the BD I and euthymic sample [fixed-effect model; SMD = 0.29; CI (−0.07 to 0.65); z = 1.59; p = 0.1121]. More detailed results are shown in the forest plot in Fig. 2. To control possible bias, we also conducted a meta-analysis without the studies that used the Continuous Performance Test as a dependent measure in Inattention. The results were very similar [fixed-effect model; SMD = 0.40; CI (0.26–0.53); z = 5.84; p < 0.0001]. More information is shown in the forest plot in online Supplementary Fig. S2.

Fig. 2. Forest plots of impulsivity (response inhibition, delay of gratification and inattention) in the total sample and in a subset of euthymic, depressed, manic and bipolar disorder I patients.
Delay of gratification
Five studies evaluated the ability to delay gratification [2,3,7,27,28]. Three of them [2,3,28] found that inability to delay gratification for a larger reward was greater among the BD patients than in the control group. One study found no significant difference between the BD patients in the euthymic phase and the healthy subjects [7]. Other found that BD manic/mixed and depressed patients exhibited more impulsive responding than healthy subjects, but found no such difference between healthy subjects and euthymic patients [27]. The risk of bias was high as three studies (60.0%) were not considered of high quality and no study had the maximum score of 9.
In the meta-analysis there was no heterogeneity between studies [τ 2 = 0.0191; H = 1.26 (1.00–2.06); I 2 = 36.9% (0.0–76.5%); Q = 6.34, p = 0.175] and the pooled effect size was 0.54 [fixed-effect model; CI (0.39–0.70); z = 6.97; p < 0.0001]. More detailed results are shown in Fig. 2.
To control possible bias, we conducted the meta-analysis without the studies that use Cambridge Gambling Task, since it had already been considered in decision-making behaviour. Results were very similar [fixed-effect model; SMD = 0.65; CI (0.46–0.84); z = 6.60; p < 0.0001]. More information is shown in online Supplementary Fig. S3.
Inattention
Inattention was measured by four studies [18,21,23,27]. Three of them found increases in omission errors in patients with BD compared with healthy subjects [18,21,23]. The remaining study found significant differences in a measure of inattention between BD manic subjects and controls. Nevertheless, no significant differences were found between depressive or euthymic BD patients and healthy subjects [27]. The risk of bias was high as two studies (50.0%) were not considered of high quality and all studies scored under 8.
In the meta-analysis there was no significant heterogeneity between studies [τ 2 = 0; H = 1.00 (1.00–1.23); I 2 = 0.0% (0.0–33.6%); Q = 0.69, p = 0.875] and the pooled effect size was 0.49 [fixed-effect model; CI (0.33–0.65); z = 5.92; p < 0.0001]. More detailed results are shown in the forest plot in Fig. 2.
Decision-making
In total, 13 of the 30 studies in this meta-analysis looked specifically at the decision-making of BD patients [1,3,5,8,13,14,18,19,21,22,25,29,30]. Eight studies found significant differences between BD patients and healthy subjects [1,3,5,18,21,24,25,30]. There were no significant differences in decision-making performance in five studies [8,13,14,19,29]. We consider that there exists a moderate risk of bias as no study had the maximum score and four studies (30.8%) were not considered of high quality in the assessment.
In the meta-analysis there was significant heterogeneity between the studies [τ 2 = 0.2847; H = 2.98 (2.39–3.70); I 2 = 88.7% (82.5–92.7%); Q = 106.26, p < 0.0001] and the pooled effect size was 0.61 [random-effects model; CI (−0.93 to −0.28); z = −3.68; p = 0.0002]. There was no evidence of publication bias from the funnel plot (online Supplementary Fig. S1) or Egger's test (p = 0.2454). In the meta-analysis, the effect size was large but not significant for patients in the depressed phase [random-effects model; SMD = −0.92; CI (−2.99 to 1.15); z = −0.87; p = 0.3828] and large and significant for patients in the manic phase [random-effects model; SMD = −1.35; CI (−2.43 to −0.27); z = −2.45; p = 0.0143] but with significant heterogeneity between studies and a high risk of publication bias. This is due to the fact that the meta-analysis only included studies with small sample size and there were important differences in effect sizes among them. There was a small but significant effect size for euthymic patients [fixed-effect model; SMD = −0.39; CI (−0.51 to −0.27); z = −6.39; p < 0.0001] and for the subgroup of BD I and euthymic patients [fixed-effect model; SMD = −0.25; CI (−0.41 to −0.10); z = −3.22; p = 0.0013]. There was no significant heterogeneity between the studies. More detailed results are shown in the forest plot in Fig. 3.

Fig. 3. Forest plots of decision-making in the total sample and in a subset of euthymic, depressed, manic and bipolar disorder I patients.
As the majority of studies used behavioural measure as the Iowa Gambling Task, we conducted a meta-analysis with only those studies and the effect size was lower [fixed-effects model; SMD = −0.43; CI (−0.55 to −0.32); z = −7.49; p < 0.0001] in comparison with the complete meta-analysis, however, heterogeneity was not significant. Detailed information is shown in online Supplementary Fig. S4.
Risk-taking behaviour
Only six of the 30 studies reported on risk-taking behaviour measures [3,10,11,17,24,26]. Four [3,10,17,26] found significantly lower risk adjustment in BD patients compared with healthy subjects; the other two showed disparate results [11,24]. The risk of bias was high as three studies (50.0%) were not considered of high quality.
In the meta-analysis there was significant heterogeneity between the studies [τ 2 = 0.2230; H = 2.31 (1.58–3.39); I 2 = 81.3% (60.0–91.3%); Q = 26.75, p < 0.0001] and the pooled effect size was 0.41 [random-effects model; CI (−0.02 to 0.84); z = 1.88; p = 0.0598] and only marginally significant for the entire sample. However, the effect size was large and statistically significant for the subgroup of BD I and euthymic patients [fixed-effect model; SMD = 0.92; CI (0.57–1.26); z = 5.24; p < 0.0001] with no significant heterogeneity. More detailed results are shown in the forest plot in Fig. 4.

Fig. 4. Forest plots of risk-taking behaviour in the total sample and in a subset of euthymic and bipolar disorder I patients.
In individual task meta-analyses, the effect size was very similar and not significant for Balloon Analogue Risk Task [random-effects model; SMD = 0.43; CI (−0.35 to 1.20); z = 1.08; p = 0.2799] and Cambridge Gambling Task [random-effects model; SMD = 0.41; CI (−0.22 to 1.04); z = 1.28; p = 0.1992]. More detailed results are shown in the forest plot in the supplementary material (online Supplementary Fig. S2).
A summary of the main results of different meta-analyses is shown in the supplementary material (online Supplementary Table S6).
Discussion
The present study synthesised and contrasted the findings of 30 case-control studies of BD individuals subjected to neuropsychological evaluations of their impulsivity, decision-making and risk-taking behaviour by way of behavioural tests. To the best of our knowledge, this is the first systematic review and meta-analysis that has simultaneously analysed these three cognitive domains in adults with BD considering behavioural measures only. Previous reviews have considered both behavioural measures and questionnaires or self-report measures.
Impulsivity
Looking at the results obtained, impulsivity was higher among BD subjects compared with healthy controls and appear to be present in all phases of the illness. Manic, depressive and euthymic bipolar patients' performance was worse than that of healthy controls on response inhibition. They also showed evidence of impairment in the delay of gratification and poorer performance on inattention tasks; however, there were not enough studies to analyse the subgroups separately here.
Our findings are consistent with those of a previous review suggesting that high impulsivity is a core feature of BD that persists even after manic and depressed symptoms are in remission (Najt et al., Reference Najt, Perez, Sanches, Peluso, Glahn and Soares2007). In contrast, a review carried out by Newman and Meyer (Reference Newman and Meyer2014) failed to detect significant differences in behavioural manifestations of impulsivity between euthymic BD patients and controls. Their findings did not coincide with those found in our study, maybe, due to the limited number of studies that used behavioural paradigms included in their review and the reduced sample size, as our meta-analysis included three cross-sectional studies [4,6,23] published after Newman and Meyer's (Reference Newman and Meyer2014) review, as well as a further two that they did not include [12,21].
This same study also reported significant differences in impulsivity between euthymic patients and healthy controls when self-report measures were considered. Indeed, an extensive body of research has observed high impulsivity in BD in all its phases using self-report measures (Henna et al., Reference Henna, Hatch, Nicoletti, Swann, Zunta-Soares and Soares2013; Saddichha & Schuetz, Reference Saddichha and Schuetz2014). Our results, therefore, support this body of research by extending similar findings to behavioural measures of impulsivity. In summary, impulsivity does appear to be affected in adult BD subjects in all phases of the disorder, suggesting that impulsive behaviour in BD is relatively independent of mood state.
Decision-making
Our meta-analyses results suggest a deficit in decision-making in BD, and the necessity to analyse if the impairment in decision-making has a state-dependent component. The differences found in decision-making were mainly related to the number of correct decisions made by subjects. Our results suggest that individuals diagnosed with BD make optimal choices significantly less often than healthy controls.
We found high heterogeneity between studies when we included the entire sample and the subgroup with symptoms of mania, indicating that the studies were not comparable and, together with the moderately risk of bias, suggested that the results should be interpreted with caution.
In manic and depressive phases, the performance of BD subjects tended to be worse than that of healthy controls with large size effect [22, 25], although for the depression phase this difference was not significant and for the manic phase heterogeneity was significant and the risk of publication bias was high. With respect to the euthymic phase, our results indicated here too that the performance of BD patients was worse but with small effect size than that of healthy controls. Nevertheless, this finding was not significant in the studies conducted by Samamé et al. (Reference Samamé, Martino and Strejilevich2012) and Edge et al. (Reference Edge, Johnson, Ng and Carver2013). This divergence in euthymic phase results might be explained by the fact that our meta-analysis included four studies published after the meta-analyses by Samamé et al. (Reference Samamé, Martino and Strejilevich2012) and Edge et al. (Reference Edge, Johnson, Ng and Carver2013). Finally, our findings are consistent with a recent analysis suggesting the existence of different cognitive profiles of decision-making in patients diagnosed with BD (Jiménez et al., Reference Jiménez, Solé, Arias, Mitjans, Varo, Reinares and Benabarre2018). Future studies should attempt to answer the question whether the heterogeneity in the results is influenced by the role of other variables, such as medication and comorbidity with other psychiatric disorders.
Risk-taking behaviour
Our meta-analysis results indicated that there are no significant differences between BD subjects and healthy controls in terms of risk-taking behaviour. A similar pattern of findings was obtained in stratified analysis for individual tasks (Balloon Analogue Risk Task and Cambridge Gambling Task). Nevertheless, these findings should be interpreted with caution as the studies included were few, showed high heterogeneity and there was a high risk of bias. This could explain the fact that the result of the meta-analysis reached only marginal significance in the comparison between BD individuals and controls, even though four of the six studies included indicated that subjects with BD had a lower risk adjustment than that of controls [3,10,17,28]. In addition, our meta-analysis showed significant differences in risk-taking when we compared the euthymic BD I group with the control group, indicating that the euthymic group was more likely to become involved in behaviours that could lead to danger or loss. To the best of our knowledge, no other systematic reviews or meta-analyses have examined this issue. Hence, our results should be considered preliminary. The profile of risk-taking impairment in BD remains to be determined until more data become available.
Limitations
Our study findings should be understood in light of its limitations. First, certain studies may have been excluded because they did not report the necessary results to allow their inclusion in the meta-analysis. Second, considerable heterogeneity was found across the studies on decision-making and risk-taking behaviour. In particular, the type and number of measures used to assess decision-making and risky behaviour varied considerably between studies. There was a lack of consensus on which neuropsychological tasks should be used to assess each cognitive domain, and the same task could be used to measure various domains since performance on most tests involved more than one cognitive process. The use of multiple outcome measures from the same task could increase bias. Heterogeneity was also reflected in the samples. Some studies included a BD I sample while others used a sample made up of BD II subjects. As for BD phases, the study samples comprised patients in different mood states (mania, depression, hypomania, mixed episode or euthymia). In an attempt to overcome this limitation, we carried out a stratified analysis based on the phase of illness (euthymia, depression or mania), an analysis based on diagnosed subtype (BD type I or other) and analysis based on the task used in decision-making (Iowa Gambling Task) and risk-taking behaviour (Balloon Analogue Risk Task or Cambridge Gambling Task). Furthermore, we used recommended methods (e.g. random-effects models) for accounting the observed heterogeneity. Third, a perusal of the effect sizes suggests that some studies may have had insufficient power to detect significant group differences. In a similar vein, the possibility of publication bias is always a concern because studies with null results are less likely than those reporting positive associations to be published. Funnel plots and Egger's test results did not suggest reporting bias on response inhibition and decision-making; but, unfortunately, publication bias could not be assessed for risk-taking, delay of gratification and inattention, as well as in the different stratified meta-analysis because the number of eligible studies with available data was <10. Lastly, there was a lack of information about variables which can interfere in the performance of subjects. Most studies did not report information regarding medical treatment used and addiction comorbidity (including substance and alcohol use).
Conclusions
The results of the study suggest that behavioural impulsivity is elevated in adults with BD in all phases of the disorder, representing a core and clinically relevant feature of BD that persists beyond mood symptoms. Decision-making also appears to be altered in BD, but heterogeneity across the studies was high. Finally, risk-taking behaviour appears to be affected in BD I and euthymic individuals; however, these findings should be viewed as preliminary, as very few studies have formally evaluated the risk-taking in BD patients and the heterogeneity between those, we did examine was high. To exclude biases resulting from high heterogeneity, the homogenisation of neuropsychological measures remains a challenge for the future. Future research should also determine which tasks are better at measuring core aspects of impulsivity, decision-making and risk-taking behaviour. In addition, our results underscore the need for longitudinal studies with larger samples that can provide more accurate results.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291720003086.
Acknowledgements
Fermin Mayoral Cleries and Jose Guzman-Parra have received funding from the Andalusia Government in the grants for human resources reinforcement in the research activity (Acción A de intesificación 2017 and Acción B de refuerzos de larga duración 2014). The authors declare no conflict of interest.