Introduction
Cognitive dysfunction is a common feature of schizophrenia spectrum disorders (SSD) (Carruthers, Van Rheenen, Gurvich, Sumner, & Rossell, Reference Carruthers, Van Rheenen, Gurvich, Sumner and Rossell2019; Van Rheenen et al., Reference Van Rheenen, Lewandowski, Tan, Ospina, Ongur, Neill and Burdick2017) and a key predictor of functional outcomes (Bowie, Reichenberg, Patterson, Heaton, & Harvey, Reference Bowie, Reichenberg, Patterson, Heaton and Harvey2006). Additionally, widespread brain morphological changes are being increasingly documented in SSD patients (Van Rheenen et al., Reference Van Rheenen, Cropley, Zalesky, Bousman, Wells, Bruggemann and Pantelis2018), and these may partially underpin the cognitive dysfunction seen in the disorder (Antonova et al., Reference Antonova, Kumari, Morris, Halari, Anilkumar, Mehrotra and Sharma2005; Karantonis et al., Reference Karantonis, Carruthers, Rossell, Pantelis, Hughes, Wannan and Van Rheenenn.d.). Cigarette smoking has been consistently associated with these factors in the general population (Durazzo, Meyerhoff, & Nixon, Reference Durazzo, Meyerhoff and Nixon2010; Elbejjani et al., Reference Elbejjani, Auer, Jacobs, Haight, Davatzikos, Goff and Launer2019; Karama et al., Reference Karama, Ducharme, Corley, Chouinard-Decorte, Starr, Wardlaw and Deary2015), and is highly prevalent in SSD. Indeed, compared to the general population, smoking rates are estimated at around 62% v. 25%, respectively (De Leon & Diaz, Reference De Leon and Diaz2005; Reitsma et al., Reference Reitsma, Fullman, Ng, Salama, Abajobir, Abate and Gakidou2017). Smokers with SSD are also reported to have higher levels of cigarette craving (Lo et al., Reference Lo, Heishman, Raley, Wright, Wehring, Moolchan and Kelly2011), reduced rates of cessation (D'Souza & Markou, Reference D'Souza and Markou2012) and take in more nicotine with each puff (Williams et al., Reference Williams, Gandhi, Lu, Kumar, Shen, Foulds and Benowitz2010) compared to smokers without SSD.
It is relatively well accepted that acute nicotine administration can result in an increase in performance in different areas of cognitive functioning within chronic smokers (Azizian, Monterosso, O'Neill, & London, Reference Azizian, Monterosso, O'Neill, London, Henningfield, London and Pogun2009; Sharma & Brody, Reference Sharma, Brody, Henningfield, London and Pogun2009). This is believed to be related, in part, to a nicotine-induced increase in activity in several brain regions, such as the dorsolateral prefrontal cortex (DLPFC) and thalamus (Azizian et al., Reference Azizian, Monterosso, O'Neill, London, Henningfield, London and Pogun2009; Sharma & Brody, Reference Sharma, Brody, Henningfield, London and Pogun2009). However, there also appears to be a simultaneous effect of long-term cigarette smoking, which acts through different mechanisms in the same population and results in decreased cognitive performance (Campos, Serebrisky, & Castaldelli-Maia, Reference Campos, Serebrisky and Castaldelli-Maia2016). Relevantly, cigarette smoking has been shown to impact neurotransmission systems and vascular endothelium, both of which have been implicated in cognitive impairment (Mackowick et al., Reference Mackowick, Barr, Wing, Rabin, Ouellet-Plamondon and George2014). Further, research has suggested that the cerebral arteries may be particularly susceptible to atherosclerosis as a result of smoking (Liu et al., Reference Liu, Lee, Jung, Koo, Kim, Hwang and Suh2014), which is argued to contribute to cognitive deficits (Dearborn et al., Reference Dearborn, Zhang, Qiao, Suri, Liu, Gottesman and Wasserman2017; Fareed et al., Reference Fareed, Zhou, Qiao, Chu, Qureshi, Mosley and Wasserman2018). Indeed, evidence from healthy cohorts suggests that smoking has a significant negative impact on cognition (Durazzo et al., Reference Durazzo, Meyerhoff and Nixon2010), grey matter volume (Brody et al., Reference Brody, Mandelkern, Jarvik, Lee, Smith, Huang and London2004; Elbejjani et al., Reference Elbejjani, Auer, Jacobs, Haight, Davatzikos, Goff and Launer2019; Gallinat et al., Reference Gallinat, Meisenzahl, Jacobsen, Kalus, Bierbrauer, Kienast and Staedtgen2006; Liao, Tang, Liu, Chen, & Hao, Reference Liao, Tang, Liu, Chen and Hao2012) and cortical thickness (Karama et al., Reference Karama, Ducharme, Corley, Chouinard-Decorte, Starr, Wardlaw and Deary2015; Kühn, Schubert, & Gallinat, Reference Kühn, Schubert and Gallinat2010; Li et al., Reference Li, Jin, Bi, Shi, Cai, von Deneen and Tian2015). Moreover, cigarette smoking has been associated with brain morphology in several clinical samples (Durazzo, Mon, Gazdzinski, & Meyerhoff, Reference Durazzo, Mon, Gazdzinski and Meyerhoff2013; Morales, Hellemann, Lee, London, & O'Neill, Reference Morales, Hellemann, Lee, London and O'Neill2012; Zorlu et al., Reference Zorlu, Cropley, Zorlu, Delibas, Adibelli, Baskin and Pantelis2017). Given the increased prevalence of smoking in SSD, it is thus plausible that cognitive and brain morphological abnormalities could be amplified in smokers with these disorders. Currently however, the effects of smoking on these factors in SSD remain unclear.
That is, while some SSD studies have demonstrated that smokers outperform non-smokers across a number of cognitive domains (Ahlers et al., Reference Ahlers, Hahn, Ta, Goudarzi, Dettling and Neuhaus2014; Hahn et al., Reference Hahn, Hahn, Dettling, Güntürkün, Tam Ta and Neuhaus2012; Morisano, Wing, Sacco, Arenovich, & George, Reference Morisano, Wing, Sacco, Arenovich and George2013; Wing, Sacco, & George, Reference Wing, Sacco and George2011b), others have reported worse cognition in SSD smokers (Depp et al., Reference Depp, Bowie, Mausbach, Wolyniec, Thornquist, Luke and Harvey2015; Iasevoli, Balletta, Gilardi, Giordano, & de Bartolomeis, Reference Iasevoli, Balletta, Gilardi, Giordano and de Bartolomeis2013; Reed, Harris, & Olincy, Reference Reed, Harris and Olincy2016; Roth, Hong, McMahon, & Fuller, Reference Roth, Hong, McMahon and Fuller2013; Stramecki et al., Reference Stramecki, Kotowicz, Piotrwoski, Frydecka, Rymaszewska, Beszlej and Misiak2018; Zhang et al., Reference Zhang, Chen, Xiu, Haile, Sun, Lu and Kosten2012). There are also reports of an absence of associations between cognition and smoking status altogether (Ekinci & Ekinci, Reference Ekinci and Ekinci2012; Sánchez-Gutiérrez et al., Reference Sánchez-Gutiérrez, Bobes, García-Portilla, Bioque, Rivero, Mané and Rabela2018; Zhang et al., Reference Zhang, Chen, Xiu, Haile, He, Luo and Kosten2013). The only two meta-analyses on this topic in SSD showed that smoking v. non-smoking patients had worse cognitive performance in some domains but not others (Coustals et al., Reference Coustals, Martelli, Brunet-Lecomte, Petillion, Romeo and Benyamina2020; Wang et al., Reference Wang, Li, Zheng, Zhong, Wang, Ng and Xiang2019). However, in one of these meta-analyses, data from just seven of 11 relevant studies were meta-analysable, and in both, sample sizes across the individual studies were relatively small. Given the inconsistent effects across the individual studies on this topic, further research is warranted.
With respect to the widespread brain changes documented in SSD patients (Van Erp et al., Reference Van Erp, Hibar, Rasmussen, Glahn, Pearlson, Andreassen and Turner2016, Reference van Erp, Iorio, Koenders, Howells, Dickie, Hong and Sommer2018), only four SSD studies have explored associations between smoking status and brain morphology. The first study showed increased grey matter volume in the superior temporal gyri and lateral prefrontal cortex in 14 SSD smokers compared to 18 SSD non-smokers (Tregellas et al., Reference Tregellas, Shatti, Tanabe, Martin, Gibson, Wylie and Rojas2007). In contrast, Schneider et al. (Reference Schneider, White, Hass, Geisler, Wallace, Roessner and Ehrlich2014) found reduced right hippocampus, right amygdala and left DLPFC volumes, as well as reduced right primary visual cortex thickness in 53 SSD smokers compared to 59 SSD non-smokers. Jørgensen et al. (Reference Jørgensen, Psychol, Skjærvø, Mørch-Johnsen, Haukvik, Lange and Agartz2015) also found thickness reductions in their transdiagnostic sample of SSD, bipolar disorder or other psychotic disorders when comparing 250 smokers to 256 non-smokers, however only in the left insula and left anterior cingulate. The single longitudinal study in SSD showed an absence of volume differences between 54 smokers and 42 non-smokers cross-sectionally, although grey matter volume reductions were evident in heavy smokers (>25 cigarettes per day) with SSD over 5 years (Van Haren et al., Reference Van Haren, Koolschijn, Cahn, Schnack, Hulshoff Pol and Kahn2010).
The results of the cognition and brain morphology studies reviewed above show inconsistencies both amongst themselves and in reference to findings in healthy cohorts. They have also been limited by the use of small samples (Morisano et al., Reference Morisano, Wing, Sacco, Arenovich and George2013; Reed et al., Reference Reed, Harris and Olincy2016; Tregellas et al., Reference Tregellas, Shatti, Tanabe, Martin, Gibson, Wylie and Rojas2007) and/or lack of appropriate control comparators (Depp et al., Reference Depp, Bowie, Mausbach, Wolyniec, Thornquist, Luke and Harvey2015; Ekinci & Ekinci, Reference Ekinci and Ekinci2012; Reed et al., Reference Reed, Harris and Olincy2016; Roth et al., Reference Roth, Hong, McMahon and Fuller2013; Schneider et al., Reference Schneider, White, Hass, Geisler, Wallace, Roessner and Ehrlich2014). It is also notable that neither of the two studies that examined cortical thickness examined surface area – the other component measure that contributes to brain volume. Cortical surface area is proposed to have more of an early neurodevelopmental origin (Budday, Steinmann, & Kuhl, Reference Budday, Steinmann and Kuhl2015; Habets, Marcelis, Gronenschild, Drukker, & Van Os, Reference Habets, Marcelis, Gronenschild, Drukker and Van Os2011), while cortical thickness has been found to be particularly influenced by changeable environmental factors (Gold et al., Reference Gold, Sheridan, Peverill, Busso, Lambert, Alves and McLaughlin2016; Jha et al., Reference Jha, Xia, Ahn, Girault, Li, Wang and Knickmeyer2019). Thus, cortical thickness may be potentially more sensitive to the subtle effects of smoking compared to surface area, but no studies have explicitly compared the influence of smoking status on these two measures of brain morphology to date.
In the current study, we aimed to overcome these limitations, by investigating differences between smoking and non-smoking SSD patients compared to smoking and non-smoking controls in the context of a range of cognition and brain morphology measures. We focused our analyses of the latter on global volume, thickness and surface area measures, as well as specific regions of interest (ROIs) including the cingulate cortex, ventrolateral prefrontal cortex (vlPFC), orbitofrontal cortex (OFC), DLPFC, superior temporal gyrus and insula (refer to Fig. 1 for visual depiction). We also examined subcortical thalamic, hippocampal and amygdala volume. These ROIs were selected given; (i) the only studies to have explored the effects of smoking on volume in SSD reported volume differences in the DLPFC, vlPFC, hippocampus, amygdala and superior temporal gyrus (Schneider et al., Reference Schneider, White, Hass, Geisler, Wallace, Roessner and Ehrlich2014; Tregellas et al., Reference Tregellas, Shatti, Tanabe, Martin, Gibson, Wylie and Rojas2007; Van Haren et al., Reference Van Haren, Koolschijn, Cahn, Schnack, Hulshoff Pol and Kahn2010); (ii) thickness differences between SSD smoking and non-smoking groups have been reported in the cingulate cortex and the insula (Jørgensen et al., Reference Jørgensen, Psychol, Skjærvø, Mørch-Johnsen, Haukvik, Lange and Agartz2015); and (iii) the cingulate cortex, OFC and thalamus are regions of reported volume and thickness reduction in healthy smokers that share some overlap with regions of reported volume and thickness reduction in SSD (Brody et al., Reference Brody, Mandelkern, Jarvik, Lee, Smith, Huang and London2004; Gallinat et al., Reference Gallinat, Meisenzahl, Jacobsen, Kalus, Bierbrauer, Kienast and Staedtgen2006; Glahn et al., Reference Glahn, Laird, Ellison-Wright, Thelen, Robinson, Lancaster and Fox2008; Rimol et al., Reference Rimol, Hartberg, Nesvåg, Fennema-Notestine, Hagler, Pung and Agartz2010; van der Kouwe et al., Reference van der Kouwe, West, McGuire, Ozawa, Kuperberg, Eddy and Salat2003). As previous studies have shown laterality effects, the ROIs for each hemisphere were analysed separately.

Fig. 1. Selected regions of interest (ROIs). Cortical map depicting the parcellated regions that were selected for the current study based on the Desikan–Killiany atlas. Subcortical regions of interest for the grey matter volume analysis (hippocampus, thalamus and amygdala) are not depicted here.
We predicted that smokers would have reduced volume and thinner cortices in these regions in both the control and SSD groups. Further, we hypothesised that no surface area differences would be present when comparing smoking and non-smoking participants, irrespective of diagnosis. We also expected cognitive performance to differ between smoking and non-smoking participants, although the direction of effects remained an open question.
Methods
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Participants
Data for the current paper were accessed through the Australian Schizophrenia Research Bank (ASRB) (Loughland et al., Reference Loughland, Draganic, McCabe, Richards, Nasir, Allen and Carr2010), representing data collected across four Australian states (New South Wales, Victoria, Queensland and Western Australia). Smoking and cognitive data were available for 754 individuals included in the study. Of this, n = 455 individuals met DSM-IV criteria for schizophrenia (n = 388) or schizoaffective disorder (n = 67) as part of the SSD group (n = 321 smokers, n = 134 non-smokers) and n = 299 individuals were healthy comparison subjects (n = 128 smokers, n = 171 non-smokers). Imaging data were also available for a subset of n = 283 participants, n = 201 of whom had an SSD (n = 132 smokers, n = 69 non-smokers) and n = 82 healthy controls (n = 26 smokers, n = 56 non-smokers).
All participants provided written informed consent for the future analysis of their stored data in accordance with the Human Research Ethics Committee of participating hospitals/institutions. Details of participant characterisation for this sample are given in the online Supplementary material.
Measures
Smoking status
Information was collected for all participants regarding smoking status through structured interviews as part of the ASRB protocol. Participants were considered non-smokers if they responded ‘no’ to the question ‘have you ever smoked cigarettes, tobacco, cigars, pipe regularly?’ and current smokers if they responded ‘yes’ to the question ‘do you currently smoke cigarettes daily?’ Participants that answered yes to the first question, but no to the second were excluded from the study, due to the limited number of participants that fell into this category both in the full sample (n = 47 SSD, n = 29 HC) and imaging subset (n = 7 SSD, n = 6 HC). Participants were also asked how many cigarettes they smoked per day and placed into one of four groups (10 cigarettes or less per day, 11–20 cigarettes per day, 21–30 cigarettes per day, 31 or more cigarettes per day). As per the methodology of Jørgensen et al. (Reference Jørgensen, Psychol, Skjærvø, Mørch-Johnsen, Haukvik, Lange and Agartz2015), participants were coded for the current study as either ‘low’ (1–10 cigarettes per day), ‘moderate’ (11–20 cigarettes per day) or ‘high’ (>21 cigarettes per day) frequency smokers.
Cognitive assessment
The Wechsler's Test of Adult Reading (WTAR) was administered as a measure of estimated premorbid IQ. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) was administered to measure cognitive functioning. Five age-adjusted domain scores (immediate memory, language, visuospatial/constructional, delayed memory and attention) and a total scaled score were calculated. Details are provided elsewhere (Loughland et al., Reference Loughland, Draganic, McCabe, Richards, Nasir, Allen and Carr2010).
Neuroimaging
Structural MRI was used to attain whole-brain T1-weighted images from a subset of the participants using Siemens Avanto 1.5-Tesla (Siemens, Erlangen, Germany) MRI scanners located in Melbourne, Sydney, Brisbane, Perth and Newcastle. Information regarding participant distribution for the scanning sites is provided in online Supplementary Table S1Footnote †Footnote 1. An optimised magnetisation-prepared rapid acquisition gradient echo (MP-RAGE) sequence with the following parameters was used: 176 sagittal slices of 1 mm thickness without gap; field of view = 250 × 250 mm2; repetition time/echo time = 1980/4.3 ms; data matrix size = 256 × 256; voxel dimensions = 0.98 × 0.98 × 1.0 mm3. The same acquisition sequence was used across all ASRB sites. An individual travelled to all five sites and was scanned at each site to quantify gross inter-site differences. A Siemens MRI phantom was also scanned at each site to enable inter-site calibration.
Both cortical reconstruction and volumetric segmentation of images were completed using the FreeSurfer image analysis suite (version 5.1.0; Martinos Centre for Biomedical Imaging, Harvard-MIT, Boston, MA, USA; http://surfer.nmr.mgh.harvard.edu/). Image processing comprised an automated volume-based and surface-based stream. The former was used to extract volume estimates for select cortical and subcortical regions using an automatic labelling system. The surface-based stream extracted cortical thickness and surface area measurements by reconstructing a three-dimensional cortical surface model. Details of the pre-processing procedure are provided in the online Supplementary material.
Data analysis
All analyses were completed using the Statistical Package for the Social Sciences (SPSS) version 25 (IBM). Details of preliminary analyses and statistical assumption checks are given in the online Supplementary material. Differences in clinical and demographic data between smokers and non-smokers in both diagnostic groups were assessed using χ2 tests for categorical variables and parametric tests (Student's t test or one-way ANOVA) for quantitative variables. Cognitive performance was analysed in a series of ANCOVAs, where the five RBANS domain scores and the total score were specified as dependent variables, and smoking status, diagnostic group and their interaction as the independent variables of interestFootnote 2. Age, sex and recruitment/scanning site were selected as covariates a priori. The total and mean regional volume, thickness and surface area estimates obtained in the imaging subset of the sample were imported into SPSS after extraction from FreeSurfer. Mean global (total grey matter volume, total mean thickness and total mean surface area) and regional volume, thickness, and surface area estimates for each participant were imported into SPSS after extraction from FreeSurfer. The global scores and selected ROIs were then analysed in a series of ANCOVAs. The analyses included the independent and covariate variables specified above, alongside either (i) global volume, thickness and surface area; or the ROIs of (ii) left and right cortical and subcortical volume; (iii) left and right cortical thickness; or (iv) left and right cortical surface area as dependent variablesFootnote 3. For the global estimates, and volume and surface area ROI analyses, intracranial volume (ICV) was added as a covariateFootnote 4.
A false discovery rate (FDR) of p < 0.05 was applied to all results to account for multiple comparisons using the Benjamini–Hochberg method (Benjamini & Hochberg, Reference Benjamini and Hochberg1995). Details concerning the methodology of the corrections are supplied in the online Supplementary material.
Results
Descriptive statistics
Demographic and clinical characteristics of the full sample and of the imaging subset are presented in Tables 1 and 2, respectively. In the full sample, SSD smokers were significantly younger than control smokers. There were also a significantly higher number of males in the SSD group compared to controls, regardless of smoking status. Further, a significant difference in the distribution of smoking frequency between smokers with and without an SSD diagnosis was evident, with significantly more high-frequency and significantly fewer low-frequency smokers in the SSD subgroups, but an equivalent number of moderate-frequency smokers in both groups.
Table 1. Demographics characteristics of the full cohort

SANS, the Scale for the Assessment of Negative Symptoms; DIP, the Diagnostic Interview for Psychoses.
Data are expressed as mean ± s.d.
a Data missing for negative symptoms (n = 12), positive symptoms (n = 42).
– Data not applicable.
*Significant at p < 0.05.
Table 2. Demographic characteristics of the subset with imaging data

ICV, intracranial volume; SANS, the Scale for the Assessment of Negative Symptoms; DIP, the Diagnostic Interview for Psychoses.
Data are expressed as mean ± s.d.
a Data missing for negative symptoms (n = 10), positive symptoms (n = 12).
– Data not applicable.
*Significant at p < 0.05.
There were no significant differences in current negative symptoms, current positive symptoms, duration of illness or age of illness onset in SSD patients who were and were not smokers. However, smokers in the SSD group had significantly lower estimated premorbid IQ scores in comparison to the other three groups, and non-smokers in the SSD group had significantly lower estimated premorbid IQ scores in comparison to the control non-smoking groupFootnote 5. In the imaging subset, group comparisons on demographic and clinical variables did not differ from that reported above, except that age did not differ significantly between the groups. ICV was also significantly higher in smokers from the SSD group than control non-smokers.
Primary analyses
As the main effects of diagnosis have been reported for all variables of interest in the ASRB data previously, the statistical values for these effects in all current analyses are reported in the online Supplementary material for brevity. Note that several main effects of smoking status were initially significant but did not survive FDR correction. Below we report on the FDR-corrected results, but details of the uncorrected results (with accompanying effect size calculations) can be found in the tables.
Cognition
Smoking group comparisons and interaction effects are reported in Table 3. There were no significant main effects of smoking or smoking status × diagnostic group interactions. However, a main effect of diagnostic group was apparent, with decreased performance in immediate memory, visuospatial/constructional, language, attention, delayed memory and total scale score, evident in SSD patients compared to controls.
Table 3. Group comparisons of cognitive domains

SSD, schizophrenia spectrum disorder; HC, healthy controls; S, smoker; NS, non-smoker.
Given the focus of this study, main effects of diagnostic group are reported in the online Supplementary material for brevity.
a Unadjusted for multiple comparisons.
b All values are adjusted for age, gender and site.
c If post-hoc relationship is not reported, finding was not significant prior or after FDR correction. SSD < HC implies significant reductions relative to HC.
d d = Cohen's d effect sizes.
*Significant at p < 0.05 after Benjamini–Hochberg FDR correction for multiple comparisons.
Bold values = significant before Benjamini–Hochberg FDR.
Brain morphology
Group comparisons and statistical values for all brain morphology ROI measures are reported in Table 4.
Table 4. Group comparisons of volume, cortical thickness and surface area regions of interest

SSD, schizophrenia spectrum disorder; HC, healthy controls; S, smoker; NS, non-smoker; LH, left hemisphere; RH, right hemisphere.
Given the focus of this study, main effects of diagnostic group are reported in the online Supplementary material for brevity.
a Unadjusted for multiple comparisons.
b All values are adjusted for age, gender, ICV and site.
c If post-hoc relationship is not reported, finding was not significant prior or after FDR correction. SSD < HC implies significant reductions relative to HC.
d d = Cohen's d effect sizes.
*Significant at p < 0.05 after Benjamini–Hochberg correction for multiple comparisons.
Bold values = significant before Benjamini–Hochberg correction.
Global morphology estimates: A main effect of diagnostic group was evident for global volume and thickness, with both measures showing significant reductions in SSD patients compared to healthy controls. Global surface area was not significantly affected by diagnostic group, nor were there any significant main effects of smoking status, and smoking status × diagnostic group interactions for any of the global measures analysed. Due to the lack of significant findings, the statistical values for these effects are reported in the online Supplementary material for brevity
Cortical and subcortical grey matter volume: There were no significant main effects of diagnosisFootnote 6 or smoking status, and no smoking status × diagnostic group interactions for any of the regions analysed.
Cortical thickness: There were no significant smoking status × diagnostic group interactions in any of the regions analysed. A main effect of diagnostic group was evident, with reduced thickness in SSD patients compared to healthy controls in the right caudal ACC and pars opercularis, and the lateral OFC, medial OFC, pars orbitalis, pars triangularis, DLPFC, superior temporal gyrus and insula bilaterally. A significant main effect of smoking status was also observed in the left posterior cingulate cortex (PCC), with reduced thickness in smokers compared to non-smokers (Fig. 2).
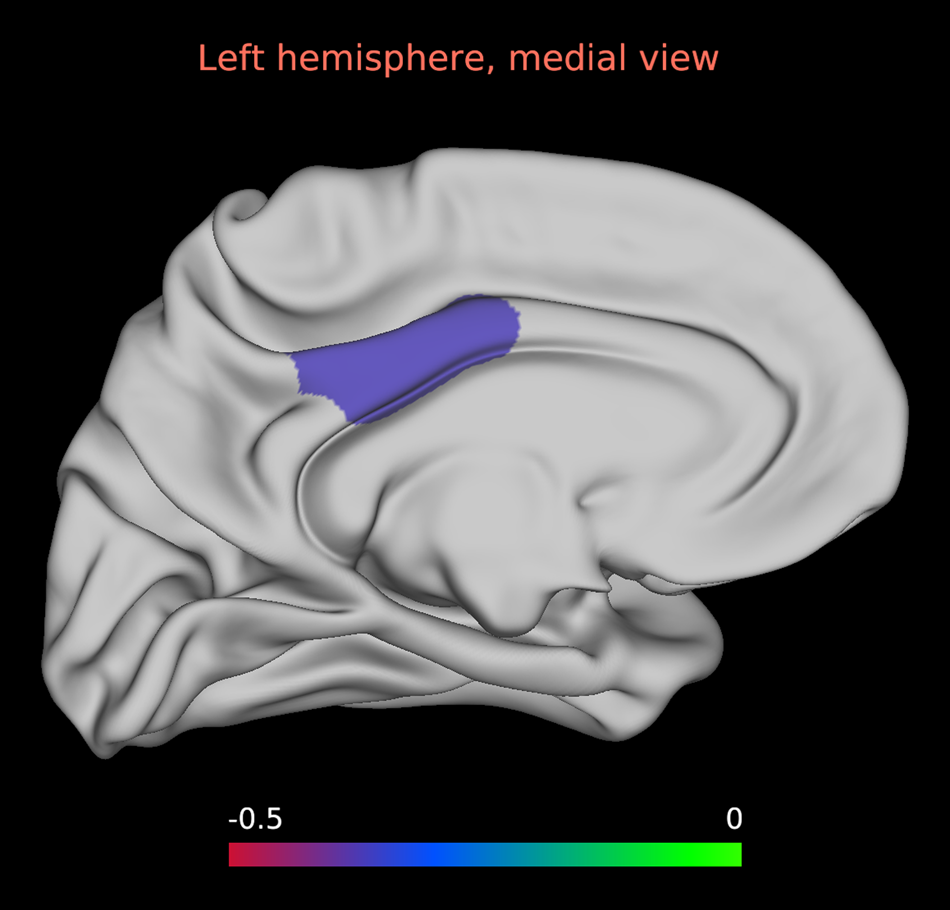
Fig. 2. Main effect of smoking status on left posterior cingulate cortex thickness. Statistical map depicting effect size of thickness reductions in smoking and non-smoking participants in the whole sample for left posterior cingulate cortex thickness. The main effect of left posterior cingulate cortex thickness was the only main effect to survive FDR correction.
Surface area: There were no significant main effects of smoking status or smoking status × diagnostic group interaction effects in any of the regions analysed. There was a main effect of diagnostic group, with reduced regional surface area in SSD patients compared to healthy controls in the right DLPFC, pars orbitalis and pars triangularis. There was a significant increase in surface area in the bilateral lateral OFC in SSD patients compared to controls.
Discussion
The findings of the current study showed no statistically significant effects of smoking on cognition, grey matter volume, cortical thickness and surface area in both SSD and healthy controls, with the exception of a thinner left PCC in smokers compared to non-smokers, irrespective of diagnostic group. Nonetheless, the effect of smoking on several cognitive and brain morphology measures was in the expected direction and was significant initially but did not survive correction. There was also a significant difference between smokers and non-smokers in premorbid IQ, and a significant difference between light and heavy smokers in the attention domain of cognition. Thus, a subtle effect of smoking does appear to be suggested by the data, but the study may not have been powered enough for the effects to reach our stringent statistical thresholds.
The absence of statistically significant smoking effects on the cognitive measures conflicts with some previous studies suggesting either worse (Depp et al., Reference Depp, Bowie, Mausbach, Wolyniec, Thornquist, Luke and Harvey2015; Iasevoli et al., Reference Iasevoli, Balletta, Gilardi, Giordano and de Bartolomeis2013; Reed et al., Reference Reed, Harris and Olincy2016; Roth et al., Reference Roth, Hong, McMahon and Fuller2013; Stramecki et al., Reference Stramecki, Kotowicz, Piotrwoski, Frydecka, Rymaszewska, Beszlej and Misiak2018; Zhang et al., Reference Zhang, Chen, Xiu, Haile, Sun, Lu and Kosten2012) or improved (Ahlers et al., Reference Ahlers, Hahn, Ta, Goudarzi, Dettling and Neuhaus2014; Hahn et al., Reference Hahn, Hahn, Dettling, Güntürkün, Tam Ta and Neuhaus2012; Morisano et al., Reference Morisano, Wing, Sacco, Arenovich and George2013; Sacco et al., Reference Sacco, Termine, Seyal, Dudas, Vessicchio, Krishnan-Sarin and George2005; Wing, Bacher, Sacco, & George, Reference Wing, Bacher, Sacco and George2011a) cognitive performance in smoking relative to non-smoking SSD patients. However, they are consistent with a number of other studies that have reported no smoking effect in SSD samples (Ekinci & Ekinci, Reference Ekinci and Ekinci2012; Iasevoli et al., Reference Iasevoli, Balletta, Gilardi, Giordano and de Bartolomeis2013; Sánchez-Gutiérrez et al., Reference Sánchez-Gutiérrez, Bobes, García-Portilla, Bioque, Rivero, Mané and Rabela2018; Zhang et al., Reference Zhang, Chen, Xiu, Haile, He, Luo and Kosten2013).
The absence of cortical surface area differences between smokers and non-smokers in either diagnostic group supported our hypothesis, although the absence of an effect of smoking on all relevant volume and most of the thickness measurements did not. Two factors related to this finding must be noted. First, our hypotheses regarding smoking and brain volume and thickness in SSD were borne from a sparse literature in which extant studies report mixed findings and encompass several limitations. Second, findings were in the expected direction for many measures (0.20–0.40 Cohen's d effect size range), with many volume and thickness regions showing reductions that were initially significant but did not survive correction for multiple comparisons.
Notably, smoking in comparison with non-smoking participants had significantly reduced thickness in the left PCC, an area previously implicated in smoking-related addiction in healthy cohorts (Jarraya et al., Reference Jarraya, Brugières, Tani, Hodel, Grandjacques, Fénelon and Palfi2010; Mondino et al., Reference Mondino, Luck, Grot, Januel, Suaud-Chagny, Poulet and Brunelin2018). Although the right PCC finding did not survive correction for multiple comparisons, mean thickness within this region was reduced in the smoking group (Cohen's d = 0.32). These data together raise the possibility of an association between the PCC and smoking behaviour. The cingulate cortices contain high densities of nicotinic acetylcholine receptors (nAChR) (Picard et al., Reference Picard, Sadaghiani, Leroy, Courvoisier, Maroy and Bottlaender2013), which are shown to be upregulated in smokers (Govind, Vezina, & Green, Reference Govind, Vezina and Green2009) and have thus been related to smoking addiction. Notably, nAChR genes have been recently linked with risk for SSD (Hong et al., Reference Hong, Yang, Wonodi, Hodgkinson, Goldman, Stine and Thaker2011). Given the significantly higher number of SSD smokers compared to HC smokers, it is plausible that the significant finding was driven by SSD smokers.
An emerging idea within recent literature is of an overlapping circuitry that may involve both smoking addiction and neurobiological mechanisms associated with SSD (Moran, Sampath, Kochunov, & Hong, Reference Moran, Sampath, Kochunov and Hong2013; Moran, Sampath, Stein, & Hong, Reference Moran, Sampath, Stein and Hong2012). Several studies have implicated the PCC as a key node in the default mode network (Buckner, Andrews-Hanna, & Schacter, Reference Buckner, Andrews-Hanna and Schacter2008; Hahn et al., Reference Hahn, Ross, Yang, Kim, Huestis and Stein2007), which has been shown to function abnormally in SSD resting-state functional MRI studies (Brennan, Harris, & Williams, Reference Brennan, Harris and Williams2013; Karbasforoushan & Woodward, Reference Karbasforoushan and Woodward2013). Moreover, the PCC has an important role in the consolidation of complex memories (Bird, Keidel, Ing, Horner, & Burgess, Reference Bird, Keidel, Ing, Horner and Burgess2015) and retrieval of episodic memories (Natu et al., Reference Natu, Lin, Burks, Arora, Rugg and Lega2019), and the disruption of these has been argued to be associated with positive symptoms in psychosis (Sharp, Tomitaka, Bernaudin, & Tomitaka, Reference Sharp, Tomitaka, Bernaudin and Tomitaka2001). Indeed, two drug models of psychosis – ketamine and psilocybin – have shown marked effects on the activity of the PCC (Leech & Sharp, Reference Leech and Sharp2014; Newell, Zavitsanou, & Huang, Reference Newell, Zavitsanou and Huang2005). Given what is known about the PCC and smoking addiction, these findings give credence to the idea of an overlapping circuitry involving both smoking addiction and SSD.
Some limitations of the current data should be considered. First, as our sample was taken from a research bank not explicitly designed to research smoking, the number of high-frequency smokers in the control sample was low (see Table 1). Although our additional analysis showed a general absence of differences between high- and low-frequency smokers in terms of cognition and brain morphology (see online Supplementary material), we cannot discount that this may have influenced the capacity to discern subtle smoking effects. In addition, the total number of control smokers in the imaging subset was low, which may have further impacted the results. It is becoming increasingly recognised that much larger sample sizes are needed to reliably identify morphological differences, and hence our sample may have been too small to accurately demonstrate differences between the groups. Further, the prevalence of smoking is significantly higher in SSDs compared to the general population, and thus the nature of smoking behaviour within the sample was not random.
Second, incomprehensive, and incomplete smoking data meant we were unable to explore the effect of several important smoking factors (duration of smoking/age of smoking initiation/smoking dependence) on the relationships analysed. Further, the collection of smoking history data relied on self-report, which may be biased by subjective recall (Gorber, Schofield-Hurwitz, Hardt, Levasseur, & Tremblay, Reference Gorber, Schofield-Hurwitz, Hardt, Levasseur and Tremblay2009). It is relatively well accepted that acute nicotine administration can improve cognitive functioning (Azizian et al., Reference Azizian, Monterosso, O'Neill, London, Henningfield, London and Pogun2009; Sharma & Brody, Reference Sharma, Brody, Henningfield, London and Pogun2009), and that chronic smokers experience withdrawal effects, including worsening of cognition (Ashare, Falcone, & Lerman, Reference Ashare, Falcone and Lerman2014). Thus, without exact measures of nicotine in the system, we were unable to control for the confounding influence of acute nicotine consumption or withdrawal. There is also a possibility that outcomes associated with chronic cigarette exposure are a result of other toxic compounds inhaled, as opposed to nicotine. As we did not include a direct measure of nicotine, this possibility cannot be excluded. Further, limited medication data also meant we were unable to control for the effects of medication, a relevant consideration given the hypothesised effects of antipsychotics on both cognition and brain morphology (Huhtaniska et al., Reference Huhtaniska, Jääskeläinen, Hirvonen, Remes, Murray, Veijola and Miettunen2017; Veselinović et al., Reference Veselinović, Scharpenberg, Heinze, Cordes, Mühlbauer, Juckel and Wobrock2019).
Third, the study was cross-sectional in nature, which precludes inferences concerning the causality of the associations. This design may also not be sensitive enough to capture associations between smoking and key variables of interest. Indeed, Van Haren et al. (Reference Van Haren, Koolschijn, Cahn, Schnack, Hulshoff Pol and Kahn2010) found that heavy smoking was related to brain volume loss over time, but it did not explain volume abnormalities in their baseline analyses. It is probable that the dynamic trajectory of smoking is more important than a single-time point, and subsequent studies should consider the effects of smoking in SSD longitudinally. Fourth, although the brain morphology ROIs were chosen based on the results of past literature, this method may have excluded some brain regions of relevance to smoking. Moreover, as the ROIs were selected partly based on literature showing changes in healthy smokers, findings may have been limited to regions that show changes in both cohorts, as opposed to SSD alone.
Finally, it must be noted that the patient group had a relatively low symptom load and long duration of illness, limiting generalisability. However, due to the multi-site method, the current dataset does constitute a representative sample of community-dwelling SSD patients. Other strengths include the large sample size for the cognitive analysis, and resulting increased statistical power compared to most previous studies on the topic. The study also provided an assessment of smoking status on several key features of SSD using a well-validated cognitive battery and measures of both cortical thickness and surface area in addition to brain volume, where only the latter has been of predominant focus in the sparse literature to date.
In sum, although the current study reported no group differences or interactions with the exception of reduced thickness in the left PCC, several results were in the expected direction and met significance initially but did not survive correction. These trends suggest the possibility of an effect that was not uncovered for the reasons mentioned above. Thus, future research on this topic is encouraged to determine if our findings replicate. Such research would do well to collect detailed smoking histories inclusive of direct nicotinergic assessment and dependency data, using large samples and employing longitudinal study designs.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291720005152
Acknowledgements
Data for this study were provided by the Australian Schizophrenia Research Bank (ASRB), which is supported by the NHMRC (enabling grant 386500), the Pratt Foundation, Ramsay Health Care, the Viertel Charitable Foundation and the Schizophrenia Research Institute. The authors thank the chief investigators and manager of the ASRB: Carr V, Schall U, Scott R, Jablensky A, Mowry B, Michie P, Catts S, Henskens F, Pantelis C and Loughland C.
Financial support
Dr Van Rheenen was supported by a National Health and Medical Research Council (NHMRC) Early Career Fellowship (1088785). Dr Cropley was supported by an NHMRC Investigator Grant (1177370) and a Brain and Behavior Research Foundation (NARSAD) Young Investigator Award (21660). Dr Zalesky was supported by an NHMRC Fellowship (1047648). Dr Bousman was supported by the Alberta Children's Hospital Foundation. Professor Shannon Weickert was supported by an NHMRC Senior Principal Research Fellowship (1117079), the Schizophrenia Research Institute (using infrastructure from the New South Wales Ministry of Health and the Macquarie Group Foundation), the University of New South Wales and Neuroscience Research Australia. Professor Pantelis was supported by an NHMRC Senior Principal Research Fellowship (628386 and 1105825) and by a NARSAD Distinguished Investigator Award. None of the funding sources played any role in the study design; in the collection, analysis or interpretation of data; in the writing of the manuscript; or in the decision to submit the manuscript for publication
Conflict of interest
Dr Van Rheenen has received grant funding (unrelated to the current paper) from Club Melbourne, the Henry Freeman Trust, Jack Brockhoff Foundation, University of Melbourne, Barbara Dicker Brain Sciences Foundation, Rebecca L Cooper Foundation and the Society of Mental Health Research. Professor Sundram has received consulting fees, advisory board fees, research support, speakers honoraria or travel support from AstraZeneca, the Australian National Health and Medical Research Council, the Australian Department of Immigration and Border Protection, Bristol-Myers Squibb, Eli Lilly, the Flack Trust, GlaxoSmithKline, Lundbeck, the One-in-Five Association, Otsuka, Pfizer, Roche and the United Nations High Commissioner for Refugees. Professor Cynthia Shannon Weickert is on an advisory board for and has received advisory board fees from Lundbeck. Over the last 4 years, Professor Pantelis has been on advisory boards for AstraZeneca, Janssen-Cilag, Lundbeck and Servier; and he has received honoraria for talks presented at educational meetings organised by AstraZeneca, Eli Lilly, Janssen-Cilag, Lundbeck, Pfizer and Shire. The other authors report no financial relationships with commercial interests.









