Introduction
Poor social functioning (SF) is ubiquitous among individuals at clinical high risk for psychosis (CHR; Ballon et al. Reference Ballon, Kaur, Marks and Cadenhead2007; Addington et al. Reference Addington, Penn, Woods, Addington and Perkins2008; Ben-David et al. Reference Ben-David, Birnbaum, Eilenberg, DeVylder, Gill, Schienle, Azimov, Lukens, Davidson and Corcoran2014) and has been found to be a predictor of development of psychotic symptoms (Cannon et al. Reference Cannon, Cadenhead, Cornblatt, Woods, Addington, Walker, Seidman, Perkins, Tsuang, McGlashan and Heinssen2008; Jang et al. Reference Jang, Shin, Shim, Park, Kim, Jang, Kwon, Hur, An and Kwon2011; Cornblatt et al. Reference Cornblatt, Carrion, Addington, Seidman, Walker, Cannon, Cadenhead, McGlashan, Perkins, Tsuang, Woods, Heinssen and Lencz2012; Velthorst et al. Reference Velthorst, Nieman, Veling, Klaassen, Dragt, Rietdijk, Ising, Wunderink, Linszen, de Haan and van der Gaag2012). However, SF difficulties have also been found to be prevalent among CHR individuals who do not transition to psychosis (Yung & McGorry, Reference Yung and McGorry2007; Addington et al. Reference Addington, Cornblatt, Cadenhead, Cannon, McGlashan, Perkins, Seidman, Tsuang, Walker, Woods and Heinssen2011; Schlosser et al. Reference Schlosser, Jacobson, Chen, Sugar, Niendam, Li, Bearden and Cannon2012a ), leading a number of authors to advocate for broadening the focus of CHR research to include functional outcomes (Yung et al. Reference Yung, Nelson, Thompson and Wood2010; Cornblatt et al. Reference Cornblatt, Carrion, Addington, Seidman, Walker, Cannon, Cadenhead, McGlashan, Perkins, Tsuang, Woods, Heinssen and Lencz2012; Addington & van der Gaag, Reference Addington and van der Gaag2015). This position is in agreement with an extensive literature pointing to SF deficits as a core liability in schizophrenia (Mueser & Tarrier, Reference Mueser and Tarrier1998). Likewise, findings from family and genetic studies of individuals with schizotypy and first-degree relatives of people with schizophrenia also suggest that SF difficulties are closely linked to the genetic vulnerability of schizophrenia (Kendler et al. Reference Kendler, Thacker and Walsh1996; Fanous et al. Reference Fanous, Gardner, Walsh and Kendler2001; Tarbox & Pogue-Geile, Reference Tarbox and Pogue-Geile2011; Fanous et al. Reference Fanous, Zhou, Aggen, Bergen, Amdur, Duan, Sanders, Shi, Mowry, Olincy, Amin, Cloninger, Silverman, Buccola, Byerley, Black, Freedman, Dudbridge, Holmans, Ripke, Gejman, Kendler and Levinson2012).
Investigations of predictors of SF in CHR individuals have highlighted the detrimental impact of emotional processing difficulties, with negative symptoms (Niendam et al. Reference Niendam, Bearden, Johnson, McKinley, Loewy, O'Brien, Nuechterlein, Green and Cannon2006; Cornblatt et al. Reference Cornblatt, Auther, Niendam, Smith, Zinberg, Bearden and Cannon2007; Svirskis et al. Reference Svirskis, Korkeila, Heinimaa, Huttunen, Ilonen, Ristkari, Hietala, Syvalahti, McGlashan, Vahlberg and Salokangas2007; Willhite et al. Reference Willhite, Niendam, Bearden, Zinberg, O'Brien and Cannon2008; Velthorst et al. Reference Velthorst, Nieman, Linszen, Becker, de Haan, Dingemans, Birchwood, Patterson, Salokangas, Heinimaa, Heinz, Juckel, von Reventlow, French, Stevens, Schultze-Lutter, Klosterkotter and Ruhrmann2010; Corcoran et al. Reference Corcoran, Kimhy, Parrilla-Escobar, Cressman, Stanford, Thompson, David, Crumbley, Schobel, Moore and Malaspina2011; Raballo & Krueger, Reference Raballo and Krueger2011; Valmaggia et al. Reference Valmaggia, Stahl, Yung, Nelson, Fusar-Poli, McGorry and McGuire2013; Meyer et al. Reference Meyer, Carrion, Cornblatt, Addington, Cadenhead, Cannon, McGlashan, Perkins, Tsuang, Walker, Woods, Heinssen, Seidman and Group2014) as well as mood symptoms being particularly relevant (Cornblatt et al. Reference Cornblatt, Auther, Niendam, Smith, Zinberg, Bearden and Cannon2007; Velthorst et al. Reference Velthorst, Nieman, Linszen, Becker, de Haan, Dingemans, Birchwood, Patterson, Salokangas, Heinimaa, Heinz, Juckel, von Reventlow, French, Stevens, Schultze-Lutter, Klosterkotter and Ruhrmann2010; Corcoran et al. 2011; Fulford et al. Reference Fulford, Niendam, Floyd, Carter, Mathalon, Vinogradov, Stuart and Loewy2013; Kim et al. Reference Kim, Song, Park, Lee, Lee, Lee, Kang, Lee, Yoo, An and Kwon2013; Meyer et al. Reference Meyer, Carrion, Cornblatt, Addington, Cadenhead, Cannon, McGlashan, Perkins, Tsuang, Walker, Woods, Heinssen, Seidman and Group2014). Results from longitudinal studies are largely consistent with these findings (Bearden et al. Reference Bearden, Wu, Caplan and Cannon2011; Eslami et al. Reference Eslami, Jahshan and Cadenhead2011; Lin et al. Reference Lin, Wood, Nelson, Brewer, Spiliotacopoulos, Bruxner, Broussard, Pantelis and Yung2011; Schlosser et al. Reference Schlosser, Pearson, Perez and Loewy2012b ; Carrion et al. Reference Carrion, McLaughlin, Goldberg, Auther, Olsen, Olvet, Correll and Cornblatt2013; Meyer et al. Reference Meyer, Carrion, Cornblatt, Addington, Cadenhead, Cannon, McGlashan, Perkins, Tsuang, Walker, Woods, Heinssen, Seidman and Group2014; Ziermans et al. Reference Ziermans, de Wit, Schothorst, Sprong, van Engeland, Kahn and Durston2014). When examined together with other symptom clusters, only negative symptoms significantly predicted poor SF (Corcoran et al. 2011; Fulford et al. Reference Fulford, Niendam, Floyd, Carter, Mathalon, Vinogradov, Stuart and Loewy2013; Kim et al. Reference Kim, Song, Park, Lee, Lee, Lee, Kang, Lee, Yoo, An and Kwon2013; Meyer et al. Reference Meyer, Carrion, Cornblatt, Addington, Cadenhead, Cannon, McGlashan, Perkins, Tsuang, Walker, Woods, Heinssen, Seidman and Group2014). Similarly, links between emotion-processing deficits (i.e. anhedonia) and SF difficulties have also been documented in individuals with schizotypy and first-degree relatives of people with schizophrenia (Kerns et al. Reference Kerns, Docherty and Martin2008; Karcher & Shean, Reference Karcher and Shean2012; Docherty et al. Reference Docherty, Sponheim and Kerns2015), supporting the view of emotional difficulties as a core component of schizophrenia-spectrum disorders.
Findings from affective science suggest two specific candidate mechanisms relevant to SF – namely, emotion awareness and emotion regulation. Emotion awareness has been shown to be important to SF because emotions provide crucial information about the significance of social situations, and help to guide potential actions to be taken to navigate such situations (Barrett et al. Reference Barrett, Gross, Christensen and Benvenuto2001). Specifically, negative emotional experiences are thought to have particular informational value in signaling the need to adjust one's current state or activity. Because different emotions may call for the use of distinct response strategies, lack of awareness or reduced clarity of experienced feelings may make it difficult for individuals to select appropriate response strategies for dealing effectively with the social situation (Barrett et al. 2001), potentially resulting in poor SF. Limited emotion awareness, in particular difficulties in identifying and describing feelings, has been linked to poor SF – individuals with poor emotion awareness have been found to have fewer acquaintances and social contacts, lower rates of marriage, as well as poorer overall SF and quality of life (Kauhanen et al. Reference Kauhanen, Kaplan, Julkunen, Wilson and Salonen1993; Salminen et al. Reference Salminen, Saarijarvi, Aarela, Toikka and Kauhanen1999; Kokkonen et al. Reference Kokkonen, Karvonen, Veijola, Laksy, Jokelainen, Jarvelin and Joukamaa2001; Henry et al. Reference Henry, Phillips, Maylor, Hosie, Milne and Meyer2006). To characterize such low emotion awareness individuals, Sifneos introduced the term alexithymia (Sifneos, Reference Sifneos1996), a multi-dimensional subclinical phenomenon that afflicts about 10% of the general population (Linden et al. Reference Linden, Wen, Poulhus, Butcher and Spielberger1994; Salminen et al. Reference Salminen, Saarijarvi, Aarela, Toikka and Kauhanen1999) and encompasses difficulties identifying and describing feelings, struggling to distinguish feelings from emotional arousal sensations, along with impaired symbolization and a tendency to focus on external events (Taylor et al. Reference Taylor, Bagby and Parker1991).
A second candidate mechanism underlying SF is emotion regulation, which has been defined as the processes that are engaged in order to influence which emotions people have, when they have them, and how these emotions are experienced or expressed (Gross, Reference Gross2007). Gross (Reference Gross1998) has proposed a process model of emotion regulation that distinguishes between antecedent- and response-focused strategies, with the former preceding the full emotional response, and the latter being initiated once the response is already underway. Among non-clinical populations, use of antecedent-focused strategies such as reappraisal has been associated with enhanced SF, greater expression of positive emotion, lower negative emotional experience and higher quality of life (Gross & Muñoz, Reference Gross and Muñoz1995; John & Gross, Reference John and Gross2004; Brackett & Salovey, Reference Brackett and Salovey2006). In contrast, response-focused strategies such as suppression have been linked to poorer SF, lower social support, lower satisfaction and sense of closeness to others, greater expression of negative emotion, as well as decreased well-being (Gross, Reference Gross1998; Gross & John, Reference Gross and John2003; Van't Wout et al. Reference Van't Wout, Chang and Sanfey2010). Consistent with these findings, clinical populations have been found to use significantly less reappraisal and more suppression (Campbell-Sills et al. Reference Campbell-Sills, Barlow, Brown and Hofmann2006; Joormann & Gotlib, Reference Joormann and Gotlib2010).
Drawing upon these affective science findings, a small but growing body of research suggests that difficulties with various aspects of emotion processing may be considered a core feature of schizophrenia (Kimhy et al. Reference Kimhy, Vakhrusheva, Jobson-Ahmed, Tarrier, Malaspina and Gross2012, Reference Kimhy, Vakhrusheva, Khan, Chang, Hansen, Ballon, Malaspina and Gross2014). Our group (Kimhy et al. Reference Kimhy, Vakhrusheva, Jobson-Ahmed, Tarrier, Malaspina and Gross2012) and others (Van't Wout et al. Reference Van't Wout, Aleman, Bermond and Kahn2007; Yu et al. Reference Yu, Li, Liu, Zheng, Ma, Chen, Chen, Yu, Lu, Pan and Wang2011; Lincoln et al. Reference Lincoln, Hartmann, Kother and Moritz2014) have documented significantly poorer emotion awareness among individuals with schizophrenia compared with healthy individuals and not-ill siblings, although these findings are not universal (Henry et al. Reference Henry, Bailey, von Hippel, Rendell and Lane2010). Similarly, individuals with schizophrenia have been found to be significantly more likely than healthy persons to suppress their emotions, as well as use less reappraisal (Van der Meer et al. Reference Van der Meer, van't Wout and Aleman2009; Kimhy et al. Reference Kimhy, Vakhrusheva, Jobson-Ahmed, Tarrier, Malaspina and Gross2012; Horan et al. Reference Horan, Hajcak, Wynn and Green2013). Some studies reported no differences from healthy participants (Henry et al. Reference Henry, Rendell, Green, McDonald and O'Donnell2008; Badcock et al. Reference Badcock, Paulik and Maybery2011; Perry et al. Reference Perry, Henry and Grisham2011), potentially related to a higher proportion of individuals with schizo-affective disorder. Germaine to these findings, indices of both emotion awareness and regulation were significantly correlated with poor SF in individuals with schizophrenia and difficulties describing feelings accounted for 35% of the variance in SF in this population, after controlling for age and neurocognition (Kimhy et al. Reference Kimhy, Vakhrusheva, Jobson-Ahmed, Tarrier, Malaspina and Gross2012).
What is not yet clear, however, is whether difficulties with emotion awareness and emotion regulation predate the onset of psychosis, and, if yes, whether such difficulties are linked to SF. Recent reports provide preliminary support for this link – Van Rijn et al. (Reference Van Rijn, Schothorst, Wout, Sprong, Ziermans, van Engeland, Aleman and Swaab2011) found that CHR individuals displayed difficulties in identifying and verbalizing their own emotions, and such difficulties were related to social inadequacy and schizotypal traits. However, the study was limited by a relatively modest and demographically narrow sample (ages 12–18 years) and SF being measured by self-report questionnaires. More recently, Van der Velde et al. (Reference Van der Velde, Swart, van Rijn, van der Meer, Wunderink, Wiersma, Krabbendam, Bruggeman and Aleman2015) found that individuals at high risk for psychosis reported greater difficulties verbalizing, identifying and analysing their own emotions compared with healthy controls (HC) and siblings. Additionally, individuals at high risk for psychosis reported lower use of reappraisal and displayed less activation in the left ventrolateral prefrontal cortex during a reappraisal task compared with HC (Van der Velde et al. Reference Van der Velde, Swart, van Rijn, van der Meer, Wunderink, Wiersma, Krabbendam, Bruggeman and Aleman2015).
To address this gap in the literature, the goals of the present study were to evaluate emotion awareness and regulation in CHR individuals. Specifically, our aims were: (1) to compare emotion awareness and regulation between CHR individuals, individuals with schizophrenia and HC; and (2) among the CHR individuals, to examine the links between emotion awareness and regulation and SF. We hypothesized that CHR individuals would display emotion awareness and regulation difficulties intermediate to the schizophrenia and HC groups. We also hypothesized that emotion awareness and regulation difficulties would predict SF in CHR individuals. We will also evaluate the influence of potential covariates previously linked to SF including intelligence, depression and anxiety.
Method
Participants
Data on individuals with schizophrenia, at CHR and HC have been obtained from baseline research assessments of two separate studies conducted at the New York State Psychiatric Institute (NYSPI) at the Columbia University Medical Center. Data on CHR help-seeking individuals (n = 54) were obtained from participants enrolled at the Center of Prevention and Evaluation, a psychosis high-risk research clinic located in the NYSPI that investigates prospectively risk and protective factors associated with the development of psychosis. Data on individuals with schizophrenia (n = 87) were collected from individuals participating in research on emotion and autonomic regulation at the NYSPI. HC (n = 50) were recruited by online and print advertisements. Both studies recruited participants from the greater New York City area. Data were collected between 2009 and 2015.
For the CHR individuals, the inclusion criteria were a CHR status as determined by the Structured Interview for Psychosis-Risk Syndromes (SIPS; Miller et al. Reference Miller, McGlashan, Rosen, Cadenhead, Cannon, Ventura, McFarlane, Perkins, Pearlson and Woods2003); age 14–30 years; English-speaking; and capacity to provide informed consent. The exclusion criteria included major medical or neurological disorder better accounting for symptoms; intelligence quotient (IQ) < 70; significant risk of harm to self and/or others; and/or ‘prodromal’ symptoms temporarily related to substance or alcohol use. For the participants with schizophrenia the inclusion criteria were age 18–50 years; English-speaking; a Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) diagnosis of schizophrenia, schizo-affective disorder or schizophreniform disorder; and capacity to provide informed consent. The exclusion criteria were use of street drugs within the past 4 weeks (confirmed via urine toxicology test), history of neurological problems or loss of consciousness; IQ < 70; and a recent history of serious suicidal/aggressive behavior. For the HC participants, the inclusion criteria were age 18–50 years and English-speaking. The exclusion criteria were a history of psychotic symptoms; a diagnosis of any DSM-IV Axis II cluster A personality disorder; IQ < 70; a first-degree family member with history of psychosis; and having been adopted.
CHR status, diagnoses and symptoms
CHR status, along with severity of attenuated psychotic symptoms, were determined by the SIPS (Miller et al. Reference Miller, McGlashan, Rosen, Cadenhead, Cannon, Ventura, McFarlane, Perkins, Pearlson and Woods2003), a semi-structured interview that assess severity of ‘prodromal’ symptoms in four categories: positive, negative, disorganized, and general symptoms. Each item is scored on a range of 0–6, with a score of 3–5 being considered prodromal and 6 indicating threshold psychosis. A doctoral-level clinician (G.B.) with 5 years of experience administering the SIPS conducted the CHR status assessments. CHR status is determined by: (1) presence of attenuated psychotic symptoms; (2) brief intermittent psychotic symptoms; and/or (3) genetic risk (first-degree family member) with a recent decline in functioning (30% decline in Global Assessment of Functioning during the previous 12 months).
Diagnoses for all participants were determined using the Diagnostic Interview for Genetic Studies (Nurnberger et al. Reference Nurnberger, Blehar, Kaufmann, York-Cooler, Simpson, Harkavy-Friedman, Severe, Malaspina and Reich1994), a semi-structured diagnostic interview and medical records review used to collect diagnostic and course of illness information for mood, psychotic and substance use DSM-IV Axis I disorders. The Beck Depression Inventory (BDI; Beck et al. Reference Beck, Steer and Brown1996) was used to measure depression. Among the participants with schizophrenia, symptoms were assessed using the Scales for Assessment of Positive and Negative Symptoms (SAPS/SANS; Andreasen & Olsen, Reference Andreasen and Olsen1982).
Emotion awareness and regulation
Emotion awareness was assessed using the Toronto Alexithymia Scale (TAS-20; Bagby et al. Reference Bagby, Taylor and Parker1994), with a higher score indicating poorer functioning. The TAS-20 is a self-report measure with three subscales: difficulty identifying feelings (DIF; seven items); difficulty describing feelings (DDF; five items); and externally oriented thinking (EOT; eight items). Participants are asked to indicate on a five-point scale (from 1 = ‘strongly disagree’ to 5 = ‘strongly agree’) to what extent they agreed with each statement. The TAS-20 has a solid internal consistency (⩾0.80) with the DIF and DDF subscales, demonstrating good reliability (r = 0.79–0.83). We elected to exclude the EOT subscale due to questionable reliability (Kooiman et al. Reference Kooiman, Spinhoven and Trijsburg2002). Emotion regulation was assessed using the Emotion Regulation Questionnaire (ERQ; Gross & John, Reference Gross and John2003), a 10-item self-report survey that provides information regarding regulation strategies of suppression (four items) and reappraisal (six items). Participants are asked to indicate on a seven-point scale (from 1 = ‘strongly disagree’ to 7 = ‘strongly agree’) to what extent they agree with each statement, with higher scores reflecting stronger endorsement of using the strategy. The ERQ has been shown to be a reliable and valid measure of emotion regulation, with an average α reliability of 0.79 for reappraisal and 0.73 for suppression. Test–retest correlations across 3 months were 0.69 for both scales (Gross & John, Reference Gross and John2003).
SF
SF was determined using the Global Functioning Scale: Social (GFS:S; Cornblatt et al. Reference Cornblatt, Auther, Niendam, Smith, Zinberg, Bearden and Cannon2007). The GFS:S assesses peer relationships in CHR participants based on age-appropriate social contacts inside and outside the family, romantic relationships, as well as the level of conflict the individual may or may not experience in these relationships. SF is rated on a 1–10 scale, with 1 being severe dysfunction (poor SF with no relationships) and 10 being highly sociable. The GFS:S displayed high inter-rater reliability (0.78–0.85), with preliminary data supporting its construct validity (Cornblatt et al. Reference Cornblatt, Auther, Niendam, Smith, Zinberg, Bearden and Cannon2007).
Additional measures
A number of potential covariates were examined including intelligence, which was indexed by the Wechsler Abbreviated Scale of Intelligence (Wechsler, Reference Wechsler1999) along with demographic and clinical information including age, sex, race, ethnicity, medication use, depression (BDI) and anxiety (Beck Anxiety Inventory; Beck & Steer, Reference Beck and Steer1993).
Procedure
Following an initial telephone screen, participants signed the informed consent forms and were assessed for eligibility. After satisfying the inclusion and exclusion criteria, participants typically completed the diagnostic and clinical assessments including measures of emotion awareness and regulation within 3 weeks of admission to their respective study as part of the baseline assessments. The CHR participants also completed the SF assessment during this period.
Data analyses
Cross-sectional group differences in emotion awareness and regulation were assessed using two-tailed t tests with significance levels set at p < 0.05, followed by one-way analyses of covariance (ANCOVAs) controlling for age. Follow-up pairwise comparisons were conducted with the Holm's sequential Bonferroni procedure used to control for type I error. Among the CHR participants, associations among emotion awareness, emotion regulation and SF were examined first using Pearson correlations, followed by partial correlations controlling for depression, anxiety and IQ. Assessment of whether emotion awareness and regulation would predict SF among the CHR participants was tested using hierarchical multiple regression analysis, with SF entered as a dependent variable, the control variables and previously identified predictors entered in block 1, the emotion awareness variables entered in block 2, and emotion regulation variables entered in block 3. We elected not to include negative symptoms as independent predictors in our analysis given such symptoms encompass to a large degree indices of SF, emotion awareness and emotion regulation, albeit with different labels. Elements of emotion awareness and emotion regulation (i.e. suppression) overlap multiple domains of negative symptoms (i.e. affective flattening, avolition–apathy). We employed a similar strategy in a previous investigation of the impact of emotion awareness and regulation on SF in individuals with schizophrenia (Kimhy et al. Reference Kimhy, Vakhrusheva, Jobson-Ahmed, Tarrier, Malaspina and Gross2012). Meyer et al. (Reference Meyer, Carrion, Cornblatt, Addington, Cadenhead, Cannon, McGlashan, Perkins, Tsuang, Walker, Woods, Heinssen, Seidman and Group2014) found that the removal of social anhedonia substantially reduced the magnitude of the relationship between overall negative symptom and SF among CHR individuals [from β = −0.422 to β = −0.252; see Meyer et al. (Reference Meyer, Carrion, Cornblatt, Addington, Cadenhead, Cannon, McGlashan, Perkins, Tsuang, Walker, Woods, Heinssen, Seidman and Group2014) online Supplementary data – Tables S1a and S1b].
Results
Demographic and clinical characteristics
The sample's demographic and clinical information is presented in Table 1. There were no significant group differences in sex, ethnicity and racial background. However, the CHR individuals were significantly younger and had lower educational level, potentially related to their younger age. Of the CHR participants, 37 were medication-free (68%), three were prescribed antipsychotic medication (5%), seven were prescribed anti-depressants (13%), and seven were prescribed both (13%).
Table 1. Demographic and clinical information (n = 191)
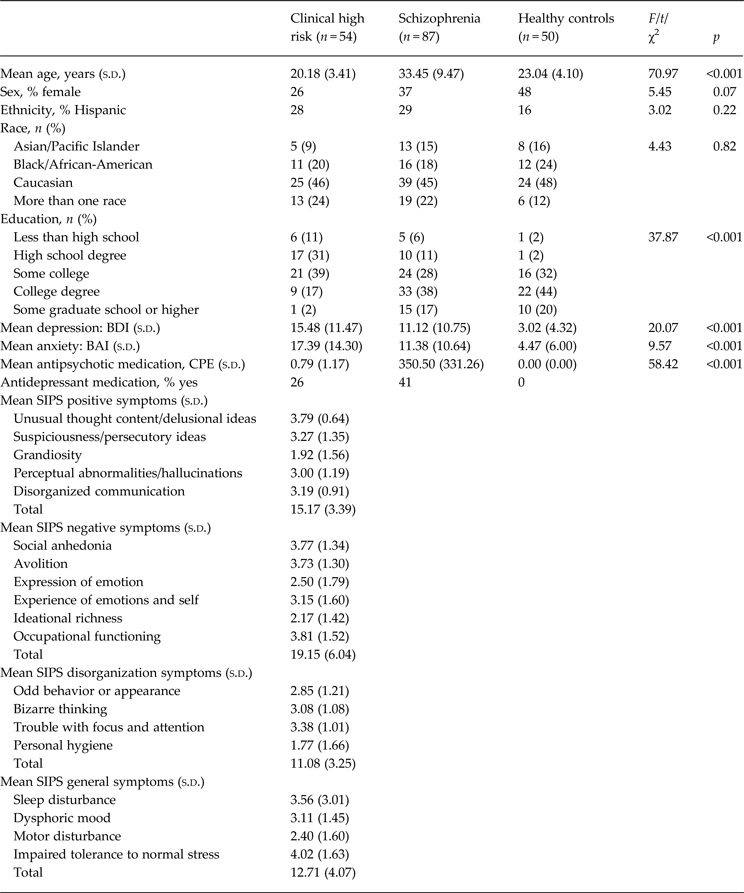
s.d., Standard deviation; BDI, Beck Depression Inventory; BAI, Beck Anxiety Inventory; CPE, chlorpromazine equivalents; SIPS, Structured Interview for Psychosis Risk Syndromes.
Data on IQ were available only for participants in the CHR group, indicating performance in the high average range (mean = 112.63, s.d. = 15.91). SF in the CHR group was not associated with age (r = 0.03, p = 0.84) or IQ (r = 0.03, p = 0.88). Likewise, there were no differences in SF in the CHR group based on sex (t = 0.38, p = 0.70), ethnicity (χ = 13.61, p = 0.06) or racial background (χ = 26.40, p = 0.19). However, suppression was significantly inversely correlated with age (r = −0.27, p = 0.05).
Among the schizophrenia group, clinical assessments indicated moderate severity of hallucinations (mean = 2.90, s.d. = 2.03) and delusions (mean = 3.00, s.d. = 1.47), along with minimal ratings of bizarre behavior and positive formal thought disorder (mean = .58, s.d. = 1.04 and mean = 1.00, s.d. = 1.28, respectively). Of the participants with schizophrenia, 37 were prescribed anti-depressants (41%) and all were prescribed antipsychotic medications, which was not significantly associated with any measures of emotion awareness or regulation.
Group comparisons of emotion awareness and regulation
Our first aim was to compare emotion awareness and regulation among individuals at CHR, with schizophrenia and HC. We conducted ANCOVAs for each of the four key variables with clinical status entered as the independent variable, the emotion awareness and regulation variables as the dependent variables, and age as a covariate. The results of these tests are presented in Table 2. For difficulties identifying feelings, there were significant group differences (F 2,187 = 27.86, p < 0.001), with clinical status accounting for 23.4% of the variance, controlling for age. Follow-up pairwise comparisons were conducted with the Holm's sequential Bonferroni procedure used to control for type I error. The CHR group had the highest average DIF, followed closely by the schizophrenia and then HC groups.
Table 2. Comparison of emotion awareness and regulation between CHR, SCZ and HC individuals (n = 191)
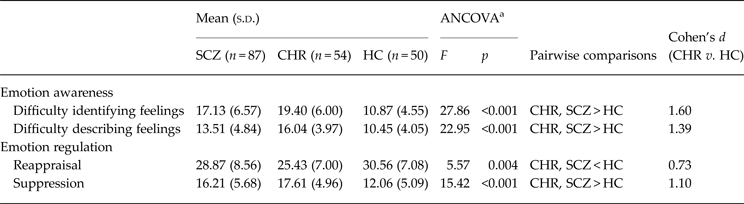
CHR, Clinical high-risk for psychosis; SCZ, schizophrenia; HC, healthy controls; ANCOVA, one-way analysis of covariance; TAS-20, Toronto Alexithymia Scale.
a With age as covariate; emotion awareness – indexed by the TAS-20; emotion regulation – indexed by the Emotion Regulation Questionnaire; lower TAS-20 and suppression scores indicate better functioning.
Similarly, for difficulties describing feelings, there were significant group differences (F 2,187 = 22.95, p < 0.001), with clinical status accounting for 20.1% of the variance, controlling for age. The CHR group had the highest mean DIF score, followed by the schizophrenia and then HC groups (Fig. 1).
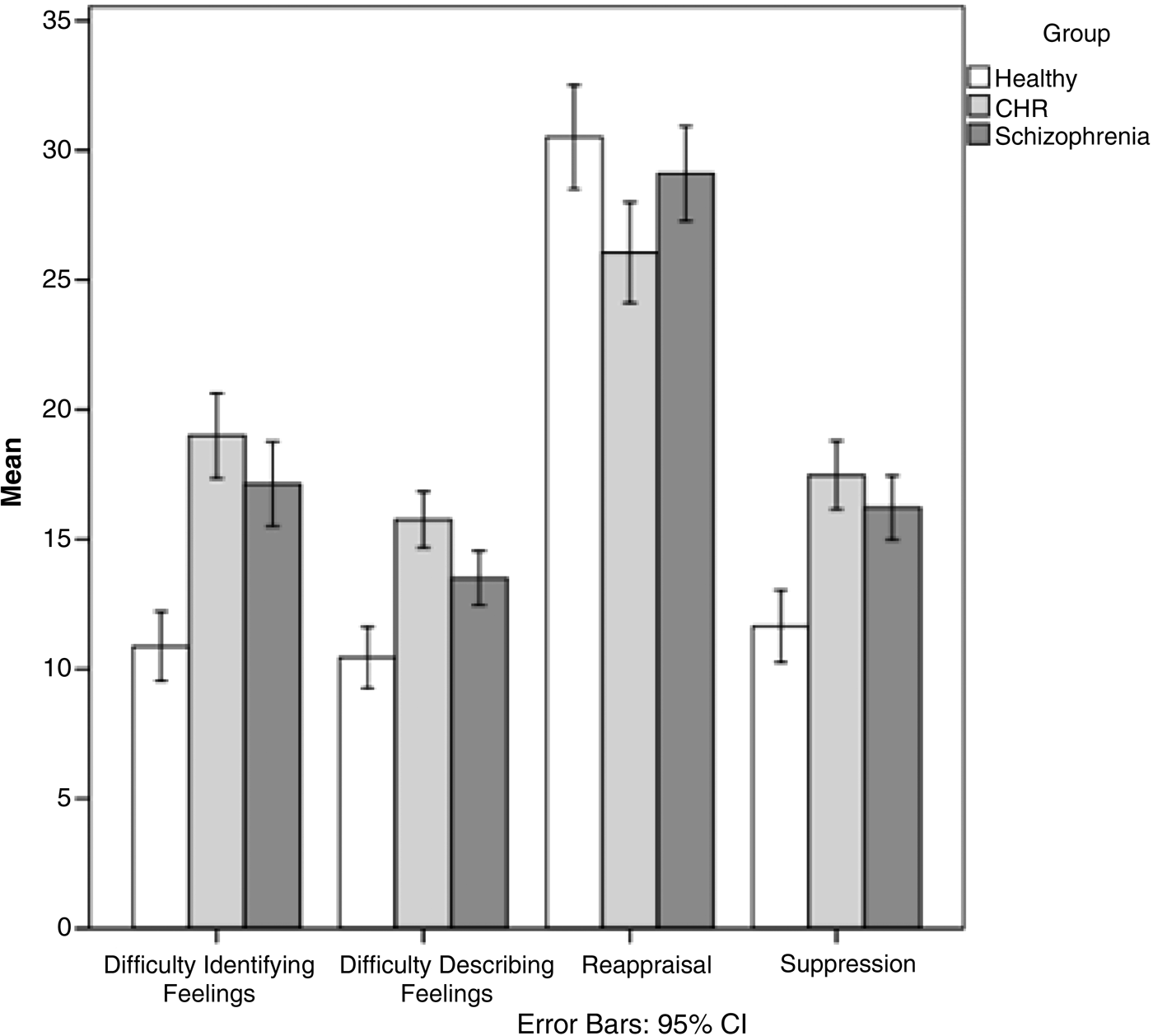
Fig. 1. Comparison of emotion awareness and regulation in individuals with schizophrenia (n = 87), at clinical high risk for psychosis (CHR; n = 54) and healthy controls (n = 50) (total n = 191). Difficulty identifying feelings – Toronto Alexithymia Scale (TAS-20); difficulty describing feelings –TAS-20; reappraisal – Emotion Regulation Questionnaire (ERQ); suppression –ERQ. Values are means, with confidence intervals (CI) represented by vertical bars.
For emotion regulation, there were significant group differences in the use of reappraisal (F 2,187 = 5.57, p = 0.004), with clinical status accounting for 5.6% of the variance, controlling for age. The HC group had significantly higher mean reappraisal scores compared with the schizophrenia and CHR groups. Likewise, for suppression there were significant group differences (F 2,187 = 15.42, p < 0.001), with clinical status accounting for 14.2% of the variance, controlling for age. The HC group had significantly higher mean scores compared with the schizophrenia and CHR groups.
The impact of emotion awareness and regulation on SF
Next, we evaluated within the CHR group the impact of emotion awareness and regulation on SF. Data on SF were available for 43 of the 54 CHR participants. There were no significant differences in age, sex, depression, and emotion awareness and regulation between participants with and without SF data. The mean SF rating was 5.60 (s.d. = 1.44, range 2–9), with 77% of CHR individuals receiving a rating of ⩽6. We conducted a hierarchical step-wise multiple regression analysis with SF entered as a dependent variable; demographic variables including age, sex and education entered in block 1; and the emotion awareness and regulation variables entered in block 2. The regression analysis indicated that after controlling for age, sex and education, the model accounted for 23.2% of the variance in SF (F 1,41 = 12.36, p = 0.001). Specifically, difficulties describing feelings contributed uniquely to the model's validity (β = −0.48, t = −3.52, p = 0.001). An exploratory analyses in the CHR group comparing emotion awareness and regulation in individuals with poor (⩽4), medium (5–6), and high (⩾7) SF revealed significant group differences, suggesting a dose–response relationship in which individuals with the poorest ability to describe feelings (F 2,40 = 5.29, p < 0.01) and highest use of suppression (F 2,40 = 3.42, p = 0.04) displayed the lowest degree of SF (see Fig. 2; Table 3).

Fig. 2. Difficulty describing feelings and use of suppression in individuals at clinical high risk for psychosis (CHR; n = 43) with poor, medium and high social functioning and healthy controls (n = 47) (total n = 90). Social functioning – Global Functioning Scale: Social [range 1 (low) to 10 (high)]; difficulty describing feelings – Toronto Alexithymia Scale; suppression – Emotion Regulation Questionnaire. Values are means, with confidence intervals (CI) represented by vertical bars.
Table 3. Emotion awareness and regulation in individuals at clinical high risk for psychosis – associations with social functioning a
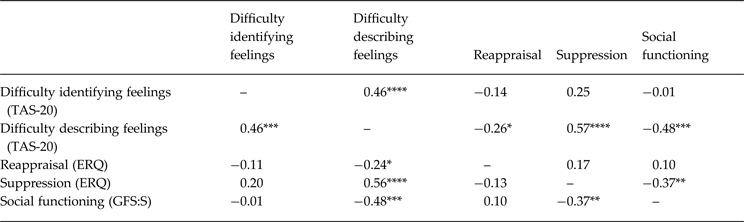
TAS-20, Toronto Alexithymia Scale; ERQ, Emotion Regulation Questionnaire; GFS:S, Global Functioning Scale: Social.
a Zero-order correlations are presented above the diagonal. Partial correlations controlling for age are presented below the diagonal. Lower TAS-20 and suppression scores indicate better functioning.
* p < 0.10, ** p < 0.05, *** p < 0.01, **** p < 0.001.
Discussion
To the best of our knowledge, the present study is the first investigation of the links between emotion awareness, emotion regulation and SF in individuals at CHR for psychosis. Our results indicate that compared with a non-clinical population, CHR individuals display substantial deficits in emotion awareness and regulation, as indicated by the large effect sizes for all measures. Specifically, CHR individuals reported significant difficulties identifying and describing their emotions, along with increased use of suppression and diminished use of reappraisal when attempting to regulate their emotions.
The most important finding of the present investigation is the identification of a link between poor emotional awareness and poor SF in CHR individuals, with difficulties in emotional awareness predicting 23.2% of the variance in SF. This association was accounted primarily by difficulties describing feeling and is consistent with results among individuals with schizophrenia (Kimhy et al. Reference Kimhy, Vakhrusheva, Jobson-Ahmed, Tarrier, Malaspina and Gross2012). Thus, our results extend findings from basic affective science in highlighting the fundamental role that emotion awareness plays in SF and psychopathology (Kauhanen et al. Reference Kauhanen, Kaplan, Julkunen, Wilson and Salonen1993; Sifneos, Reference Sifneos1996; Salminen et al. Reference Salminen, Saarijarvi, Aarela, Toikka and Kauhanen1999; Kokkonen et al. Reference Kokkonen, Karvonen, Veijola, Laksy, Jokelainen, Jarvelin and Joukamaa2001; Henry et al. Reference Henry, Phillips, Maylor, Hosie, Milne and Meyer2006). A second important finding of the present investigation relates to the severity of emotion awareness and regulation deficits in the CHR group. Specifically, the degree of CHR difficulties in these domains was comparable with those found in the schizophrenia group. These results are in agreement with recent reports (Van der Velde et al. Reference Van der Velde, Swart, van Rijn, van der Meer, Wunderink, Wiersma, Krabbendam, Bruggeman and Aleman2015). Together, these findings suggest that emotion awareness deficits are present in full severity well before the onset of full psychotic symptoms. Thus, such deficits may potentially reflect an affective behavioral marker of early SF difficulties in this population. This early presentation of emotional deficits also raises key questions about their developmental origins in CHR individuals. Data from a large population-based study of alexithymia among 8785 twin-pairs indicate that DDF was attributed primarily to environmental effects, with 69% of the variance explained by shared and non-shared environmental effects (15 and 54%, respectively), with genetic effects accounting for the remaining 31% (Jorgensen et al. Reference Jorgensen, Zachariae, Skytthe and Kyvik2007). Given the substantial impact of emotion awareness on SF in CHR individuals, as well as those with schizophrenia, future studies should investigate the relative effects of environmental and genetics factors on emotional awareness in CHR individuals. Likewise, the results are in agreement with findings from clinical, family and genetic studies of individuals with schizotypy, schizophrenia, and their first-degree relatives which support the view of emotion (i.e. anhedonia) and SF difficulties as core components of the schizophrenia spectrum vulnerability (Kendler et al. Reference Kendler, Thacker and Walsh1996; Fanous et al. Reference Fanous, Gardner, Walsh and Kendler2001; Kerns et al. Reference Kerns, Docherty and Martin2008; Tarbox & Pogue-Geile, Reference Tarbox and Pogue-Geile2011; Fanous et al. 2012; Karcher & Shean, Reference Karcher and Shean2012; Docherty et al. Reference Docherty, Sponheim and Kerns2015).
Our findings invite a discussion about the putative neurobiological mechanism underlying poor emotional awareness in CHR individuals and the prospect of ameliorating them. Evidence from imaging studies of individuals with schizophrenia has linked alexithymia to white matter fractional anisotropy, in particular in the corpus callosum, left superior and inferior longitudinal fasciculi, inferior occipitofrontal fasciculus, anterior and posterior thalamic radiation, and the precuneus (Kubota et al. Reference Kubota, Miyata, Sasamoto, Kawada, Fujimoto, Tanaka, Sawamoto, Fukuyama, Takahashi and Murai2012). Alexithymia has also been linked to lower gray matter volume in the left supramarginal gyrus (Kubota et al. Reference Kubota, Miyata, Hirao, Fujiwara, Kawada, Fujimoto, Tanaka, Sasamoto, Sawamoto, Fukuyama, Takahashi and Murai2011), a region involved in language processing. Consistent with these findings, lower gray matter volume in this region has been documented among CHR individuals (Koutsouleris et al. Reference Koutsouleris, Schmitt, Gaser, Bottlender, Scheuerecker, McGuire, Burgermeister, Born, Reiser, Moller and Meisenzahl2009) along with reduced activation (Fusar-Poli et al. Reference Fusar-Poli, Broome, Woolley, Johns, Tabraham, Bramon, Valmaggia, Williams and McGuire2011), suggesting a potential region of interest underlying poor emotional awareness in this population. Increasing patients’ abilities to identify and describe emotions are an important component of many contemporary cognitive and behavioral psychotherapies (Greenberg & Pascual-Leone, Reference Greenberg and Pascual-Leone2006) and preliminary evidence suggests feasibility and efficacy of improving alexithymia (Fukunishi et al. Reference Fukunishi, Kikuchi and Takubo1997; Beresnevaite, Reference Beresnevaite2000; Honkalampi et al. Reference Honkalampi, Hintikka, Saarinen, Lehtonen and Viinamaki2000). Consistent with this view, evidence from basic affective science studies demonstrates that the act of labeling emotions in response to affective stimuli attenuates emotional responses (Lieberman et al. Reference Lieberman, Eisenberger, Crockett, Tom, Pfeifer and Way2007; Niles et al. Reference Niles, Craske, Lieberman and Hur2015). Specifically, among non-clinical individuals, labeling one's emotional experiences has been found to activate the right ventrolateral prefrontal cortex, resulting in reduced amygdala activation (Hariri et al. Reference Hariri, Bookheimer and Mazziotta2000; Narumoto et al. Reference Narumoto, Yamada, Iidaka, Sadato, Fukui, Itoh and Yonekura2000; Gorno-Tempini et al. Reference Gorno-Tempini, Pradelli, Serafini, Pagnoni, Baraldi, Porro, Nicoletti, Umita and Nichelli2001; Hariri et al. Reference Hariri, Mattay, Tessitore, Fera and Weinberger2003). Future studies should aim to develop and test treatments to address emotion awareness difficulties in CHR individuals.
Our results have additional implications for future research of schizophrenia and CHR individuals. To date, research of social cognition in these clinical populations has focused primarily on a number of domains including perception of emotion in others, theory of mind, social perception and attribution style (Pinkham et al. Reference Pinkham, Penn, Green, Buck, Healey and Harvey2014). In contrast, the way individuals perceive their own emotions and how they process and regulate them have received relatively little attention. Given our results, along with previous reports (Kimhy et al. Reference Kimhy, Vakhrusheva, Jobson-Ahmed, Tarrier, Malaspina and Gross2012, Reference Kimhy, Vakhrusheva, Khan, Chang, Hansen, Ballon, Malaspina and Gross2014; O'Driscoll et al. Reference O'Driscoll, Laing and Mason2014; Van der Velde et al. Reference Van der Velde, Swart, van Rijn, van der Meer, Wunderink, Wiersma, Krabbendam, Bruggeman and Aleman2015), emotion awareness and regulation may serve as promising targets for investigations relating to social cognition and SF in individuals with CHR and schizophrenia. The strengths of the present investigation include the rigorous diagnostic and clinical assessments and the use of a psychometrically sound clinician-based measure that assess SF separately from role functioning and symptom severity (Cornblatt et al. Reference Cornblatt, Auther, Niendam, Smith, Zinberg, Bearden and Cannon2007). One potential limitation of the present study is the moderate CHR sample size. Another limitation is the use of self-report measures to index emotion regulation and the focus on only two emotion-regulation strategies. Also, data on a number of potential covariates were available only for the CHR group (e.g. intelligence).
In summary, our results indicate that CHR individuals display substantial emotion-awareness and emotion-regulation deficits, at severity comparable with those observed in individuals with schizophrenia. Such deficits, in particular difficulties describing feelings, predate the onset of psychosis and contribute significantly to poor SF in this population.
Acknowledgements
This work was supported by grants 1K23MH077653 and 1R21MH096132 (to D.K.) and 1R01MH093398 from the National Institute of Mental Health, Bethesda, MD, USA.
Declaration of Interest
None.







