Introduction
The DSM-IV (American Psychiatric Association, 2000) and the subsequent DSM-5 (American Psychiatric Association, 2013) diagnostic criteria define autism and schizophrenia as two distinct disorders, but this phenotypic dichotomy has been challenged by empirical evidence for the autism–schizophrenia continuum (King & Lord, Reference King and Lord2011; Sasson et al. Reference Sasson, Pinkham, Carpenter and Belger2011). The two disorders share notable similarities in genetic, neuroanatomical, neuropsychological and behavioural aspects. At the genetic level, two large case–control studies have shown that parental schizophrenia is a significant risk factor for autism (Larsson et al. Reference Larsson, Eaton, Madsen, Vestergaard, Olesen, Agerbo, Schendel, Thorsen and Mortensen2005; Daniels et al. Reference Daniels, Forssen, Hultman, Cnattingius, Savitz, Feychting and Sparen2008). Genetic studies have shown that disruption of several genomic regions such as the DISC1 gene (Marx, Reference Marx2007) and NRXN1 gene (Viñas-Jornet et al. Reference Viñas-Jornet, Esteba-Castillo, Gabau, Ribas-Vidal, Baena, San, Ruiz, Coll, Novell and Guitart2014) are associated with both autism and schizophrenia. At the neuroanatomical level, autism and schizophrenia share similar grey matter volume reduction in the limbic–striato–thalamic circuitry (Cheung et al. Reference Cheung, Yu, Fung, Leung, Wong, Li, Sham, Chua and McAlonan2010). At the neuropsychological level, executive dysfunctions are prevalent in both autism patients (Lai et al. Reference Lai, Lau, Lui, Lok, Tam, Chan, Cheng, Lam and Cheung2016) and schizophrenia patients (Evans et al. Reference Evans, Chua, McKenna and Wilson1997; Chan et al. Reference Chan, Chen and Law2006). At the behavioural level, features such as flattened affect, impoverished speech, concrete thinking and impaired social functioning are prevalent in both clinical populations (Volkmar & Cohen, Reference Volkmar and Cohen1991). In fact, high-functioning autism could easily be conflated with schizophrenia (Sheitman et al. Reference Sheitman, Kraus, Bodfish and Carmel2004; Bastiaansen et al. Reference Bastiaansen, Meffert, Hein, Huizinga, Ketelaars, Pijnenborg, Bartels, Minderaa, Keysers and de Bildt2011), in particular when reliable developmental history and positive symptoms of schizophrenia are not conspicuous.
Theory of mind (ToM) refers to the ability to infer the mental state of other people (Baron-Cohen, Reference Baron-Cohen1995), and is one of the more complex neuropsychological functions. Schizophrenia and autism are both known to be associated with ToM impairments (Bora et al. Reference Bora, Yucel and Pantelis2009; Chung et al. Reference Chung, Barch and Strube2014; Bliksted et al. Reference Bliksted, Ubukata and Koelkebeck2016). On the other hand, it has been postulated that, whereas autistic features such as social isolation and poor mentalising ability are related to the inability to represent the mental state of other people, schizophrenic symptoms such as paranoid delusions are related to the erroneous representations of mental state of other people (Frith, Reference Frith1992; Pickup & Frith, Reference Pickup and Frith2001). In the context of empirical evidence supporting the autism–schizophrenia continuum, it is worthy to examine the similarities and differences of ToM between schizophrenia and autism. Although a recent meta-analytic review (Chung et al. Reference Chung, Barch and Strube2014) concluded that the magnitude of ToM impairments in autism patients was comparable with schizophrenia patients, a few studies have directly compared the ToM ability in the two clinical populations. To our knowledge, only seven previous studies (see Table 1) directly compared ToM ability of schizophrenia patients with autism patients, and the results were conflicting. For instance, using verbal-based ToM tasks, one study (Craig et al. Reference Craig, Hatton, Craig and Bentall2004) reported that schizophrenia patients and patients with Asperger's syndrome were both impaired in ToM relative to controls, but they were not significantly different from each other. Another study (Pilowsky et al. Reference Pilowsky, Yirmiya, Arbelle and Mozes2000), which also used a verbal-based ToM paradigm, i.e. the false belief task, found no significant difference between childhood-onset schizophrenia patients and high-functioning autism patients. Bowler (Reference Bowler1992)’s and Murphy (Reference Murphy2006)’s studies reported that verbal- and visual-based ToM impairments in patients with Asperger's syndrome were comparable with schizophrenia patients. On the other hand, Ozguven et al. (Reference Ozguven, Oner, Baskak, Oktem, Olmez and Munir2010) reported that, although the two clinical groups shared similar impairments on second-order false belief tests, patients with Asperger's syndrome performed poorer than schizophrenia patients on first-order false belief tests. Couture et al. (Reference Couture, Penn, Losh, Adolphs, Hurley and Piven2010) reported that both high-functioning autism patients and schizophrenia patients did not exhibit any significant ToM impairment compared with controls on the ‘Eyes test’, a visual-based ToM task. Lugnegård et al. (Reference Lugnegård, Unenge Hallerbäck, Hjärthag and Gillberg2013) utilised the verbal-based false belief task and found that schizophrenia patients were more severely impaired in ToM than autism patients.
Table 1. Summary of previous studies comparing ToM impairments in autism spectrum disorders and schizophrenia

AS, Asperger's Syndrome; SCZ, Schizophrenia; HC, Healthy Control; ABC, Autism Behaviour Checklist; KSADS, Kiddie-Schedule for Affective Disorders and Schizophrenia for school age children; PSE, Present State Examination; PD, Personality Disorder; HFA, High-Functioning Autism; IQ, Intelligence Quotient; N.A., not available.
The discrepant findings of these empirical studies might be due to several notable methodological issues. For instance, Bowler (Reference Bowler1992)’s study did not recruit a healthy comparison group. The majority of these studies (Bowler, Reference Bowler1992; Pilowsky et al. Reference Pilowsky, Yirmiya, Arbelle and Mozes2000; Craig et al. Reference Craig, Hatton, Craig and Bentall2004; Murphy, Reference Murphy2006; Ozguven et al. Reference Ozguven, Oner, Baskak, Oktem, Olmez and Munir2010) were limited by very small sample size, and several studies (Murphy, Reference Murphy2006; Couture et al. Reference Couture, Penn, Losh, Adolphs, Hurley and Piven2010; Lugnegård et al. Reference Lugnegård, Unenge Hallerbäck, Hjärthag and Gillberg2013) did not match schizophrenia patients and autism patients in terms of intelligence quotient (IQ). Although Chung et al.’s (Reference Chung, Barch and Strube2014) meta-analysis found IQ to have no impact on the magnitude of ToM impairments in schizophrenia and autism, this notion might not be conclusive, because only seven among the 37 studies included in the meta-analysis had reported IQ scores. Moreover, several previous studies (Bowler, Reference Bowler1992; Pilowsky et al. Reference Pilowsky, Yirmiya, Arbelle and Mozes2000; Ozguven et al. Reference Ozguven, Oner, Baskak, Oktem, Olmez and Munir2010) only utilised verbal-based ToM paradigms, even though autism is associated with limited verbal ability. More importantly, none of the previous studies had examined, whether schizophrenia and autism would have differential impairments in affective and cognitive ToM. Whereas affective ToM refers to the ability to infer emotions of other people, cognitive ToM refers to the ability to infer beliefs of other people (Shamay-Tsoory et al. Reference Shamay-Tsoory, Aharon-Peretz and Perry2009). Evidence in the non-clinical population supports a two-facetted model of ToM, that affective ToM but not cognitive ToM processing recruits the ventromedial prefrontal cortex (PFC) (Sebastian et al. Reference Sebastian, Fontaine, Bird, Blakemore, De brito, Mccrory and Viding2012). Evidence in individuals with traumatic brain injury and neurodegenerative diseases (Hynes et al. Reference Hynes, Baird and Grafton2006; Poletti et al. Reference Poletti, Enrici and Adenzato2012) also supports the unique role of the ventromedial PFC in affective ToM. A recent study (Ho et al. Reference Ho, Lui, Hung, Wang, Li, Cheung and Chan2015) has demonstrated that schizophrenia patients are more impaired in affective rather than cognitive ToM. Notably, this ‘affective/cognitive’ model is different from the ‘verbal/visual’ model of ToM (Chung et al. Reference Chung, Barch and Strube2014). In the latter two-facetted model of ToM, verbal (‘cognitive–linguistic’) but not visual mentalising is strongly influenced by individuals’ language ability and cognitive processing of contextual details (Chung et al. Reference Chung, Barch and Strube2014). However, the distinction of affective v. cognitive ToM does not necessarily involve language ability or processing of contextual details (Shamay-Tsoory et al. Reference Shamay-Tsoory, Shur, Barcai-Goodman, Medlovich, Harari and Levkovitz2007a). To reconcile the discrepant findings and to address limitations of the previous studies, it is necessary to comprehensively examine mentalising ability in a larger and well-matched sample with high-functioning autism and schizophrenia patients, using tasks which tap into affective and cognitive domains of ToM.
This study therefore investigated both verbal-based and visual-based ToM in high-functioning autism patients and schizophrenia patients, and utilised a paradigm to tap into the affective and cognitive ToM constructs. This study aimed to: (1) compare ToM ability of schizophrenia patients with age- and IQ-matched autism patients; and (2) investigate whether there are shared or divergent ToM impairments between the two disorders, using both verbal and visual tasks as well as the affective and cognitive ToM models. Based on earlier findings (Chung et al. Reference Chung, Barch and Strube2014), we hypothesised that high-functioning autism patients and schizophrenia patients would exhibit similar verbal and visual ToM impairments. Based on Frith's theoretical framework (Pickup & Frith, Reference Pickup and Frith2001) and the recent findings on affective and cognitive ToM in schizophrenia (Ho et al. Reference Ho, Lui, Hung, Wang, Li, Cheung and Chan2015), we hypothesised that high-functioning autism patients would show severe impairments in both affective and cognitive ToM, whilst schizophrenia patients would show severe impairments in affective ToM but mild impairments in cognitive ToM.
Methods
Participants
Thirty participants with high-functioning autism and 30 participants with clinically-stable schizophrenia were recruited from outpatient clinics in a large psychiatric hospital in Hong Kong. The inclusion criteria for high-functioning autism participants were: (1) a DSM-IV diagnosis of autistic disorder, Asperger's disorder, or pervasive developmental disorder not otherwise specified; (2) the estimated intelligence should not be lower than 70, which defines high-functioning in autism patients; (3) aged from 16–19; and (4) ethnic Chinese. The inclusion criteria for schizophrenia participants were (1) a DSM-IV diagnosis of schizophrenia; (2) aged from 16 to 19; and (3) clinical stabilisation as reported by the treating psychiatrists. The exclusion criteria for both clinical groups were: (1) presence of any co-morbid DSM-IV disorder; (2) mental retardation; (3) severe hearing or visual impairment; (4) history of head injury; (5) history of neurological disorder; and (6) history of substance abuse in the past 24 months. The diagnosis of high-functioning autism was ascertained by trained child psychiatrists using best clinical judgement, based on the clinical history documented in medical records and the Autism Diagnostic Observation Schedule (ADOS) (Bastiaansen et al. Reference Bastiaansen, Meffert, Hein, Huizinga, Ketelaars, Pijnenborg, Bartels, Minderaa, Keysers and de Bildt2011; Volkmar et al. Reference Volkmar, Siegel, Woodbury-Smith, King, McCracken and State2014). The diagnosis of schizophrenia was ascertained by trained general adult psychiatrists using the Structured Clinical Interview for DSM-IV Axis I disorders (SCID-I) (First et al. Reference First, Spitzer, Gibbon and Williams1996). In terms of DSM-IV diagnosis, the high-functioning autism group consisted of four participants with autism, 18 participants with Asperger's syndrome and eight participants with pervasive developmental disorder not otherwise specified. According to the DSM-IV, one schizophrenia participant was diagnosed with the disorganised subtype, and the remaining 29 participants were with the paranoid subtype. Thirty healthy controls were recruited from youth centres, and assessed by qualified psychiatrists using structured interviews to ensure the absence of any past or current DSM-IV disorders.
Demographics, duration of untreated psychosis (Norman & Malla, Reference Norman and Malla2001), duration of illness, current medications and chlorpromazine equivalence (Gardner et al. Reference Gardner, Murphy, O'Donnell, Centorrino and Baldessarini2010) of current antipsychotic medications were gathered from medical records and clinical interviews. All schizophrenia participants were receiving antipsychotic medications, including both second-generation (clozapine: n = 4, risperidone: n = 8, amisulpride: n = 5, aripiprazole: n = 7, olanzapine: n = 8, paliperidone palmitate: n = 2) and first-generation (haloperidol, n = 1) antipsychotics. Amongst the high-functioning autism participants, six of them were receiving antipsychotics (aripiprazole: n = 3, olanzapine: n = 2, risperidone: n = 1) and the remaining were medication-free. Schizophrenia participants’ clinical symptoms were assessed by trained psychiatrists using the Positive and Negative Syndrome scale (PANSS) (Kay et al. Reference Kay, Fiszbein and Opler1987). Cerebral dominance was ascertained using the Annett Handedness scale (Annett, Reference Annett1970). Intelligence was estimated using a prorating method, based on the Chinese version of the Arithmetic, Similarities and Digit Span subscales of the Wechsler Adult Intelligence Scale-Revised (WAIS-R) (Gong, Reference Gong1992). All participants provided written informed consent. The local ethics committee approved this project.
As shown in Table 2, the three groups did not differ in age (p = 0.277), gender ratio (p = 0.530), handedness (p = 0.770), years of education (p = 0.194) and estimated IQ (p = 0.214).
Table 2. Demographics, clinical characteristics, and neurocognitive profile of the participants

IQ, Intelligence Quotient; PANSS, Positive and Negative Syndrome Scale.
Measurements
ToM: the Yoni Task
The Yoni Task is a computerised task developed by Shamay-Tsoory et al. (Reference Shamay-Tsoory, Shur, Barcai-Goodman, Medlovich, Harari and Levkovitz2007a). This cartoon-based non-verbal task measures an individual's ability to judge others’ mental state, based on the eye gaze and facial expression cues on the face of ‘Yoni’, a cartoon character of this task. In this study, we utilised the Chinese version of the Yoni Task (Ho et al. Reference Ho, Lui, Hung, Wang, Li, Cheung and Chan2015). It taps into first- and second-order ToM, each was further differentiated into cognitive and affective ToM conditions, as well as physical (control) condition, resulting in a total of six different conditions, namely first-order cognitive (Cog1), first-order affective (Aff1), first-order physical (Phy1), second-order cognitive (Cog2), second-order affective (Aff2) and second-order physical (Phy2). The first-order ToM conditions involve Yoni and objects, while the second-order conditions involve Yoni and another character. This paradigm has been used in autism patients (Shamay-Tsoory, Reference Shamay-Tsoory2008) and schizophrenia patients (Shamay-Tsoory et al. Reference Shamay-Tsoory, Shur, Barcai-Goodman, Medlovich, Harari and Levkovitz2007a, Reference Shamay-Tsoory, Aharon-Peretz and Levkovitzb; Ho et al. Reference Ho, Lui, Hung, Wang, Li, Cheung and Chan2015); it has the advantage of tapping into both cognitive and affective ToM using highly comparable conditions, and is unlikely to be affected by the poor verbal ability of autism participants.
ToM: the Faux Pas Task
To assess higher-order verbal-based ToM, we utilised the Chinese version (Zhu et al. Reference Zhu, Lee, Li, Jing, Wang and Wang2007) of the Faux Pas Task (Stone et al. Reference Stone, Baron-Cohen and Knight1998). It consists of 10 different stories; each involving two or three characters (a speaker and 1–2 listeners) in a hypothetical social encounter. Each story presents a social faux pas, in which a character unintentionally speaks something socially inappropriate (because of absence of knowledge regarding the situations of other characters in the story) that would have induced unpleasant emotions in another character in the story. Participants were required to recognise which character in the story committed a social faux pas, and to infer how the other character in the story who listened to the social faux pas would experience. The interviewer read out all the stories to the participants, who needed to answer five questions: (1) whether one of the characters has spoken inappropriately (thus committing a social faux pas); (2) which character in the story has committed a social faux pas; (3) explain why her/his words would constitute a social faux pas; (4) why he/she committed a social faux pas (i.e. the intention of the character); and (5) control questions to ascertain participants’ understanding of the stories. Participants scored one mark if they answered the questions correctly. The Faux Pas Task thus generated four scores, i.e. faux pas recognition, which is the sum of the number of correct answers to the first question, and faux pas understanding, which is the sum of the number of correct answers to the second question; faux pas inference of emotion, which is the sum of the number of correct answers to the third question, and faux pas inference of intention, which is the sum of the number of correct answers to the fourth question.
Statistical analysis
Statistical analyses were conducted using the Statistical Package for Social Science (SPSS) version 17.0 for Windows. The level of significance was set at p < 0.05 (two-tailed) unless otherwise specified. Normality of data was examined using the Kolmogorov–Smirnov test. The demographics and neurocognitive performance between participants with high-functioning autism, schizophrenia and controls were compared using analyses of variance (ANOVAs) and Chi-square (χ2) test, depending on data normality.
Given that two different paradigms were utilised to examine verbal-based and visual-based ToM, it was necessary to examine the level of task/item difficulty of the paradigms for all participants (Chapman & Chapman, Reference Chapman and Chapman1973). We combined the three groups of participants, and calculated the mean percentage of accuracy for items of the ToM paradigms. In addition, the percentage of accuracy for items of the ToM paradigms in the entire sample were entered into a mixed model ANOVAs (within-group variables: Aff1, Cog1, Aff2, Cog2, faux pas recognition, understanding, inference of emotion and inference of intention). The levels of task/item difficulty in the Yoni Task and the Faux Pas Task were compared using post-hoc comparisons with Bonferroni corrections.
The group difference in ToM performance was examined using multivariate and univariate ANOVAs with post-hoc comparisons and Bonferroni corrections. To examine whether schizophrenia participants and high-functioning autism participants have differential impairments of cognitive–affective ToM, the performance in the Yoni Task were entered into a mixed model of ANOVAs, with group as the between-subject factor and ToM condition as the within-subject factor.
Results
Task difficulty of the two paradigms
When the three groups were combined together, the participants’ mean percentage of accuracy for Aff1, Cog1, Aff2 and Cog2 of the Yoni Task were 93.1% (s.d. = 17.4%), 91.7% (s.d. = 21.5%), 80.5% (s.d. = 17.2%) and 78.0% (s.d. = 21.2%), respectively, and that for faux pas recognition, understanding, inference of emotion and inference of intention of the Faux Pas Task were 83.6% (s.d. = 17.0%), 79.6% (s.d. = 19.1%), 56.0% (s.d. = 32.3%) and 27.7% (s.d. = 24.3%), respectively.
The mixed model ANOVAs (within-group variables: Aff1, Cog1, Aff2, Cog2, faux pas recognition, understanding, inference of emotion and inference of intention) found that the items of ToM paradigms significantly differed in the overall task/item difficulty (F[7,83] = 107.030, p < 0.001, partial eta squared = 0.900). Post-hoc pairwise comparisons (for the within-group variables) found that the faux pas recognition was more difficult than Aff1 (p < 0.001, survived Bonferroni correction) and Cog1 (p = 0.002, survived Bonferroni correction), but comparable with Aff2 (p = 0.176) and Cog2 (p = 0.368, Bonferroni corrected) of the Yoni Task. Similarly, the faux pas understanding was more difficult than Aff1 (p < 0.001, survived Bonferroni correction) and Cog1 (p < 0.001, survived Bonferroni correction), but comparable with Aff2 (p = 0.651) and Cog2 (p = 0.513) of the Yoni Task. However, both the faux pas inference of emotion and the faux pas inference of intention were more difficult than all items of the Yoni Task (p < 0.001, survived Bonferroni corrections). Taken together, these results corroborated that first-order ToM (Aff1 and Cog1) have the lower level of task/item difficulty than higher-order ToM. Among items measuring higher-order ToM, Aff2 and Cog2 and the faux pas recognition and understanding have comparable levels of task/item difficulty. However, the faux pas inference of emotion and the faux pas inference of intention are more difficult than other items of the paradigms.
Performance on the Yoni Task
Table 3 and Fig. 1 show participants’ performance in the Yoni Task. As expected, the groups did not differ in the control (physical) conditions (Phy1: F[2,87] = 0.908, p = 0.407; Phy2: F[2,87] = 0.884, p = 0.417), suggesting that participants were able to understand and follow the instructions of the task. While the groups performed comparably well in the first-order conditions (Aff1: F[2,87] = 1.781, p = 0.175; Phy2: F[2,87] = 2.344, p = 0.102), there were significant group differences in the second-order affective (Aff2) (F[2,87] = 5.280, p = 0.007, survived Bonferroni correction) and cognitive (Cog2) conditions (F[2,87] = 5.467, p = 0.006, survived Bonferroni correction). Post-hoc comparisons found that the group differences were attributable to high-functioning autism participants’ poorer performance in Aff2 (p = 0.016) and Cog2 (p = 0.020) conditions, as well as schizophrenia participants’ poorer performance in Aff2 (p = 0.021) and Cog2 (p = 0.012) conditions, compared with controls. Notably, schizophrenia participants and high-functioning autism participants did not differ in Aff2 (p < 1.000) and Cog2 (p = 0.995) conditions. When Aff1, Aff2, Cog1 and Cog2 performances were entered into a mixed model ANOVA, with Group (schizophrenia, high-functioning autism, controls) as the between-subject variable, and ToM-order (first-order, second-order) and ToM-type (affective, cognitive) as the within-subject variables, the Group main effect was significant (F[2,87] = 4.514, p = 0.014, partial eta squared = 0.094). The ToM-order main effect (F[1,87] = 57.798, p < 0.001, partial eta squared = 0.399) and the ToM-type main effect (F[1,87] = 6.759, p = 0.011, partial eta squared = 0.072) both reached statistical significance. However, the Group-by-ToM-order interaction (F[2,87] = 1.173, p = 0.314, partial eta squared = 0.026), the Group-by-ToM-type interaction (F[2,87] = 1.936, p = 0.150, partial eta squared = 0.043), and the three-way interaction (F[2,87] = 0.221, p = 0.802, partial eta squared = 0.005) all failed to reach statistical significance.

Fig. 1. Mean scores of The Yoni Task (max 1.0; error bars represent standard errors). Aff1, first-order affective ToM condition; Aff2, second-order affective ToM condition; Cog1, first-order cognitive ToM condition; Cog2, second-order cognitive ToM condition; Phy1, control condition in the first-order ToM task; Phy2, control condition in the second-order ToM task.
Table 3. Participants’ performances in the Yoni Task

Aff1, First degree affective theory of mind; Aff2; Second degree affective theory of mind; Cog1, First degree cognitive theory of mind; Cog2, Second degree cognitive theory of mind; Phy1, control condition in first degree task; Phy2, control condition in second degree task. p-values in bold are statistically significant at 0.05.
Performance on the Faux Pas Task
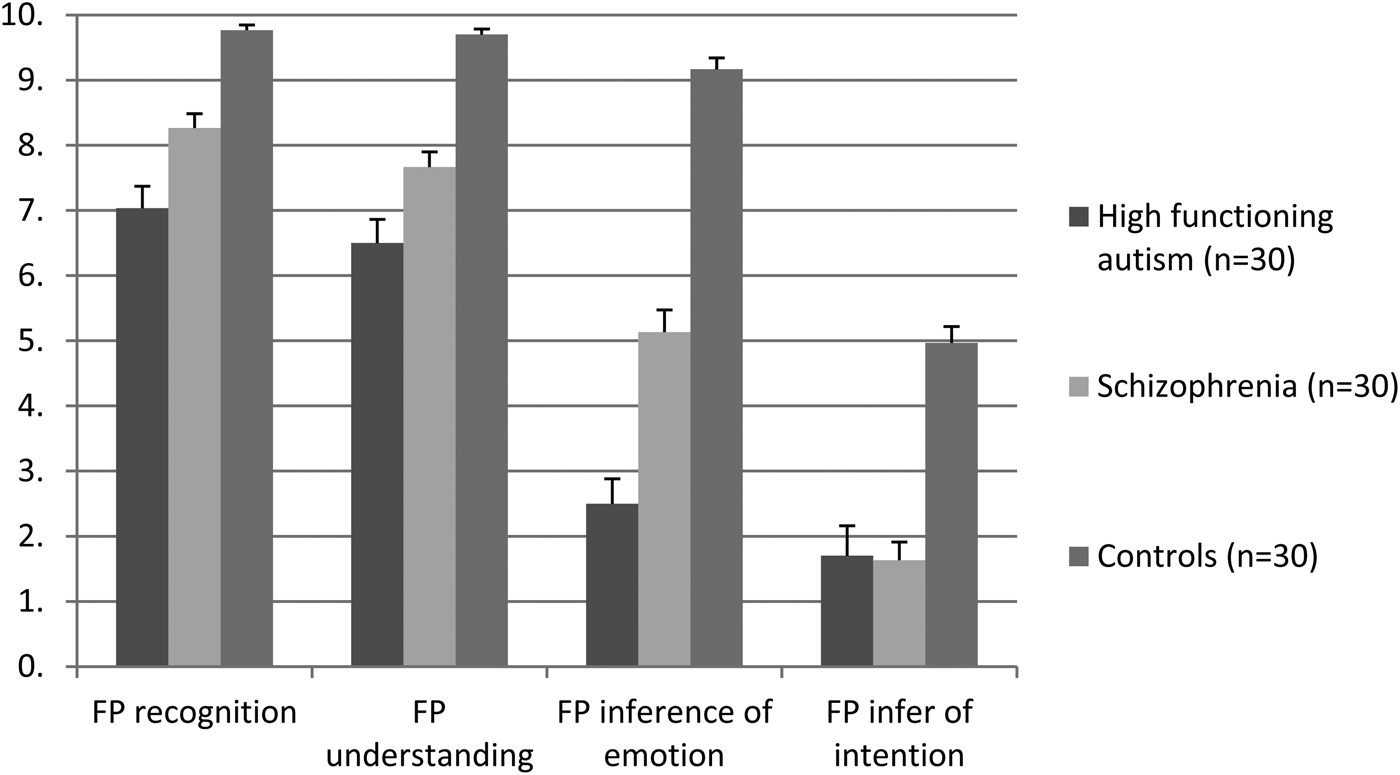
Table 4 and Fig. 2 show participants’ performance in the Faux Pas Task. While all participants scored full marks in the control questions, the group difference was statistically significant in faux pas recognition (F[2,87] = 33.450, p < 0.001, survived Bonferroni correction), understanding (F[2,87] = 40.631, p < 0.001, survived Bonferroni correction), inference of emotion (F[2,87] = 117.174, p < 0.001, survived Bonferroni correction) and inference of intention (F[2,87] = 108.933, p < 0.001, survived Bonferroni correction). Post-hoc comparison found that high-functioning autism participants exhibited the worse ToM in both faux pas recognition, understanding, inference of emotion and inference of intention, and schizophrenia participants exhibited milder impairments in ToM in terms of faux pas recognition, understanding and inference of emotion. Both clinical groups performed poorer than controls in the Faux Pas Task. High-functioning autism participants and schizophrenia participants performed similarly poorly in faux pas inference of intention. When ToM performances in the four faux pas variables were entered into a mixed model ANOVA, with Group as the between-subject variables (schizophrenia, autism, controls) and Faux Pas Condition as the within-subject variable (recognition, understanding, inference of emotion, inference of intention), the Group main effect was statistically significant (F[2,87] = 91.871, p < 0.001, partial eta squared = 0.679), so was the Faux Pas Condition main effect (F[3,85] = 284.797, p < 0.001, partial eta squared = 0.910). The Group-by-Faux Pas Condition interaction was also significant (F[6261] = 18.852, p < 0.001, partial eta squared = 0.302).

Fig. 2. Mean scores of the four items of the Faux Pas Task (max 10; error bars represent standard errors). FP, Faux Pas.
Table 4. Participants’ performance in the Faux Pas Task

FP, the Faux Pas Task. p-values in bold are statistically significant at 0.05.
Discussion
The present study showed that high-functioning autism patients shared similar extent of visual ToM impairments with schizophrenia patients, consistent with earlier meta-analytic findings (Chung et al. Reference Chung, Barch and Strube2014). In the verbal-based Faux Pas Task, high-functioning autism patients showed greater magnitude of ToM impairments than schizophrenia patients, contrary to earlier meta-analytic findings (Chung et al. Reference Chung, Barch and Strube2014). Importantly, autism and schizophrenia appeared to be similarly impaired in affective and cognitive ToM. Taken together, these findings support the notion of an autism–schizophrenia continuum, by demonstrating the existence of ToM impairments across the two phenotypes. Historically, autism is considered part of the schizophrenia spectrum; and autistic thinking, lack of reciprocity and mutism are regarded as symptoms of schizophrenia. Diagnostic classification systems and symptoms clustering studies (Kolvin, Reference Kolvin1971; Kolvin et al. Reference Kolvin, Ounsted, Humphrey and McNay1971) succeeded in separating the two neurodevelopmental disorders. Nevertheless this phenotypic dichotomy is not fully supported by neuropsychological and genetic studies (Sasson et al. Reference Sasson, Pinkham, Carpenter and Belger2011). Our findings contribute further evidence to support that autism and schizophrenia exhibit shared social cognitive impairments, implicating shared neuropathology of the ‘mentalising network’ (Völlm et al. Reference Völlm, Taylor, Richardson, Corcoran, Stirling, McKie, Deakin and Elliott2006) for the two neurodevelopmental disorders.
Despite our refined paradigm which attempted to differentiate the different extent of affective v. cognitive ToM impairments between autism and schizophrenia, the two diagnostic groups showed comparable impairments in the two-facetted model of ToM, comparable with earlier meta-analytic findings (Chung et al. Reference Chung, Barch and Strube2014). On the other hand, the ability to infer emotions of other people is more severely impaired in high-functioning autism patients than schizophrenia patients, even though the two clinical groups showed comparable impairments in the ability to infer intentions of other people. This finding generally concurs with the notion that misperception of others’ intention is related to delusions in schizophrenia, while the inability to infer emotions of other people is related to autistic features such as social isolation and lack of reciprocity. Our findings that autism patients and schizophrenia patients shared similar impairments in visual-based ToM are generally consistent with previous studies, which directly compared the mentalising ability of the two disorders. For instance, our findings of shared visual ToM impairments concur with earlier studies (Craig et al. Reference Craig, Hatton, Craig and Bentall2004; Murphy, Reference Murphy2006; Couture et al. Reference Couture, Penn, Losh, Adolphs, Hurley and Piven2010), which utilised the Eyes Test, a non-verbal ToM paradigm. Our findings on the Faux Pas Task also resemble a previous study (Craig et al. Reference Craig, Hatton, Craig and Bentall2004), which utilised the Hints Test, which assesses the ability to infer the embedded intention of the speaker in the stories, and found high-functioning autism and schizophrenia patients to have similar magnitude of impairments in the ability to infer other's intentions.
The magnitude of visual ToM impairments appeared to be considerably less than the magnitude of verbal ToM impairment in both disorders. Moreover, when the two disorders were compared directly, autism patients performed poorer than schizophrenia patients in verbal but not visual ToM. However, our findings do not necessarily indicate that autism patients, when compared to schizophrenia patients, have differential impairments in verbal rather than visual ToM. Chapman & Chapman (Reference Chapman and Chapman1973) had previously illustrated that it is incorrect to conclude the existence of differential impairments based only on participants’ performance in two different paradigms. It is necessary to take into account the level of task difficulty of paradigms, i.e. whether they could easily discriminate the pathological individuals from the healthy individuals. Differential impairments can only be firmly concluded when the following conditions are satisfied: (1) the sample shows greater impairments in one type of ability than another; and (2) the paradigms measuring the two abilities of interest have comparable levels of task difficulty (Chapman & Chapman, Reference Chapman and Chapman1973). Our findings suggest that the levels of task/item difficulty in faux pas inference of emotion and inference of intention are substantially greater than those in the other two items of the Faux Pas Task and the Yoni Task. On the other hand, the task/item difficulty between faux pas recognition and understanding of the Faux Pas Task, and Aff2 and Cog2 of the Yoni Task were comparable. To elucidate whether autism patients, compared with schizophrenia patients, show differential impairments in verbal rather than visual ToM, further research should match the discriminative power of verbal-based and visual-based ToM paradigms.
The Yoni Task only requires basic ToM skills such as eye gaze processing and facial emotion recognition, which typically developing children are able to master at the age of 4 (Baron-Cohen, Reference Baron-Cohen2001). Moreover, the Yoni Task mainly utilises simplified cartoon faces with eyes and mouths only. While the eye-gaze direction and mouth shape of the characters are relatively simple for making emotional inferences, they are very different from real-life social situations when facial emotion expressions are often subtle and ambiguous. In contrast, the stories in the Faux Pas Task describe social situations simulating real-life encounters, and may be more ecologically valid.
Compared with previous studies, our study has several important advantages. First, our study but not others (Murphy, Reference Murphy2006; Couture et al. Reference Couture, Penn, Losh, Adolphs, Hurley and Piven2010; Lugnegård et al. Reference Lugnegård, Unenge Hallerbäck, Hjärthag and Gillberg2013) had matched participants’ age and estimated IQ, which might be confounders of ToM performance (Sasson et al. Reference Sasson, Pinkham, Carpenter and Belger2011). In this study, the diagnoses of ASD and schizophrenia were stringently verified using standardised diagnostic methods (Volkmar et al. Reference Volkmar, Siegel, Woodbury-Smith, King, McCracken and State2014). Furthermore, we attempted to minimise the effect of poor verbal skills on ToM performance in both schizophrenia and autism patients, using a visual-based paradigm. Importantly, this study utilised a unique sample of adolescent patients, contrary to previous studies which recruited childhood (Pilowsky et al. Reference Pilowsky, Yirmiya, Arbelle and Mozes2000) and adult (Craig et al. Reference Craig, Hatton, Craig and Bentall2004; Bowler, Reference Bowler1992; Murphy, Reference Murphy2006; Ozguven et al. Reference Ozguven, Oner, Baskak, Oktem, Olmez and Munir2010; Couture et al. Reference Couture, Penn, Losh, Adolphs, Hurley and Piven2010; Lugnegård et al. Reference Lugnegård, Unenge Hallerbäck, Hjärthag and Gillberg2013) patients with autism and schizophrenia. During adolescence, the social brain demonstrates structural and functional developments (Blakemore, Reference Blakemore2008; Burnett et al. Reference Burnett, Sebastian, Cohen Kadosh and Blakemore2011). Our findings represent the social cognitive deficits in this critical period among patients with the two neurodevelopmental disorders, relatively unaffected by long-term medication and disease chronicity. Lastly, we attempted to differentiate affective and cognitive ToM in autism and schizophrenia patients, a topic seldom studied in previous studies.
However, several limitations of this study must be considered. First, although our sample was larger than previous studies (Bowler, Reference Bowler1992; Pilowsky et al. Reference Pilowsky, Yirmiya, Arbelle and Mozes2000; Craig et al. Reference Craig, Hatton, Craig and Bentall2004; Murphy, Reference Murphy2006; Ozguven et al. Reference Ozguven, Oner, Baskak, Oktem, Olmez and Munir2010), we were still unable to detect a significant Group-by-ToM-type interaction effect in the Yoni Task, and Type II error could be a concern. Secondly, all our schizophrenia participants and a minority of our high-functioning autism participants were medicated at the time of assessments. Therefore, medication effect might have affected ToM performance. Importantly, schizophrenia is a heterogeneous disorder, and this study did not distinguish between the different subtypes of schizophrenia, although previous evidence (Couture et al. Reference Couture, Penn, Losh, Adolphs, Hurley and Piven2010) suggests that the paranoid subtype and the hebephrenic subtype of schizophrenia could have different ToM profiling. Although the Yoni Task has the advantage of capturing and differentiating the affective and cognitive facets of ToM, this visual-based paradigm may be less effective in eliciting subtle impairments of mentalising ability than other more complicated visual-based paradigm, such as the animated task used in a recent study (Lugnegård et al. Reference Lugnegård, Unenge Hallerbäck, Hjärthag and Gillberg2013). In addition, the Faux Pas Task does not tap into the two-facetted model of ToM. Importantly, the verbal-based and visual-based paradigms utilised in this study have different levels of task/item difficulty. Although our findings of shared social cognitive impairments apparently implicate shared neuropathology of the mentalising network (Völlm et al. Reference Völlm, Taylor, Richardson, Corcoran, Stirling, McKie, Deakin and Elliott2006), more evidence from functional neuroimaging studies is needed. Lastly, future studies should address several inherent problems of ToM studies directly comparing autism patients with schizophrenia patients, i.e. (1) the difference in gender ratio between autism and schizophrenia, and (2) the gender difference in ToM ability in both healthy and clinical populations. In fact, Lugnegård et al. (Reference Lugnegård, Unenge Hallerbäck, Hjärthag and Gillberg2013) found a higher degree of similarities in ToM between male autism patients and schizophrenia patients than between female autism patients and schizophrenia patients. Replication of our findings in larger samples using stratification-by-gender analysis is needed.
To conclude, this study is one of the few studies that directly compares ToM impairments in schizophrenia patients with high-functioning autism patients. Our findings suggest that high-functioning autism shared similar but more severe ToM impairments than schizophrenia patients, and support the notion of the schizophrenia–autism continuum.
Acknowledgements
This study was supported by a donation from the Philip K. H. Wong Foundation granted to Eric F. C. Cheung of Castle Peak Hospital. Raymond Chan was supported by the Beijing Municipal Science & Technology Commission Grant (no. Z161100000216138), and the Beijing Training Project for the Leading Talents in S & T (Grant no. Z151100000315020). Yi Wang was supported by a National Science Fund China (Grant no. 31400884). The funding agents had no role in the study design; collection, analysis and interpretation of the data; writing of the manuscript; or decision to submit the paper for publication.
Declaration of Interest
None.